Abstract
Background
Cardiovascular disease is the leading cause of death in Argentina and the U.S. Argentina is 92% urban, with cardiovascular disease risk factor levels approximating the U.S.
Methods
The Coronary Heart Disease (CHD) Policy Model is a national-scale computer model of CHD and stroke. Risk factor data were obtained from the Cardiovascular Risk Factor Multiple Evaluation in Latin America Study (2003–04), Argentina National Risk Factor Survey (2005) and U.S. national surveys. Proportions of cardiovascular events over 2005–2015 attributable to risk factors were simulated by setting risk factors to optimal exposure levels [systolic blood pressure (SBP) 115 mm Hg, low-density lipoprotein cholesterol (LDL) 2.00 mmol/l (78 mg/dl), high-density lipoprotein cholesterol (HDL) 1.03 mmol/l (60 mg/dl), absence of diabetes, and smoking]. Cardiovascular disease attributable to body mass index (BMI) > 21 kg/m2 was assumed mediated through SBP, LDL, HDL, and diabetes.
Results
Cardiovascular disease attributable to major risk factors was similar between Argentina and the U.S., except for elevated SBP in men (CHD 8 % points higher in Argentine men, 6% higher for stroke). CHD attributable to BMI > 21 kg/m2 was substantially higher in the U.S. (men 10–11 % points higher; women CHD 13–14% higher).
Conclusions
Projected cardiovascular disease attributable to major risk factors appeared similar in Argentina and the U.S., though elevated BMI may be responsible for more of U.S. cardiovascular disease. A highly urbanized middle-income nation can have cardiovascular disease rates and risk factor levels comparable to a high income nation, but fewer resources for fighting the epidemic.
Keywords: coronary heart disease, stroke, risk factors, Argentina, United States
Introduction
Cardiovascular disease is the leading cause of death in both Argentina and the United States. Coronary heart disease (CHD) mortality rates in Argentina are lower than in the U.S., and mortality from CHD has declined to a similar degree in both nations since the 1970’s.[1] Stroke mortality rates are higher in Argentina compared with the U.S. and have declined comparatively less. The incidence of CHD and stroke and proportion attributable to risk factors in Argentina have not been estimated on a national level, as prospective cohort data are not yet available, and only recently have population-based directly measured risk factor data been available.[2] Argentina’s population is 92% urban, and prevalence of cardiovascular disease risk factors in Buenos Aires adults approximates that of the U.S.,[2] except that U.S. obesity prevalence is >50% higher. Knowing the amount of cardiovascular disease preventable by controlling risk factors can inform implementation of cardiovascular disease primary prevention policies in middle income nations like Argentina.[3] The CHD Policy Model, a national-scale, Markov style computer model of cardiovascular disease, was used to project CHD and stroke incidence in Argentina and the U.S., and estimate the proportion attributable to selected major risk factors.
Materials and Methods
The Coronary Heart Disease Policy Model
The CHD Policy Model is a computer-simulation, state-transition (Markov cohort) model of national scale CHD incidence, prevalence, mortality, and costs in adults aged 35–84 years.[4] The CHD Policy Model is comprised of three submodels: the demographic-epidemiologic submodel, the bridge submodel, and the disease history submodel. The demographic-epidemiologic submodel predicts CHD incidence and non-CHD mortality among the population without CHD, stratified into cells by age, sex, and up to six additional categorized risk factors: systolic blood pressure (SBP, <130, 130–139.9, ≥140 mmHg), smoking status (active smoker, non-smoker with exposure to environmental tobacco smoke, non-smoker without environmental exposure), high density lipoprotein (HDL) cholesterol [<1.00, 1.00–1.53, or >1.54 mmol/L (<40, 40–59.9, ≥60mg/dL)], low-density lipoprotein (LDL) cholesterol [<2.6, 2.6–3.3, or ≥3.4 mmol/L ( <100, 100–129.9, ≥130 mg/dL)], body mass index (BMI, <25, 25–29.9, ≥30 kg/m2), and diabetes mellitus (yes or no). After CHD develops, the bridge submodel characterizes the initial CHD event and its sequelae for 30 days. Then, the disease history submodel predicts subsequent CHD events, revascularization procedures, CHD mortality, and non-CHD mortality among patients with CHD. All population distributions, risk factor levels, coefficients, event rates, and case fatality rates can be modified for forecasting simulations. Population weighted means and proportions of cardiovascular risk factors in U.S. adults were estimated from pooled U.S. National Health and Nutrition Examination Surveys (NHANES) 1999–2004.[5] For this analysis, the main projected outcomes were first-ever and repeat, fatal and nonfatal CHD events [including stable and unstable angina pectoris, acute myocardial infarction (MI), and cardiac arrest due to CHD) and total stroke (ischemic and hemorrhagic) events.
