Abstract
The tumor-vascular disrupting agent (VDA) vadimezan (5,6-dimethylxanthenone-4-acetic acid, DMXAA) has been shown to potentiate the antitumor activity of photodynamic therapy (PDT) using systemically administered photosensitizers. Here, we characterized the response of subcutaneous syngeneic Colon26 murine colon adenocarcinoma tumors to PDT using the locally applied photosensitizer precursor aminolevulinic acid (ALA) in combination with a topical formulation of vadimezan. Diffuse correlation spectroscopy (DCS), a non-invasive method for monitoring blood flow, was utilized to determine tumor vascular response to treatment. Additionally, correlative CD31-immunohistochemistry to visualize endothelial damage, ELISA assays to measure induction of tumor necrosis factor-alpha (TNF-α) and tumor weight measurements were also examined in separate animals. In our previous work, DCS revealed a selective decrease in tumor blood flow over time following topical vadimezan. ALA-PDT treatment also induced a decrease in tumor blood flow. The onset of blood flow reduction was rapid in tumors treated with both ALA-PDT and vadimezan. CD31-immunostaining of tumor sections confirmed vascular damage following topical application of vadimezan. Tumor weight measurements revealed enhanced tumor growth inhibition with combination treatment compared to ALA-PDT or vadimezan treatment alone. In conclusion, vadimezan as a topical agent enhances treatment efficacy when combined with ALA-PDT. This combination could be useful in clinical applications.
INTRODUCTION
Photodynamic therapy (PDT), a clinically useful curative or palliative treatment for a variety of solid malignancies acts through multiple mechanisms, including vascular damage, direct killing of tumor cells and stimulation of host anti-tumor immune responses (1). Initial PDT studies used the now FDA-approved, intravenously administered exogenous photosensitizer porfimer sodium (Photofrin®) but a major disadvantage of this agent was the prolonged cutaneous sensitivity that followed administration (2). Conversely, the prodrugs aminolevulinic acid (ALA) and its methyl ester form methyl aminolevulinate (Metvix™, MAL) can be applied topically and cause cells, in particular tumor cells, to accumulate increased amounts of the endogenous photosensitizer protoporphyrin IX (PpIX), which does not cause prolonged photosensitivity (3,4). Topical PDT using both ALA and MAL has gained considerable acceptance in the clinical setting due to the convenience of application, rapid clearance of the photosensitizer and the decreased period of skin photosensitivity following application compared to first generation photosensitizers (5). Furthermore, topical PDT was as effective as surgical excision of superficial dermal lesions, was not invasive, caused less cosmetic damage and had the advantage of treating multiple lesions simultaneously (6–10). While both ALA and MAL are used for treatment of dermatological lesions, our studies focused on ALA as the prodrug precursor for PpIX synthesis because of our greater experience with ALA-PDT in approved protocols in pre-clinical studies (11–13) and in clinical settings for treatment of BCC at Roswell Park Cancer Institute (14,15). Kuijpers et al found similar short-term therapeutic efficacy between ALA and MAL-PDT in treatment of nodular BCC lesions and multiple reports showed that there was no difference in photosensitizer distribution after topical application of either prodrug (16–18). Furthermore, a recent report by the British Association of Dermatologists of the guidelines for management of BCC recommended the use of ALA or MAL-PDT for treatment of superficial lesions (19). While ALA-PDT has demonstrated efficacy in treatment of numerous superficial skin lesions and malignancies (20–22), its efficacy in treatment of BCCs was highly dependent on lesion size and surgical excision of nodular BCC was found more effective than ALA-PDT (23,24). Therefore, it would be advantageous to find mechanisms that increases ALA-PDT efficacy in nodular lesions to maintain the advantages of PDT and reduce the need for surgical excision.
The presence of a functioning vascular network in tumors is essential to meet their metabolic demands for oxygen and nutrient supply. Indeed, it is well recognized that vascular destruction is one of the major consequences of PDT when performed using most systemically administered photosensitizers (25,26). Studies of the response to ALA-PDT suggest that the antitumor activity is primarily mediated by direct tumor cell toxicity rather than effects on the tumor vasculature (11,27). We therefore investigated the combination of topical ALA-PDT with topical application of vadimezan, a vascular disrupting agent. We previously showed that vadimezan caused a steady and rapid decrease in blood flow over time, resulting in a decrease to 50–55% of baseline at 2 hours post application and further decreasing to 65–70% of baseline at 3 hours post application, confirming that vadimezan causes a rapid disruption of tumor blood flow when used as a topical agent (12). Here, we expanded our studies to examine blood flow after combination treatment with ALA-PDT and vadimezan to test our hypothesis that direct cytotoxicity of ALA-PDT in conjunction with the potent vascular disruptive effects of vadimezan would enhance the antitumor activity in vivo.
