Abstract
Neurobiological theories posit that schizophrenia relates to disturbances in connectivity between brain regions. Resting-state functional magnetic resonance imaging is a powerful tool for examining functional connectivity and has revealed several canonical brain networks, including the default mode, dorsal attention, executive control, and salience networks. The purpose of this study was to examine changes in these networks in schizophrenia. 42 patients with schizophrenia and 61 healthy subjects completed a RS-fMRI scanning session. Seed-based region-of-interest correlation analysis was used to identify the default mode, dorsal attention, executive control, and salience networks. Compared to healthy subjects, individuals with schizophrenia demonstrated greater connectivity between the posterior cingulate cortex, a key hub of the default mode, and the left inferior gyrus, left middle frontal gyrus, and left middle temporal gyrus. Interestingly, these regions were more strongly connected to the executive control network in healthy control subjects. In contrast to the default mode, patients demonstrated less connectivity in the executive control and dorsal attention networks. No differences were observed in the salience network. The results indicate that resting-state networks are differentially affected in schizophrenia. The alterations are characterized by reduced segregation between the default mode and executive control networks in the prefrontal cortex and temporal lobe, and reduced connectivity in the dorsal attention and executive control networks. The changes suggest that the process of functional specialization is altered in schizophrenia. Further work is needed to determine if the alterations are related to disturbances in white matter connectivity, neurodevelopmental abnormalities, and genetic risk for schizophrenia.
Keywords: Schizophrenia, Resting-state, fMRI, Default Mode, Dorsal Attention, Executive Control
1. Introduction
Dysconnectivity hypotheses of schizophrenia postulate that the disorder relates to abnormalities in neuronal connectivity (Stephan et al., 2009; Friston, 1999; Bullmore et al., 1997; Andreasen et al., 1998). Contemporary dysconnectivity theories posit that disturbed neural connectivity results from a combination of genetic and environmental risk factors that impinge upon normal neurodevelopment (Karlsgodt et al., 2008; Maynard et al., 2001; Bullmore et al., 1997). Reduced dendritic length and spine density, altered coherence in brain activity across cortical regions during task performance, and identification of schizophrenia susceptibility alleles and increased copy number variants in genes related to neuronal signaling and neurodevelopment are all consistent with dysconnectivity theories (Glessner et al., 2010; Walsh et al., 2008; Woodward et al., 2009; Lewis and Sweet, 2009; Uhlhaas and Singer, 2010).
Resting-state Functional magnetic resonance imaging (fMRI) has revealed that spontaneous neural activity, inferred on the basis of blood-oxygen-level dependence (BOLD) response time-series data, correlates across brain regions and is organized into spatially segregated functional connectivity networks (e.g. Fox et al., 2005). In addition to basic sensory and motor networks, RS-fMRI has identified several, ‘higher-order’ resting-state networks (RSNs) consisting primarily of interconnections between heteromodal association cortical regions (Biswal et al., 1995; Biswal et al., 1997; Li et al., 2000; Fox et al., 2005; Vincent et al., 2008; Greicius et al., 2003). They include; 1) the well known default mode network (DMN), which is comprised of posterior cingulate cortex (PCC)/precunues, ventro-medial prefrontal cortex (vmPFC), lateral parietal cortex, and mesial temporal lobe structures; 2) a dorsal attention network (DAN) consisting of the intraparietal sulcus (IPS)/superior parietal lobule (SPL), frontal eye fields (FEF), and extrastriate visual areas (middle temporal: area MT+); 3) a dorsolateral prefrontal cortex (dlPFC)-parietal executive control network (ECN); and 4) the ‘salience’ network that includes inferior frontal gyrus/anterior insular cortex and the anterior cingulate. The relevance of resting-state connectivity to individual differences in behavior and neuropsychiatric disorders is an area of intense investigation. The DMN, DAN, and ECN have been linked to memory, attention, and executive cognitive functions, respectively (Hampson et al., 2006; Seeley et al., 2007; Wang et al., 2010a; Wang et al., 2010b; Carter et al., 2010). Consequently, these networks may be particularly relevant to schizophrenia given their association with cognitive functions known to be impaired in schizophrenia.
