Abstract
Objective
Obesity is related to increased risk of several health complications, including depression. Many studies have reported improvements in mood with weight loss, but results have been equivocal. The present meta-analysis examined changes in symptoms of depression that were reported in trials of weight loss interventions. Between-groups comparisons of different weight loss methods (e.g., lifestyle modification, diet alone, pharmacotherapy) were examined, as were within-group changes for each treatment type.
Method
MEDLINE was searched for articles published between 1950 and January 2009. Several obesity-related terms were intersected with terms related to depression. Results were filtered to return only studies of human subjects, published in English.
Of 5971 articles, 394 were randomized controlled trials. Articles were excluded if they did not report mean changes in weight or symptoms of depression, included children or persons with psychiatric disorders (other than depression), or provided insufficient data for analysis. Thirty-one studies (n = 7937) were included. Two authors independently extracted a description of each study treatment, sample characteristics, assessment methods, and changes in weight and symptoms of depression. Treatments were categorized as: lifestyle modification, non-dieting, dietary counseling, diet-alone, exercise-alone, pharmacotherapy, placebo, or control interventions.
Results
Random effects models found that lifestyle modification was superior to control and non-dieting interventions for reducing symptoms of depression, and marginally better than dietary counseling and exercise-alone programs. Exercise-alone programs were superior to controls. No differences were found for comparisons of pharmacologic agents and placebos. Within-group analyses found significant reductions in symptoms of depression for nearly all active interventions. A meta-regression found no relationship between changes in weight and changes in symptoms of depression in lifestyle modification interventions.
Conclusions
On average, obese individuals in weight loss trials experienced reductions in symptoms of depression. Future studies should examine incidence and resolution of clinically significant depressive disorders with weight loss interventions.
Keywords: Depression, Meta-Analysis, Diet, Exercise, Lifestyle, Pharmacotherapy, Obesity
Introduction
Over the past two decades, obesity has emerged as a serious public health concern. Approximately one-third of American adults are now obese, defined by a body mass index (BMI) ≥ 30 kg/m2 (1). Obesity is an independent risk factor for diabetes, cardiovascular disease, and several cancers (2), leading to significantly increased mortality rates (3).
Depression is another health complication that is commonly associated with obesity. Odds of ever having met criteria for major depressive disorder are 20–50% higher among obese individuals than normal weight persons (4–6). Extremely obese persons are at even greater risk (5). The relationship between obesity and depression appears to be bi-directional; some longitudinal studies have shown that depression is associated with subsequent weight gain and obesity (7–12), whereas others have found that obesity is associated with the development of depression (13–14). Obese women are particularly vulnerable to depression (15–17).
Behavioral interventions for obesity induce mean weight losses of 5% to 10% of initial weight (18). Despite patients’ remaining obese, these modest reductions result in significant improvements in metabolic risk factors for cardiovascular disease (19–22). Patients also experience improvements in body image (23–24), physical functioning (25–26), and quality of life (27–28). Moreover, intentional weight loss is often accompanied by improvements in mood (29). These favorable psychological findings stand in sharp contrast to early reports that linked dieting and weight loss to depression (30–31).
Weight loss medications may present an exception to the generally favorable changes in mood associated with weight loss. Rimonabant, a cannabinoid-1 receptor (CB1R) inverse agonist, was removed from the market in Europe in 2008 after it was found to be associated with unexpectedly high rates of serious adverse psychiatric effects that included depression, anxiety, and suicidal ideation and behavior (32–33). The U.S. Food and Drug Administration now requires that all new weight loss medications be routinely evaluated for their risk of depression, suicidal ideation, and related complications (34).
The present meta-analysis sought to examine whether different types of weight loss interventions (including lifestyle modification, non-dieting, dietary counseling, exercise-alone, and pharmacotherapy) are associated with differential changes in symptoms of depression among obese individuals. We also assessed, through meta-regression, the relationship between weight loss and changes in symptoms of depression. We are careful not to use the term “depression” per se because most weight loss studies have excluded persons with major depressive disorder or failed to use a validated diagnostic interview to assess for the presence of clinically significant depression.
Method
Data Sources
We searched the Ovid MEDLINE database for studies published between 1950 and January 2009. The search strategy was to intersect several terms related to obesity and weight change (obese, obesity, body mass index, adipose, adiposity, overweight, weight loss, weight gain, weight change, weight reduction, weight increase) with terms related to depression (mood, depressed, depression, depressive, suicide, suicidal). Results were filtered to return only studies of human subjects, published in English. Additionally, we reviewed studies of intentional weight loss interventions that we knew reported changes in symptoms of depression, despite their not being identified by the search strategy.
Study Selection
The MEDLINE search returned 8799 publications, of which 5971 were published in English and included human subjects. Only randomized controlled trials (n = 394) were considered for inclusion. In addition, weight and symptoms of depression must have been assessed at baseline and post-treatment. Trials were excluded if they: included only persons with binge eating disorder; included children or adolescents; reported weight change with antidepressant or other psychotropic therapies; or manipulated individual macro- or micronutrients without weight loss as an intended outcome. These criteria yielded 39 studies for potential inclusion.
Data Extraction
Each included article was reviewed by two authors (ANF and AH) to extract the duration of treatment, the method of assessing symptoms of depression, and a description of each study intervention (see Table 1). An intervention was coded as lifestyle modification if its description included: mention of counseling related to diet and exercise; mention of diet and exercise prescriptions plus behavioral interventions; or use of a known lifestyle modification intervention (e.g., the LEARN manual) (35). We defined non-dieting interventions as those that were described as such, or that focused on health and self-acceptance rather than weight loss. We coded interventions that included counseling and instruction to achieve a reduction in calorie intake, without increasing energy expenditure, as dietary counseling. Interventions that manipulated energy level and content of participants’ diets, but included no counseling, were coded as diet-alone interventions. Exercise-alone programs were defined as interventions in which increased physical activity was prescribed or supervised in the absence of instructions to reduce calorie intake. Pharmacologic interventions were those that included administration of orlistat, sibutramine, or rimonabant without an accompanying lifestyle modification program. Nearly all pharmacotherapy studies included a placebo group. Control groups included persons who were placed on a waiting list for one of the above active treatments, or who received only standard advice/printed materials, an attention-control intervention, or no treatment. (No studies of bariatric surgery met inclusion criteria.)
Table 1.
Summary of studies and treatment groups included in analyses.