We chose to use the subcutaneous Colon26 murine colon adenocarcinoma in BALB/c, rather than the transgenic Gli transcription factor overexpressing model used in our previous study (12) which develops spontaneous BCC-like tumors with a similar morphology to human BCC (28). Growth of subcutaneously inoculated Colon26 tumors is very reproducible and the blood supply is evenly distributed within the tumor. Tumor growth in the K5-Gli-2 mice is sporadic, sizes vary considerably and the blood supply of the tumors tends to be confined to the connective tissue surrounding the tumor nodules. For proof of principle, and to reproducibly demonstrate changes in blood flow, we decided to use a model in which differences in size and blood vessel distribution were minimal.
Vadimezan is currently in Phase III clinical trials after successful Phase II trials in combination with standard chemotherapy for treatment of non-small cell lung cancer (29,30) and castration-refractory metastatic prostate cancer (31). Vadimezan has shown antivascular effects in both murine and human tumors, increasing vascular permeability and hemorrhagic necrosis (32,33). The local synthesis of cytokine TNF-α within murine tumors was found in part to cause the tumor destruction (34,35). Our earlier studies indicated that addition of intraperitoneal vadimezan to PDT using the systemically-administered photosensitizers porfimer sodium (Photofrin™) and HPPH (Photochlor™) dramatically enhanced the destruction of solid tumors implanted in mice (34,36).
It is well established that to achieve the maximal benefit from photodynamic treatments, sufficient oxygen levels need to be present in the tumor to form the damaging reactive oxygen species. Therefore, the time of topical vadimezan administration must be optimized to ensure that the induced vascular collapse does not impede the photodynamic reaction. Based on the rapid decrease of tumor blood flow and tumor perfusion after topical vadimezan treatment observed in our previous study, we demonstrate that the combination of ALA-PDT and topical vadimezan decreases tumor size when vadimezan is applied 1 hour prior to irradiation (12).
MATERIALS AND METHODS
Reagents
5-Aminolevulinic acid hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). Solid 5,6-Dimethylxanthenone-4-acetic acid (courtesy of Gordon Rewcastle, University of Auckland, New Zealand) was stored at room temperature in the dark.
Tumor model, treatment groups, vadimezan treatment and photodynamic therapy
All experimental protocols were approved by Roswell Park Cancer Institute Institutional Animal Care and Use Committee. Six to eight-week-old female BALB/cAnNCr mice were obtained from Jackson Laboratory (Bar Harbor, ME). After removing mouse fur by shaving and use of a depilatory cream, mice were subcutaneously inoculated with 1 × 106 of the murine colon adenocarcinoma Colon26 on the upper right back. When tumors reached 5–6 mmin diameter, mice were randomly placed in each of the treatment groups: untreated controls, ALA-PDT alone, ALA-PDT plus topical DMSO, topical vadimezan alone and ALA-PDT plus topical vadimezan. ALA was prepared as a 20% (w/v) topical solution in the proprietary vehicle DUSA (DUSA Pharmaceuticals, Inc., Wilmington, MA). Twenty uL of the 20% ALA solution was applied topically to the tumor in 5 uL increments 3 hours prior to light treatment. The ALA incubation time was based on our prior experience with topical ALA in this tumor model at the sizes used in this study in which 3 hours is sufficient for PpIX synthesis throughout tumors of 1.5–2.5 mm thickness to the deepest part, the reported depth (1 to 2 mm) of measureable drug concentration in human BCC tumor biopsies after topical ALA application (18,37). Also, studies by Ericson et al showed a maximal gradient between normal skin and tumor tissue fluorescence at 3 hours after ALA application (38). OpSite transparent medical dressing (Smith & Nephew Medical Limited, Hull HU3 2BN England) was placed over the tumor to protect the area. Vadimezan solution, 1 mg vadimezan dissolved in 40 uL of DMSO was applied to the tumor in a similar fashion in 5 uL increments at one hour before laser treatment and OpSite was placed over the tumor. Three hours after application of ALA, mice were anesthetized with Ketamine/Xylazine (K/X, 100/10 mg/kg) and placed on a heated pad to maintain body temperature. A light spot diameter of 1.1 cm from a dye laser pumped by an argon ion laser tuned to 635 nm was used to irradiate the tumor at a fluence of 80 J/cm2 and a fluence rate of 75 mW/cm2. When tumors in the control group grew to the endpoint of 400 mm3, all mice were sacrificed and tumors were removed and weighed.