Argentina-specific assumptions
Argentina-specific CHD Policy Model assumptions are listed in Appendix Table 1. The estimated population of Argentina aged 35–84 years in the year 2005, by age and sex, and 35-year olds arriving 2006–2015 were obtained from the Argentina National Statistics and Census Institute (Instituto Nacional Estadística y Census, INDEC, http://www.indec.gov.ar). Cause-specific mortality data by year, age, and sex were obtained from the Ministry of Health for the years 1997–2007 (Statistics and Information Department, Ministry of Health, Argentina, Appendix). Argentina-specific model inputs for CHD and total stroke incidence, case-fatality, and prevalence were obtained for the CHD Policy Model-Argentina whenever possible from published data from population-based studies[6,7,8,9] and national hospital administrative data (personal communication, Dr. Daniel Ferrante, Ministry of Health, Argentina).
Age and sex-specific means of SBP, LDL cholesterol, HDL cholesterol, BMI were obtained from the Buenos Aires portion of the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) Study,[10,11] a stratified random sample of men and women aged 25–64 years during 2003–2004.[2] CARMELA data were for ages 35–64 years, so estimates for ages 65–84 years in Argentina were imputed based on the CARMELA values for ages 35–64 years and risk factor age trends observed in the U.S. NHANES. Estimates of self-reported active and passive smoking prevalence in adults aged 35–83 years were obtained from the 2005 Argentine National Risk Factor Survey (Encuesta Nacional de Factores de Riesgo, ENFR) using a questionnaire adapted from World Health Organization and Pan American Health Organization instruments and validated for Argentina.[12]
Model Calibration
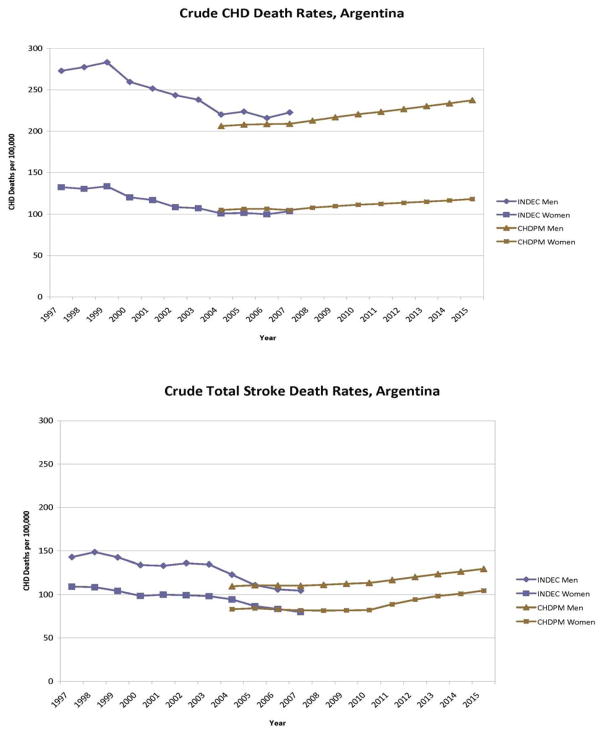
The CHD Policy Model-Argentina predicted deaths were compared with CHD and stroke deaths observed from Argentina vital statistics for the years 1997–2007 (Figure 1). Vital statistic reporting estimated that crude rates of CHD deaths declined between 1999 and 2004 despite the fact that the population aged 65–84 years increased by 10%. Because of the change in CHD and stroke mortality 2000–2004, the model’s incidence rates were adjusted to reproduce the total number of deaths reported by the Argentine Ministry of Health for 2005 and maintain constant projected age-specific CHD death rates over the years 2005–2040 under fixed case-fatality and risk factor conditions.
Figure 1.
Crude CHD and stroke mortality rates from Argentina’s national vital statistics (Instituto Nacional de Estatdistica y Censos, INDEC) and projected by the CHD Policy Model-Argentina (CHDPM). The Policy Model was calibrated to match vital statistics mortality in the base year of 2005.
Cardiovascular disease event prediction
For the main simulations comparing Argentina with the U.S., multivariate risk equations were estimated from U.S. Framingham Heart Study data[13] with CHD (including stable or unstable angina, nonfatal MI, fatal MI, or arrest) or stroke events (ischemic stroke, including transient ischemic attack, plus hemorrhagic stroke)[14] as the outcome. Risk coefficients for age, sex, SBP, smoking status, LDL, HDL, diabetes, and BMI were estimated in the CHD prediction model and age, sex, SBP, smoking status, and diabetes in the total stroke model.[14] Statistically significant (P<0.05) age*risk factor interactions were incorporated into age-specific risk factor coefficients. Risk factor beta coefficients were estimated from examinations 9 to 13, 24, and 25 from the original Framingham Heart Study cohort and 1–6 from the Framingham offspring cohort, for whom adequate data were available for a time-dependent logistic regression analysis. In order to model competing mortality risk, a separate non-CHD death equation was also estimated from Framingham data including age, sex, SBP, diabetes, and smoking status.
