SUMMARY
The objective was to engineer an inexpensive intraoral removable denture system for rodents that can be utilized in numerous oral health research applications. At the forefront is biofilm research related to Candida-associated denture stomatitis. Previously described intraoral devices are primitive and inadequate. The denture system was engineered consisting of a fixed part that is anchored to the posterior palate by orthodontic wires and acrylic resin, and a removable part fitted to the anterior palate that is retained by magnets embedded in the fixed part. Both parts are custom-fitted to the rodent palate by impression making and cast fabrication.
Rats fitted with the intraoral denture system maintained body weight and normal activity with the device maintaining integrity and durability for upwards of 8 weeks. The denture system was used successfully to establish a working model of denture stomatitis. This newly engineered inexpensive intraoral removable denture system for rodents can be utilized in numerous oral health research applications, including denture-associated infections, biofilms, and a variety of biomaterial applications. The removable portion is advantageous for longitudinal analyses and charging/discharging of biomaterials.
Keywords: denture, oral biofilm, animal models, denture stomatitis, Candida albicans
INTRODUCTION
In vivo studies of denture-associated biofilms require some type of intraoral device in an animal model. Such devices in animal models have been reported in the literature (1–5) but with substantive limitations, mostly relating to the ability to conduct longitudinal analyses effectively and a custom fit of the device. Furthermore, existing devices have not been exploited for studies related to biofilms. Biofilms are communities of microbial cells that are irreversibly attached to a substratum or interface or to each other, and are embedded in a self-made matrix of extracellular polymeric substances (extracellular matrix) (6). In the oral cavity, biofilms are suggested to play a major role in many infectious diseases, including dental caries (7), periodontitis (8), and prosthesis-related infection (denture stomatitis) (9). Denture stomatitis is a common oral mucosal disease primarily caused by the fungal organism, Candida albicans. Prevalence is reported from 27 to 50% of denture-wearers depending on the population surveyed (10–12). Because biofilms contribute to persistent infections through significant antimicrobial resistance (13), the study of Candida biofilms could yield significant improvement in prevention and treatment of denture stomatitis.
An animal model using an intraoral device is suitable and advantageous for the study of Candida biofilms in denture stomatitis. Important questions include the role of the biofilm in pathogenesis and host responses, as well as the presence and distribution between the abiotic denture surface and the biotic mucosal tissue. Some specific requirements of the intraoral device for the Candida biofilm study are as follows: 1) should be retained in the oral cavity long enough for the biofilm to form and affect the oral mucosa, 2) should be removable so that longitudinal observations can be performed, 3) should be biostable, 4) should not interfere animal’s daily activities such as eating and drinking, 5) should be easy to fabricate at a reasonable cost, and 6) should be custom fitted to the palate to ensure adequate contact and without altering the rat dental architecture. The existing models incorporating intraoral devices are outdated and do not meet these requirements. Hence we sought to engineer an innovative fixed and removable intraoral device for a rodent (rat) that meets all these criteria. The hypothesis is that an improved intraoral device will be more efficient and effective for numerous oral health research applications, including denture-associated infections, biofilms, and a variety of biomaterial applications.
MATERIALS AND METHODS
Rats
Male Wistar retired breeder rats weighing 400 to 600 g were purchased from Charles River Laboratories at the National Cancer Institute (NCI), Frederick, Md were used. All animals were housed and handled according to institutionally recommended guidelines. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the LSU Health Sciences Center. Experiments were carried out in accordance with the guidelines specified by the National Institutes of Health (NIH) regarding the care and use of animals for experimental procedures.