Immunohistochemical analysis
BALB/c mice bearing Colon26 tumors and mice without tumors were left untreated or were treated with ALA-PDT, topical vadimezan or the combination as described above. Twenty-four hours later mice were sacrificed and tumors removed. The tumors were cut in half to expose the deepest part of the tumor and placed in Zinc fixative (Formalin-free, BD Pharmigen, San Diego, CA) overnight, then transferred to 70% ethanol. The samples were then processed and embedded in paraffin. Sections were stained for CD31, an endothelial cell marker as described previously by Henderson et al (39). The mean tumor thickness of control (untreated) tumors at the time of treatment was 2.02 +/−0.20 (mean+/− standard deviation, range 1.8–2.3 mm) and the mean thickness of all tumors 24 h after treatment was 1.97 +/− 0.16 mm.
Optical blood flow measurements (Diffuse Correlation Spectroscopy)
The DCS technique was used to non-invasively and continuously monitor blood flow. The DCS instrument was previously detailed in our published work (15,40–42). The DCS technique was also previously described in detail (43–49). Briefly, the instrument had a 785 nm long coherence length laser (CrystaLaser), a single photon-counting detector (Perkin-Elmer) and a custom built autocorrelator board (Correlator.com). The source-detector Photodetector outputs were fed into the correlator board with the resulting intensity autocorrelation functions and photon arrival times recorded by a computer (43). The normalized intensity autocorrelation function allowed for the diffuse electric field temporal autocorrelation function to be obtained (43), which was shown to satisfy the diffusion equation (46–48). Brownian or random flow models can be applied to extract information about the particle motion by solving the diffusion equation (50).
Blood flow was monitored by DCS in Colon26 tumors on the upper right back of BALB/c mice. Tumors for DCS were slightly larger (6.5 – 7.5 mm in diameter) than those for histology and the PDT treatment tumor response. Tumors are measured with calipers which includes the overlying skin so the tumor tissue itself is somewhat smaller than this. To ensure that the projections onto the tumor of the source and detection fibers with the largest separation distances used could be positioned to monitor only tumor tissue, the slightly larger tumor size was used. Hypoxic areas (although not necrotic) are found within tumors at all the sizes we have used in this study, but do not appear to be greater in the larger tumors (possibly because the vasculature is slightly more mature). The slightly increased diameter of tumors used the DCS may have slightly changed the tumor vascular dynamics, and if the tumors from the DCS monitored groups had been grown to the endpoint, they may well have had a poorer response.
However, we believe that the differences in the conclusions drawn would also be minimal. The depth of penetration of the diffuse light corresponds to approximately one-third to one-half of the separation distance between the source and detector fibers (50). In these studies, 2 separation distances of 2 and 3.5 mm were used, which correlate to tumor depths of 1 to 1.33 mm and 1.75 to 2.33 mm, respectively. Two depths within the tumor were examined to monitor whether ALA-PDT and vadimezan caused vascular effects both close to the surface where the drugs were topically applied and at the deepest aspects of the tumor where topically applied drugs may be present at lower concentrations. Mice were anesthetized during DCS measurements and placed on a heating pad at 34.5°C to maintain body temperature. A thermal couple was inserted under the skin on the right flank of the mouse to monitor body temperature throughout DCS. To keep the mice immobilized, booster injections of K/X at 0.25 of the original dose (25/2.5 mg/kg) were administered every 30 minutes. Mice were sacrificed immediately after DCS was completed. To monitor normal tissue blood flow and the effect of anesthesia, data was collected from source and detector fibers placed on depilated skin over the hip of the mouse and protected from light. For all DCS data, blood flow fluctuations in the tumor were first normalized to baseline measurements of tumor blood flow taken prior to treatment. The blood flow measurements were further normalized to the blood flow from the non-tumor, skin tissue region. Once the baseline measurements were made, treatment and DCS monitoring began.