A number of studies have examined RSNs in schizophrenia, especially the DMN. There is strong evidence that the DMN is abnormal in schizophrenia; although, findings are mixed with reports of both increased connectivity between brain regions comprising the DMN (Whitfield-Gabrieli et al., 2009; Zhou et al., 2007b), and even expansion of the DMN to include additional brain regions (Skudlarski et al., 2010; Salvador et al., 2010; Mannell et al., 2010), and decreased connectivity (Camchong et al., 2009; Bluhm et al., 2007; Rotarska-Jagiela et al., 2010). Considerably less is know about the integrity of other RSNs (Seeley et al., 2007; Zhang et al., 2009; Carter et al., 2010). Altered connectivity within a fronto-parietal network has been reported in several studies (Zhou et al., 2007a; Rotarska-Jagiela et al., 2010; Skudlarski et al., 2010); although one study did not find abnormal dlPFC connectivity in antipsychotic naïve first episode patients (Lui et al., 2009).
The lack of definitive conclusions may relate to the relatively small number of patients included in most studies (20 or fewer in many cases), limited data on networks other than the DMN, and the diversity of methods used to quantify connectivity. Moreover, it’s unclear if some RSNs are differentially affected in schizophrenia as most studies focused on just one network. The purpose of this investigation was to examine the effects of schizophrenia on four canonical RSNs: the default mode, dorsal attention, executive control, and salience networks.
2. Methods
2.1. Participants
42 patients with schizophrenia (n=28) and schizoaffective disorder (n=14) and 61 healthy control subjects matched for age, gender, ethnicity, and parental education participated in this study. Subject demographics are presented in Table 1. With the exception of age at illness onset being earlier in schizoaffective patients (17.7 vs. 22.8; t=2.24, p=.031), no significant differences in demographics or clinical symptoms were observed between the schizophrenia and schizoaffective patient groups. We will refer to the patient group as the schizophrenia group throughout the remainder of the paper. Patients were recruited from inpatient and outpatient services at the Vanderbilt Psychiatric Hospital in Nashville, Tennessee. This study was approved by the Vanderbilt University Institutional Review Board and all subjects provided written informed consent prior to participating in the study. Subjects were administered the Structured Clinical Interview for Diagnosing DSM-IV Disorders (SCID: First et al., 1996) to confirm diagnoses in patients and rule out current or past psychiatric illness in control subjects. Schizoaffective patients were also administered the schizoaffective module of the Diagnostic Interview for Genetic Studies (DIGS: Nurnberger, Jr. et al., 1994) to confirm the presence of mood symptoms for 30% or more of the total duration of illness. Clinical symptoms in patients were quantified with the Positive and Negative Syndrome Scale (PANSS: Kay et al., 1987). Pre-morbid IQ was estimated using the Wechsler Test of Adult Reading (WTAR: Wechsler, 2001). Exclusion criteria included: estimated pre-morbid IQ less than 80, age less than 16 or greater than 65, presence of a systemic medical illness (i.e. diabetes, cardiovascular disease) or central nervous system disorder (i.e. multiple sclerosis, epilepsy) that would affect study participation, history of significant head trauma, reported pregnancy or lactation, substance abuse within last three months (patients), or lifetime history of substance abuse/dependence (controls), psychotropic drug use (controls), and MRI contra-indicators (i.e. metal implants, claustrophobia). With the exception of one patient who was not taking an antipsychotic drug (APD), patients were taking either one second generation APD (n=33); a combination of second generation APDs (n=4); a first generation APD (n=1); or a combination of first and second generation APDs (n=3).
Table 1.
