Abstract
Radiation-induced neurotoxicity is a well-characterized phenomenon. However, the underlying mechanism of this toxicity is poorly understood. In the central nervous system (CNS), excitotoxic mechanisms are implicated in many neurodegenerative disease processes. Pivotal to the excitotoxic pathway is dysfunction of glutamate signaling. We reported previously that exposure to low-LET γ radiation results in altered glutamate transport in neurons and astrocytes. In the present study, we sought to investigate the effects of various particle radiations of differing LET on glutamate transport as a measure of the neurochemical vulnerability of the CNS. NTera2-derived neurons and astrocytes isolated as pure and mixed cultures were exposed to doses of 10 cGy, 50 cGy or 2 Gy of 250 MeV protons, 290 MeV/nucleon carbon ions, or 1000 MeV/nucleon iron ions. Transporter function was assessed at 3 h, 2 days and 7days after exposure. Functional assessment of glutamate transport revealed that neurons and astrocytes respond in a reciprocal manner after exposure to particle radiation. Uptake activity in neurons increased after particle irradiation. This effect was evident as late as our last time (7 days) after exposure (P < 0.05). In astrocytes, transporter activity decreased after exposure. The decrease in uptake observed in astrocytes was evident 7 days after exposure to carbon and iron ions. Uptake in mixed cultures after exposure to all three forms of radiation revealed a muted interactive response suggestive of the individual responses of each cellular phenotype acting in opposition.
INTRODUCTION
Future manned space missions will be increasingly longer in duration, and therefore astronauts will be exposed to significant levels of galactic cosmic radiation. In deep space, galactic cosmic radiation is composed primarily of protons and high charge and energy (HZE) particles with abundance and energy spectra that are different than what is encountered during shuttle missions near the Earth. Exposure to HZE particles produces decreased neurogenesis, neurochemical alterations and behavioral changes similar to those of many age-related diseases (1-4). Thus thorough investigations into the effects of particle radiation are warranted to assess the potential radiation hazards to which astronauts will be exposed. These potential hazards will affect the planning of future space missions.
Clinical data and animal models demonstrate that exposure of normal brain tissue to any form of ionizing radiation results in behavioral, cognitive and motor deficits that manifest themselves over months or even years. The underlying cellular and molecular mechanisms of this neurotoxic response are poorly understood. However, given the highly integrated nature of the central nervous system (CNS), it has been theorized that the pathogenesis of this neurotoxic radioresponse involves multiple cellular targets (5). The emerging model of radiation-induced pathogenesis maintains that interactions between the various CNS cellular phenotypes determine the systemic CNS radioresponse (5).
The current view of the dynamic nature of the CNS is radically different than in previous years, wherein the CNS was considered relatively radioresistant (3, 6-9). Many transformative processes have come to be recognized as inherent elements in the function and characterization of the CNS. Processes such as neurogenesis and synaptic plasticity underlie changes in the brain that have long-term structural and functional consequences. Neural precursor cells have been shown to be particularly sensitive to both photon and particle radiations. Dose- and LET-dependent reductions of neural precursors have been reported in mice after exposure to 1–3 Gy iron and carbon ions (10). In general, impairments in neurogenesis are dependent on dose (4, 10-12). The decrease in neurogenesis is most evident at doses less than 50 cGy (12). However, it appears that the ultimate deficits observed in the CNS are the result of more than cell loss. Monje et al. proposed that alterations in local signaling lead to chronic dysfunction of the neurogenic microenvironment (13). Radiation of differing quality and dose can alter factors in the microenvironment affecting various cellular programs including regulation of oxidative states, neuronal excitability and cellular differentiation (14, 15). Based on these observations, the pathogenesis of the CNS radioresponse is complex and involves diverse pathways. This response appears to be dependent on LET for certain distinct but possibly interrelated processes such as neurogenesis, synaptic remodeling and free radical production.
In the mammalian CNS, glutamate is the principal excitatory neurotransmitter and is essential for normal neurotransmission because it mediates most excitatory synaptic transmission. It is involved in synaptic plasticity and is therefore believed to play an integral role in learning and memory processes. However, glutamate is also implicated in many neurodegenerative disorders and neurotoxic processes such as ischemia, stroke and seizure. Because glutamate is required for normal brain function, its release and clearance from the extracellular space are carefully controlled. After synaptic release, extracellular glutamate must be cleared quickly, because high concentrations of glutamate are known to initiate neurotoxic cascades, principally excitotoxicity and oxidative glutamate toxicity. Therefore, injury or an aberration within the glutamatergic system would have a profound impact on the function of associated circuits. For example, deterioration of astrocytic glutamate uptake by radiation might adversely affect excitatory synaptic transmission in the hippocampus, which relies on low concentrations of extracellular glutamate in the synaptic cleft. Glutamate transporters represent the only significant mechanism for the uptake of extracellular glutamate, and their importance in the long-term maintenance of low non-toxic glutamate concentrations is well documented (16). Reuptake of glutamate is carried out primarily by five isoforms of high-affinity, sodium-dependent glutamate transporters (EAATs 1–5). Astrocytes and neurons each express four of five isoforms to varying degrees. The transporters localized on astrocytes are believed to be responsible for the majority of glutamate uptake.