Fabrication of the intraoral device
The procedure employed to fabricate a custom-fitted denture system for the rat palate was as follows (patent pending). Under anesthesia (ketamine) the rat was placed in a custom-made dental unit with adjustable loops to restrain the mouth open. An impression was made using light-body VPS impression material (Aquasil Ultra LV; Dentsply Caulk, Milford, Del) directly on the palate and all maxillary cheek teeth using a manual gun dispenser (Cartridge Dispensing Gun; Dentsply Caulk), with an automix and intraoral tip (Mix Tips; Dentsply Caulk) (Fig. 1A). The impression was poured in type III dental stone (Quickstone; Whip Mix Corp, Louisville, Ky) to make a cast. The fixed part was made to fit in the posterior portion of the palate, while the removable part was made to fit the anterior portion. On the stone cast, the fixed part was made using autopolymerizing acrylic resin (Jet Tooth Shade Acrylic; Lang Dental, Wheeling, Ill). Caution was exercised not to cover the occlusal surfaces of the cheek teeth. Two magnets (diameter 1/8 inch, thickness 1/16 inch; K&J Magnetics, Inc, Jamison, Pa) were embedded in the fixed part of the device (Fig. 1B). In the removable part a stainless steel strip (3 × 12 mm) was embedded so that it would extend to the magnets in the fixed part (Fig. 1C) when together as a single unit. Orthodontic ligature wire (Ligature Wire, 0.25 mm diameter; Masel, Bristol, Pa) was placed on both right and left interproximal spaces between the first and second cheek tooth (Fig. 2) to provide scaffolding. The fixed part was then positioned on the posterior palate in the anesthetized rat and secured with a mixture of the autopolymerizing acrylic resin covering the occlusal surfaces. The removable part was then positioned on the anterior palate via the attachment to the magnets on the fixed part (Fig. 3).
Figure 1.
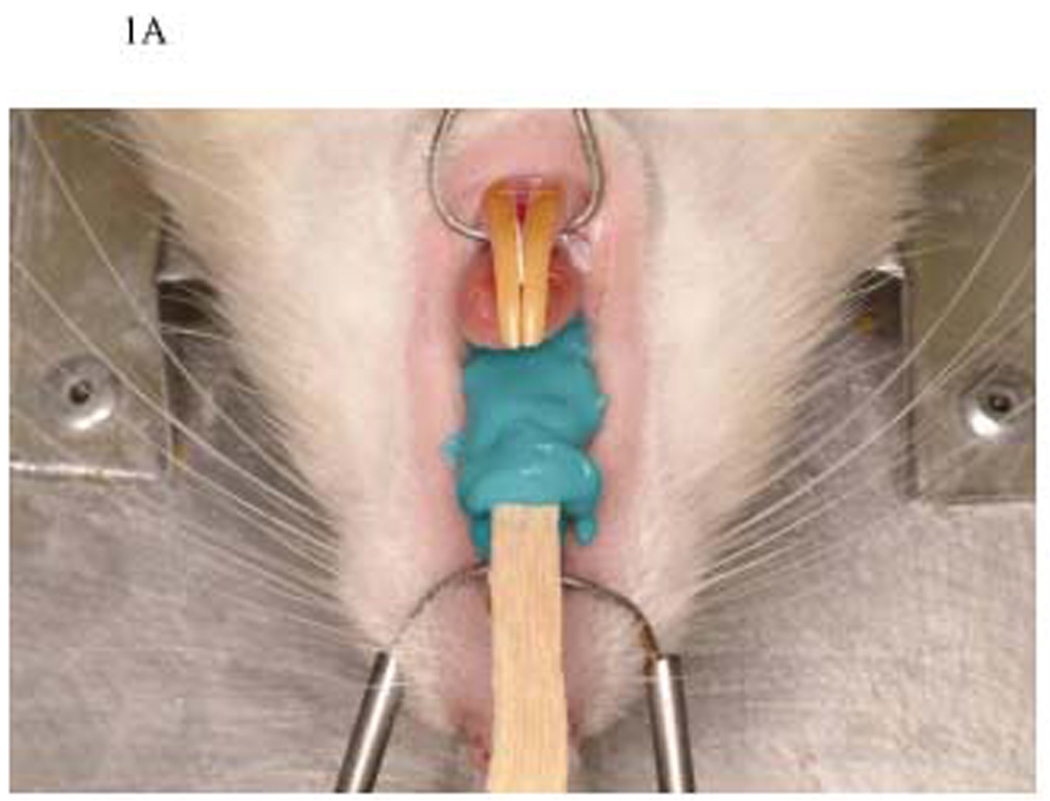
Figure 1A. Taking an impression. Impression of the maxillary arch was done using vinyl polysiloxane impression material and wooden tongue depressor.
