Abstract
Purpose
Inflammatory breast cancer (IBC), the most lethal form of breast cancer, has characteristics linked to higher risk of contralateral breast cancer. However, no large studies have examined risk of contralateral breast cancer following IBC.
Methods
We calculated absolute risk of invasive contralateral breast cancer among 5,631 IBC and 174,634 comparably staged non-IBC first breast cancer cases who survived at least 2 months following diagnosis and were reported to 13 Surveillance, Epidemiology, and End Results (SEER) registries between January 1, 1973 and December 31, 2006. We considered that contralateral cancers occurring within 2–23 months of first cancer diagnosis may more likely be metastatic/recurrent disease and those occurring 2 or more years after diagnosis independent primaries.
Results
Absolute risk of contralateral breast cancer was generally greater following IBC than regional/distant non-IBC, regardless of age and hormone receptor status of first cancer diagnosis. Much of the increase in absolute risk following IBC occurred within 2–23 months of first cancer diagnosis, while the risk for non-IBC occurred more gradually over time since diagnosis. For instance, among women first diagnosed before age 50, absolute risks following IBC and non-IBC were 4.9% vs. 1.1% at 2 years, 6.0% vs. 2.2% at 5 years, and 7.7% vs. 6.1% at 20 years after diagnosis. However, patterns of higher risk following IBC than non-IBC were also evident for at least 10–15 years in the subcohort of women who survived at least 24 months without a contralateral cancer.
Conclusion
Our results suggest that IBC has higher risk of cancer in the contralateral breast than comparably staged non-IBC, possibly due to both metastasic/recurrent disease and independent primaries.
Keywords: Inflammatory Breast Cancer, Contralateral Breast Cancer
Introduction
Inflammatory breast cancer (IBC) is a form of locally advanced breast cancer characterized by redness, edema, and peau d’orange of the breast, often with no underlying tumor mass [1]. The clinical characteristics are attributed to the presence of tumor emboli in the breast dermal lymphatics. Although rare, constituting 1–5% of newly diagnosed breast cancers in the United States, IBC is the most lethal form of breast cancer [1]. It is by definition either regional or distant stage at diagnosis and is characterized by rapid metastasis and poor survival [1]. IBC has an earlier age at diagnosis on average than non-IBC and a higher percentage is hormone-receptor negative [1]. Earlier age at diagnosis [2, 3, 4], hormone-receptor-negative status [4, 5], and later stage at diagnosis [2] have all been linked to higher risk of contralateral breast cancer. Risk of contralateral breast cancer following IBC has been reported in only one study, with higher risk noted for patients with distant metastases and IBC, particularly within the first two years after diagnosis [6].
Although most molecular data from small series of cases suggests that contralateral breast cancers are independent primaries [7], in the absence of these data it is difficult to definitively distinguish new primaries from recurrent or metastatic disease, particularly when the initial breast cancer is of more advanced stage. In epidemiologic studies, variable times since first breast cancer diagnosis have been used to separate probable metastatic or recurrent disease from independent second primaries. According to SEER rules, all metachronous cancers occurring 2 or more months after first cancer diagnosis are considered independent primaries unless the medical records indicate that the tumor is recurrent or metastatic disease [8]. Others have determined that 2 years after first cancer diagnosis is the most appropriate single cutoff to separate probable recurrent or metastatic disease (those diagnosed within 2 years of the first cancer) from probable independent primaries (those diagnosed 2 or more years after first cancer diagnosis) [6]. Concordance status between primary and contralateral disease according to prognostic factors, such as hormone receptor status, has also been used to determine the relationship between primary and contralateral disease [9].
Determining risk of contralateral breast cancer and whether such a cancer is metastatic disease or an independent primary has implications for both prognosis, treatment [9,10], and risk management strategies, such as increased surveillance, chemoprevention with drugs such as tamoxifen, oophorectomy, or prophylactic mastectomy [11].
In this manuscript, we calculate absolute risk of contralateral breast cancer following IBC according to age at diagnosis, interval since the first primary tumor, and type of contralateral breast cancer using data from the population-based registries in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. We also calculate absolute risk according to hormone receptor status of the first tumor. For comparison, we include estimates for comparably staged non-IBC as well as absolute risks of a first breast cancer in the general population. We attempt to distinguish between recurrent/metastatic disease and independent second primaries by carefully examining risk according to time since diagnosis, type of contralateral cancer, and concordance on several prognostic factors between first and contralateral breast cancers.