In our laboratory we observed alterations in glutamate transport after exposure to γ rays and found that neurons and astrocytes responded reciprocally in terms of glutamate uptake (17 ). In general, neurons increased glutamate uptake after exposure, whereas glutamate uptake in astrocytes decreased. Given the current understanding of the neurobiological response after exposure to HZE-particle radiation and our previous findings of altered neurotransmitter uptake after γ-ray exposure, we investigated the premise that particle radiation would differentially alter glutamate transport in astrocytes and neurons more dramatically. In the present study we sought to gain a better understanding of the cellular mechanism(s) that underlies the neuropathology observed in the mature brain after exposure to particle radiation of varying LET. To achieve this objective, we investigated the effect of HZE-particle radiation on mixed cocultures as well as isolated pure cultures of postmitotic neurons and astrocytes derived from the NTera2/D1 cell line. Changes in transporter function were assessed 3 h, 2 days and 7 days after exposure to 10 cGy, 50 cGy and 2 Gy of 250 MeV protons, 290 MeV/nucleon carbon ions, or 1000 MeV/nucleon iron ions.
MATERIALS AND METHODS
Cell Culture
Culture of NT2/D1 cells was carried out as described previously (18, 19). NT2 cells were plated at a density of 2.5 ×106 cells per T-75 flask in 13 ml complete medium (DMEM/F12 medium supplemented with 10% FBS, 5% penicillin-streptomycin, and 2 mM l-glutamine). Cells were incubated at 37°C in 95% air/5% CO2 until they reached approximately 80% confluence (usually 2 to 3 days) prior to initiating differentiation. Differentiation: During the differentiation period, cells were cultured in 12 ml of complete medium containing 10 μM retinoic acid (RA). Medium was replaced three times a week for a total of 4 weeks to induce the neural phenotype. Replate 1: After 4 weeks, cells were split 1:6 in complete medium and cultured for 48 h. Replate 2 (neuron and astrocyte harvesting): Neurons (NT2/N) were separated from the underlying glial layer by mechanically dislodging neurons by gently striking the flask. Harvested neurons were then plated onto Matrigel-coated plates (BD Biosciences, San Jose, CA), while astrocytes (NT2/A) were replated 1:3 onto new T-75 flasks. “Neuron medium” was replenished every 3 or 4 days with “inhibition medium” (DMEM/F12 medium, 5% FBS, 1% penicillin-streptomycin, 10 μM FrdU, 10 μM Urd, 1 μM araC) for a maximum of 2 weeks. Astrocytes were maintained in complete DMEM/F12 medium and replated every 7–10 days.
Neurons and astrocytes were maintained in culture for 7 and 2 days, respectively, prior to use in the various experimental procedures. Cells were plated at a density of 2.0 × 105 cells per well in six-well plates. Mixed cultures of neurons and astrocytes were plated at the same density but at a ratio of 1:10 neurons to astrocytes.
Radiation Conditions
External-beam proton irradiations were performed using 250 MeV protons (LET 0.4) from the Loma Linda University Proton Research Facility synchrotron accelerator. The dose rate delivered was in the range of 65 cGy/min, and the proton beam was 20 cm in diameter. The proton doses delivered were 10 cGy, 50 cGy and 2 Gy. High-energy 56Fe- and 12C-ion exposures were conducted at the Brookhaven National Laboratory (BNL) NASA Sponsored Research Laboratory during NSRL runs 7c and 8a. A single fraction of 10 cGy, 50 cGy or 2 Gy 56Fe (1000 MeV/nucleon, LET 148 keV/μm) or 12C ions (290 MeV/nucleon, LET 13 keV/μm) was delivered to cell cultures. Radiation was delivered at a dose rate of 20 cGy/min, 50 cGy/min and 1 Gy/min for doses of 10 cGy, 50 cGy and 2 Gy, respectively. The total width of the particle beam was 20 cm with 18 cm of usable area. Immediately before delivery of the beam, cell culture vessels (six-well plates) were flipped such that the surface on which cells were grown was perpendicular to the plane of the beam. Cell cultures were assayed at 3 h, 2 days or 7 days after exposure.
3H-Glutamate Uptake
3H-glutamate uptake in isolated NT2/N and NT2/A cells and mixed cultures was measured as described previously (17, 20, 21). After irradiation, cells were washed twice in PBS and equilibrated in Krebs buffer (Sigma-Aldrich, St. Louis, MO) for 20 min at 37°C. Uptake was initiated by the addition of 1 ml l-[3,4-3H]glutamate [0.5 μCi (1.5 kBq)/ml] (GE Healthcare, Piscataway, NJ). Uptake was terminated after 20, 40 and 60 min by adding 2 ml ice-cold PBS. The cells were then washed in ice-cold PBS and trypsinized. Triplicate fractions were collected, and incorporated radioactivity was determined using a liquid scintillation counter (model LS 6500, Beckman Coulter, Fullerton, CA) at Loma Linda University and a Packard Tri-Carb 2500TR liquid scintillation analyzer at BNL. Uptake of 3H-glutamate was calculated as counts per minute per milligram of protein. Protein quantification was carried out using the Bradford method.
Image Capture and Analysis
Cells were viewed through an Olympus IX-70-based microscope (Horizon Optical, Temecula, CA), and images were captured with ImagePro (Media Cybernetics, Silver Springs, MD). Cellular morphometry measurements were obtained using the image analysis software Ellipse 2.0.5.1 (ViDiTo, Kosice, Slovakia). Cell area was determined based on defined soma perimeter with exclusion of processes.
Statistical Analysis
Two-way repeated-measures analysis of variance (ANOVA) with one within-subjects factor (time) and one between-subjects factor (dose) and their interaction term was used to compare the effect of different doses over time. ANOVA with the Tukey test for further multiple comparisons was used to compare the different doses with each other if the dose response was significant. A P value of <0.05 was taken to indicate significant differences among groups. The analyses were performed using SigmaStat 2.03 (Systat Software, Inc., San Jose, CA).