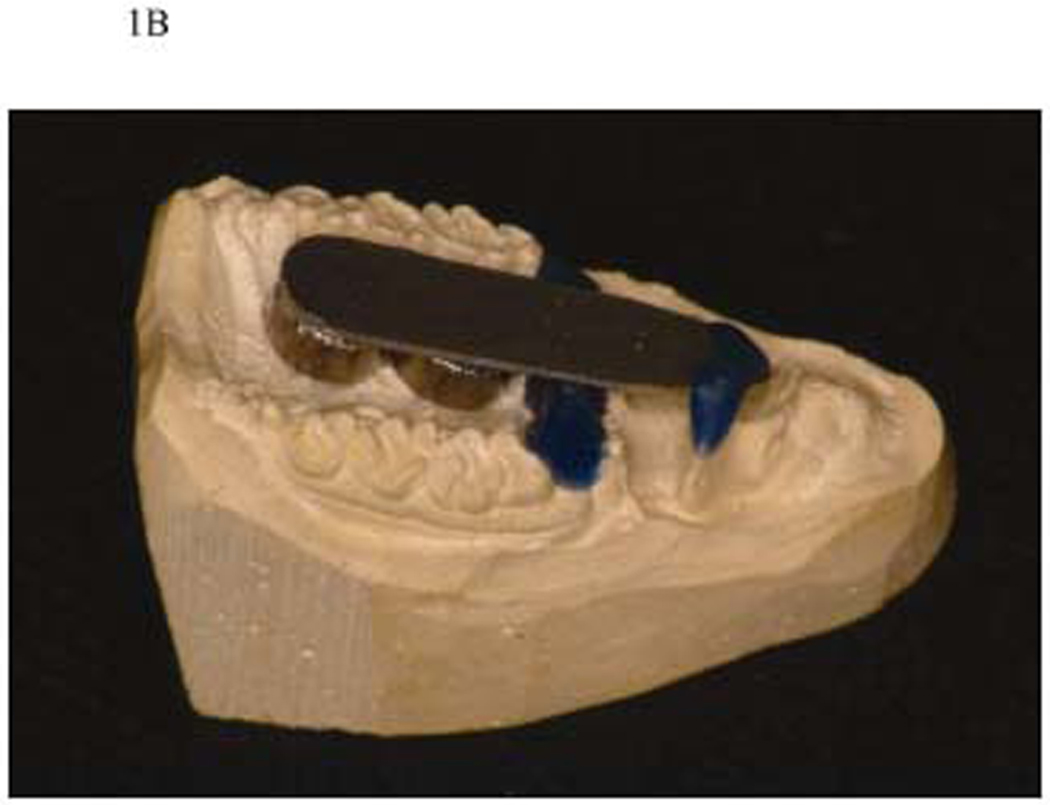
Figure 1B. Creating a stone cast. A stone cast was made with two magnets embedded and a metal bar placed and secured using wax.