| Study | Sample | Depression Measure |
Post- treatment Assessment |
Treatment Group | Description | Included in Between- Groups Analysis* |
Included in Within- Groups Analysis |
|---|---|---|---|---|---|---|---|
| Andersen (36) | N = 40 100% F Age = 42.9 BMI = 32.9 |
BDI | 16 weeks | LM + structured activity | Standard group-based LM with weekly sessions. Three supervised step aerobics classes per week. | -- | LM |
| LM + lifestyle activity | Same LM as above. Instructions to increase activity through the day in lieu of supervised exercise classes. | -- | LM | ||||
| Annesi (37) | N = 111 100% F Age = 43.9 BMI = 36.6 |
POMS subscale | 6 months | LM | Set in community center. Six group nutrition sessions, six cognitive-behavioral treatment sessions with wellness professional, three 30-minute exercise sessions per week. | LM vs. C | LM |
| Control | Wait-list. | LM vs. C | -- | ||||
| Bacon (38) | N = 78 100% F Age = 39.3 BMI = 36.3 |
BDI | 1 year | LM | Standard group-based LM with 24 weekly sessions and six monthly aftercare visits. | LM vs. ND | LM |
| Non-dieting | Same number and schedule of visits as above, but sessions focused on body acceptance, eating behavior, activity, nutrition, and social support. Participants were encouraged to improve diet quality and eat in response to hunger/satiety cues, rather than given specific goals for energy intake or diet composition. | LM vs. ND | ND | ||||
| Cabioglu (39) | N = 165 100% F Age = 36.4 BMI = 33.2 |
SCL-90 subscale | 20 days | Diet-alone | Diet was prescribed at 1450 kcal/day and patients were instructed to maintain normal physical activity. | DA | |
| Diet + electro-acupuncture | Same as above, plus three 30-minute sessions of electroacupuncture per week, with stimulation at sites related to weight loss. | -- | -- | ||||
| Diet + placebo electro-acupuncture | Same as above, however, needles were inserted superficially at sites unrelated to weight loss. | -- | -- | ||||
| Carels (40) | N = 44 100% F Age = 54.7 BMI = 35.9 |
BDI | 24 weeks | LM | Standard group-based LM with 24 weekly sessions lasting 60–75 minutes each. | -- | LM |
| LM + Self-control therapy | Standard group-based LM with 24 weekly sessions lasting 90–120 minutes each. Sessions also included cognitive and behavioral coping and self-control skills. | -- | LM | ||||
| Dennis (41) | N = 39 0% F Age = 31.2 BMI = 33.5 |
CES-D | 16 weeks | Exercise alone | Set aboard deployed U.S. Navy ship. Navy-mandated exercise program, including 1 hour of exercise 4 days per week. | LM vs. EX | EX |
| LM + exercise | Same as above plus standard group-based LM with weekly sessions. | LM vs. EX | LM | ||||
| Evangelista (42) | N = 99 with advanced heart failure 25% F Age = 53.3 BMI = 30.6 |
MAACL subscale | 12 weeks | Exercise | Home-based, graduated, low-level exercise protocol consisting of light aerobic exercise and resistive training. Participants instructed to complete protocol at least 4 times per week. | EX vs. C | EX |
| No treatment | Participants were instructed to maintain their usual levels of activity. | EX vs. C | -- | ||||
| Faulconbridge (43) | N = 194 84% F Age = 43.3 BMI = 37.7 |
BDI | 1 year | Sibutramine | 5–15 mg/day of sibutramine and pamphlet on healthy eating and physical activity. Eight visits (10–15 minutes each) with physician. | -- | RX |
| LM | Standard group-based LM with weekly sessions through 18 weeks and every-other-week sessions thereafter through week 40. | -- | LM | ||||
| Sibutramine + LM | Combined the two treatments described above. | -- | -- | ||||
| Sibutramine + Brief LM | Combined the sibutramine treatment described above plus brief individual LM sessions (10–15 minutes each), delivered by physician in 8 visits. | -- | -- | ||||
| Fontaine (44) | N = 38 66% F Age = 36.5 BMI = 31.1 |
BDI | 13 weeks | LM + structured activity | Standard group-based LM with weekly sessions. Participants instructed to complete an aerobic exercise of choice three to four times per week. | -- | LM |
| LM + lifestyle activity | Same LM as above. Participants instructed to increase lifestyle activity (rather than aerobic exercise). | -- | LM | ||||
| Galletly (45) | N = 28 with polycystic ovarian syndrome 100% F Age = 32.5 BMI = 37.4 |
HADS | 16 weeks | High-protein, low-carbohydrate LCD | Weekly group exercise sessions, instructions to exercise two additional times per week, dietary counseling every 2 weeks, and 3-day food records monthly. Instruction to consume 30% of calories from protein, 40% from carbohydrate, and 30% from fat at restricted level for 12 weeks and weight-maintaining level for 4 weeks. | -- | LM |
| Low-protein, high-carbohydrate LCD | Same as above, except prescribed diet provided 15% of calories from protein, 55% from carbohydrate, and 30% from fat. | -- | LM | ||||
| Hainer (46) | N = 80 100% F Age = 43.9 BMI = 36.9 |
BDI | 4 months | LCD + placebo | LCD: Detailed instruction on self-monitoring, eating regularly (4–5 meals/day), restricting calorie intake (to 5–6 MJ/day), and increasing physical activity (150 minutes of walking per week). Monthly checkups that “encouraged changes in lifestyle according to cognitive behavioral modification techniques.” | RX vs. PBO | -- |
| LCD + sibutramine | Same LCD as above plus 10 mg/day of sibutramine. | RX vs. PBO | RX | ||||
| Halyburton (47) | N = 93 with abdominal obesity + ≥ 1 additional metabolic risk factor 60% F Age = 50.2 BMI = 33.5 |
BDI | 8 weeks | High-carbohydrate LCD | Prescribed LCD, providing 24% of calories from protein, 46% from carbohydrate, and 30% from fat. Key foods were supplied at every-other-week visits with a dietitian. | -- | DA |
| Low-carbohydrate LCD | Isocaloric dietary prescription and visits, but LCD provided 35% of calories from protein, 4% from carbohydrate, and 61% from fat. | -- | DA | ||||
| Kerr (48) | N = 401 100% F Age = 41.2 BMI = 32.3 |
CES-D | 12 months | Internet-based LM | One face-to-face goal-setting session, completion of 12 internet-based educational modules with feedback monthly by email and quarterly by phone. | LM vs. C | LM |
| Control | Standard advice and materials on weight from usual care provider. | LM vs. C | -- | ||||
| Kiortsis (49) | N = 60 100% F Age = 44.7 BMI = 34.4 |
HAM-D | 3 months | LCD + placebo | LCD: “Low-calorie diet, promoting a 500–1000 kcal reduction in daily energy.” | RX vs. PBO | -- |
| LCD + sibutramine | Same LCD as above plus 10 mg/day of sibutramine. | RX vs. PBO | RX | ||||
| LCD + orlistat | Same LCD as above plus 120 mg of orlistat three times daily. | RX vs. PBO | RX | ||||
| Klem (50) | N = 535 100% F Age = 47 BMI = 25.1 |
BDI | 20 weeks | LM | Standard group-based LM. | LM vs. C | LM |
| Control | Assessment only. | LM vs. C | -- | ||||
| Melanson (51) | N = 90 86% F Age = 42.5 BMI = 31.2 |
POMS subscale | 24 weeks | Diet and exercise counseling | Dietary counseling in weekly sessions, incorporating use of meal replacements to achieve 500 kcal/day deficit. “Simple, progressive walking program” delivered in weekly meetings with an exercise physiologist. | LM vs. EX | LM |
| Exercise counseling | Same walking program as above with no dietary counseling. | LM vs. EX | EX | ||||
| Neiman (52) | N = 91 100% F Age = 45.6 BMI = 33.1 |
GWBS subscale | 12 weeks | Diet + Exercise | Weekly groups on nutrition and weight loss skills. Specific calorie goals set and meal plans provided. Four supervised aerobic activity (i.e., walking) sessions per week. |
LM vs. C, LM vs. DC, LM vs. EX |
LM |
| Diet-alone | Same weekly groups as above. Four supervised sessions per week of mild stretching and range-of-motion exercises (vs. aerobic activity). | LM vs. DC | DC | ||||