RESULTS
Established cultures of astrocytes and neurons were derived from NTera2/D1 embryonal carcinoma cells in which exposure to retinoic acid induces differentiation along a neuroectodermal lineage (22, 23) and gives rise to cells of two distinct lineages that exhibit mature phenotypes (18, 19, 24). We followed the procedure described by Sandhu et al. (19) to differentiate these stem cells into neurons and astrocytes. Previous immunocytochemical analysis confirmed that the differentiation process yielded two types of cells displaying mature neuronal phenotype and full expression of glutamate transporters (17 ).
Functional Effects of Proton Radiation on Glutamate Transport
The functional effect of particle radiation on glutamate transport activity was determined directly by measuring the collective capacity of the transporters to take up labeled glutamate.
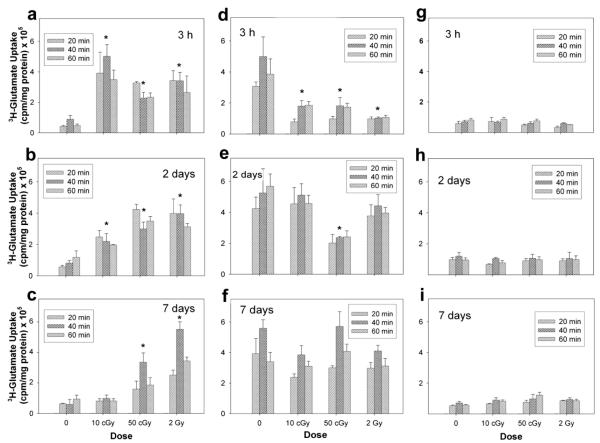
Unirradiated neurons exhibited a low basal level of glutamate uptake (Fig. 1a–c). Maximal uptake of 3H-glutamate was generally observed to occur 40 min after 3H-glutamate incubation. In irradiated neurons, a statistically significant interaction was found between radiation dose and time after irradiation (P < 0.05). Pairwise multiple comparisons indicated a significant increase in uptake at 3 h and 2 days after exposure to each of the three doses of protons used (P < 0.05, n = 3). At 3 h after exposure, uptake increased between 1.6- and 4.7-fold relative to unirradiated control cultures (Table 1). Two days after irradiation, the increase in uptake was still present at all three doses. By 7 days after exposure, only those cultures exposed to 50 cGy and 2 Gy displayed a statistically significant increase in uptake of 4.7- and 8.4-fold, respectively.
FIG. 1.
Functional assessment of glutamate transporters in isolated neuron (panels a–c) and astrocyte cultures (panels d–f) and mixed cocultures (panels g–i) after exposure to 250 MeV protons. Functional activity was assessed directly by measuring the uptake of 3H-glutamate 3 h, 2 days and 7 days after irradiation. Data are the average values of three replicate experiments ± SD.
TABLE 1.
Effects of Protons and HZE Particles on Glutamate Uptake in Astrocytes and Neurons
| Cell type | Radiation type | Dose-averaged LET (keV/μm) |
Dose | Fluence (particles/cm2) |
Average hits/cell |
Glutamate uptakea at 3 h |
Glutamate uptakea at 2 days |
Glutamate uptakea at 7 days |
|---|---|---|---|---|---|---|---|---|
| Astrocytes | Gamma raysb (1.17 and 1.33 MeV) |
0.25 | 10 cGy | n/a | n/a | – | – | – |
| 50 cGy | n/a | n/a | decreased | – | – | |||
| 2 Gy | n/a | n/a | decreased | – | – | |||
| Protons (250 MeV) | 0.4 | 10 cGy | 1.6 × 108 | 11,098 | decreased | – | – | |
| 50 cGy | 7.8 × 108 | 55,494 | decreased | decreased | – | |||
| 2 Gy | 3.1 × 109 | 221,976 | decreased | – | – | |||
| Carbon ions (290 MeV/ nucleon) |
13 | 10 cGy | 4.8 × 106 | 342 | decreased | – | decreased | |
| 50 cGy | 2.4 × 107 | 1708 | decreased | – | decreased | |||
| 2 Gy | 9.6 × 107 | 6830 | decreased | decreased | decreased | |||
| Iron ions (1000 MeV/ nucleon) |
148 | 10 cGy | 4.2 × 105 | 30 | – | decreased | decreased | |
| 50 cGy | 2.1 × 106 | 150 | – | decreased | decreased | |||
| 2 Gy | 8.4 × 106 | 600 | – | decreased | – | |||
| Neurons | Gamma rays (1.17 and 1.33 MeV) |
0.25 | 10 cGy | 2.5 × 108 | 445 | increased | – | – |
| 50 cGy | 1.2 × 109 | 2224 | increased | increased | increased | |||
| 2 Gy | 5.0 × 109 | 8895 | increased | increased | increased | |||
| Protons (250 MeV) | 0.4 | 10 cGy | 1.6 × 108 | 278 | increased | increased | – | |
| 50 cGy | 7.8 × 108 | 1390 | increased | increased | increased | |||
| 2 Gy | 3.1 × 109 | 5560 | increased | increased | increased | |||
| Carbon ions (290 MeV/ nucleon) |
13 | 10 cGy | 4.8 × 106 | 9 | increased | increased | increased | |
| 50 cGy | 2.4 × 107 | 43 | increased | – | – | |||
| 2 Gy | 9.6 × 107 | 171 | increased | – | – | |||
| Iron ions (1000 MeV/ nucleon) |
148 | 10 cGy | 4.2 × 105 | 1 | – | increased | – | |
| 50 cGy | 2.1 × 106 | 4 | increased | increased | increased | |||
| 2 Gy | 8.4 × 106 | 15 | increased | increased | increased |
Dashes indicate that there was no significant change in uptake after irradiation.