Based on an analysis of Framingham Heart Study data, no independent effect of BMI was assumed, but rather elevated BMI was assumed to exert its effect on disease risk via changes in associated risk factors. The effect of a one kg/m2 increase in BMI was: SBP (males: 1.43 mm Hg, females: 1.24 mm Hg),[15] LDL [males: 0.07 mmol (2.76 mg/dl), females: 0.6 mmol (2.24mg/dl)],[16] HDL cholesterol [males: −0.4 mmol/l (−1.55 mg/dl),[16] females −0.02 mmol/l (−0.77 mg/dl)]. A range of diabetes prevalence was assumed for persons with ideal BMI (21 kg/m2): a low prevalence characteristic of ideal BMI (4% in ages ≥18 years),[17,18] and a moderated prevalence found in persons with ideal BMI but moderate family history of diabetes (8% in ages ≥18 years, family history acting as a rough proxy for genetic and environmental diabetes risks independent of body weight). Because of uncertainty regarding BMI’s effect on diabetes, proportions of CHD and stroke attributable to elevated BMI are reported as ranges.
Attributable Risk Analysis
Annual risk for CHD is calculated for each model cell by a multivariate logistic regression equation. Therefore annual risk for events is determined by the age, sex, and risk factor relative levels assigned to that cell, and the combined multiplicative effect of the risk factor coefficients. Minimum risk exposure levels[19] were defined based on observations from large epidemiologic studies [SBP 115 mm Hg, [20,21] LDL 2.00 mmol/l (78 mg/dl),[22,23] HDL 1.03 mmol/l (60 mg/dl), BMI 21 kg/m2,[24] absence of diabetes and active and passive smoking]. The proportion of CHD and stroke attributable to unfavorable risk factor exposures was obtained by first simulating a base case scenario for the years 2005–2015 with risk factors fixed at 2005 levels and comparing this to a scenario simultaneously setting risk factors at optimal exposure levels for the entirety of the 11-year simulation. The resulting attributable proportions reflect a hypothetical scenario in which all risk factor exposures are removed at the same time (and combined effects constrained to explain less than 100% of the outcome), rather than removing the effect of single risk factors one at a time.[25] Events attributable to elevated BMI were simulated and reported separately because of the assumption that BMI effects are mediated exclusively through downstream risk factors already quantified in the main analysis.
Uncertainty and Sensitivity Analyses
The range of uncertainty surrounding attributable risk estimates due to error in risk factor risk coefficient estimates was simulated by repeating the attributable risk analysis after substituting the lower and upper bounds of the risk factor relative risks estimated from the Framingham Heart Study. Because the CHD Policy Model assumes a higher proportion of incident CHD is allocated to MI at older ages and risk factor proportions or means and relative risks may differ by age category, the proportion of the outcome attributable to a specific risk factor may differ between MI and total CHD. Additionally, the Policy Model assumes that for smokers, MI comprises a greater proportion of incident CHD (Appendix Table 3). Therefore, proportion of MI explained by each risk factor in the main simulations was compared with the proportion of total CHD explained. Attributable proportions that were significantly different between CHD and MI outcomes were reported (significance defined as no overlap in the 95% confidence intervals of the attributable proportion estimates).
Because active smokers in the Argentine National Risk Factor survey reported a lower average number of cigarettes smoked daily compared with U.S. averages (Appendix Table 4), a sensitivity analysis estimated the proportion of CHD attributable to active smoking assuming this lower average number of daily cigarettes smoked. In order to investigate differences in the proportion of MI attributed to smoking in the Argentina portion of the INTERHEART Latin America study[26] (smoking defined as past or current smoking) and CHD Policy Model-Argentina projections (current smoking only), a sensitivity analysis substituted the approximate active smoking odds ratio for MI from INTERHEART into the Policy Model-Argentina after adjusting the effect size to reflect risk for total CHD including angina (original MI odds ratio for active smokers without former smokers, 3.6, total CHD relative risk assumed, 1.6).
The proportion of total stroke that is hemorrhagic is approximately 14% in the Framingham Heart Study,[27] and the proportion in Argentina likely approximately 30%.[28] Because of the stronger association of SBP with hemorrhagic stroke compared with ischemic stroke in younger adults,[29] the proportion of total stroke attributable to SBP was re-estimated after weighting the total stroke SBP relative risk to reflect contributions of ischemic and hemorrhagic stroke-specific and age-specific SBP relative risks from the Asia Pacific Cohort Studies Collaboration[29] assuming a ratio of ischemic stroke to hemorrhagic stroke of seven to three.
Role of the funding source
The funding source had no role in study design, analysis, data interpretation, or writing of the report.