| Exercise-alone | Four supervised aerobic activity (i.e., walking) sessions per week. | LM vs. EX, EX vs. C |
EX | ||||
| Control | Four supervised sessions per week of mild stretching and range-of-motion exercises (vs. aerobic activity). | LM vs. C EX vs. C |
-- | ||||
| Pi-Sunyer (53) | N = 3045 with hypertension and/or dyslipidemia 80.7% F Age = 45.0 BMI = 37.6 |
HADS | 1 year | LCD + placebo | LCD: Prescribed energy deficit of 600 kcal/day. At visits (every 14 days for 1 month and every 28 days thereafter), participants received dietary counseling and were encouraged to increase physical activity. | RX vs. PBO | -- |
| LCD + rimonabant, 5mg | Same LCD as above plus 5 mg/day of rimonabant. | RX vs. PBO | RX | ||||
| LCD + rimonabant, 20mg | Same LCD as above plus 20 mg/day of rimonabant. | RX vs. PBO | RX | ||||
| Rapoport (54) | N = 75 100% F Age = 47.5 BMI = 35.7 |
BDI | 10 weeks | LM | Standard group-based LM with weekly sessions. Specific goals set for energy intake and diet composition. | LM vs. ND | LM |
| Non-dieting | Cognitive-behavioral intervention with weekly group sessions. Restrictive dieting was discouraged, but goals set for diet composition. | LM vs. ND | ND | ||||
| Sarsan (55) | N = 76 100% F Age = 42.6 BMI = 34.9 |
BDI | 12 weeks | Aerobic exercise | Progressive program of walking and leg cycle exercise at 50–85% heart rate reserve, in three to four supervised sessions per week. | EX vs. C | EX |
| Resistance exercise | Supervised upper and lower body exercises with increasing resistance, three days per week. | EX vs. C | EX | ||||
| Control | No treatment. | EX vs. C | -- | ||||
| Sbrocco(56) | N = 24 100% F Age = 41.3 BMI = 32.6 |
BDI | 13 weeks | LM | Standard group-based LM, based on Schlundt, with weekly sessions. | LM vs. ND | LM |
| Non-dieting | Cognitive-behavioral intervention with weekly sessions. Restrictive dieting was discouraged and participants taught to view eating and exercise behavior “as a choice.” | LM vs. ND | ND | ||||
| Scheen (57) | N = 1045 with type 2 diabetes 51% F Age = 55.6 BMI = 34.2 |
HADS | 1 year | LCD + placebo | See Pi-Sunyer. | RX vs. PBO | -- |
| LCD + rimonabant, 5mg | See Pi-Sunyer. | RX vs. PBO | RX | ||||
| LCD + rimonabant, 20mg | See Pi-Sunyer. | RX vs. PBO | RX | ||||
| Smith (58) | N = 133 with hypertension 56% F Age = 47.5 Weight = 94.2 kg |
BDI | 6 months | LM + exercise | Standard group-based LM with 26 sessions. Exercise goal of 35 minutes of activity at 70%–85% of maximal heart rate, with 10 minutes of warm-up and cool-down, four days perweek. | LM vs. C, LM vs. EX | LM |
| Exercise-alone | Identical exercise goal. Participants instructed to maintain their usual diet. | LM vs. EX, EX vs. C |
EX | ||||
| Control | Wait-list. | LM vs. C, EX vs. C |
-- | ||||
| Surwit (59) | N = 42 100% F Age = 40.3 BMI = 35.4 |
BDI | 6 weeks | Low-fat high-sucrose LCD | LCD providing 11% of energy as fat, 19% as protein, and 71% as carbohydrate, provided for 6 weeks. Sucrose accounted for 43% of daily energy intake. | -- | DA |
| Low-fat low-sucrose LCD | Same as above, except sucrose accounted for 4% of daily energy intake. | -- | DA | ||||
| Tanco (60) | N = 60 100% F BMI = 39.5 |
BDI | 8 weeks | LM | Standard group-based LM with weekly sessions. |
LM vs. ND, LM vs. C |
LM |
| Non-dieting | Cognitive intervention with weekly sessions, “aimed at fostering insight into maladaptive behaviors, enhancing emotional well-being, and promoting regular physical exercise and nondisordered eating in the absence of any attempt at weight reduction.” | LM vs. ND | ND | ||||
| Control | Wait-list. | LM vs. C | -- | ||||
| Van Gaal (61) | N = 1057 with hypertension and/or dyslipidemia 79% F Age = 45.0 BMI = 36.6 |
HADS | 2 years | LCD + placebo | See Pi-Sunyer. | RX vs. PBO | -- |
| LCD + rimonabant, 5mg | See Pi-Sunyer. | RX vs. PBO | RX | ||||
| LCD + rimonabant, 20mg | See Pi-Sunyer. | RX vs. PBO | RX | ||||
| Vander Wal (62) | N = 166 73% F Age = 46.7 BMI = 37.0 |
CES-D | 4 weeks | Cereal substitution | Instruction to replace two meals per day with ready-to-eat cereal. | -- | DA |
| Cereal substitution + nutrient bar | Same as above, plus instruction to replace one snack per day with a nutrition bar. | -- | DA | ||||
| Cereal and waffle substitution + nutrient bar | Instruction to replace one meal per day with ready-to-eat cereal, one meal per day with a waffle, and one snack per day with a nutrition bar. | -- | DA | ||||
| Control | Wait-list with instruction to maintain normal dietary routines. | -- | -- | ||||
| Wadden 1990 (63) | N = 15 100% F Age = 44.6 BMI = 42.5 |
BDI | 18 weeks | LM with LCD | Standard group-based LM with weekly sessions. Calorie goal set at 1000–1200 kcal/day, with specific goals for diet composition and energy expenditure. | -- | LM |
| LM with VLCD | Same as above, except protein-sparing modified fast (500 kcal/day) was implemented from weeks 5–12. | -- | LM | ||||
| Wadden 2004 (64) | N = 123 100% F Age = 44.2 BMI = 35.9 |
BDI | 40 weeks | LM with 1200–1500 kcal/day | Standard group-based LM with weekly then every-other-week sessions. Calorie intake goal set at 1200–1500/day, with specific goals for diet composition and energy expenditure. | LM vs. ND | LM |
| LM with 1000 kcal/day | Same LM program described above. Liquid formula diet provided at 1000 kcal/day, with return to self-selected diet at 1200–1500 kcal/day beginning at week 14. Specific goals were set for diet composition and energy expenditure. | LM vs. ND | LM | ||||
| Non-dieting | Same schedule of contact described above, but participants were explicitly instructed not to reduce calorie intake. Instead, they were instructed to eat regularly in response to hunger/satiety cues. Specific goals for energy expenditure were identical to those in the two behavioral weight loss interventions. | LM vs. ND | ND | ||||
| Williamson (65) | N = 48 58% F Age = 37.5 BMI = 28.3 |
BDI | 12 months | LM + Calorie restriction | Weekly group sessions with psychologist (emphasizing standard LM skills) and at least two individual sessions with a dietitian or exercise physiologist per month in the first 26 weeks. Provided diet and calorie intake goals set at 25% below weight-maintaining level. Participants were not encouraged to increase exercise. | LM vs. DC | DC |
| LM + Calorie restriction and exercise | Same schedule of sessions as above. Provided diet and calorie intake goals set at 12.5% below weight-maintaining level, and energy expenditure goals set at 12.5% above weight-maintaining levels. |
LM vs. C, LM vs. DC |
LM | ||||
| LM with VLCD | Same schedule of sessions as above. A liquid formula diet was provided at 890 kcal/day to achieve 15% reduction in initial weight, followed by provided and self-selected diets at (reduced) weight-maintaining levels. Participants were not encouraged to increase exercise. | LM vs. DC | DC | ||||
| Control | Provided diet and calorie intake goals were set at weight-maintaining level. | LM vs. C | -- | ||||
| Wing (66) | N = 33 76% F Age = 51.3 Weight = 103.2 kg |
BDI | 20 weeks | LM with LCD | Standard group-based LM with weekly sessions. Calorie goal set at 1000–1500 kcal/day, with specific goals for diet composition and energy expenditure. | -- | LM |
| LM with VLCD | Same as above, except calorie goal set at 400 kcal/day during months 2–3 and gradually increasing during month 4 to 1000–1500 kcal/day in month 5. | -- | LM |
The group described in a given row represented the treatment printed in boldface in the between-group comparison(s) listed.