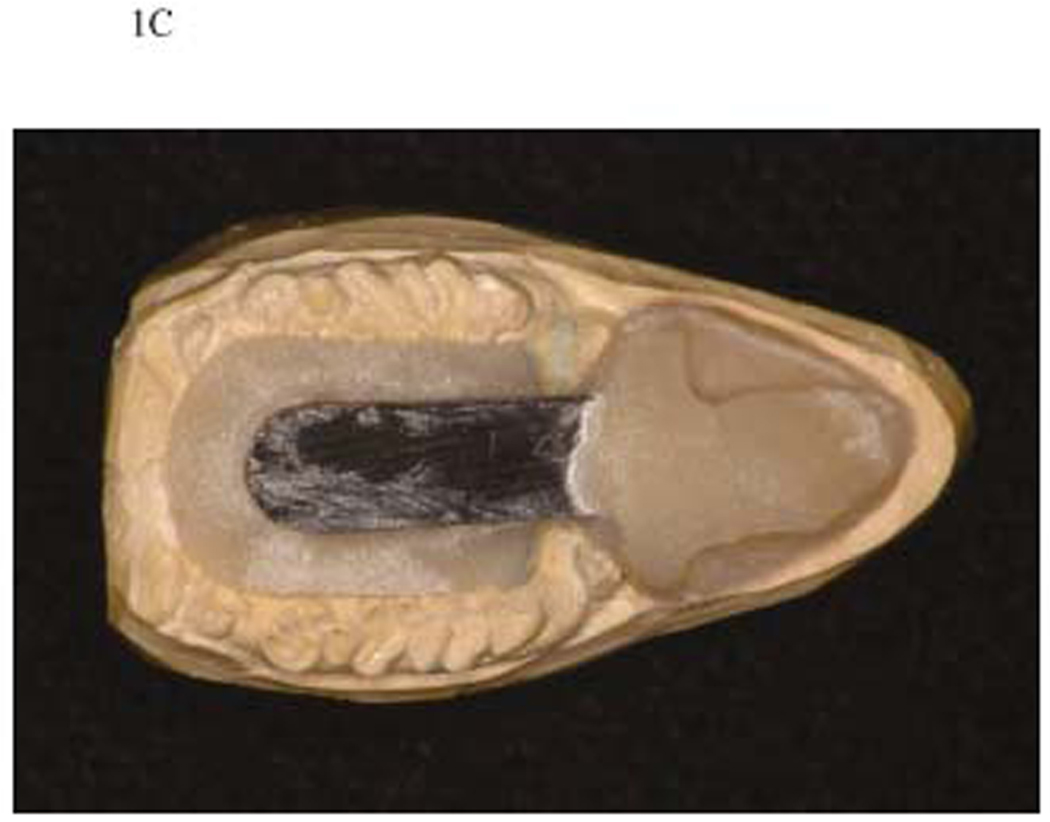
Figure 1C. The finished device on cast. The finished cast with the fixed portion devoid of covering the occlusal surface of cheek teeth.
Figure 2.
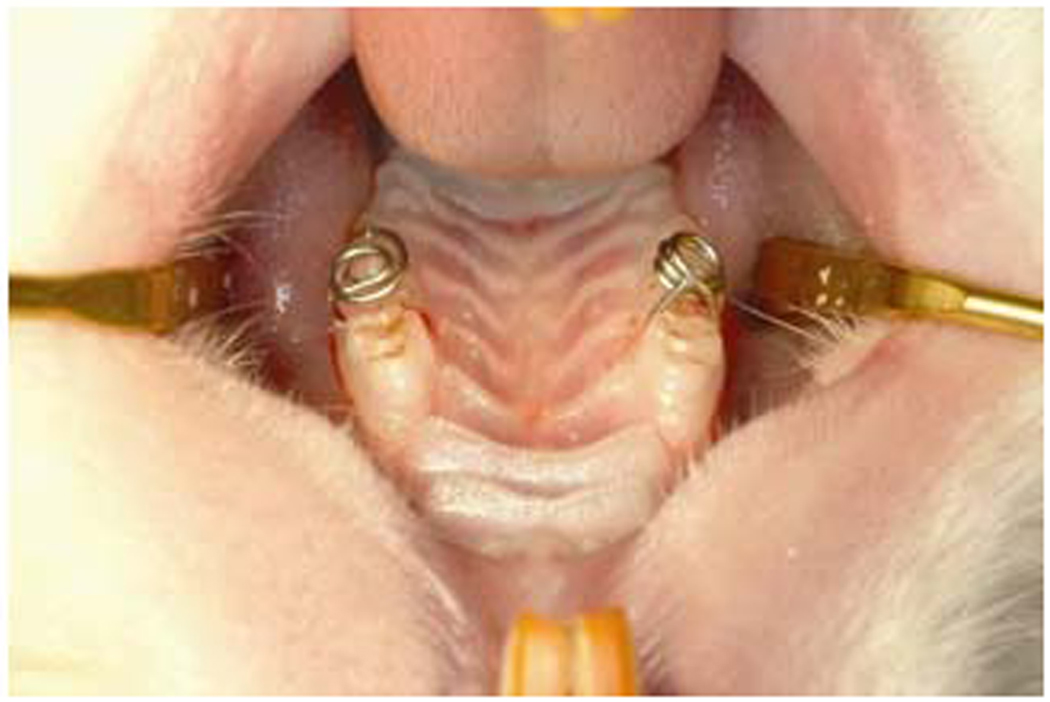