Demographic Characteristics
| Variable | Controls | Schizophrenia | x2 | p | ||
|---|---|---|---|---|---|---|
| N | 61 | 42 | ||||
| Gender (male:female) | 33:28 | 27:15 | 1.06 | .303 | ||
| Ethnicity (White:AA:Other) | 50:10:1 | 28:13:1 | 3.20 | .202 | ||
|
| ||||||
| Mean | SD | Mean | SD | t | p | |
|
| ||||||
| Age | 33.1 | 11 | 36.9 | 11.9 | −1.70 | .092 |
| Parental ED | 14.0 | 2.0 | 13.5 | 2.8 | 0.63 | .530 |
| Education | 4.4 | 1.5 | 2.9 | 1.5 | 4.97 | .000 |
| Premorbid IQ | 111.3 | 10.4 | 103.0 | 12.8 | 3.46 | .001 |
| Age Illness Onset | -- | -- | 21.3 | 6.9 | -- | -- |
| Duration of Illness | -- | -- | 15.3 | 11.1 | -- | -- |
| PANSS Positive | -- | -- | 19.2 | 7.4 | -- | -- |
| PANSS Negative | -- | -- | 13.8 | 6.2 | -- | -- |
| PANSS General | -- | -- | 32.2 | 7.8 | -- | -- |
2.2. Imaging Data Acquisition and Analysis
Complete details of the imaging data acquisition and analysis are presented in the Supplemental Materials. Briefly, a 7 minute resting-state echo-planar imaging (EPI) scan (28 axial slices, matrix=80×80, 3.0 mm × 3.0 mm in-plane resolution, slice thickness=4.0 mm, 203 volumes, TR/TE=2000/35 ms) was acquired on each subject. Subjects were instructed to rest quietly with their eyes closed and not to fall asleep during the scan. A high resolution T1-weighted fast field echo (FFE) structural scan (170 sagital slices, matrix=256×256, 1.0 mm isovoxel resolution, TR/TE=8.0/3.7 ms) was also acquired. Preprocessing of the functional data included motion correction, slice timing correction, band-pass filtering (0.01 Hz < f < 0.1 Hz), coregistration to structural scan, spatial normalization to MNI space, and spatial smoothing (8 mm Gaussian kernel). Each subject’s structural scan was segmented into grey matter, white matter, and CSF tissue classes using the unified segmentation approach implemented in SPM5 with default settings.
2.2.1. Resting-state fMRI: seed-to-voxel analysis
The CONN-fMRI Functional Connectivity toolbox v1.2 (http://www.nitrc.org/projects/conn cited in Whitfield-Gabrieli et al., 2011) was used to create individual subject seed-to-voxel connectivity maps. The seed ROIs consisted of 6 mm radius spheres centered on MNI coordinates used to identify the corresponding networks in prior studies (Seeley et al., 2007; Vincent et al., 2008; Vincent et al., 2006). Each RSN and their corresponding seed ROI (in MNI coordinates) were as follows: DMN (PCC: 1 −55 17); DAN (left and right IPS/SPL: −25 −53 52/25 −57 52); ECN (left and right dlPFC: −42 34 20/44 36 20); salience network (left and right fronto-insular cortex: −32 26 −14/38 22 −10). The mean time series from each ROI was used as a predictor in a multiple regression general linear model (GLM) at each voxel. Regressors corresponding to the 6 motion correction parameters, and their first temporal derivatives, global grey matter, white matter, and CSF were also included to remove variance related to motion, the global, white matter, and CSF signals, respectively. Regressors for the global, white matter, and CSF signals were created by extracting the BOLD time-courses from the tissue class segmented images (grey matter, white matter, CSF), averaged across all voxels within each tissue class. Consistent with prior studies (Vincent et al., 2008; Vincent et al., 2006), for networks with bilateral ROI seeds the connectivity maps derived from the left and right ROI were averaged to create a single connectivity map.
Second level random effects analyses were used to create within group statistical parametric maps (SPMs) for each network and to examine connectivity differences between groups. For the within group analyses, the SPMs generated for each network were thresholded at the whole-brain cluster-level corrected alpha level .05 for voxel-wise p=.001 to show regions positively correlated with the seed ROI. For each network, the within group thresholded maps of positive correlation were combined across patient and control groups to create a single mask containing voxels that positively correlated with the seed ROI at the a-priori threshold in either the patient or control groups. These were used to restrict the between groups analysis to only those voxels that positively correlated with the respective network seeds in either the control or patient groups. The between groups SPMs were thresholded at the whole-brain cluster-level corrected alpha .05 for voxel-wise p-value of .005.