Materials and Methods
We evaluated female patients of white, black, and other known races diagnosed between January 1, 1973 and December 31, 2006 with unilateral invasive breast cancer (ICD-0-3 = C500-C509, excluding histologies 9590–9989, 9050–9055, and 9140) with known breast side and age at diagnosis who survived at least 2 months following diagnosis and were reported to population-based registries in the SEER program. The original nine SEER registries (SEER 9) are located in the metropolitan areas of Atlanta (1975–2006), Detroit (1973–2006), San-Francisco-Oakland (1973–2006), and Seattle-Puget Sound (1974–2006) and the states of Connecticut (1973–2006), Hawaii (1973–2006), Iowa (1973–2006), New Mexico (1973–2006), and Utah (1973–2006). Data from 1992–2006 are available for four additional areas (the California metropolitan areas of San Jose-Monterey and Los Angles, Rural Georgia, and the Alaska Native Tumor Registry) which in combination with the SEER 9 comprise the SEER 13 [12].
Among these women, we identified all IBC cases using the following SEER codes: {Site and Morphology.Histologic Type ICD-O-3} = 8530 OR {Extent of Disease.EOD 10 - extent (1988+)} = 70 OR {Extent of Disease.CS extension} = 71–73). Site and Morphology.Histologic Type ICD-O-3} = 8530 identifies “inflammatory carcinoma”. Extent of Disease EOD 10 refers to “inflammatory carcinoma, including diffuse (beyond that directly overlying the tumor) dermal lymphatic permeation or infiltration”. Extent of disease code 71 refers to “diagnosis of inflammatory breast cancer without a clinical description of inflammation, erythema, edema, or peau d’orange of more than 50% of the breast, with or without dermal lymphatic infiltration”. Extent of disease codes 72 and 73 refer to diagnosis of inflammatory breast cancer with a clinical description of inflammation, erythema, edema, or peau d’orange, with or without dermal lymphatic infiltration, of 50% or less of the breast and more than 50% of the breast, respectively. We identified regional/distant staged non-IBC based on the SEER Stage A coding scheme (local, regional, distant) [12].
We identified first contralateral invasive breast cancers with known breast side and age at diagnosis occurring at least 2 months after diagnosis of the first breast cancer and classified them as IBC or non-IBC. Case records were followed until the earliest of the following dates: diagnosis of first contralateral breast cancer, death, last contact if before December 31, 2006; or December 31, 2006, if date of last contact was after 2006.
We also classified first and contralateral breast cancers diagnosed during 1988–2006 as hormone receptor (HR) positive (ER or PR is positive) or HR negative (ER and PR are negative or one is negative and the other unknown). We further classified first and contralateral breast cancers as lobular (histology codes 8520 and 8522) or non-lobular (other histology codes).
To distinguish earlier from later onset breast cancer, we classified age at diagnosis of the first cancer as less than 50 and 50 years of age or older.
We calculated absolute risk (reported as a percent) and pointwise 95% confidence intervals of contralateral breast cancer accounting for competing causes [13]. Competing causes were defined as follows: death in analyses of any contralateral breast cancer; death and the other type of contralateral breast cancer in analyses of type-specific contralateral breast cancer. We also calculated absolute risk of death from all causes, with contralateral breast cancer as the competing risk. We did similar analyses in the subcohort of women who survived for 24 months or more without having had a contralateral breast cancer. Finally, we calculated absolute risk of developing a first breast cancer, a first IBC, and first non-IBC for women who were 45 and 65 years old (the approximate median ages in our <50 and ≥ 50 age groups [14].
We calculated percent agreement for ER- and PR-status and tumor histology (lobular, non-lobular) between first and contralateral IBC and non-IBC breast cancers. Chi-square tests were used to test for statistical significance; a p-value of less than .05 was considered statistically significant.
Results
A total of 5,631 first IBCs and 174,634 first regional/distant non-IBCs were included in the analysis; 274 contralateral invasive breast cancers were identified among the IBC cases and 6,019 among non-IBC cases. Eighty-one percent of first IBC cases were white, 13% black, and 6% other known races. The comparable percentages among non-IBC cases were 84%, 10%, and 6%. The median age at diagnosis was 56 years for IBC cases and 58 years for non-IBC cases. Follow-up time ranged from 2 months to 396 months. Table 1 presents number of first and contralateral cancers by age at diagnosis and type of first breast cancer, type of contralateral breast cancer, and months since diagnosis of first breast cancer.