Orthodontic ligature wires used for scaffold support. Placement of orthodontic ligature wire on the right and left interproximal spaces between the first and second cheek tooth
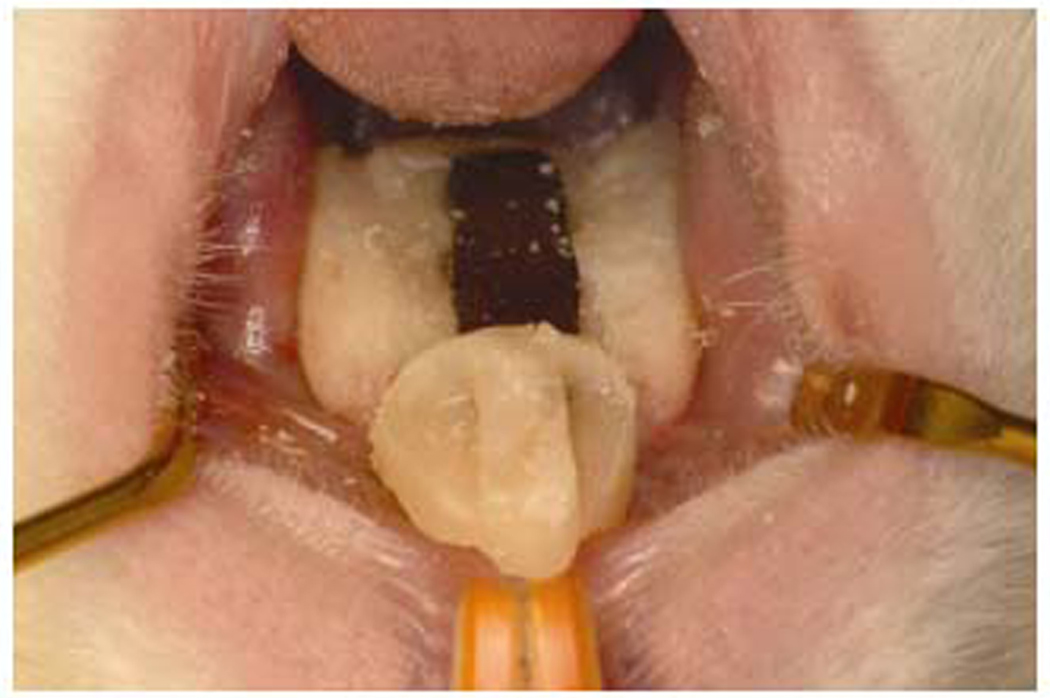
Figure 3.