2.2.2. Resting-state fMRI: ROI-to-ROI analysis
To further determine if connectivity in patients differed from the “normal” pattern of connectivity, the BOLD time series was extracted from each cluster in a network and correlated with the BOLD time-series signal of every other cluster in the network to create a correlation matrix showing connectivity between each region within the network. Mean network connectivity (mean of all pair-wise correlations within a network) was calculated and compared between groups. Because we were interested in the extent to which connectivity differed from the normal pattern and determining if the changes observed in patients could be detected by applying network maps derived from a control group, the regions used in this analysis were extracted from the control group. We used a stringent threshold (FWE=.01 for the DMN, ECN, and salience networks, and t=8.5 for the DAN) in order delineate separate clusters within larger regions that showed connectivity to the original network seed ROI. This was particularly relevant to the DAN which included a large swath of cortex that enveloped the IPS/SPL and area MT+. The higher threshold used for the DAN allowed us to separate this region into two separate ROIs. The regions included in each network, along with their anatomical labels, are presented in Supplemental Figure 1.
3. Results
3.1. Seed-to-Voxel Analysis
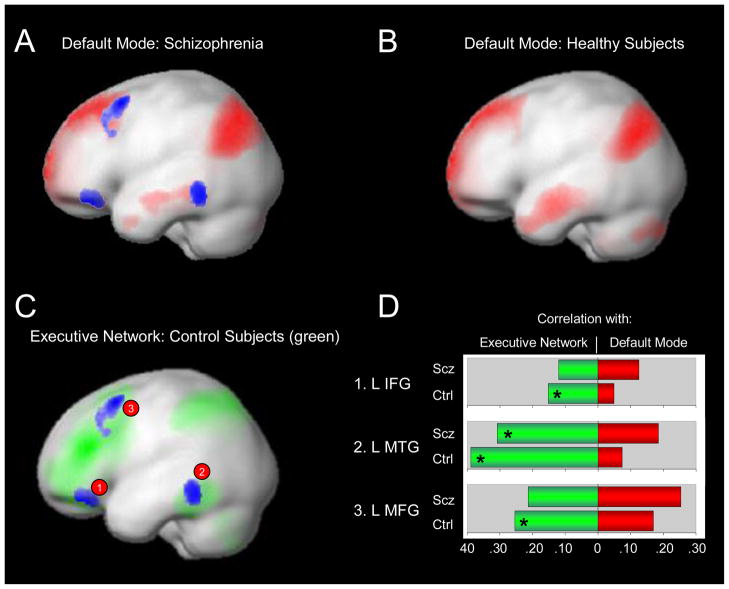
Visual inspection of the RSNs indicated that the connectivity maps for both groups were consistent with prior findings (see Figure 1). Second level analyses revealed a number of differences between patients and controls in the DMN, DAN, and ECN, but not salience networks (see Table 2). Patients demonstrated greater connectivity between the PCC seed ROI and the left inferior gyrus, left middle frontal gyrus, and left middle temporal gyrus (See Figure 2A). These regions were not part of the DMN in controls, based on connectivity with the PCC, suggesting that the spatial topography of the DMN may be altered in schizophrenia. Prior work has shown that there is very little spatial overlap between the DMN and other networks and that even adjacent cortical regions show markedly different connectivity patterns (Vincent et al., 2008). Remarkably, the clusters showing increased connectivity with the DMN PCC seed ROI in patients overlapped with the ECN in controls (see Figure 3) suggesting that these regions were not showing the normal pattern of functional specialization. To follow up on this speculation, we examined connectivity between the three clusters and the ROIs comprising the DMN and ECN used in the ROI-to-ROI analysis described below. We calculated each cluster’s mean connectivity with the DMN and ECN and performed paired t-tests to see if each cluster showed greater connectivity with the DMN or ECN. As expected, in controls, each cluster was more strongly connected to the ECN than DMN (all paired t-tests>3.27, p<.003). However, in patients, only the left middle temporal gyrus cluster was more strongly connected with the ECN than DMN (t(41)=3.75, p<.002). The remaining two clusters, left inferior frontal gyrus and left middle frontal gyrus did not show greater connectivity with either the DMN or ECN (t(41)=0.19, p>.849; and t(41)=1.51, p>.138, respectively). These findings suggest that the functional specialization of prefrontal cortical regions is altered in schizophrenia.