Results
Base case risk factors and cardiovascular event rate projections, Argentina and U.S
Risk factor means and proportions measured in the Buenos Aires CARMELA sample and the Argentina National Risk Factor Survey were similar to values from the U.S. NHANES for adults ages 35–64 years, with the exception that active and passive smoking and mean LDL were higher in Argentina, diabetes was less prevalent in Argentine women, mean SBP was higher in Argentine men, and mean BMI higher in the U.S. (Table 1). Compared with national vital statistics, model calibration for CHD and stroke deaths was good for the years 2004–2007 (Figure 1). The population of Argentine adults aged 35–84 years was 14.4 million in 2005, 11% of the U.S. population of the same ages (Table 2). Estimated age-standardized 2005 CHD incidence rates were lower in Argentina compared with the U.S. (4.7/1000 in Argentina vs. 6.6/1000 in the U.S.), and stroke event rates were higher (4.2/1,000 in Argentina vs. 3.0/1000 in the U.S.).
Table 1.
Means and percentages (standard errors) of Argentine and U.S. adults age 35–64 years with selected major cardiovascular risk factors. U.S. estimates are from the 1999–2006 National Health and Nutrition Examination Surveys (NHANES). Except for smoking prevalence (from Argentine National Risk Factor Survey, 2005) Argentina data are from the Buenos Aires sample of the CARMELA study (2003–2004). Data are age standardized to the World Health Organization standard population.[43]
| Risk Factor | Men | Women | ||
|---|---|---|---|---|
| Argentina | United States | Argentina | United States | |
| Smoking | ||||
| Active (%) | 36.3 (2.0) | 28.6 (1.2) | 28.3 (1.6) | 23.7 (1.1) |
| Passive (%) | 42.4 (2.6) | 10.4 (0.8) | 37.4 (2.3) | 7.5 (0.7) |
|
| ||||
| HDL cholesterol mg/dl [mean, mg/dl]* | 46.5 (0.9) | 46.6 (0.4) | 57.6 (0.9) | 57.6 (0.5) |
|
| ||||
| LDL cholesterol [mean, mg/dl]* | 137.7 (2.7) | 126.3 (1.4) | 127.0 (2.9) | 121.3 (1.3) |
|
| ||||
| Diabetes (%)† | 8.7 (0.0) | 12.2 (0.8) | 4.6 (0.0) | 9.0 (0.8) |
|
| ||||
| Systolic Blood Pressure (SBP) mm Hg (mean) | 129.6 (1.1) | 123.7 (0.5) | 121.2 (1.2) | 121.7 (0.5) |
|
| ||||
| Body Mass Index (BMI) kg/m2 (mean) | 27.6 (0.3) | 28.7 (0.2) | 26.3 (0.5) | 29.0 (0.2) |
Diabetes defined as fasting plasma glucose ≥7.0 mmol/l (126 mg/dl) or self-reported history of diabetes diagnosis.
To convert from mg/dl to mmol/l, divide by 39.
Table 2.
Population and projected CHD and stroke events (age-standardized rates per 1,000 persons) in 2005, U.S. and Argentina adults age 35–84 years. Age standardization using the World Health Organization standard population.[43]
| Parameter | Men | Women |
|---|---|---|
| Population | ||
| Argentina | 6 596 000 | 7 831 000 |
| United States | 63 126 000 | 71 595 000 |
|
| ||
| CHD events | ||
| Argentina | 70 000 (10.75 per 1000) | 32 000 (3.44 per 1000) |
| United States | 810 000 (13.07 per 1000) | 460 000 (5.83 per 1000) |
|
| ||
| Total stroke events | ||
| Argentina | 32 000 (5.12 per 1000) | 68 000 (3.61 per 1000) |
| United States | 214 000 (3.54 per 1000) | 204 000 (2.56 per 1000) |
Incident CHD and stroke attributable to risk factors in Argentina and the U.S., 2005–2015
The proportion of CHD attributable to selected major risk factors was largely similar between Argentina and the U.S. (Table 3) except the proportion attributable to elevated SBP was lower in U.S. men. The proportions of stroke events attributable to the CHD Policy Model’s risk factors were also similar, except a smaller proportion of stroke was attributed to elevated SBP in U.S. men. A large proportion (>50%) of stroke was not explained by SBP, smoking, or diabetes exposures in Argentine women and U.S. men and women. CHD attributable to BMI > 21 kg/m2 (mediated through SBP, LDL, HDL, and diabetes) was substantially higher in the U.S. (38.2-34.9% men, 29.0–32.7% women) compared with Argentina (24.4–27.9% men, 18.2-15.7% women). Albeit less dramatically, more of stroke was attributable to BMI in the U.S. as well (20.3–21.9% in U.S. men, 12.2–19.5% in U.S. women compared with 15.4–16.7% in Argentine men and 9.5–11.0% in Argentine women).
Table 3.