Table 1.
Number of First and Contralateral Breast Cancers by Age at Diagnosis, Type of Cancer, and Months Since First Cancer Diagnosis - SEER data (1973–2006)
| Age at 1st breast cancer diagnosis | < 50 years | ≥ 50 years | ||
|---|---|---|---|---|
| Type of 1st breast cancer | IBC | Regional/distant Non-IBC | IBC | Regional/distant Non-IBC |
| # 1st breast cancers | 1,884 | 50,323 | 3,747 | 124,311 |
| # contralateral breast cancers | 119 | 1,958 | 155 | 4,061 |
| <24 months since 1st cancer dx | 75 | 429 | 83 | 958 |
| 24–60 months since 1st cancer dx | 32 | 534 | 47 | 1,154 |
| > 60 months since 1st cancer dx | 12 | 995 | 25 | 1,949 |
| # contralateral IBC | 30 | 72 | 40 | 90 |
| <24 months since 1st cancer dx | 20 | 29 | 28 | 35 |
| 24–60 months since 1st cancer dx | 8 | 17 | 11 | 28 |
| > 60 months since 1st cancer dx | 2 | 26 | 1 | 27 |
| # contralateral Non-IBC | 89 | 1,886 | 115 | 3,971 |
| <24 months since 1st cancer dx | 55 | 400 | 55 | 923 |
| 24–60 months since 1st cancer dx | 24 | 517 | 36 | 1,126 |
| > 60 months since 1st cancer dx | 10 | 969 | 24 | 1,922 |
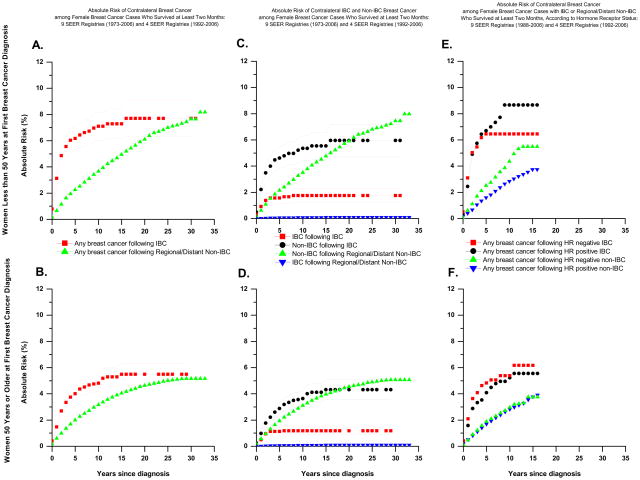
Absolute risks of any contralateral breast cancer, contralateral IBC, and contralateral non-IBC according to age at diagnosis and type of first breast cancer are presented in Table 2 and graphically in Figure 1A., B., C., and D. For comparison, absolute risks for a first breast cancer in either breast among similarly aged women in the general population are presented in Table 2. Among women less than age 50, absolute risks of developing a contralateral breast cancer after IBC or non-IBC were higher than absolute risks of developing a first breast cancer in either breast; this was not generally true among older women.
Table 2.