C = Control, DA = Diet-Alone, DC = Dietary Counseling, EX = Exercise-Alone, LM = Lifestyle Modification, ND = Non-Dieting, PBO = Placebo, RX = Pharmacotherapy.
% F = percentage of sample that was female; Age is expressed in mean years; BMI (body mass index) is expressed in mean kg/m2; Weight is expressed in kg if BMI was not reported.
BDI = Beck Depression Inventory (67) or Beck Depression Inventory-II (68); CES-D = Center for Epidemiologic Studies Depression scale (69); GWBS = General Well-Being Schedule (70); HADS = Hospital Anxiety and Depression Scale (71); HAM-D = Hamilton Rating Scale for Depression (72); MAACL = Multiple Affect Adjective Checklist (73); POMS = Profile of Mood States (74); SCL-90 = Symptom Checklist – 90 (75).
LCD = Low-calorie diet; VLCD = Very low-calorie diet; kcal = kilocalories.
For each study group, the same two authors also extracted sample size and participants’ mean (± standard deviation or standard error) weights and depression scores at baseline and at post-treatment. BMI was extracted when weight was not reported. Data from intent-to-treat samples and analyses were used when available. When only change values were reported for weight or symptoms of depression, or when results were collapsed across multiple groups, we contacted the corresponding authors to request mean values (and a measure of variability) for each study group. Inability to ascertain the necessary data led to exclusion of eight studies. For the final 31 included studies (36–66) (total n = 7937), we requested the correlations between pre- and post-treatment values for weight and symptoms of depression. When the correlations were not available, a conservative default value of 0.50 was used.
Two additional variables – presence/absence of supervised exercise sessions and intensity of counseling – were extracted for each treatment group. Interventions that included at least 16 counseling visits in the first 6 months (as in the Diabetes Prevention Program) (76) or before treatment ended (whichever occurred first) were coded as high-intensity-counseling. Those with fewer than 16 sessions in 6 months were coded as moderate-intensity-counseling. Interventions that included advice, provision of materials, or assessments with no mention of counseling were coded as low-intensity-counseling.
Statistical Analyses
The variety of interventions precluded a single meta-analysis. Thus, we computed separate between-groups effects for each of the following comparisons, which commonly occurred in the included studies: 1) lifestyle modification vs. control; 2) lifestyle modification vs. non-dieting; 3) lifestyle modification vs. dietary counseling; 4) lifestyle modification vs. exercise-alone; 5) exercise-alone vs. control; and 6) pharmacologic agent vs. placebo. In addition, we computed within-groups effects (i.e., changes from baseline) for each type of active treatment, as well as for diet-alone interventions.
A random effects model was computed for each of the six between-groups comparisons described above. For each analysis, we report the standardized mean difference (SMD) accompanied by its 95% confidence intervals (CI) and associated Z and p values. We assessed the statistical significance and magnitude of heterogeneity of effects using the Q test and I2 statistic, respectively. (The latter measures the proportion of the observed variance that is accounted for by true differences in effect size.) Funnel plots were reviewed and a fail-safe N (the number of studies with null findings that would need to be included in the analysis to render a significant effect non-significant) was computed for each analysis to examine the possibility of publication bias. For the within-groups comparisons (of baseline vs. post-treatment symptoms of depression), a random effects model was computed for each active treatment type. When possible, meta-regressions also were conducted to determine the relationships of group-level covariates (e.g., mean weight change, treatment duration, counseling intensity, and the presence of supervised exercise sessions) to the effect size for changes in depressive symptoms. All analyses were conducted using Comprehensive Meta-Analysis software (77).
Results
Of the 31 included studies, 7 and 9 reported results from intent-to-treat and completers’ analyses, respectively. Handling of missing data was not specified in the remaining 15 studies. Correlations between pre- and post-treatment values for weight and for depressive symptoms were unattainable for 65.8% of comparisons. The mean observed correlation was 0.54 (suggesting that the imputation of 0.50 for missing correlations was appropriate).
Between-Groups Comparisons of Active Treatments
The 31 included studies contributed a total of 34 comparisons to the six between-groups analyses. There were seven comparisons of lifestyle modification vs. control (total n = 1216), six of lifestyle modification vs. non-dieting (n = 364), three of lifestyle modification vs. dietary counseling (n = 95), four of lifestyle modification vs. exercise-alone (n = 281), and five of exercise-alone vs. control (n = 320). The nine comparisons of pharmacotherapy vs. placebo (n = 6075) included one group treated with orlistat, two with sibutramine, three with 5 mg of rimonabant, and three with 20 mg of rimonabant.
As shown in Figure 1, lifestyle modification programs were found to induce significantly greater reductions in symptoms of depression than control (Panel A) and non-dieting (Panel B) interventions. Fail-safe N analyses revealed that 26 and 6 comparisons with null findings would need to have been added to the 7 and 6 comparisons, respectively, of lifestyle modification vs. control and lifestyle modification vs. non-dieting interventions to render the meta-analytic results non-significant.
Figure 1.
Results of meta-analyses comparing reductions in symptoms of depression with lifestyle modification vs. control (Panel A), non-dieting (Panel B), dietary counseling (Panel C), and exercise-alone (Panel D) interventions. A positive standardized mean difference (SMD) indicates a greater reduction in symptoms of depression with lifestyle modification. The sizes of individual study markers are proportional to the weight of the corresponding study in the analyses. The midpoint of the diamond marker in each analysis represents the overall SMD, and the width of the diamond corresponds to the 95% CI. Note, Wadden (64) (Panel B) compared a non-dieting program to lifestyle modification programs with daily calorie targets of 1000 kcal/day (a) and 1200–1500 kcal/day (b). Williamson (65) (Panel C) compared a lifestyle modification program with dietary counseling interventions that induced a 25% energy deficit (a) and one that provided 890 kcal/day.
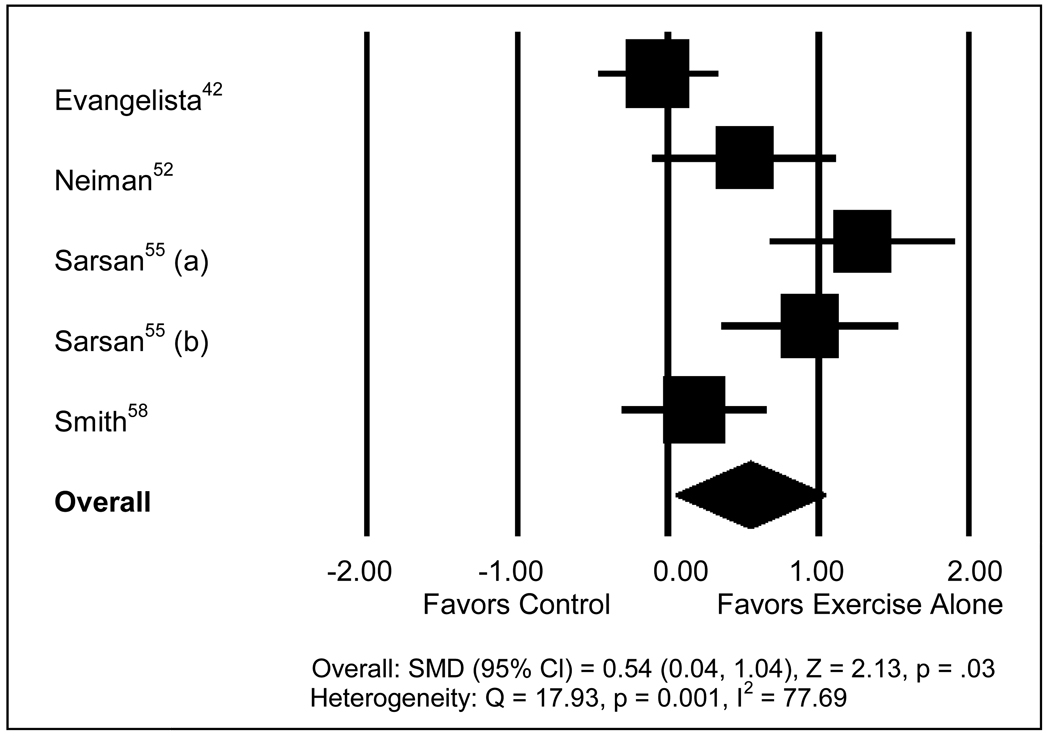
Reductions in symptoms of depression were marginally greater with lifestyle modification than with dietary counseling (Panel C, p = 0.053) and exercise-alone (Panel D, p = 0.054). The largest effect size was found for the comparison of exercise-alone with control (Figure 2), which significantly favored exercise for greater reductions in symptoms of depression. Eighteen comparisons, all with null findings, would need to have been added to the five comparisons of exercise-alone vs. control interventions in order to render the meta-analytic result non-significant. Pharmacologic interventions, as a whole, produced similar changes in symptoms of depression, compared with placebos (Figure 3).