Main analysis: percent of predicted incident CHD and total stroke attributable to selected risk factors in Argentina and the United States, 2005–2015. All estimates were based on relative risks (± 95% confidence interval) estimated from the Framingham Heart Study.
| Risk Factor and exposure level | Men | Women | ||
|---|---|---|---|---|
| Argentina | United States | Argentina | United States | |
| Coronary Heart Disease | ||||
| SBP > 115 mm Hg | 28.8 (23.9—33.2) | 21.2 (17.3—24.9) | 27.3 (25.1—35.2) | 29.5 (27.6—37.8) |
| Current active Smoking | 3.7 ( 2.0— 5.3) | 3.2 ( 1.7— 4.7) | 2.8 ( 1.7— 3.4) | 3.3 ( 2.3— 4.1) |
| Passive Smoking | 1.7 ( 1.0— 2.3) | 2.4 ( 1.5— 3.3) | 4.0 ( 1.6— 4.0) | 2.3 ( 1.6— 3.1) |
| Diabetes mellitus* | 12.9 (12.0—13.6) | 15.0 (14.0—15.8) | 13.8 (12.7—14.8) | 16.7 (15.6—17.7) |
| LDL > 78 mg/dl (2.00 mmol/l) | 27.9 (23.4—31.9) | 28.6 (24.5—32.4) | 24.9 (24.8—29.5) | 26.9 (27.0—31.4) |
| HDL <60 mg/dl (1.03 mmol/l) | 11.4 ( 6.4—17.0) | 15.6 ( 9.9—21.8) | 4.7 ( 2.3— 7.4) | 4.3 ( 6.6— 2.5) |
| Not explained by these risk factor levels | 14.0 | 14.2 | 22.2 | 16.6 |
|
| ||||
| Total stroke | ||||
| SBP > 115 mm Hg | 41.8 (37.5—47.3) | 36.0 (31.1—40.6) | 34.4 (29.4—40.9) | 38.0 (32.1—43.2) |
| Current active Smoking | 10.9 ( 8.0—13.7) | 9.5 ( 6.8—12.1) | 4.6 ( 0.4— 6.1) | 5.4 ( 3.9— 6.9) |
| Passive Smoking | 0.6 ( 0.3— 0.4) | 0.6 ( 0.5— 0.6) | 0.6 ( 0.4— 0.6) | 0.5 ( 0.4— 0.6) |
| Diabetes mellitus* | 4.7 ( 0.0—10.3) | 5.0 ( 0.0—10.9) | 8.4 ( 3.7—14.2) | 10.6 ( 4.7—17.1) |
| Not explained by these risk factor levels | 42.4 | 48.7 | 52.0 | 46.0 |
Diabetes defined as self-report of physician diagnosis of diabetes, fasting glucose ≥ 7.0 mmol/l (126 mg/dl) or taking diabetes medications.
Sensitivity analyses
When the CHD outcome was restricted to MI, the proportion of events attributable to active smoking in Argentina was more than two fold higher (Table 4). Compared with the analysis with total CHD as the outcome, the proportion of MI attributable to diabetes was relatively higher and LDL relatively lower. The same pattern of difference between total CHD and MI as the outcome was found for U.S. adults (data not shown). Substituting MI odds ratio for active smokers from INTERHEART raised the proportion of MI attributed to active smoking to 15.8% in men and 8.0% in women. Assuming a lower number of cigarettes smoked daily in active smokers did not much effect the proportion of CHD explained. Adjusting the SBP relative risk for total stroke to reflect a higher proportion of incident hemorrhagic stroke in Argentina increased the proportion of total stroke explained by SBP >115 mm Hg by 12.1 percentage points in men and 14.6 percentage points in women.
Table 4.
Sensitivity analyses: percent of projected cardiovascular outcomes attributable to selected risk factors in Argentina, 2005–2015. Risk factor risk coefficients for the main estimates were estimated from the Framingham Heart Study and estimates include projected 95% confidence intervals based on the 95% confidence intervals of the relative risk estimates.
| Outcome | Argentina | |
|---|---|---|
| Men | Women | |
| Acute Myocardial Infarction | ||
| Current active Smoking | ||
| Main estimate (95% confidence interval) | 10.1 (7.6—12.8) | 7.2 (5.6— 8.6) |
| INTERHEART odds ratio | 15.8 | 8.0 |
| Diabetes | ||
| Main estimate (95% confidence interval) | 19.8 (14.8—17.2) | 16.9 (15.5—18.2) |
| LDL > 78 mg/dl (2.00 mmol/l) | ||
| Main estimate (95% confidence interval) | 16.0 (12.3—19.6) | 15.6 (15.1—19.7) |
|
| ||
| Coronary Heart Disease | ||
| Current active Smoking | ||
| Main estimate (95% confidence interval) | 3.7 (2.0— 5.3) | 2.8 (1.7— 3.4) |
| Lower number of cigarettes/day | 3.0 | 2.3 |
| INTERHEART odds ratio | 7.1 | 6.3 |
|
| ||
| Total stroke | ||
| SBP > 115 mm Hg | ||
| Main estimate (95% confidence interval) | 41.8 (37.5—47.3) | 34.4 (29.4—40.9) |
| Relative risk weighted for higher proportion hemorrhagic stroke in Argentina | 53.9 | 49.1 |
Discussion
Using the CHD Policy Model, a Markov-style computer model of CHD and stroke, we estimated that the proportion of CHD and stroke attributable to SBP, active and passive smoking, LDL cholesterol, HDL cholesterol, diabetes and BMI were in most cases similar in Argentina and the United States. CHD attributable to elevated BMI was considerably more in the U.S. compared with Argentina (>10 percentage points higher in men and women). Adjusting blood pressure relative risks to reflect a higher propotion of hemorrhagic stroke in Argentina led to a >12 percentage point higher proportion of total stroke attributed to elevated systolic blood pressure.