Installed removable intraoral device. The complete device shown as installed on the rat palate
RESULTS
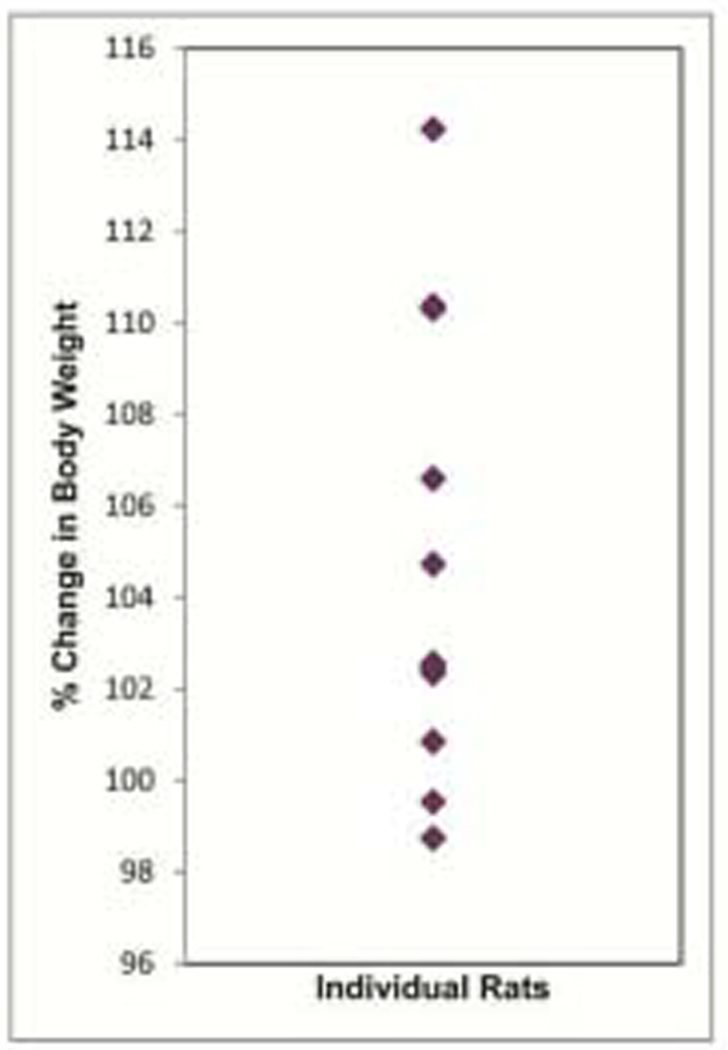
The rats with the intraoral device were monitored for normal activity and feeding over a 4 week period. The rats all maintained body weight with most increasing over time (Fig. 4). The rats were ultimately placed on a gel diet to avoid food debris gathering under the denture system, which interfered with the ability to adequately evaluate biofilm on the denture. Again body weight was maintained or increased. The denture system maintained its integrity and durability for upwards of 8 weeks. If any fixed part became loose it could be secured with additional resin. Hence, the intraoral denture system did not compromise the animal’s normal activity.
Figure 4.
Monitoring of rat activity. Change in body weight of rats with removable intraoral device over a 4 week period.
DISCUSSION
A literature search revealed only one other removable device for the rat that combined a fixed and removable part into a single device (4). The device consisted of a cast metal framework that was permanently cemented on the cheek teeth and an acrylic base that was retained by orthodontic tube and wire. In the current device, a simpler design and manufacturing process was applied in which the removable part was retained by 2 magnets that were permanently located in the middle of fixed part, and secured by acrylic resin and wire. The use of magnets was advantageous in that it made the entire design small, simple, and inexpensive. Also, the removable part could be removed and replaced with ease, not requiring any special tool for handling. A limitation, however, is that a large area of the palate was required for the fixed part of the device, which resulted in a reduced area for observation of the interaction between the removable part and the oral mucosa. Corrosion of the magnets was also a concern, but coating the surfaces of the magnets using a gypsum hardener (Surface Hardener; Renfert GmbH, Hilzingen, Germany) prevented any such corrosion.
The new intraoral denture system was subsequently used to initiate a study of denture stomatitis. To this end, a contemporary rat model of denture stomatitis was established. Following the inoculation of C. albicans applied as a cell pellet paste to the removable device, weekly oral swabs showed consistent colonization of C. albicans on the removable device and associated palatal tissue over an 8 week period. Significant biofilm formation was detected on the removable device at 4 weeks post-inoculation and on the associated palate at 6 weeks post-inoculation. Clinical disease Stage 1 of denture stomatitis (pin-point erythema) was detected on the palate at 6 weeks post-inoculation and Stage 2 (diffuse erythema) and 3 (hyperplasia) (14) at 8 weeks post-inoculation (Fidel and Noverr, manuscript in preparation). These results support the hypothesis that the improved intraoral device provided an effective and efficient means to conduct biofilm research related to Candida-associated denture stomatitis. Additional studies on biofilm formation and pathogenesis are in progress.