Figure 2.
Meta-analysis comparing exercise-alone with control interventions. A positive standardized mean difference (SMD) indicates a greater reduction in symptoms of depression with exercise. The size of individual study markers is proportional to the weight of that study in the analysis. Sarsan (55) compared aerobics (a) and resistance (b) training programs to controls. The midpoint of the diamond marker represents the overall SMD, and the width of the diamond corresponds to the 95% CI.
Figure 3.
Meta-analysis comparing pharmacologic interventions with placebo. The abbreviations “Sib,” “Orl,” and “Rim” refer to sibutramine, orlistat, and rimonabant, respectively. The dosage of rimonabant (mg/d) also is shown. A positive standardized mean difference (SMD) indicates a greater reduction in symptoms of depression with pharmacotherapy. The size of individual study markers is proportional to the weight of that study in the analysis. The midpoint of the diamond marker represents the overall SMD, and the width of the diamond corresponds to the 95% CI.
Within-Groups Changes Observed in Each Treatment Type
Effect sizes for the within-groups changes in symptoms of depression (from baseline) could be computed for a total of 60 treatment groups. Among those, 27 groups received lifestyle modification, five received a non-dieting intervention, three received dietary counseling, eight received diet-alone, and seven received exercise-alone, for a total of 50 groups treated with non-pharmacologic interventions. Among 10 pharmacotherapy groups, one received orlistat, three received sibutramine, and three each received rimonabant at 5 mg and 20 mg.
As shown in Table 2, non-pharmacologic interventions, as a whole, were associated with significant reductions in symptoms of depression (p < 0.001). Examination of results from specific categories of non-pharmacologic interventions revealed significant changes in symptoms of depression in groups treated with lifestyle modification (p < 0.001), non-dieting interventions (p = 0.002), diet-alone (p = 0.002), and exercise-alone (p = 0.006), but not with dietary counseling (p = 0.30). The non-significant effect of dietary counseling may be due to the small number of studies included in that analysis or to an increase in symptoms of depression that was found in one group whose calorie intake was restricted by as much as 67%. Pharmacologic interventions, generally, induced a marginally significant reduction in symptoms of depression (p = 0.07). Separate analyses of rimonabant and non-rimonabant groups found no significant change in groups treated with rimonabant (p = 0.53), but a significant reduction in symptoms in groups treated with orlistat or sibutramine (p = 0.007). Further examination revealed that significant reductions were seen in the three groups treated with sibutramine (p ≤ 0.02), but not in the group treated with orlistat (p = 0.16).
Table 2.
Summary of random effects meta-analyses examining within-group changes in symptoms of depression, by treatment type.
| Treatment Type | Groups | Subjects | SMD | 95% CI | Z, p | Q, p | I2 | Fail-Safe N |
|---|---|---|---|---|---|---|---|---|
| Non-Pharmacologic (All) | 50 | 1789 | 0.50 | 0.40 to 0.59 | 10.33, < 0.001 | 179.9, < 0.001 | 72.8 | 4170 |
| Lifestyle Modification | 27 | 1103 | 0.60 | 0.47 to 0.72 | 9.30, < 0.001 | 96.9, <0.001 | 73.2 | 1765 |
| Non-Dieting | 5 | 139 | 0.37 | 0.14 to 0.59 | 3.17, 0.002 | 6.3, 0.18 | 36.9 | 17 |
| Dietary Counseling | 3 | 45 | 0.40 | −0.36 to 1.15 | 1.03, 0.30 | 6.8, 0.03 | 70.8 | 1 |
| Diet-Alone | 8 | 262 | 0.37 | 0.14 to 0.60 | 3.10, 0.002 | 10.2, 0.07 | 50.9 | 62 |
| Exercise Program | 7 | 240 | 0.50 | 0.14 to 0.85 | 2.75, 0.006 | 46.7, <0.001 | 87.2 | 74 |
| Pharmcologic (All) | 10 | 3890 | 0.07 | −0.01 to 0.15 | 1.83, 0.07 | 38.8, <0.001 | 76.8 | 0 |
| Rimonabant | 6 | 3763 | 0.02 | −0.03 to 0.06 | 0.62, 0.53 | 10.3, 0.07 | 51.3 | 0 |
| Orlistat/Sibutramine | 4 | 123 | 0.52 | 0.14 to 0.89 | 2.68, 0.007 | 11.1, 0.01 | 73.1 | 15 |
Effects of Covariates
Only the analysis of changes in depressive symptoms within groups treated with lifestyle modification was large enough to support post hoc meta-regression analyses. Weight change (B = 0.16), duration of treatment (B = −0.01), and counseling intensity (B = 0.03) were not significantly related to changes in symptoms of depression (ps ≥ 0.33). The inclusion of supervised exercise sessions, however, was related to significantly greater reductions in symptoms of depression (B = −0.28, p = 0.04).
Discussion
This meta-analysis examined the effects of different weight loss interventions on symptoms of depression, and compared those effects across different types of treatment. In general, we found that nearly all non-pharmacologic weight loss approaches resulted in a significant reduction in symptoms of depression, again contradicting early reports that dieting and weight loss precipitated mood disturbance. Comparisons of treatment types found that lifestyle modification induced significantly greater reductions in symptoms of depression than control and non-dieting interventions. Reductions in symptoms of depression were marginally greater with lifestyle modification than with alternative weight loss interventions, including dietary counseling and exercise-alone. Exercise-alone also was superior to control for reducing symptoms of depression. The significant between-groups effect sizes were in the small to medium range (78).
Two findings from the present analyses indicate that the reductions in symptoms of depression cannot be fully explained by weight loss. First, there were significant reductions in symptoms of depression with non-dieting programs, which were not intended to – and did not – induce weight loss. The beneficial effect of non-dieting on symptoms of depression may be due to the cognitive-behavioral strategies that encourage self-acceptance regardless of body weight. The same strategies may also reduce perceptions of stigma and of the severity of weight-related impairments, while fostering a sense of mastery and self-control that may have been previously limited in obese participants. Second, our meta-regression analysis of the lifestyle modification groups revealed that the within-groups effect for weight change was unrelated to the within-group effect for changes in symptoms of depression. That is, weight loss was not associated with increased or decreased symptoms of depression. Furthermore, neither the duration of treatment nor the intensity of the counseling intervention was related to the magnitude of change in depressive symptoms. Thus, other elements of treatment were likely responsible for the favorable effect on mood. Like those who received non-dieting interventions, obese individuals in lifestyle modification also may have achieved cognitive or behavioral changes that improved mood independently of the weight loss that resulted from those changes. They also may have benefited from the social support of their fellow group members and treatment providers. (Nearly all lifestyle modification programs studied here were delivered in group format.)