The higher mean SBP and higher proportion of cardiovascular disease attributed to elevated blood pressure in Argentine men may be due to the 10% rate of hypertension control in Argentina[30] compared with >34% in U.S. men,[31,32] and lack of the same difference in women due to low rates of hypertension control in U.S. women ≥60 years old.[32] National blood pressure surveys and treatment guidelines have achieved a measurable degree of success in the U.S.[33] Argentina implemented a national hypertension control program based on World Health Organization (WHO) guidelines in 2008–2009, but the effect of this program on blood pressure treatment and control rates has yet to be measured.
Apparent similarities in the proportion of cardiovascular disease attributable to elevated SBP, dyslipidemia, and diabetes in Argentine and U.S. women may mask underlying differences. The U.S. has a high prevalence of obesity compared with nations like Argentina, presumably due to both higher caloric intake and less physical activity,[34] though obesity prevalence may be rising in Argentina among lower income and education status groups.[12] The much higher proportion of cardiovascular disease (particularly CHD) attributable to elevated BMI in the U.S. suggests that a large proportion of cardiovascular disease is associated with “secondary” high BP, dyslipidemia and diabetes (downstream of elevated BMI), while in Argentina BMI is lower on average and “primary” high BP, dyslipidemia and diabetes are more common. The approach of this analysis was to shift risk factor distributions toward a lower risk level, but it did not account for differences in risk factor distributions in the two nations. For example, while mean SBP and BMI were similar (Table 1), SBP ≥140 mm Hg is more prevalent in Argentine men aged 35–64 years (22.5% Argentina and 12.9% U.S.), and obesity (BMI ≥30 kg/m2) more prevalent in U.S. men of the same ages (31.7% U.S. and 16.0% Argentina).
CARMELA reported that prevalence of hypertension (29%), elevated total cholesterol (≥240 mg/dl, 19%), and active smoking (39%) in Buenos Aires were among the highest of the seven Latin American cities sampled, while it was in the ‘middle of the pack’ regarding obesity (BMI ≥30 kg/m2, 19.7%) and diabetes (6.2%).[2] In the U.S., since 1990 active cigartette smoking, high total cholesterol, and hypertension have all declined in prevalence, while prevalence of obesity and diabetes have increased.[35] National survey data from Argentina indicate that active smoking has declined by an absolute 5% since 1990.[12,36] Argentina’s trend trajectories for SBP, LDL and HDL cholesterol, passive smoking, diabetes, and BMI are not known, and will only be known when results from the 2009–2010 National Survey and additional population-based surveys become available. Despite a more constrained ardiovascular disease prevention budget, Argentina has proposed several programs aimed at controllig cardiovascular disease risk factors, such as the “Argentina Saludable” program (http://www.msal.gov.ar/argentina_saludable/).
While future risk factor secular trends remain uncertain in Argentina, its cardiovascular disease mortality is lower than in most other Latin American nations and has definitely been declining since 1970.[1] The explantion for the mortality declines could be favorable trends in risk factors, improved acute care and/or secondary prevention,[37] or a background of progressive economic development.[38] Nonetheless, Argentina’s cardiovascular mortality has declined less than mortality due to infectious and maternal/child diseases and noncommunicable diseases now constitute nearly 65% of total deaths.[39] It is therefore unclear if Argentina is entering an “epidemiologic transition” toward relatively higher cardiovascular disease mortality experienced a century ago in high income nations like the United States[40] and occurring in other middle income nations,[41] or if its cardivascular disease mortality trend will be more benign. Our projected age-standardized stroke event rates were higher in Argentina compared with the U.S. and the ratio of stroke to CHD higher, a pattern that appears to be true for most Latin American nations.[28]
The proportion of CHD attributable to unfavorable exposures from selected risk factors in CHD Policy Model simulations (approximately 85% of men and 80% of women) compares reasonably well with the overall proportion of MI attributable to risk factors in INTERHEART Latin America study using more and different risk factors (overall proportion explained 86–88%),[26] though less well with INTERHEART overall (proportion of MI explained 90% in men and 95% in women).[25] Estimated proportions of CHD and MI attributable to high BP and diabetes were similar to MI attributable risks reported by INTERHEART Latin America for Argentina, but our estimated proportion of MI attributable to smoking in Argentina was considerably smaller ( 10.1% in men and 7.2% in women compared with 42.5% in men and 25.7% in women in INTERHEART Latin America).[26] Eliminating active smoking in isolation (and not in concert with other risk factors) would bring the proportion of MI attributable to active smoking in Argentina in our analysis to 24% in men and 10% in women. Using only active smoking prevalence, we approximate MI smoking attributable risk to be between 23–37% in men and 12–21% in women in INTERHEART Latin America, depending on assumed number of cigarettes smoked per day.