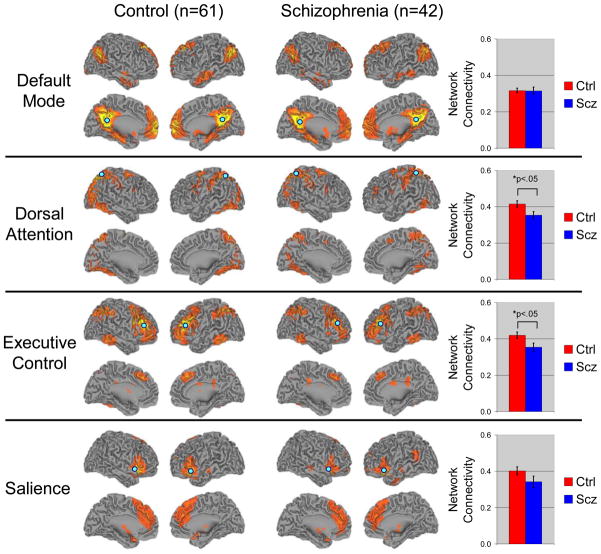
Figure 1.
Resting-state networks are differentially affected in schizophrenia. Based on the normal topography of the selected resting-state networks, mean connectivity in the dorsal attention and executive control networks was reduced in schizophrenia. Note: seed regions used to identify each network are shown as blue circles. Abbreviations: Ctrl=Control subjects; Scz=Schizophrenia
Table 2.
Resting-state network alterations in schizophrenia.
| Network | Contrast | Brain Region | MNI Coordinates | Peak T-value | Cluster Size (Voxels)* | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Defualt Mode | Ctrl>Scz | No Significant Differences Found | -- | -- | -- | -- | -- |
| Scz>Ctrl | L. Middle Frontal Gyrus (BA 10) | −34 | 42 | −16 | 4.64 | 228 | |
| L. Inferior Frontal Gyrus (BA 47) | −28 | 34 | −14 | 4.50 | |||
| L. Middle Temporal Gyrus (BA 20) | −64 | −46 | −12 | 4.00 | 230 | ||
| L. Middle Frontal Gyrus (BA 6) | −38 | 14 | 54 | 3.85 | 271 | ||
| L. Middle Frontal Gyrus (BA 9) | −46 | 18 | 32 | 3.15 | |||
| L. Middle Frontal Gyrus (BA 8) | −38 | 24 | 44 | 2.91 | |||
| Dorsal Attention | Ctrl>Scz | R. Superior Parietal Gyrus (BA 7) | 22 | −54 | 48 | 4.97 | 331 |
| L. Fusiform Gyrus (BA 19) | −24 | −76 | −16 | 4.15 | 1643 | ||
| L. Fusiform Gyrus (BA 19) | −18 | −84 | −16 | 4.00 | |||
| L. Middle Occipital Gyrus (BA 19) | −32 | −92 | 20 | 3.91 | |||
| R. Lingual Gryus (BA 17) | 24 | −82 | 6 | 4.06 | 1336 | ||
| R. Cuneus (BA 30) | 32 | −76 | 12 | 3.78 | |||
| R. Fusiform (BA 19) | 34 | −72 | −8 | 3.75 | |||
| Scz>Ctrl | No Significant Differences Found | -- | -- | -- | -- | -- | |
| Executive Control | Ctrl>Scz | R. Precentral Gyrus (BA 6) | 44 | 8 | 26 | 4.92 | 706 |
| R. Middle Frontal Gyrus (BA 9) | 38 | 18 | 24 | 3.90 | |||
| R. Middle Frontal Gyrus (BA 9) | 40 | 0 | 34 | 3.60 | |||
| Salience | Ctrl>Scz | No Significant Differences Found | -- | -- | -- | -- | -- |
| Scz>Ctrl | No Significant Differences Found | -- | -- | -- | -- | -- | |
Table shows up to 3 local maxima within a cluster more than 8.0mm apart
Voxel size = 2 × 2 × 2 mm
Abbreviations: BA=Brodmann’s Area; Ctrl=Control; L=Left; R=Right; Scz=Schizophrenia
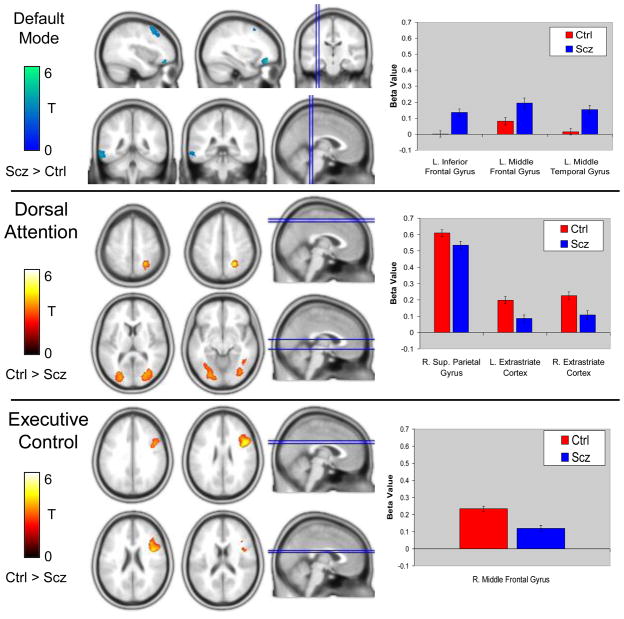
Figure 2.
Resting-state functional connectivity differences between schizophrenia patients and healthy control subjects in the default mode, dorsal attention, and executive control networks. Compared to control subjects, patients demonstrated greater connectivity between the PCC seed ROI and the left inferior frontal gyrus, left middle frontal gyrus, and left middle temporal gyrus (Top Panel). In contrast, patients demonstrated reduced connectivity between the dorsal attention IPS/SPL seed ROI and right superior parietal gyrus and bilateral extrastriate visual areas (Middle Panel). Similarly, connectivity between the executive control dlPFC seed ROI and right middle frontal gyrus was reduced in schizophrenia (Bottom Panel). Abbreviations: Ctrl=Control group; L=Left; R=Right; Scz=Schizophrenia group.