The meta-regression revealed that inclusion of a supervised exercise component was favorably related to changes in symptoms of depression among lifestyle modification participants. Several studies support a direct link between increased physical activity and improvements in mood (79–80). An alternative explanation for the added benefit of supervised physical activity sessions (which are typically done in groups) is that they carried many of the same benefits of the group counseling sessions, described above. Clinically, many obese patients report embarrassment about exercising at health clubs because they assume they will be unfavorably compared with others whom they perceive to be more fit, more attractive, more coordinated, or generally more competent to exercise. Exercising in the company of fellow participants in weight loss programs may have helped to normalize participants’ perceptions of themselves in relation to others.
Our generally favorable findings contradict those of a previous meta-analysis that reported little improvement in quality of life (including depression) in randomized controlled weight loss trials (81). Maciejewski et al. analyzed results from eight studies that measured symptoms of depression with the BDI and found that the random-effects comparison of treatment and control interventions yielded a nonsignificant pooled effect size of 0.07 (95% CI = −0.32 to 0.46) (81). However, there are substantive differences between that analysis and ours, particularly with respect to the definitions of treatment and control arms. In only three of the eight studies included in the previous meta-analysis did the control participants receive no treatment (60, 82–83), and in one of those, the active treatment was acupuncture (83). In three other studies, there were few differences between the interventions that were defined as treatment and control: group-based vs. individual lifestyle modification (84); cognitive-behavioral counseling plus nutrition education vs. cognitive-behavioral counseling alone (85); and lifestyle modification vs. dietary counseling (86). In two other studies (which also were included in the present analysis), the comparisons appeared to be between lifestyle modification and non-dieting interventions. However, Maciejewski et al. classified the lifestyle modification program as the treatment in one study (38) and as the control in the other (54). By contrast, the present study provided operational definitions of treatment types and included separate analyses for each comparison that appeared frequently enough in our search results to support a meta-analysis.
Our findings directly contradict the notion that intentional weight loss interventions adversely affect psychological well-being. Keys’ report in 1950 on the induction of neurotic – and even psychotic – symptoms with calorie restriction (30) remains of concern to some researchers and clinicians. We note, however, that the participants in Keys’ classic starvation study were normal weight volunteers whose energy intake was restricted by 50%, and whose weight was reduced 26% (rendering participants clinically anorectic). Thus, the adverse effects observed should not be generalized to overweight and obese persons who are prescribed more modest reductions in calorie intake and lose approximately 10% of initial weight. In 1957, Stunkard described “dieting depression” as a constellation of negative affective and psychomotor symptoms that was found in some obese persons who engaged in weight loss therapy (31). Certainly, some people experience depression and other psychological distress while trying to lose weight. However, results of the Look AHEAD study, a large randomized controlled trial that is examining the effects of intentional weight loss in overweight and obese patients with type 2 diabetes, found that the incidence of significant symptoms of depression was significantly lower among those who received an intensive lifestyle intervention than among controls, who received usual care (29). These findings, as well as those from the present meta-analysis should allay any remaining concerns that attempting to lose weight with diet, exercise, and behavior therapy may be harmful to the psychosocial status of obese patients without pre-existing psychopathology.
Concerns, however, remain about the psychiatric side effects of pharmacologic agents. Christensen et al. reported that patients treated with rimonabant were significantly more likely to discontinue treatment due to mood disorders (odds ratio = 2.5) and anxiety disorders (odds ratio = 3.0) than those who received placebo (33). Our analysis, which found no difference in changes in symptoms of depression between pharmacologic interventions and placebo, must be interpreted cautiously for two reasons. First, our analyses only included mean changes on measures of depressive symptoms. The incidence of clinically significant distress and discontinuation rates were not included. Second, baseline and post-treatment depression scores were only reported for 79% to 82% of patients in the three studies of rimonabant that were included in the present meta-analysis. Thus, the analyzed data were incomplete. Separate examination of groups treated with sibutramine found significant improvements in symptoms of depression, suggesting that all pharmacologic weight loss interventions should not be assumed to have similar effects on mood.
Four additional limitations of the present meta-analysis must be noted. First, we were unable to include all relevant studies due to unavailability of necessary data. In particular, studies published before 2000 are underrepresented. Second, at least 9 of the included studies reported results of completers’ analyses, rather than the more conservative intent-to-treat analyses. The type of analysis was not specified in nearly half of the studies. Third, the applicability of the present findings to the obese treatment-seeking population, as a whole, is questionable. Trials of weight loss interventions routinely exclude persons with significant psychological distress at screening. Given the elevated rates of psychopathology in obese individuals (4–6, 15–17), the samples in the included studies must be considered highly selected. As a result, this analysis does not fully address the potential adverse effects of dieting and weight loss on mood in obese individuals who suffer from depression, binge eating disorder, or other psychiatric disorders, prior to undertaking weight reduction.
Collectively, our findings suggest that the effects of most weight loss interventions – particularly non-pharmacologic therapies – on mood are favorable when weight loss is undertaken by obese individuals who are generally free of depression and other psychopathology. While exercise-alone interventions had the largest within-treatment pooled effect size, lifestyle modification programs, which incorporated exercise with dietary instruction and behavior therapy, were found to induce marginally or significantly greater reductions in symptoms of depression than other non-pharmacologic treatments. Future research on the effects of intentional weight loss on mood would benefit from including persons with higher levels of baseline distress, using diagnostic measures (based on the latest edition of the Diagnostic and Statistical Manual of Mental Disorders) rather than symptom inventories to assess depression, and expanding outcomes to include incidence and resolution of clinically significant distress among participants in weight loss trials.
Acknowledgements
This work was supported, in part, by a grant from Merck to Fabricatore and by National Institutes of Health (NIH) grants K23 DK070777 and K24 DK065018 to Fabricatore and Wadden, respectively.