LET based on microdosimetry electron spectra.
Unirradiated astrocytes displayed higher uptake than unirradiated neurons. However, in marked contrast to irradiated neuronal cultures, astrocytes exposed to proton radiation responded with significant decreases (Fig. 1d–f). At 3 h, an approximate 0.7-fold decrease in uptake was detected after all three doses. At 2 days after exposure only those astrocytes irradiated with 50 cGy protons continued to exhibit a 0.6-fold decrease in uptake. Unlike neurons, exposure of astrocytes to protons did not result in any significant alteration in uptake at 7 days after exposure.
Mixed cultures of neurons and astrocytes did not exhibit any statistically significant differences in uptake after exposure to protons. Mixed cultures displayed a muted response in uptake (Fig. 1g–i) that seemed unaffected by dose or time.
Functional Effects of HZE-Particle Radiation on Glutamate Transport
1. Carbon-ion effects on glutamate uptake
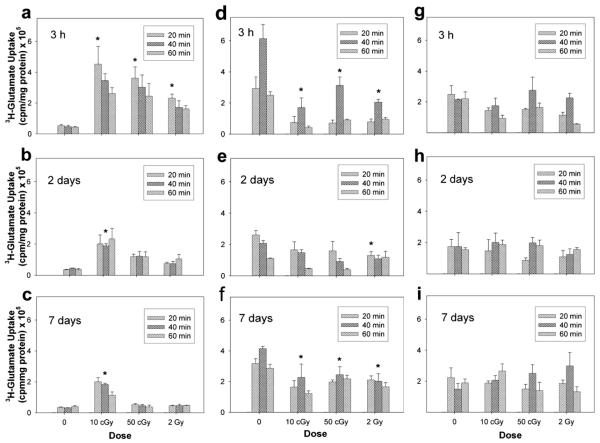
Similar to what we observed with protons, uptake in neurons increased in response to carbon-ion exposure. Lower doses of carbon ions produced a greater increase in uptake. Significant increases in glutamate uptake were measured at 3 h after irradiation (Fig. 2a–c). At 2 days after exposure only those cultures irradiated with 10 cGy continued to exhibit alterations in uptake. This effect was still present at 7 days after exposure.
FIG. 2.
Functional assessment of glutamate transporters after exposure to 290 MeV/nucleon carbon ions in isolated neuron (panels a–c) and astrocyte cultures (panels d–f) and mixed cocultures (panels g–i). Functional activity was assessed directly by measuring the uptake of 3H-glutamate in cultures 3 h, 2 days and 7 days after irradiation. Data are the average values of three replicate experiments ± SD.
Exposure of astrocytes to carbon ions produced a significant decrease in uptake activity at 3 h, 2 days and 7 days after irradiation (Fig. 2d–f ). Fluctuations in uptake were observed over the 7-day time course. At 3 h there was on average a 0.6-fold reduction in uptake that was not linear. By 2 days, astrocytes displayed significantly reduced uptake only after exposure to 2 Gy carbon ions. By 7 days after exposure, all irradiated cultures exhibited decreases in uptake that were approximately 0.5-fold lower than those in unirradiated control cultures.
Uptake in irradiated mixed cultures of neurons and astrocytes was not significantly different than uptake measured in unirradiated control cultures (Fig. 2g–i). There was no effect of radiation on the mixed cultures for any of the doses and times used in this study.
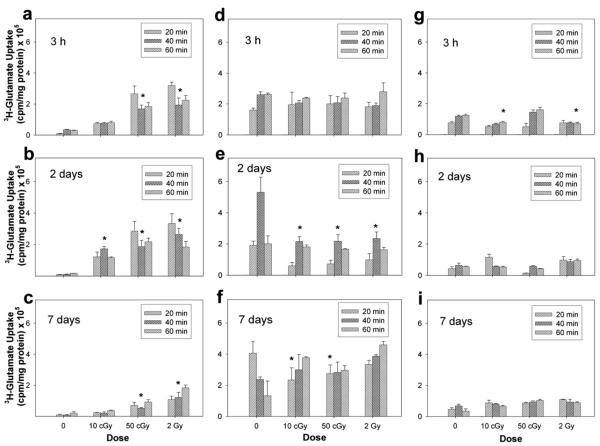
2. Iron-ion effects on glutamate uptake
Exposure to iron ions (Fig. 3) produced effects on glutamate transport similar to those with carbon ions. At 3 h after exposure to 1000 MeV/nucleon iron ions, glutamate uptake in neurons increased significantly in cultures exposed to 50 cGy and 2 Gy. At 2 days after exposure, there was a significant increase in uptake in all irradiated cultures. Transporter activity increased approximately 19.1-fold relative to unirradiated control cultures. By 7 days, cultures exposed to 50 cGy and 2 Gy showed an uptake similar to that seen at 3 h.
FIG. 3.
Functional assessment of glutamate transporters in isolated neuron (panels a–c) and astrocyte cultures (panels d–f ) and mixed cocultures (panels g–i) after exposure to 1000 MeV/nucleon iron ions. Functional activity was assessed directly by measuring the uptake of 3H-glutamate in cultures 3 h, 2 days and 7 days after irradiation. Data are the average values of three replicate experiments ± SD.
In astrocytes there was no change in uptake at 3 h after exposure to iron ions (Fig. 3d–f ). At 2 days, there was a detectable decrease across all three doses. By 7 days after exposure, only those cultures exposed to 10 and 50 cGy iron ions continued to display significant differences in uptake.