Figure 3.
Functional segregation between the default mode and executive controls networks is reduced in schizophrenia. Default mode functional connectivity, based on correlation with a PCC seed ROI, in schizophrenia and healthy subjects is shown in Panels A and B. The blue clusters indicate regions where patients demonstrated greater connectivity than controls with the PCC. As shown in Panel C, these regions overlapped with the executive control network observed in healthy subjects (based on connectivity with a dlPFC seed ROI). The connectivity profiles of these regions differed between groups (Panel D). In healthy subjects, these regions correlated more strongly with executive control network regions than default mode regions. In contrast, only one of the clusters, left middle temporal gyrus, demonstrated greater connectivity with executive control network regions than the default mode in schizophrenia (* within group paired t-test p<.05).
Abbreviations: Ctrl=Control subjects; dlPFC=Dorsolateral prefrontal cortex; IFG=Inferior frontal gyrus; L=Left; MFG=Middle frontal gyrus; MTG=Middle temporal gyrus; PCC=Posterior cingulate cortex; ROI=Regions-of-interest; Scz=Schizophrenia.
In contrast to the DMN, connectivity with the DAN and ECN seed ROIs was reduced in schizophrenia. In the DAN, patients exhibited less connectivity between the seed IPS/SPL ROI and the right posterior parietal gyrus, and a large swath of extrastriate cortex, bilaterally, that included portions of area MT+, lingual gyrus, middle occipital gyrus, and fusiform gyrus. For the ECN, connectivity between the seed ROI and a region of the right prefrontal cortex corresponding to the right middle frontal gyrus was reduced in schizophrenia patients. No differences in connectivity with the fronto-insular salience network seed ROI were detected. Although the sample sizes after stratification were small, we examined differences between schizophrenia and schizoaffective patients in each cluster identified in the between groups analysis (controls vs. schizophrenia). No differences were found between schizophrenia and schizoaffective patients (all p-values>.05). Moreover, ANOVA analysis indicated that both schizophrenia and schizoaffective groups differed from controls in each of the seven clusters (all p-values<.008).