Footnotes
Conflicts of Interest
Fabricatore has served as a consultant for Pfizer, Merck, and Ethicon-Endosurgery, and has received research support (including funding for this study) from Merck. Although he is now employed by Nutrisystem, Inc., Fabricatore was employed full-time at the University of Pennsylvania (where he retains an adjunct appointment) at the time the study was completed. Wadden serves on the Advisory Boards of Novo Nordisk and Orexigen and has received research support from Orexigen and Pfizer. Nguyen is employed by Merck and Heymsfield was employed by Merck at the time the work was completed. Faith has served as a consultant to, and has received research support from, Merck. The other authors have no potential conflicts to declare.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--The Evidence Report. Obes Res. 1998;6 suppl 2:51S–209S. [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Impact of smoking and preexisting illness on estimates of the fractions of deaths associated with underweight, overweight, and obesity in the US population. Am J Epidemiol. 2007;166:975–982. doi: 10.1093/aje/kwm152. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 6.Mather AA, Cox BJ, Enn MW, Sareen J. Associations of obesity with psychiatric disorders and suicidal behaviors in a nationally representative sample. J Psychosom Res. 2009;66:277–285. doi: 10.1016/j.jpsychores.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- 8.Pine DS, Cohen P, Brook J, Coplan JD. Psychiatric symptoms in adolescence as predictors of obesity in early adulthood: a longitudinal study. Am J Public Health. 1997;87:1303–1310. doi: 10.2105/ajph.87.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med. 2006;160:285–291. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- 11.Stice E, Presnell K, Shaw H, Rohde P. Psychological and behavioral risk factors for obesity onset in adolescent girls: a prospective study. J Consult Clin Psychol. 2005;73:195–202. doi: 10.1037/0022-006X.73.2.195. [DOI] [PubMed] [Google Scholar]
- 12.Murphy JM, Horton NJ, Burke JD, Jr, Monson RR, Laird NM, Lesage A, et al. Obesity and weight gain in relation to depression: findings from the Stirling County Study. Int J Obes. 2009;33:335–341. doi: 10.1038/ijo.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Läksy K, et al. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes. 2006;30:520–527. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000;90:251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes. 2006;30:513–519. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- 17.Barry D, Pietrzak RH, Petry NM. Gender differences in associations between body mass index and DSM-IV mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Ann Epidemiol. 2008;18:458–466. doi: 10.1016/j.annepidem.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12 suppl:151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 20.Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Prevention Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 23.Cash TF. Self-help for a negative body image: A comparison of components of a cognitive-behavioral program. Behav Ther. 2002;33:235–251. [Google Scholar]
- 24.Foster GD, Wadden TA, Vogt RA. Body image in obese women before, during, and after weight loss treatment. Health Psychol. 1997;16:226–229. doi: 10.1037//0278-6133.16.3.226. [DOI] [PubMed] [Google Scholar]
- 25.Ackermann RT, Edelstein SL, Venkat Narayan KM, Zhang P, Engelgau M, Herman WH, et al. Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity. 2009;17:2176–2181. doi: 10.1038/oby.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaukua J, Pekkarinen, Sane T, Mustajoki Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–694. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 27.Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health-related quality of life and weight loss. Obes Res. 2001;9:564–571. doi: 10.1038/oby.2001.73. [DOI] [PubMed] [Google Scholar]
- 28.Kolotkin RL, Norquist SM, Crosby RD, Suryawanshi S, Teixeira PJ, Heymsfield SB, et al. One-year health-related quality of life outcomes in weight loss trial participants: comparison of three measures. Health Qual Life Outcomes. 2009;7:53. doi: 10.1186/1477-7525-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faulconbridge LF, Wadden TA, Rubin RA, Walkup AP, Fabricatore AN, Coday M, et al. One-year changes in weight and symptoms of depression in depressed vs. non-depressed individuals in the Look AHEAD study. Obesity. 2009;17 suppl 2:576. [Google Scholar]
- 30.Keys A. Biology of human starvation. Minneapolis, MN: University of Minnesota Press; 1950. [Google Scholar]
- 31.Stunkard AJ. The dieting depression; incidence and clinical characteristics of untoward responses to weight reduction regimens. Am J Med. 1957;23:77–86. doi: 10.1016/0002-9343(57)90359-5. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration Advisory Committee. FDA briefing document: Zimulti (rimonabant) Tablets, 20 mg. [accessed January 12, 2010];Rockville, MD: FDA; 2007 Available at http://www.fda.gov/OHRMS/DOCKETS/AC/07/briefing/2007-4306b1-00-index.htm.
- 33.Christensen R, Kristensen KP, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 34.Department of Health and Human Service, Public Health Service, Food and Drug Administration Center for Drug Evaluation and Research Memorandum. Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC) [accessed August 24, 2009];Appendix 2: Request to Sponsors - Advice for the pharmaceutical industry in exploring their placebo-controlled clinical trials databases for suicidality and preparing data sets for analysis by FDA. 2006 November 16; Available at http://www.fda.gov/ohrms/dockets/ac/briefing/2006-4272b1-01-FDA.pdf.
- 35.Brownell KD. The LEARN program for weight management. 10th ed. Dallas, TX: American Health Publishing Company; 2004. [Google Scholar]
- 36.Andersen RE, Wadden TA, Bartlett, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs. structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281:335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 37.Annesi JJ, Unruh JL. Relations of exercise, self-appraisal, mood changes and weight loss in obese women: Testing propositions based on Baker and Brownell's model. Am J Med Sci. 2008;335:198–204. doi: 10.1097/MAJ.0b013e318152010c. [DOI] [PubMed] [Google Scholar]
- 38.Bacon L, Keim NL, Van Loan MD, Derricote M, Gale B, Kazakz A, et al. Evaluating a 'non-diet' wellness intervention for improvements of metabolic fitness, psychological well-being and eating and activity behaviors. Int J Obes. 2002;26:854–865. doi: 10.1038/sj.ijo.0802012. [DOI] [PubMed] [Google Scholar]
- 39.Cabioglu MT, Ergene N, Tan U. Electroacupuncture treatment of obesity with psychological symptoms. Int J Neurosci. 2007;117:579–590. doi: 10.1080/00207450500535545. [DOI] [PubMed] [Google Scholar]
- 40.Carels RA, Darby LA, Caccapaglia HM, Douglass OM. Reducing cardiovascular risk factors in postmenopausal women through a lifestyle change intervention. J Women's Health. 2004;13:412–426. doi: 10.1089/154099904323087105. [DOI] [PubMed] [Google Scholar]
- 41.Dennis KE, Pane KW, Adams BK, Qi BB. The impact of a shipboard weight control program. Obes Res. 1999;7:60–67. doi: 10.1002/j.1550-8528.1999.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 42.Evangelista LS, Doering LV, Lennie T, Moser DK, Hamilton MA, Fonarow GC. Usefulness of a home-based exercise program for overweight and obese patients with advanced heart failure. Am J Cardiol. 2006;97:886–890. doi: 10.1016/j.amjcard.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Faulconbridge LF, Wadden TF, Berkowitz RI, Sarwer DB, Womble LG, Hesson LA, et al. Changes in symptoms of depression with weight loss: results of a randomized trial. Obesity. 2009;17:1009–1016. doi: 10.1038/oby.2008.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontaine KR, Barofsky I, Anderson RE, Bartlett SJ, Wiersema L, Cheskin LJ, et al. Impact of weight loss on health-related quality of life. Qual LifeRes. 1999;8:275–277. doi: 10.1023/a:1008835602894. [DOI] [PubMed] [Google Scholar]
- 45.Galletly C, Moran L, Noakes M, Clifton P, Tomlinson L, Norman R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome-a pilot study. Appetite. 2007;49:590–593. doi: 10.1016/j.appet.2007.03.222. [DOI] [PubMed] [Google Scholar]