Unlike mixed cultures irradiated with proton or carbon ions, exposure of mixed cocultures to iron ions produced an initial change in glutamate uptake after exposure to iron ions (Fig. 3g–i). At 3 h, there was approximately a 0.4-fold decrease in uptake after 10 cGy and 2 Gy. Transporter activity in irradiated cultures returned to baseline values by 2 days after exposure, and at 7 days there were no significant differences in uptake measured between irradiated mixed cultures and un-irradiated controls.
Effect of Paraquat-Induced ROS on Glutamate Uptake
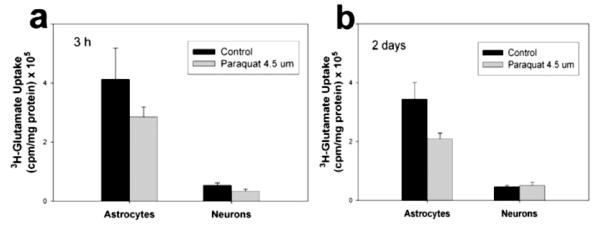
To ascertain whether radiation mediated the observed changes in transporter activity through a redox-regulated mechanism, we investigated the regulation of transporter activity via changes in oxidant status. Glutamate transporters possess redox-sensing cysteine residues that regulate transport rate. Therefore, we wanted to know whether the observed changes in transporter activity after radiation exposure could be modulated by the increased ROS load known to result from radiation exposure. If radiation were modulating transporter activity principally via an ROS-mediated mechanism, then any other ROS stimulus should initiate this mechanism. Investigation of transporter activity regulation via redox mechanisms was determined by chemically increasing ROS levels. Paraquat (N,N’-dimethyl-4,4′-bipyridinium dichloride), a potent generator of superoxide ions, was selected because 4 μM paraquat generates the same level of ROS that is measured postirradiation. A time-course study of 3H-glutamate uptake revealed that exposure to ROS resulting from Paraquat application produced a maximum decrease in glutamate uptake at 40 min (Fig. 4). Paraquat-induced ROS produced a slight decrease in uptake that was not statistically significant. In neurons, chemically induced ROS production failed to evoke any significant changes in transporter activity after 3 h or 2 days of application. These findings indicate that an increased ROS load, in particular superoxide, does not significantly alter glutamate uptake in astrocytes or neurons after Paraquat treatment or account for the reciprocal responses in these cell types when the stressor encountered is radiation.
FIG. 4.
Glutamate uptake after Paraquat treatment. Transporter activity was assessed by measuring 3H-glutamate uptake in astrocytes and neurons at 3 h (panel a) and 2 days (panel b) after inducing ROS production with 4.5 μM Paraquat. Data represent the average values of three replicate experiments ± SD.
DISCUSSION
Alterations in glutamate transport are commonly observed as a consequence of a variety of adverse conditions such as hypoxia or exposure to ROS generators. Transporter dysfunction is believed to be a precursor of neurotoxic cascades. We investigated the effects of particle radiation on isolated and mixed cell types of the CNS. Neurons and astrocytes produced reciprocal glutamate transport profiles when exposed to 250 MeV protons, 290 MeV/nucleon carbon ions, and 1000 MeV/nucleon iron ions. Exposure of neuronal transporters resulted in increased transporter activity, whereas astrocyte transporters responded with a decrease in transporter activity. These results resemble those we reported previously with γ rays. This strongly suggests that alterations in glutamate transporter activity are a common response to ionizing radiations. Based on observations by other investigators, particle radiation is known to have a greater impact on biological end points of neurotoxicity in NT2 cells (25) and oxidative stress in neural precursors (26). We expected to see responses that would reflect the differences in radiation quality and LET. We did not observe a clear LET dependence with protons and heavy ions. However, consistent with observations made by other investigators, we observed a longer biological effect with particle radiation than with γ rays (17 ).
The increase in glutamate uptake in neurons after exposure to protons and carbon and iron ions was surprising because glutamate transporters localized on neurons are generally believed not to participate significantly in the clearance of extracellular glutamate. It is not currently understood what the main function is of the transporters expressed on neurons. However, it has been suggested that their function may involve regulation of cellular oxidative stress and synaptic remodeling, because neuronal glutamate transport is associated with cysteine uptake (27, 28), and the activity and expression of EAAT3 have been shown to be highly regulated by neuronal activity (29, 30). The increase in transporter activity we measured in neurons after particle exposure was not unexpected because we had previously noted this response using γ rays (17 ). However, the changes in neuron transporter activity were much greater than those measured in astrocytes, and the changes observed in neurons were greater at all times and for all types of radiation used here. We did not detect a simple LET dependence of modulated transporter activity in neurons. In astrocytes, the general response to protons and carbon- and iron-ion radiation was a decrease in transporter activity similar to that observed using γ rays. In astrocytes at 3 h, differences after exposure could be attributed to fluence. However, unlike the recovery time observed previously with γ radiation, transporter activity in astrocytes after particle irradiation did not return to baseline by 2 days after exposure. The alterations in astrocyte transporter activity were evident at 2 days after exposure to all three radiations and were still evident at 7 days after carbon- and iron-ion irradiation. Overall, the biological response of neurons and astrocytes measured in terms of glutamate uptake was more pronounced and longer-lasting with HZE-particle radiation than with γ rays. These findings are consistent with those of other investigators in which exposure of neural precursors to particle radiation resulted in more rapid and persistent changes (26, 31).
In the brain, neurons and glia do not exist in isolation. Rather they comprise a functional unit. To better understand the collective response resulting from neuroglial interactions, we plated mixed cultures of neurons and astrocytes at similar ratios to those found in the brain. Coculturing of neurons and astrocytes produced cultures that exhibited a robust and radioresistant phenotype in which the uptake response did not differ from that of unirradiated mixed cultures in response to proton and carbon-ion radiation. However, irradiation of cultures with high-LET iron ions produced an initial change in transporter activity at 3 h after exposure.