3.2. Correlations between functional connectivity and clinical symptoms
We extracted the connectivity beta weights from the seven clusters identified in the between groups analysis and ran correlations between functional connectivity in these clusters and PANSS positive, negative, and general symptoms. Of the three clusters demonstrating greater connectivity with the DMN seed ROI (PCC) in schizophrenia, a significant positive correlation between PCC-left middle frontal gyrus connectivity and PANSS general symptoms was observed (r=.38, p=.013) indicating that the expansion of the DMN observed in schizophrenia was associated with worse overall psychopathology. No additional correlations were found.
3.3. ROI-to-ROI Analysis
Consistent with the seed-to-voxel analysis, mean connectivity was reduced in the DAN (t(101)=2.32, p=.022) and ECN (t(101)=2.36, p=.020), but not salience network (see Figure 1). Moreover, we did not observe any group differences in overall connectivity within the DMN (t(101)=0.06, p=.956). Again, this is consistent with the seed-to-voxel analysis as the regions showing increased connectivity in patients in the voxelwise analysis were outside the “normal” DMN and, therefore, not included in this analysis. Consistent with the seed-based voxelwise analysis, mean network connectivity did not differ between schizophrenia and schizoaffective patients (all p-values>.419). The connectivity matrices for each network with pair-wise connectivity color coded by strength are presented in Supplemental Figure 2. Between group comparisons of the pair-wise connections for the DAN and ECN networks indicated that interhemispheric connections between homotopic brain regions, IPS/SPL and MT+, appeared to be particularly affected in the DAN, while dlPFC connectivity with temporal and parietal regions was reduced in the ECN. Complete results of this analysis are depicted graphically in Supplemental Figure 3.
4. Discussion
Our findings indicate that resting-state functional connectivity disturbances vary by network in schizophrenia. Specifically, patients demonstrated greater connectivity between the PCC, the key hub of the DMN, and regions of the prefrontal and temporal lobe not normally considered part of the DMN. Conversely, connectivity was markedly reduced in the DAN and ECN networks. In the DAN, connectivity between the IPS/SPL seed ROI and parietal and extrastriate visual areas was reduced. Similarly, connectivity between the ECN seed ROI and the right middle frontal gyrus was reduced in patients. These findings support dysconnectivity models of schizophrenia, but indicate that the alterations in connectivity are characterized by both qualitative and quantitative changes that vary across networks rather than a global increase or decrease in connectivity.
Increased connectivity in the DMN has been previously reported in schizophrenia. In some cases patients demonstrated hyper-connectivity between regions that are normally part of the DMN, such as the PCC and mPFC (Whitfield-Gabrieli et al., 2009), whereas other groups reported qualitative changes in the DMN similar to our results (Skudlarski et al., 2010; Salvador et al., 2010; Mannell et al., 2010). For example, Skudlarski et al. (2010), Salvador et al. (2010), and Mannel et al. (2010) found that the DMN is expanded in schizophrenia to include inferior frontal/orbitofrontal and lateral temporal regions. Similarly, Garrity et al. (2007) reported increased DMN connectivity in frontal and lateral temporal cortical areas in patients during a passive auditory oddball paradigm.
Our findings build upon prior studies by showing that the regions demonstrating greater connectivity with the DMN seed ROI in patients overlap with the ECN in control subjects, and that connectivity within the ECN and DAN is reduced. There are prominent changes in the topography of RSNs and interactions between networks over the course of normal brain maturation (Fair et al., 2008; Fair et al., 2009; Stevens et al., 2009). Broadly speaking, between childhood and adulthood there is a shift from diffuse to local processing characterized by strengthening of long range connections and increased segregation between networks (Fair et al., 2007; Fair et al., 2009; Stevens et al., 2009; Uddin et al., 2010). The fact that: 1) the regions showing greater connectivity with the PCC overlapped with the normal topography of the ECN in controls; 2) two of the three clusters, left inferior and middle frontal gyrus, failed to show the normal pattern of greater connectivity with the ECN; and 3) connectivity in the ECN and DAN was reduced suggest that the normal segregation between networks and increasing within network connectivity that occurs during development may be compromised in schizophrenia. It is also interesting in this regard that connectivity between homotopic brain regions within the DAN was reduced in patients. Homotopic connectivity is present in infants and is highly dependent on trans-collosal white matter tracts (Honey et al., 2009; Johnston et al., 2008; Fransson et al., 2007). Consequently, in contrast to the alterations observed in the DMN, which may reflect abnormalities in experience dependent maturation of cortical functional specialization, reduced homotopic connectivity may reflect disturbances in white matter connectivity as a consequence of genetic risk and/or pre/peri-natal insults.