- 46.Hainer V, Kunesova M, Bellisle F, Hill M, Braunerova R, Wagenknecht M. Psychobehavioral and nutritional predictors of weight loss in obese women treated with sibutramine. Int J Obes. 2005;29:208–216. doi: 10.1038/sj.ijo.0802850. [DOI] [PubMed] [Google Scholar]
- 47.Halyburton AK, Brinkworth GD, Wilson CJ, Noakes M, Buckley JD, Keogh JB, Clifton PM. Low- and high-carbohydrate weight loss diets have similar effects on mood but not cognitive performance. Am Journal Clin Nutr. 2007;86:580–587. doi: 10.1093/ajcn/86.3.580. [DOI] [PubMed] [Google Scholar]
- 48.Kerr J, Patrick K, Norman G, Stein MB, Calfas K, Zabinski M, et al. Randomized controlled trial of a behavioral intervention for overweight women: impact on depressive symptoms. Depress Anxiety. 2008;25:555–558. doi: 10.1002/da.20320. [DOI] [PubMed] [Google Scholar]
- 49.Kiortsis DN, Tsouli S, Filippatos TD, Konitsiotis S, Elisaf MS. Effects of sibutramine and orlistat on mood in obese and overweight subjects: a randomised study. Nutr Metab Cardiovasc Dis. 2008;18:207–210. doi: 10.1016/j.numecd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Klem ML, Wing RR, Simkin-Silverman L, Kuller LH. The psychological consequences of weight gain prevention in healthy, premenopausal women. Int J Eat Disord. 1997;21:167–174. doi: 10.1002/(sici)1098-108x(199703)21:2<167::aid-eat7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 51.Melanson KJ, Dell’Olio J, Carpenter MR, Angelopoulous TJ. Changes in multiple health outcomes at 12 and 34 weeks resulting from 12 weeks of exercise counseling with or without dietary counseling in obese adults. Nutrition. 2004;20:849–856. doi: 10.1016/j.nut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Nieman DC, Custer WF, Butterworth DE, Utter AC, Henson DA. Psychological response to exercise training and/or energy restriction in obese women. J Psychosom Res. 2000;48:23–29. doi: 10.1016/s0022-3999(99)00066-5. [DOI] [PubMed] [Google Scholar]
- 53.Pi-Sunyer XF, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients RIO-North America: a randomized controlled Trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 54.Rapoport L, Clark M, Wardle J. Evaluation of a modified cognitive-behavioural programme for weight management. Int J Obes Relat Metab Disord. 2000;24:1726–1737. doi: 10.1038/sj.ijo.0801465. [DOI] [PubMed] [Google Scholar]
- 55.Sarsan A, Ardic F, Ozgen M, Topuz O, Sermez Y. The effects of aerobic and resistance exercises in obese women. Clin Rehabil. 2006;20:773–782. doi: 10.1177/0269215506070795. [DOI] [PubMed] [Google Scholar]
- 56.Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. Behavioral choice treatment promotes continuing weight loss: preliminary results of a cognitive –behavioral decision-based treatment for obesity. J Consul Clin Psychol. 1999;67:260–266. doi: 10.1037//0022-006x.67.2.260. [DOI] [PubMed] [Google Scholar]
- 57.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. RIO-Diabetes Study. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 58.Smith PJ, Blumenthal JA, Babyak MA, Georgiades A, Hinderliter A, Sherwood A. Effects of weight loss on depressive symptoms among men and women with hypertension. J Psychosom Res. 2007;63:463–469. doi: 10.1016/j.jpsychores.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surwit RS, Feinglos MN, McCaskill CC, Clay SL, Babyak MA, Brownlow BS, et al. Metabolic and behavioral effects of a high-sucrose diet during weight loss. Am J Clin Nutr. 1997;65:908–915. doi: 10.1093/ajcn/65.4.908. [DOI] [PubMed] [Google Scholar]
- 60.Tanco S, Linden W, Earle T. Well-being and morbid obesity in women: a controlled therapy evaluation. Int J Eat Disord. 1998;23:325–329. doi: 10.1002/(sici)1098-108x(199804)23:3<325::aid-eat10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 61.Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O, et al. Long-term effect of CB1 blockade with rimonabant on cardiometabloic risk factors: two year results from the RIO-Europe Study. Eur Heart J. 2008;29:1761–1771. doi: 10.1093/eurheartj/ehn076. [DOI] [PubMed] [Google Scholar]
- 62.Vander Wal JS, McBurney MI, Cho S, Dhurandhar NV. Ready-to-eat cereal products as meal replacements for weight loss. Int J Food Sci Nutr. 2007;58:331–340. doi: 10.1080/09637480701240802. [DOI] [PubMed] [Google Scholar]
- 63.Wadden TA, Mason G, Foster GD, Stunkard AJ, Prange AJ. Effects of a very low calorie diet on weight, thyroid hormones and mood. Int J Obes. 1990;14:249–258. [PubMed] [Google Scholar]
- 64.Wadden TA, Foster GD, Sarwer DB, Anderson DA, Gladis M, Sanderson RS, et al. Dieting and the development of eating disorders in obese women: results of a randomized controlled trial. Am J Clin Nutr. 2004;80:560–568. doi: 10.1093/ajcn/80.3.560. [DOI] [PubMed] [Google Scholar]
- 65.Williamson DA, Martin CK, Anton SD, York-Crowe E, Han H, Redman L, et al. Is caloric restriction associated with development of eating-disorder symptoms? Results from the CALERIE trial. Health Psychol. 2008;27 suppl 1:S32–S42. doi: 10.1037/0278-6133.27.1.S32. [DOI] [PubMed] [Google Scholar]
- 66.Wing RR, Marcus MD, Blair EH, Burton LR. Psychological responses of obese type II diabetic subjects to very-low-calorie diet. Diabetes Care. 1991;14:596–599. doi: 10.2337/diacare.14.7.596. [DOI] [PubMed] [Google Scholar]
- 67.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 68.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 69.Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 70.Fazio AF. A concurrent validation study of the NCHS' general well-being schedule. Hyattsville, MD: US Department of Health, Education, and Welfare; 1977. Vital and Health Statistics, Series 2, no. 73. [PubMed] [Google Scholar]
- 71.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 72.Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41:21–24. [PubMed] [Google Scholar]
- 73.Zuckerman M, Lubin B. Manual for the Revised Multiple Affect Adjective Check List. San Diego, CA: Educational and Industrial Testing Service; 1985. [Google Scholar]
- 74.McNair DM, Lorr M, Droppleman LF. Educational and Trial Testing Service Manual: Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1970. [Google Scholar]
- 75.Derogatis LR. The Symptom Checklist-90-Revised. Minneapolis, MN: NCS Assessments; 1992. [Google Scholar]
- 76.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis (Version 2.2.023) [Computer software] Englewood Cliffs, NJ: Biostat; 2005. [Google Scholar]
- 78.Cohen J. Statistical Power for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1988. [Google Scholar]
- 79.Greer TF, Trivedi MH. Exercise in the treatment of depression. Curr Psychiatry Rep. 2009;11:466–472. doi: 10.1007/s11920-009-0071-4. [DOI] [PubMed] [Google Scholar]
- 80.Blake H, Mo P, Malik S, Thomas S. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;23:873–887. doi: 10.1177/0269215509337449. [DOI] [PubMed] [Google Scholar]
- 81.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol. 2005;58:568–578. doi: 10.1016/j.jclinepi.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 82.Wardle J, Rogers P, Judd P, Taylor MA, Rapoport L, Green M, et al. Randomized trial of the effects of cholesterol-lowering dietary treatment of psychological function. Am J Med. 2000;108:547–553. doi: 10.1016/s0002-9343(00)00330-2. [DOI] [PubMed] [Google Scholar]
- 83.Mazzoni T, Mannucci E, Rizzello SM, Ricca V, Rotella CM. Failure of acupuncture in the treatment of obesity: a pilot study. Eat Weight Disord. 1999;4:198–202. doi: 10.1007/BF03339737. [DOI] [PubMed] [Google Scholar]
- 84.Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RI, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717–721. [PubMed] [Google Scholar]
- 85.Painot D, Jotternad S, Kammer A, Fossatin M, Goaly A. Simultaneous nutritional cognitive-behavioural therapy in obese patients. Patient Educ Couns. 2001;42:47–52. doi: 10.1016/s0738-3991(00)00092-6. [DOI] [PubMed] [Google Scholar]
- 86.Kiernan M, King AC, Stefanick ML, Killen JD. Men gain additional psychological benefits by adding exercise to a weight-loss program. Obes Res. 2001;9:770–777. doi: 10.1038/oby.2001.106. [DOI] [PubMed] [Google Scholar]