Approximately half of stroke events remained unexplained by elevated SBP, smoking, or diabetes in our simulations. When we weighted blood pressure relative risks for stroke to reflect a higher proportion of hemorrhagic stroke in Argentina, approximately 30% of total stroke in men and 37% in women remained unexplained by the blood pressure, tobacco smoking, and diabetes. Our stroke analysis was limited by not including universal stroke risk factors not measured in Argentine surveys, such as alcohol intake and atrial fibrillation, additional cardiovascular risk factors common to CHD[25] and stroke,[42] or stroke risk factors specific to Argentina (such as Chagas disease).[28] Our estimates of total stroke attributable to elevated SBP and diabetes are in the range reported by INTERSTROKE.[42] Relatively lower proportions attributed to smoking are likely mostly a function of overall higher smoking prevalence in INTERSTROKE.
A limitation of this analysis was that active and passive smoking prevalence in Argentina was obtained from a national survey, but other risk factor estimates used for Argentina were from CARMELA, which sampled adults in Buenos Aires city only. Because CARMELA sampled Buenos Aires only, estimates based on CARMELA measurements may not be generalizable to Argentina as a whole. Risk factor means and proportions for U.S. adults are representative of the overall population so this analysis is limited in that U.S. race/ethnic subgroups and urban and rural populations were not analyzed separately.
Conclusions
Computer modeling analysis of cardiovascular disease in Argentina and the U.S. suggests that proportions of CHD and stroke attributable to major risk factors are overall similar in the two countries. There may be underlying differences pointing to different prevention objectives in Argentina and the U.S. For example, dietary and pharmacologic control of blood pressure should be a high priority for Argentina, and primary prevention of obesity a high priority in the U.S. On the whole, we projected that reducing risk factor levels to ideal levels would reduce cardiovascular disease event rates by a least a half in Argentina and the U.S. A highly urbanized middle-income nation can have cardiovascular disease risk factor levels and event rates approaching those of a high-income nation, but fewer resources for fighting the epidemic.
Supplementary Material
Acknowledgments
We thank Dr Albert Shen for his assistance with managing and analyzing U.S. National Health and Nutrition Examination Survey data. We thank participants in the CARMELA Study and other past studies of cardiovascular disease in Argentina who contributed to this research. The Framingham Heart Study (FHS) and Framingham Offspring Study (FOS) are conducted and supported by the U.S. National Heart, Lung, and Blood Institute (NHLBI) in collaboration with FHS and FOS investigators. Portions of this manuscript were prepared using a limited access dataset obtained by the NHLBI and the content does not necessarily reflect the opinions or views of the FHS, FOS, or the NHLBI. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [39]
Funding
Supported by a Mentored Career Development Award number K08HL089675 from the United States National Heart, Lung, and Blood Institute of the NIH (to Dr Moran). Drs Ferrante, Mejia, Coxson and Pérez-Stable were supported by grant No. TW05935 from the Tobacco Research Network Program, Fogarty International Center, National Cancer Institute, National Institute of Drug Abuse.
Footnotes
Conflict of Interest Statement: None declared
Supplementary data are available at International Journal of Cardiology online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodriguez T, Malvezzi M, Chatenoud L, Bosetti C, Levi F, et al. Trends in mortality from coronary heart and cerebrovascular diseases in the Americas: 1970–2000. Heart. 2006;92:453–460. doi: 10.1136/hrt.2004.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schargrodsky H, Hernandez-Hernandez R, Champagne BM, Silva H, Vinueza R, et al. CARMELA: assessment of cardiovascular risk in seven Latin American cities. Am J Med. 2008;121:58–65. doi: 10.1016/j.amjmed.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Effects of Modest Reductions in Dietary Salt on Cardiovascular Disease. New England Journal of Medicine. 2010 doi: 10.1056/NEJMoa0907355. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Centers for Disease Control and Prevention/National Center for Health Statistics. National Health and Nutrition Examination Surveys. [Google Scholar]
- 6.Caccavo A, Alvarez A, Facundo HB, Ferrari AE, Carrique AM, Lasdica SA, Esandi ME. Population incidence of ST-elevation myocardial infarction or left bundle branch block over 11 years in a community in Buenos Aires Province. Rev Argent Cardiol. 2007;75:185–188. [Google Scholar]
- 7.Blanco P, Gagliardi J, Higa C, Dini A, Guetaa J, Di Toro D, Botto F, Sarmiento RA. Acute myocardial infarction. Results of the Argentine Society of Cardiology Survey of 2005 in the Republic of Argentina. Rev Argent Cardiol. 2007;75:163–170. [Google Scholar]
- 8.Sposato LA, Esnaola MM, Zamora R, Zurru MC, Fustinoni O, et al. Quality of ischemic stroke care in emerging countries: the Argentinian National Stroke Registry (ReNACer) Stroke. 2008;39:3036–3041. doi: 10.1161/STROKEAHA.108.521062. [DOI] [PubMed] [Google Scholar]
- 9.Lavados PM, Sacks C, Prina L, Escobar A, Tossi C, et al. Incidence, 30-day case-fatality rate, and prognosis of stroke in Iquique, Chile: a 2-year community-based prospective study (PISCIS project) Lancet. 2005;365:2206–2215. doi: 10.1016/S0140-6736(05)66779-7. [DOI] [PubMed] [Google Scholar]
- 10.Dr Carlos Boissonnet, personal communication.