Absolute risk of contralateral breast cancer according to years since first cancer diagnosis and absolute risk of a first breast cancer at 2 ages in the general population without breast cancer – SEER data (1973–2006)
| Age at diagnosis and type of first breast cancer | Absolute risk of contralateral breast cancer according to years since first cancer diagnosis | Absolute risk of contralateral IBC according to years since first cancer diagnosis | Absolute risk of contralateral non-IBC according to years since first cancer | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 yrs | 5 yrs | 10 yrs | 15 yrs | 20 yrs | 2 yrs | 5 yrs | 10 yrs | 15 yrs | 20 yrs | 2 yrs | 5 yrs | 10 yrs | 15 yrs | 20 yrs | |
| Age <50 yrs | |||||||||||||||
| IBC | 4.9% | 6.0% | 7.1% | 7.3% | 7.7% | 1.4% | 1.6% | 1.8% | 1.8% | 1.8% | 3.5% | 4.6% | 5.3% | 5.5% | 5.9% |
| Non-IBC(reg/dist) | 1.1% | 2.2% | 3.7% | 4.9% | 6.1% | 0.1% | 0.1% | 0.2% | 0.2% | 0.2% | 1.1% | 2.1% | 3.5% | 4.8% | 5.9% |
| Age ≥ 50 yrs | |||||||||||||||
| IBC | 2.7% | 3.7% | 4.7% | 5.3% | 5.5% | 0.9% | 1.1% | 1.2% | 1.2% | 1.2% | 1.8% | 2.9% | 3.6% | 4.3% | 4.3% |
| Non-IBC (reg/dist) | 1.0% | 2.0% | 3.2% | 4.1% | 4.6% | 0.0% | 0.1% | 0.1% | 0.1% | 0.1% | 1.0% | 1.9% | 3.1% | 4.0% | 4.5% |
| General population without breast cancer | Absolute risk of 1st breast cancer according to years since specified age | Absolute risk of 1st IBC according to years since specified age | Absolute risk of 1st non-IBC according to years since specified age | ||||||||||||
| 5 yrs | 10 yrs | 15 yrs | 20 yrs | 5 yrs | 10 yrs | 15 yrs | 20 yrs | ||||||||
| 45-year old | 0.9% | 2.1% | 3.6% | 5.2% | 0.0% | 0.0% | 0.1% | 0.1% | 0.9% | 2.1% | 3.5% | 5.1% | |||
| 65-year old | 2.0% | 4.1% | 5.9% | 7.3% | 0.0% | 0.0% | 0.1% | 0.1% | 2.0% | 4.0% | 5.8% | 7.2% | |||
Figure 1.
Among IBC and non-IBC cases diagnosed before age 50, absolute risks of a contralateral breast cancer were similar 30 years after diagnosis (7.7%), but for most of the follow-up period the absolute risk following an IBC was higher than that following a non-IBC (Table 2 and Figure 1.A). Much of the increase in absolute risk among IBC cases occurred within 2 (4.9%) and 5 (6.0%) years of diagnosis, whereas the absolute risk following non-IBC increased more gradually over time (1.1% at 2 years and 2.2% at 5 years). Among cases diagnosed at 50 years or older, absolute risks of a contralateral breast cancer after 30 years were 5.5% and 5.2% following IBC and non-IBC, respectively, with much of the risk following IBC occurring within 2 to 5 years (Table 2 and Figure 1.B.). Similar patterns were seen for absolute risk of a contralateral IBC and non-IBC (Table 2 and Figure 1.C. and D.).
These patterns most likely reflect the effect of competing risks, which influences the number of women remaining at risk for a contralateral breast cancer. Among younger IBC cases, the absolute risks of death at 5, 10, and 30 years were 58%, 68%, and 84%, respectively. Among similarly aged non-IBC cases, the corresponding estimates were 28%, 40%, and 66%. Among older IBC cases, the corresponding absolute risk estimates were 68%, 79%, and 95% as compared to 40%, 58% and 88% among older non-IBC cases. Thus, a considerably higher proportion of non-IBC than IBC cases remained at risk of a contralateral breast cancer 5 or more years after first cancer diagnosis.
In the cohort of women who survived at least 24 months without having had a contralateral breast cancer, absolute risks of contralateral breast cancer at 5, 10, 15, and 30 years after first cancer diagnosis among IBC cases diagnosed before age 50 were 3.2%, 4.7%, 5.0%, and 5.6% (data not shown in figure). Comparable estimates for similarly aged non-IBC cases were 1.6%, 3.2%, 4.9%, and 7.6%. Estimates at 5, 10, 15, and 30 years among older IBC cases were 2.9%, 4.2%, 5.4%, and 5.4% and among older non-IBC cases were 1.5%, 2.9%, 4.0%, and 5.3%. Among IBC cases the absolute risk of a subsequent IBC was 1 percent or less; among non-IBC cases it was 0.2% or less.