We used 250 MeV protons with a corresponding LET of 0.4 keV/μm, which is not numerically that different than 0.25 keV/μm for γ rays. However, irradiation with protons resulted in changes in transporter activity that were more dramatic at 3 h compared to γ radiation and that persisted at later times that were not detectable after γ irradiation (Table 1). A similar response pattern was observed with carbon and iron ions. This appears to indicate that track structure with more clustering of ions is significant in transporter activity. The magnitude of change for a given dose decreased with time. Iron-ion irradiation of astrocytes failed to produce initial changes in uptake at 3 h; however, at 2 days after exposure, astrocytes exhibited decreased uptake and continued to do so at 7 days after 10 and 50 cGy. It may be that the subtle differences in the response patterns of neurons and astrocytes to different ions result from the complex interplay of track structure for a particular ion species and cytoarchitectural influence (32).
Glutamate uptake is regulated at several levels, primarily via expression of transporter protein and functional activity of the expressed transporters. Previous investigations led us to conclude that changes in glutamate uptake after radiation exposure are not regulated by changes in transporter protein expression levels but rather are regulated at the level of transporter activity (17). Glutamate transporters appear to be susceptible to changes in cellular redox state, because they possess redox-sensing cysteine residues that regulate transport rate via thiol-disulfide redox interconversion. Three transporter subtypes [EAAT-1 (GLAST), EAAT-2 (GLT1), and EAAT-3] have been shown to be equally inhibited by oxidants (33). If radiation were in fact modulating transporter activity principally via an ROS-mediated mechanism, then it seems logical that any ROS stimulus could initiate this mechanism to modulate transporter activity. Chemically increasing ROS levels using Paraquat failed to evoke long-term uptake changes or to mirror the changes in transporter activity observed after radiation exposure. Therefore, the significant and persistent changes in transporter activity observed in astrocytes and neurons after irradiation are not principally mediated by redox modulatory mechanisms. As such, it does not appear that secondary reactive processes, like increased oxidant levels after exposure, regulate transporter activity long term, nor do they account for the reciprocal responses in these particular cell types in the context of radiation-induced ROS.
Current knowledge of transporter regulation suggests that the transporters are differentially regulated wherein each transporter subtype is responsive to multiple and at times opposing factors. An example of this is PKC activation, which is known to increase EAAT-1 and EAAT-2 expression but decrease EAAT3 trafficking to the cell surface (34). Furthermore, the predominant transporters EAAT-1 and EAAT-2 can be reciprocally regulated by the same stimulus. Examples of this are the up-regulation EAAT-1 but not EAAT-2 with cAMP and the opposing effects of glutamate on EAAT-1 and EAAT-2 expression (35). When assessing transporter activity, we must keep in mind that we are measuring the collective uptake activity of all transporter subtypes present on a given cell type. Astrocytes and neurons can express any combination of transporter subtypes at any given time. Given this, it is probable that radiation may be activating a signaling cascade that preferentially activates a molecular target with opposing effects in neurons and astrocytes. Such a mechanism appears more likely to involve regulation of glutamate transporter activity rather than a strictly redox mechanism and also helps explain the reciprocal effects observed in the two cell types.
Other investigators have proposed that cell type-specific metabolic and signaling pathways may be responsible for differences in the glial uptake response after injury (36, 37 ). Astrocytes express several enzymes involved in glutamate metabolism and transport that neurons do not. An example of this is that astrocytes preferentially metabolize acetate compared to neurons. These cell-specific differences become evident when a toxin such as fluoroacetate, a selective inhibitor of the TCA cycle in astrocytes, impairs not only synthesis of glutamate precursor but also glutamate uptake (38, 39). Such differences in the cellular metabolic machinery could account for the uptake response observed in cultures of isolated neurons and astrocytes.
In our mixed cultures we measured low uptake activity in both irradiated and unirradiated mixed cultures. Cocultures of neurons and astrocytes exhibited an uptake response distinct from that observed in isolated neurons or astrocytes. The basal transporter activity in mixed cultures was generally low. In unirradiated mixed cultures, neurons seemed to influence the uptake response given the relatively low level of transporter activity and ratio of neurons to astrocytes. In trying to understand this response pattern we cannot ignore the bidirectional crosstalk that takes place between neurons and astrocytes. Nakajima et al. investigated the influence of neuronal conditioned medium on the activity and level of glutamate transporters in rat microglia. They demonstrated that a neuronal stimulus appeared to promote the uptake of glutamate in activated microglia (40). Similar to this finding, we observed that coculturing neurons and astrocytes produced a basal uptake that was low and that appeared to be influenced by neurons. When mixed cultures were irradiated, we did not see characteristic changes in uptake that were predominantly neuron- or astrocyte-driven. That is, irradiated mixed cultures did not exhibit uptake responses expected of either irradiated astrocytes or neurons. Because the response profile observed in irradiated mixed cultures did not differ significantly from unirradiated cell cultures, this suggests a resistance to perturbations such as radiation. We believe the blunted response was indicative of a homeostatic effect composed of interactive neuron-astrocyte responses. This effect can be interpreted to mean that the overall uptake of glutamate from the extracellular space is not expected to be compromised after radiation exposure. Furthermore, when the individual response of each cell type was examined, we found that in neuron cultures uptake increased after exposure, whereas in astrocytes uptake decreased but was not completely abolished. Therefore, it seems reasonable to surmise that radiation-induced neurotoxic cascades would not be initiated by altered glutamate uptake at the level of glutamatergic receptor overactivation because the net concentration of extracellular glutamate should be the same in irradiated and unirradiated mixed cultures. Alterations in glutamate uptake, however, could result in abnormal intracellular glutamate concentrations. Intracellular changes in neurotransmitter concentrations could potentially trigger aberrant signaling between neurons and astrocytes because intercellular signaling between these cells is partly mediated by glutamate and calcium. Calcium-dependent glutamate release from astrocytes is known to modulate neuronal excitability (41) and synaptic transmission (42). Changes in these fundamental CNS functions may ultimately translate to dysfunction in cognitive and behavioral output. Our data indicate that glutamate is involved in the radioresponse of the CNS, perhaps not at the level of receptor-mediated excitotoxicity but rather as a signaling mediator of radiation injury.