- 11.Hernandez-Hernandez R, Silva H, Velasco M, Pellegrini F, Macchia A, et al. Hypertension in seven Latin American cities: the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study. J Hypertens. 2010;28:24–34. doi: 10.1097/HJH.0b013e328332c353. [DOI] [PubMed] [Google Scholar]
- 12.Ferrante D, Virgolini M. The 2005 National Risk Factor Survey: principal results. Prevalence of cardiovascular disease risk factors in Argentina. Rev Argent Cardiol. 2007;75:20–29. [Google Scholar]
- 13.Framingham Heart Study CD-ROM. Department of Health and Human Services; 2005. [Google Scholar]
- 14.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 15.Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986–1995. Arch Intern Med. 2000;160:2847–2853. doi: 10.1001/archinte.160.18.2847. [DOI] [PubMed] [Google Scholar]
- 16.Wilsgaard T, Arnesen E. Change in serum lipids and body mass index by age, sex, and smoking status: the Tromso study 1986–1995. Ann Epidemiol. 2004;14:265–273. doi: 10.1016/j.annepidem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U.S. population: the 6-year results from the National Health and Nutrition Examination Survey (1999–2004) Diabetes Care. 2007;30:2517–2522. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- 18.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 19.Ezzati M, Hoorn SV, Rodgers A, Lopez AD, Mathers CD, et al. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet. 2003;362:271–280. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- 20.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 21.Blood pressure, cholesterol, and stroke in eastern Asia. Eastern Stroke and Coronary Heart Disease Collaborative Research Group. Lancet. 1998;352:1801–1807. [PubMed] [Google Scholar]
- 22.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Peto R, Collins R, MacMahon S, Lu J, et al. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. Bmj. 1991;303:276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu D, He J, Duan X, Reynolds K, Wu X, et al. Body weight and mortality among men and women in China. Jama. 2006;295:776–783. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 26.Lanas F, Avezum A, Bautista LE, Diaz R, Luna M, et al. Risk factors for acute myocardial infarction in Latin America: the INTERHEART Latin American study. Circulation. 2007;115:1067–1074. doi: 10.1161/CIRCULATIONAHA.106.633552. [DOI] [PubMed] [Google Scholar]
- 27.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, et al. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40:1032–1037. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavados PM, Hennis AJ, Fernandes JG, Medina MT, Legetic B, et al. Stroke epidemiology, prevention, and management strategies at a regional level: Latin America and the Caribbean. Lancet Neurol. 2007;6:362–372. doi: 10.1016/S1474-4422(07)70003-0. [DOI] [PubMed] [Google Scholar]
- 29.Lawes CM, Rodgers A, Bennett DA, Parag V, Suh I, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21:707–716. doi: 10.1097/00004872-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Silva H, Hernandez-Hernandez R, Vinueza R, Velasco M, Boissonnet CP, et al. Cardiovascular Risk Awareness, Treatment, and Control in Urban Latin America. Am J Ther. 2010;17:159–166. doi: 10.1097/MJT.0b013e3181a84ec5. [DOI] [PubMed] [Google Scholar]
- 31.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 33.Cutler DM, Long G, Berndt ER, Royer J, Fournier AA, et al. The value of antihypertensive drugs: a perspective on medical innovation. Health Aff (Millwood) 2007;26:97–110. doi: 10.1377/hlthaff.26.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Weinsier RL, Hunter GR, Heini AF, Goran MI, Sell SM. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med. 1998;105:145–150. doi: 10.1016/s0002-9343(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 35.Health, United States. 2009 with Chartbook on Trends in the Health of Americans. National Center for Health Statistics; [Google Scholar]
- 36.Encuesta Epidemiologica de Consumo de Sustancias Psicoactivas en Argentina. 1999 [Google Scholar]
- 37.Hunink MG, Goldman L, Tosteson AN, Mittleman MA, Goldman PA, et al. The recent decline in mortality from coronary heart disease, 1980–1990. The effect of secular trends in risk factors and treatment. Jama. 1997;277:535–542. [PubMed] [Google Scholar]
- 38.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National vital statistics. Argentina: National Institute for Statistics and Censuses (INDEC), Republic of Argentina; [Google Scholar]
- 40.Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 41.Zhao D, Liu J, Wang W, Zeng Z, Cheng J, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008;39:1668–1674. doi: 10.1161/STROKEAHA.107.502807. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010 doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. GPE Discussion Paper Series: No. 31. Geneva: World Health Organization; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.