HR status was available for 3,554 (63%) first IBC cases, of which 2,023 (57%) were HR-positive and 1,531 (43%) were HR-negative. HR status was available for 87,271 (50%) non-IBC cases, of which 67,571 (77%) were HR-positive and 19,700 (23%) were HR-negative. Among IBC cases with known receptor status, 173 contralateral breast cancers were diagnosed (40 following HR-positive breast cancer in women less than 50, 58 following HR-positive breast cancer in women 50 years or older, 34 following HR-negative breast cancer in younger women, and 41 following HR-negative breast cancer in older women); the corresponding number among non-IBC cases was 1,850 (398 following HR-positive breast cancer in women less than 50, 966 following HR-positive breast cancer in women 50 years or older, 238 following HR-negative breast cancer in younger women, and 248 following HR-negative breast cancer in older women).
The higher absolute risk of any breast cancer following IBC than regional/distant non-IBC was evident for both HR-positive and HR-negative first cancers (Figure 1E., F.). For instance, among younger women, absolute risks following HR-positive IBC at 5, 10, and 16 years (the last year of follow up) were 6.7%, 8.7%, and 8.7%, respectively. Among comparably aged HR-positive non-IBC breast cancers the corresponding absolute risks were 1.6%, 2.8%, and 3.7%. Absolute risks following HR-negative IBC were 6.4%, 6.4%, and 6.4% and those following HR-negative non-IBC were 2.5%, 4.4%, and 5.5%. Absolute risks following HR-positive and HR-negative breast cancer in older women were similar to each other, but were higher following IBC than non-IBC.
Both ER- and PR- status were available for 117 (68%) contralateral breast cancers following IBC and for 1,349 (73%) contralateral breast cancers following non-IBC. Concordance on both ER- and PR-status for first and contralateral breast cancers was 59% among IBC cases and 53% among regional/distant non-IBC cases (chi-square p-value = .24). The percent agreement of these variables for IBC followed by IBC was 74% and IBC followed by non-IBC was 54%. The percent agreement on these variables following non-IBC was the same whether the subsequent tumor was non-IBC or IBC (53%) (Table 3). When contralateral breast cancer cases were divided into those that occurred within 23 months after diagnosis and those that occurred 24 or more months after diagnosis, the percent agreement on ER and PR status was higher for the tumors diagnosed within the first 23 months after diagnosis and was similar for first IBC and non-IBC cases (Table 3).
Table 3.
Percentage agreement on both ER and PR status for first and contralateral breast cancers according to type of contralateral breast cancer and time since diagnosis.
| Type of first breast cancer | Type of contralateral breast cancer | Time since first cancer diagnosis | ||
|---|---|---|---|---|
| Contralateral IBC | Contralateral Non-IBC | 2–23 months | ≥ 24 months | |
| IBC | 74% (20/27) | 54% (49/90) | 67% (50/75) | 45% (19/42) |
| Non-IBC | 53% (26/49) | 53% (687/1300) | 69% (248/362) | 51% (505/987) |
When tumor histology (lobular vs. non-lobular) was examined for first and contralateral breast cancers, the percent agreement was 89% for IBC cases and 81% for non-IBC cases (chi-square p-value = .001).
Discussion
We found that the absolute risk of contralateral breast cancer following IBC increased rapidly in the first five years after diagnosis, while that of non-IBC increased more gradually over time. These patterns may reflect the much higher absolute risk of death following IBC than non-IBC, leaving a much lower percentage of IBC cases at risk of a contralateral breast cancer for longer periods of time since first cancer diagnosis. Thus, it is particularly notable that the absolute risk of cancer in the contralateral breast was higher for many years following a diagnosis of IBC than non-IBC in women of similar age, stage, and hormone-receptor status at diagnosis.
The particularly aggressive nature of IBC raises the possibility that cancers in the contralateral breast reflect recurrent/metastatic disease rather than independent primaries, even though, according to SEER rules, breast cancers designated as contralateral second primaries must not be described as metastases in the medical records [12]. We attempted to address this possibility in three ways: by examining results according to time since first cancer diagnosis, by type of contralateral breast cancer, and by assessing whether concordance of several tumor characteristics for first and contralateral cancers differed by IBC status of the first breast cancer.
If we assume that cancers in the contralateral breast diagnosed within 2 years of first cancer diagnosis are most likely recurrent/metastatic disease and those diagnosed after 2 years are most likely independent second primaries, our results suggest higher risk of recurrent/metastatic disease and independent second primaries in the contralateral breast following IBC than similarly staged non-IBC. Although lymphatic drainage from the breast is primarily to the axillary nodes, connections may cross the median plane to the contralateral breast [15]. IBC, in particular, is characterized by early lymphatic and vascular invasion by tumor emboli [1] and by genes enriched in mediators of cell motility [16]. Thus, it is possible that IBC may be more likely to metastasize to the contralateral breast than non-IBC, although patterns of recurrence in the opposite breast have not been specifically noted for IBC [17].