An additional component to consider in the regulation of glutamate-mediated transmission through neuron-glial interactions is the role of the vasculature, which is paramount in this process by supplying the basic energy components required to sustain such interactions. Increased neuronal activity places an energetic demand that is met by a continuous vascular supply of glucose and oxygen (43). Data suggest that glutamate coordinates both metabolic and vascular responses to neuronal activity, with astrocytes playing a key role in coupling synaptic activity to glucose use (44). Several mechanisms have been suggested to be involved in neurometabolic and neurovascular coupling during activation. These models are supported by anatomical studies that demonstrate that, in addition to being surrounded by astrocytic processes, blood vessels are also in direct contact with nerve terminals in some cases. Different subtypes of receptors for select neurotransmitters and neuropeptides have been identified on specific cellular components of the microvascular unit (i.e., endothelial and smooth cells and their associated perivascular astrocytes) (43, 45-47 ). Furthermore, bidirectional calcium signaling can occur between astrocytes and endothelial cells via gap junctions and paracrine signaling (48). Clearly, a significant degree of intercellular signaling takes place among the various CNS cell types. The relationship between radiation-induced glutamate changes and neuroglial and vascular intercellular signaling represents a potential area of study in understanding radiation-induced pathophysiology within the CNS.
ACKNOWLEDGMENTS
This work was supported by NASA grants 06RAD2-61 (LMG) and NAG 9-1452 (LMG), NASA Cooperative Agreement NNJ06HD78A (JMS), and NIH award R25GM060507. We are grateful to Drs. Marcelo Vazquez and Peter Guida at Brookhaven National Laboratory (BNL) for kindly providing the NTera2 cells. We also wish to acknowledge Leticia Ortloff and Brandon Bianski for their technical assistance as well as the staff at BNL’s NASA Space Radiation Laboratory for their technical and logistical support.
REFERENCES
- 1.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 2.Shukitt-Hale B, Casadesus G, Cantuti-Castelvetri I, Rabin BM, Joseph JA. Cognitive deficits induced by 56Fe radiation exposure. Adv. Space Res. 2003;31:119–126. doi: 10.1016/s0273-1177(02)00878-5. [DOI] [PubMed] [Google Scholar]
- 3.Joseph JA, Shukitt-Hale B, McEwen J, Rabin BM. CNS-induced deficits of heavy particle irradiation in space: the aging connection. Adv. Space Res. 2000;25:2057–2064. doi: 10.1016/s0273-1177(99)01013-3. [DOI] [PubMed] [Google Scholar]
- 4.Rola R, Otsuka S, Obenaus A, Nelson GA, Limoli CL, VandenBerg SR, Fike JR. Indicators of hippocampal neurogenesis are altered by 56Fe-particle irradiation in a dose-dependent manner. Radiat. Res. 2004;162:442–446. doi: 10.1667/rr3234. [DOI] [PubMed] [Google Scholar]
- 5.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat. Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Chapman JD, Allalunis-Turner MJ. Cellular and molecular targets in normal tissue radiation injury. In: Gutin P, Leibel S, Sheline G, editors. Radiation Injury to the Nervous System. Raven Press; New York: 1991. pp. 6–7. [Google Scholar]
- 7.Noel F, Tofilon PJ. Astrocytes protect against X-ray-induced neuronal toxicity in vitro. Neuroreport. 1998;20:1133–1137. doi: 10.1097/00001756-199804200-00032. [DOI] [PubMed] [Google Scholar]
- 8.Blakely EA, Chang PY. A review of ground-based heavy ion radiobiology relevant to space radiation risk assessment: Cataracts and CNS effects. Adv. Space Res. 2007;40:1307–1319. [Google Scholar]
- 9.Nojima K, Ando K, Fujiwara H, Ando S. Effects of carbon ions on primary cultures of mouse brain cells. Adv. Space Res. 2000;25:2051–2056. doi: 10.1016/s0273-1177(99)01016-9. [DOI] [PubMed] [Google Scholar]
- 10.Rola R, Sarkissian V, Obenaus A, Nelson GA, Otsuka S, Limoli CL, Fike JR. High-LET radiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat. Res. 2005;164:556–560. doi: 10.1667/rr3412.1. [DOI] [PubMed] [Google Scholar]
- 11.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 12.Rola R, Fishman K, Baure J, Rosi S, Lamborn KR, Obenaus A, Nelson GA, Fike JR. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with 56Fe particles. Radiat. Res. 2008;169:626–632. doi: 10.1667/RR1263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 14.Vlkolinsky R, Krucker T, Nelson GA, Obenaus A. 56Fe-particle radiation reduces neuronal output and attenuates lipopolysaccharide-induced inhibition of long-term potentiation in the mouse hippocampus. Radiat. Res. 2008;169:523–530. doi: 10.1667/RR1228.1. [DOI] [PubMed] [Google Scholar]
- 15.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin. Radiat. Oncol. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez MC, Benitez A, Ortloff L, Green LM. Alterations in glutamate uptake in NT2-derived neurons and astrocytes after exposure to gamma radiation. Radiat. Res. 2009;171:41–52. doi: 10.1667/RR1361.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pleasure SJ, Page C, Lee VM. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J. Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandhu JK, Sikorska M, Walker PR. Characterization of astrocytes derived from human NTera-2/D1 embryonal carcinoma cells. J. Neurosci. Res. 2002;68:604–614. doi: 10.1002/jnr.10236. [DOI] [PubMed] [Google Scholar]