This represents just one of several applications for such an intraoral denture system for research purposes. Others include testing of antimicrobial-impregnated denture materials, drug-releasing delivery systems, pathogenesis of other denture-associated oral diseases, and polymicrobial biofilms related to dentures and oral health. With these multi-faceted uses of the intraoral denture system significant advances in oral health research can be achieved with an avenue to major discoveries to treat/cure/understand oral infectious disease and biofilms, as well as new applications for oral biomaterials.
In conclusion, an inexpensive intraoral removable denture system for rodents has been engineered that can be utilized in numerous oral health research applications, including denture-associated infections, biofilms, and a variety of biomaterial applications.
ACKNOWLEDGEMENTS
This work was supported by NIH NCRR CoBRE grant ARRA supplement (P20RR20160-S1) and in part by the South Louisiana Institute for Infectious Disease Research and the Louisiana Vaccine Center sponsored by the Louisiana Board of Regents.
REFERENCES
- 1.Barclay SC, MacDonald DG, Watson IB. The effect of diet on palatal prosthetic coverage in rats. J Dent. 1997;25:71–78. doi: 10.1016/0300-5712(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 2.Barclay SC, MacDonald DG, Watson IB. The effect of chairside relining materials on rat palatal mucosa. J Dent. 1997;25:251–255. doi: 10.1016/s0300-5712(96)00005-x. [DOI] [PubMed] [Google Scholar]
- 3.Imai Y, Sato T, Mori S, Okamoto M. A histomorphometric analysis on bone dynamics in denture supporting tissue under continuous pressure. J Oral Rehabil. 2002;29:72–79. doi: 10.1046/j.1365-2842.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- 4.Mori S, Sato T, Hara T, Nakashima K, Minagi S. Effect of continuous pressure on histopathological changes in denture-supporting tissues. J Oral Rehabil. 1997;24:37–46. doi: 10.1046/j.1365-2842.1997.00443.x. [DOI] [PubMed] [Google Scholar]
- 5.Tsuruoka M, Ishizaki K, Sakurai K, Matsuzaka K, Inoue T. Morphological and molecular changes in denture-supporting tissues under persistent mechanical stress in rats. J Oral Rehabil. 2008;35:889–897. doi: 10.1111/j.1365-2842.2008.01883.x. [DOI] [PubMed] [Google Scholar]
- 6.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fejerskov O. Changing paradigms in concepts on dental caries: Consequences for oral health care. Caries Res. 2004;38:182–191. doi: 10.1159/000077753. [DOI] [PubMed] [Google Scholar]
- 8.Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Periodontitis: An archetypical biofilm disease. J Am Dent Assoc. 2009;140:978–986. doi: 10.14219/jada.archive.2009.0307. [DOI] [PubMed] [Google Scholar]
- 9.Ramage G, Tomsett K, Wickes BL, López-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Pires FR, Santos EB, Bonan PR, De Almeida OP, Lopes MA. Denture stomatitis and salivary Candida in Brazilian edentulous patients. J Oral Rehabil. 2002;29:1115–1119. doi: 10.1046/j.1365-2842.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- 11.Shulman JD, Rivera-Hidalgo F, Beach MM. Risk factors associated with denture stomatitis in the United States. J Oral Pathol Med. 2005;34:340–346. doi: 10.1111/j.1600-0714.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Zissis A, Yannikakis S, Harrison A. Comparison of denture stomatitis prevalence in two population groups. Int J Prosthodont. 2006;19:621–625. [PubMed] [Google Scholar]
- 13.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 14.Newton AV. Denture sore mouth: a possible aetiology. Br Dent J. 1962;112:357–360. [Google Scholar]