Risk of contralateral non-IBC was generally greater after IBC than non-IBC, even two or more years after first cancer diagnosis. High throughput gene expression profiling has shown IBC to be genetically heterogeneous, having the same cell-of-origin subtypes as non-IBC (e.g. luminal A, luminal B, non-luminal-HER2-positive, non-luminal HER2-negative) but with more being non-luminal than for non-IBC [18]. Results suggest that molecular subtype and IBC phenotype are determined by independent gene sets, which remain to be identified [18]. A number of markers not related to cell-of-origin subtypes have been associated with IBC, including p53 mutations, over-expression of RhoC guanosine triphosphatase (GTPase), loss of expression of WISP 3, a breast tumor suppressor gene located at 6q22-q23, and expression of E-cadherin, and lympho angiogenic factors such as vascular endothelial growth factor (VEGF)-C, (VEGF)-D, and VEGFR-3. However, none of these are specific to IBC [19]. Based on these characteristics of IBC and non-IBC, it seems plausible that contralateral non-IBCs following IBC are more likely to be independent primaries than metastatic disease.
Although overall concordance on ER and PR status was similar for first IBC and non-IBC cases, concordance was highest when an IBC was followed by a contralateral IBC and when tumors were diagnosed within the 2–23 months after first cancer diagnosis regardless of first tumor type. Concordance on histology was greater than 80 percent for both IBC and non-IBC cases, but was statistically significantly higher for IBC than non-IBC. Although these results may suggest that a contralateral IBC following an IBC and tumors diagnosed within the first 2–23 months after first cancer diagnosis are more likely to be recurrent or metastatic disease, concordance on tumor characteristics could also reflect multiple primary tumors arising in a common milieu [9].
Strengths of our study include the large number of IBC cases identified through the SEER program, the relatively large number of subsequent contralateral breast cancers, standardized rules for classifying contralateral breast cancers, the systematic recording of basic demographic information and tumor characteristics for both first and contralateral breast cancers, and information on follow-up, including cause of death. To our knowledge, this is the largest and most comprehensive study to date of contralateral breast cancer following IBC.
We must note several methodologic issues that need consideration in interpreting our results. The number of contralateral breast cancers following IBC was insufficient to allow adjustment for treatment effects. Since the early 1980s, IBC has generally been treated with neoadjuvant chemotherapy, mastectomy, and then postmastectomy radiation therapy, and possibly hormone therapy [20, 21]. Most of the chemotherapeutic regimens are similar to those used to treat non-IBC [21]. Similar to other analyses using SEER data [4], we were unable to adjust for adjuvant tamoxifen treatment of the first breast cancer, which is associated with reduced risk of contralateral ER-positive breast cancer in women with a hormone-receptive positive first breast cancer [22]. This is unlikely, however, to be a major shortcoming of our analysis because we saw consistent patterns of higher risk of contralateral breast cancer following IBC than non-IBC among those with HR-positive and HR-negative first tumors. We were also unable to adjust for family history of breast cancer or specific rare germline mutations, such as BRCA1, BRCA2, and TP53, associated with hereditary breast cancer, but these syndromes are rare [23]. Moreover, we are unaware of any data suggesting that hereditary breast cancer is more likely to be IBC. Most other breast cancer risk factors have not been consistently associated with contralateral breast cancer [24, 25]. We also can not rule out the possibility that medical surveillance is heightened following a first IBC compared to non-IBC, thus accounting for the higher risks of a contralateral breast cancer early on following IBC. To our knowledge, however, there are no data to support this suggestion.