Altered connectivity within the DMN may have implications for cognition and task-related brain activity. Gabrieli-Whitfield et al. (2007) found that increased connectivity in the DMN was associated with abnormal working memory related activity in the dorsolateral prefrontal cortex. Moreover, the normal inverse association between components of the DMN and dorsolateral PFC was absent in patients; an observation that is reminiscent of our findings showing that the expanded DMN regions in schizophrenia are normally part of the ECN. Given that abnormal prefrontal activation during performance of a wide range of cognitive tasks is ubiquitous in schizophrenia (Minzenberg et al., 2009); DMN dysfunction may be a central neurobiological feature of the disorder with important implications for task-evoked brain activity and cognitive functioning. However, such alterations in the normally antagonistic relationship between the default and executive networks may not be unique to schizophrenia (e.g. Daniels et al., 2010).
Reduced connectivity within the DAN may also be relevant to cognitive impairment and task-evoked brain activation in schizophrenia. Resting-state connectivity within the DAN correlates with visual attention deficits in stroke patients and individuals with temporal lobe epilepsy (Carter et al., 2010; Zhang et al., 2009). Moreover, combined task-evoked BOLD response and resting-state fMRI studies have revealed important relationships between spontaneous fluctuations and task performance related brain activity. Specifically, Mennes and colleagues (2010) found that the magnitude of BOLD response observed during performance of a visual attention task was related to the degree to which the region correlated with key nodes of the DAN, IPS and area MT+ in particular. It is tempting to hypothesize that reduced resting-state connectivity within the DAN will be associated with deficits in visual attention and reduced task-evoked BOLD response in schizophrenia.
In conclusion, schizophrenia is associated with marked changes in connectivity within key RSNs. The changes are characterized by topographical expansion of the DMN, and reduced connectivity in the DAN and ECN. Future studies with larger sample sizes, including enough subjects to carry out meaningful comparisons between schizophrenia and schizoaffective disorders, and abundant phenotypic data will be required to fully characterize RSN alterations in schizophrenia and determine their functional consequences.
Supplementary Material
Acknowledgments
The authors would like to thank Alfonso Nieto-Castanon for his assistance using the Functional Connectivity (CONN-fMRI) toolbox. The authors would also like to thank all of the subjects who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Van KJ, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels JK, McFarlane AC, Bluhm RL, Moores KA, Clark CR, Shaw ME, Williamson PC, Densmore M, Lanius RA. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35:258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinical Version (SCID-CV) American Psychiatric Press Inc; Washington, D.C: 1996. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Reilly MP, Kim CE, Takahashi N, Albano A, Hou C, Bradfield JP, Zhang H, Sleiman PM, Flory JH, Imielinski M, Frackelton EC, Chiavacci R, Thomas KA, Garris M, Otieno FG, Davidson M, Weiser M, Reichenberg A, Davis KL, Friedman JI, Cappola TP, Margulies KB, Rader DJ, Grant SF, Buxbaum JD, Gur RE, Hakonarson H. Strong synaptic transmission impact by copy number variations in schizophrenia. Proc Natl Acad Sci U S A. 2010;107:10584–10589. doi: 10.1073/pnas.1000274107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31:424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- Salvador R, Sarro S, Gomar JJ, Ortiz-Gil J, Vila F, Capdevila A, Bullmore E, McKenna PJ, Pomarol-Clotet E. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang L, LaViolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010a;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Negreira A, LaViolette P, Bakkour A, Sperling RA, Dickerson BC. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010b;20:345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading Pearson. 2001. [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, Laviolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Waldie B, Rogers B, Tibbo P, Seres P, Purdon SE. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr Res. 2009;109:182–190. doi: 10.1016/j.schres.2008.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.