- 20.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J. Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandhu JK, Pandey S, Ribecco-Lutkiewicz M, Monette R, Borowy-Borowski H, Walker PR, Sikorska M. Molecular mechanisms of glutamate neurotoxicity in mixed cultures of NT2-derived neurons and astrocytes: protective effects of coenzyme Q10. J. Neurosci. Res. 2003;72:691–703. doi: 10.1002/jnr.10579. [DOI] [PubMed] [Google Scholar]
- 22.Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- 23.Lee VM, Andrews PW. Differentiation of NTERA-2 clonal human embryonal carcinoma cells into neurons involves the induction of all three neurofilament proteins. J Neurosci. 1986;6:514–521. doi: 10.1523/JNEUROSCI.06-02-00514.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bani-Yaghoub M, Bechberger JF, Underhill TM, Naus CC. The effects of gap junction blockage on neuronal differentiation of human NTera2/clone D1 cells. Exp. Neurol. 1999;156:16–32. doi: 10.1006/exnr.1998.6950. [DOI] [PubMed] [Google Scholar]
- 25.Guida P, Vazquez ME. Cytotoxic and cell cycle effects in human neuronal progenitor cells exposed to 1 GeV/n Fe ions. Adv. Space Res. 2007;39:1004–1010. [Google Scholar]
- 26.Limoli CL, Giedzinski E, Baure J, Rola R, Fike JR. Redox changes induced in hippocampal precursor cells by heavy ion irradiation. Radiat. Environ. Biophys. 2007;46:167–172. doi: 10.1007/s00411-006-0077-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J. Neurochem. 2003;84:1332–1339. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- 28.Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 29.Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat. Neurosci. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- 30.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur. J. Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 31.Giedzinski E, Rola R, Fike JR, Limoli CL. Efficient production of reactive oxygen species in neural precursor cells after exposure to 250 MeV protons. Radiat. Res. 2005;164:540–544. doi: 10.1667/rr3369.1. [DOI] [PubMed] [Google Scholar]
- 32.Hamada N. Recent insights into the biological action of heavyion radiation. J. Radiat. Res. (Tokyo) 2009;50:1–9. doi: 10.1269/jrr.08070. [DOI] [PubMed] [Google Scholar]
- 33.Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J. Biol. Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- 34.Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J. Neurosci. 1998;18:2475–2485. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem. Int. 2000;37:163–170. doi: 10.1016/s0197-0186(00)00019-x. [DOI] [PubMed] [Google Scholar]
- 36.Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch. Neurol. 2001;58:365–370. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- 37.Kugler P, Schmitt A. Complementary neuronal and glial expression of two high-affinity glutamate transporter GLT1/EAAT2 forms in rat cerebral cortex. Histochem. Cell Biol. 2003;119:425–435. doi: 10.1007/s00418-003-0530-7. [DOI] [PubMed] [Google Scholar]
- 38.Szerb JC, Issekutz B. Increase in the stimulation-induced overflow of glutamate by fluoroacetate, a selective inhibitor of the glial tricarboxylic cycle. Brain Res. 1987;410:116–120. doi: 10.1016/s0006-8993(87)80030-6. [DOI] [PubMed] [Google Scholar]
- 39.Keyser DO, Pellmar TC. Synaptic transmission in the hippocampus: critical role for glial cells. Glia. 1994;10:237–243. doi: 10.1002/glia.440100402. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima K, Yamamoto S, Kohsaka S, Kurihara T. Neuronal stimulation leading to upregulation of glutamate transporter-1 (GLT-1) in rat microglia in vitro. Neurosci. Lett. 2008;436:331–334. doi: 10.1016/j.neulet.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 41.Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J. Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur. J. Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 43.Bonvento G, Sibson N, Pellerin L. Does glutamate image your thoughts? Trends Neurosci. 2002;25:359–364. doi: 10.1016/s0166-2236(02)02168-9. [DOI] [PubMed] [Google Scholar]
- 44.Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol. Sci. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- 45.Cohen Z, Bouchelet I, Olivier A, Villemure JG, Ball R, Stanimirovic DB, Hamel E. Multiple microvascular and astroglial 5-hydroxytryptamine receptor subtypes in human brain: molecular and pharmacologic characterization. J. Cereb. Blood Flow Metab. 1999;19:908–917. doi: 10.1097/00004647-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Abounader R, Elhusseiny A, Cohen Z, Olivier A, Stanimirovic D, Quirion R, Hamel E. Expression of neuropeptide Y receptors mRNA and protein in human brain vessels and cerebromicrovascular cells in culture. J. Cereb. Blood Flow Metab. 1999;19:155–163. doi: 10.1097/00004647-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Elhusseiny A, Cohen Z, Olivier A, Stanimirovic DB, Hamel E. Functional acetylcholine muscarinic receptor subtypes in human brain microcirculation: identification and cellular localization. J. Cereb. Blood Flow Metab. 1999;19:794–802. doi: 10.1097/00004647-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Braet K, Cabooter L, Paemeleire K, Leybaert L. Calcium signal communication in the central nervous system. Biol. Cell. 2004;96:79–91. doi: 10.1016/j.biolcel.2003.10.007. [DOI] [PubMed] [Google Scholar]