In summary, risk of contralateral breast cancer was generally higher following IBC than non-IBC, particularly in the first five years after diagnosis. This was true both for contralateral cancers diagnosed within the first 2 years of diagnosis, which may be more likely to be metastatic disease [6], as well as for cancers diagnosed 2 or more years after first cancer diagnosis, possibly more likely to be independent primaries. Our findings point to intriguing areas for future research, including whether cancers in the contralateral breast following IBC are true second primaries or rather reflect an unusual propensity for IBC to metastasize to the contralateral breast. In either case, our results underscore the importance of vigilant screening of the opposite breast following a diagnosis of IBC as well as other types of breast cancer. Magnetic Resonance Imaging has been shown to be the most accurate imaging technique for detecting a breast parenchymal lesion in inflammatory breast cancer patients [26]. Both the high probability of death from IBC and the higher probability of a contralateral breast cancer compared to other types of breast cancer should be considered in evaluating risk management strategies for contralateral breast cancer following IBC [27].
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and contracts from the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
The authors thank Nathan Appel at IMS, Inc. and David Check at the National Cancer Institute for computer support.
References
- 1.Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH. Epidemiology of inflammatory breast cancer. Breast Dis. 2005–2006;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein JL, Lapinski RH, Thakore SS, Doucette JT, Thompson WD. The descriptive epidemiology of second primary breast cancer. Epidemiology. 2003;14:522–558. doi: 10.1097/01.ede.0000072105.39021.6d. [DOI] [PubMed] [Google Scholar]
- 3.Berrington de Gonzalez A, Curtis RE, Gilbert E, Berg CD, Smith SA, Stovall M, Ron E. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102:220–226. doi: 10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurian AW, McClure LA, John EM, Horn-Ross PL, Ford JM, Clarke CA. Second primary breast cancer occurrence according to hormone receptor status. J Natl Cancer Inst. 2009;101:1058–1065. doi: 10.1093/jnci/djp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchardy CB, Benhamou S, Fioretta G, Verkooijen HM, Chappuis PO, Neyroud-Caspar I, Castiglione M, Vinh-Hung V, Vlastos G, Rapiti E. Risk of second breast cancer according to estrogen receptor status and family history. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1137-z. Published online: 29 September 2010. [DOI] [PubMed] [Google Scholar]
- 6.Rubino C, Arriagada R, Delaloge S, Lê MG. Relation of risk of contralateral breast cancer to the interval since the first primary tumor. Br J Cancer. 2010;102:213–219. doi: 10.1038/sj.bjc.6605434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imyanitov EN, Hanson KP. Molecular pathogenesis of bilateral breast cancer. Cancer Letters. 2003;191:1–7. doi: 10.1016/s0304-3835(02)00523-2. [DOI] [PubMed] [Google Scholar]
- 8.Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr, editors. NIH Publ. No. 05–5302. National Cancer Institute; Bethesda, MD: 2006. New malignancies among cancer survivors: SEER Cancer Registries, 1973–2000. [Google Scholar]
- 9.Swain SM, Wilson JW, Mamounas EP, Bryant J, Wickerham D, Fisher B, Paik S, Wolmark N. Estrogen receptor status of primary breast cancer is predictive of estrogen receptor status of contralateral breast cancer. J Natl Cancer Inst. 2004;96:516–23. doi: 10.1093/jnci/djh097. [DOI] [PubMed] [Google Scholar]
- 10.Banelli B, Casciano I, Di Vinci A, Gatteschi B, Levaggi A, Carli F, Bighin C, Salvi S, Allemanni G, Ghiorzo P, Pronzato P, Venturini M, Romani M, Del Mastro L. Pathological and molecular characteristics distinguishing contralateral metastatic from new primary breast cancer. [Advanced Access, Oct 29];Ann Oncol. 2009 doi: 10.1093/annonc/mdp470. [DOI] [PubMed] [Google Scholar]
- 11.Tuttle T, Habermann E, Abraham A, Emory T, Virnig B. Contralateral prophylactic mastectomy for patients with unilateral breast cancer. Expert Rev Anticancer Ther. 2007;7:1117–1122. doi: 10.1586/14737140.7.8.1117. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2005 Counties. ( www.seer.cancer.gov) released April 2008, based on the November 2007 submission. [Google Scholar]
- 13.Klein JP, Moeschberger ML. Survival analysis; techniques for censored and truncated data. Springer Verlag; 2003. pp. 127–128. [Google Scholar]
- 14.Software Citation: DevCan: Probability of Developing or Dying of Cancer Software, Version 6.5.0. Statistical Research and Applications Branch, National Cancer Institute; 2010. http://srab.cancer.gov/devcan. [Google Scholar]
- 15.online text is a revision of the text “Basic Human Anatomy” which was published in 1983 by W.B. Saunders. Copyright c O'Rahilly 2008 Author: Ronan O'Rahilly, MD. Site editor: Rand Swenson, DC, MD, PhD With: Fabiola Muller, Dr. rer. nat. and Stanley Carpenter, PhD Contributors: Brian Catlin, MD John Lyons, MD Dartmouth Medical School
- 16.Van Laere S, Beissbarth T, Van der Auwera I, Van den Eynden G, Trinh XB, Elst H, Van Hummelen P, van Dam P, Van Marck E, Vermeulen P, Dirix L. Relapse-free survival in breast cancer patients associated with a gene expression signature characteristic for inflammatory breast cancer. Clin Cancer Res. 2008;14:7452–7460. doi: 10.1158/1078-0432.CCR-08-1077. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M, Valero V, Buzdar AU, Kau S-W, Broglio KR, Gonzalez-Angulo AM, Sneige N, Islam R, Ueno NT, Buchholz TA, Singletary SE, Hortobagyi GN. Inflammatory breast cancer (IBC) and patterns of recurrence. Cancer. 2007;110:1436–1444. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 18.Jaiyesimi IA, Buzdar AU, Hortobagyi G. Inflammatory breast cancer: A review. J Clin Oncol. 1992;10:1014–1024. doi: 10.1200/JCO.1992.10.6.1014. [DOI] [PubMed] [Google Scholar]
- 19.Bertucci F, Finetti P, Birnbaum D, Viens P. Gene expression profiling of inflammatory breast cancer. Cancer. 2010;116 (11 Suppl):2783–93. doi: 10.1002/cncr.25165. [DOI] [PubMed] [Google Scholar]
- 20.Dawood S, Merajver SD, Viens P, Vermeulen PB, Swain SM, Buchholz TA, Dirix LY, Levine PH, Lucci A, Krishnamurthy S, Robertson FM, Woodward WA, Yang WT, Ueno NT, Cristofanilli M. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2010 Aug 9; doi: 10.1093/annonc/mdq345. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawood S. Biology and management of inflammatory breast cancer. Expert Rev Anticancer Ther. 2010;10:209–220. doi: 10.1586/era.09.90. [DOI] [PubMed] [Google Scholar]
- 22.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 23.Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, Froster UG, Schlehe B, Bechtold A, Arnold N, Preisler-Adams S, Nestle-Kraemling C, Zaino M, Loeffler M, Kiechle M, Meindl A, Varga D, Schmutzler RK. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27:5887–5892. doi: 10.1200/JCO.2008.19.9430. [DOI] [PubMed] [Google Scholar]
- 24.Largent JA, Capanu M, Bernstein L, Langholz B, Mellemkjaer L, Malone KE, Begg CB, Haile RW, Lynch CF, Anton-Culver H, Wolitzer A, Bernstein JL. Reproductive history and risk of second primary breast cancer: the WECARE Study. Cancer Epidemiol Biomarkders Prev. 2007;16:906–911. doi: 10.1158/1055-9965.EPI-06-1003. [DOI] [PubMed] [Google Scholar]
- 25.Figueiredo JC, Bernstein L, Capanu M, Malone KE, Lynch CF, Anton-Culver H, Stovall M, Bertelsen L, Haile RW, Bernstein JL. Oral contraceptives, postmenopausal hormones, and risk of asynchronous bilateral breast cancer: The WECARE Study Group. J Clin Oncol. 2008;26:1411–1418. doi: 10.1200/JCO.2007.14.3081. [DOI] [PubMed] [Google Scholar]
- 26.Yang WT, Le-Petross HT, Macapinlac H, Carkaci S, Gonzalez-Angulo AM, Dawood S, Resetkova E, Hortobagyi GN, Cristofanilli M. Inflammatory breast cancer: PET/CT, MRI, mammography, and sonography findings. Breast Cancer Res Treat. 2008;109:417–426. doi: 10.1007/s10549-007-9671-z. [DOI] [PubMed] [Google Scholar]
- 27.Lostumbo L, Carbine NE, Wallace J, Ezzo J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database of Systematic Reviews. 2004;(4):Art. No.:CD002748. doi: 10.1002/14651858.CD002748.pub2. [DOI] [PubMed] [Google Scholar]