Enzyme-linked immunosorbent assay
TNF-α protein expression in tumor tissue from BALB/c mice inoculated with Colon26 tumors treated with DMSO or vadimezan was determined using a Quantikine mouse TNF-α/TNFSF1A ELISA kit purchased from R&D Systems (Minneapolis, MN). Tumor tissues were excised at various time points after treatment and homogenized in 1ml per gram of tumor tissue of CelLytic MT Mammalian Tissue Lysis/Extraction Reagent (Sigma-Aldrich, St. Louis, MO) plus protease inhibitor cocktail (P-8340, Sigma-Aldrich, St. Louis, MO) at a ratio of 400:1. Supernatants were isolated by centrifugation and their protein concentration was determined using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Samples containing 40 μg of protein were analyzed for cytokine expression. Four to six mice were used for each test group (except 1 h DMSO, 2 mice only, since vehicle alone showed no histological or blood flow effects at 1 hour post application).
Statistical analysis
Unpaired two-tailed t-test analyses were carried out on parametric data using the GraphPad Prism software, version 5.01. (GraphPad Software, Inc.)
RESULTS
ALA-PDT caused a decrease in tumor blood flow
The reported literature shows that the extent of ALA-PDT induced vascular damage is highly variable. The vascular response can be altered by parameters which include but are not limited to route of ALA delivery, light dosimetry and wavelength of activating light used (8,11,51–53). We showed previously that ALA-PDT in tumor bearing mice led to a decrease in tumor blood flow immediately following the start of light delivery, but blood flow remained constant at 50 to 60% of baseline for the hour following irradiation (12). To confirm and extend these observations of the effect of topical ALA-PDT on blood flow, DCS was employed in the current study to non-invasively monitor tumor blood flow in vivo continuously before, during and after completion of light delivery (80 J/cm2 delivered at a rate of 75 mW/cm2), but for a prolonged period of 3 hours. The average relative tumor blood flow was measured at two depths within the tumor of approximately 1 and 1.75 mm (n=2) (Figure 1). Immediately at the start of light delivery a drop in tumor blood flow was observed at both monitored depths within the tumor tissue, culminating in a maximal drop to approximately 50 to 70% of baseline levels during light delivery. Following completion of light delivery, the relative tumor blood flow did not recover to baseline levels or decrease further decrease for the 3 hours monitored. This demonstrates that ALA-PDT alone causes a measureable decrease in tumor blood flow, similar at both depths which is maintained for 3 hours but does not lead to complete stasis of tumor blood flow. Therefore, addition of the vascular disrupting agent vadimezan to the ALA-PDT treatment was proposed which may further enhance the antivascular effect and tumor response.
Figure 1. Diffuse correlation spectroscopy measurement after ALA-PDT.
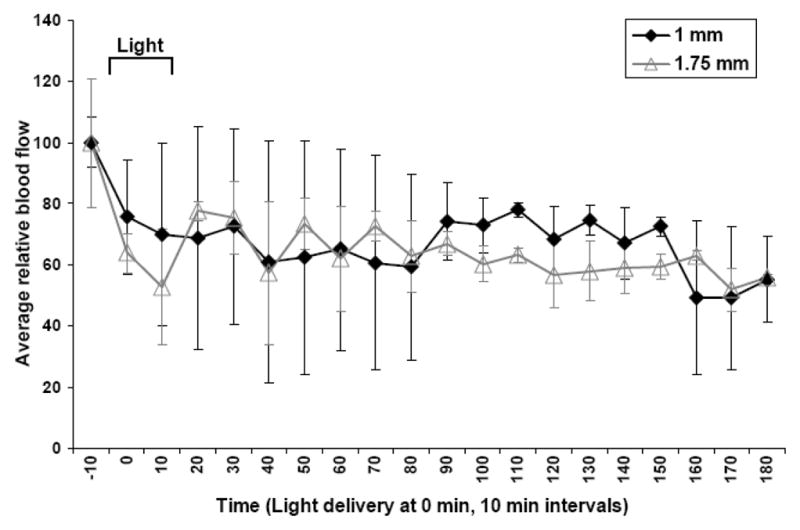
Tumor blood flow was monitored by DCS in response to ALA-PDT treatment before, during and after light delivery (80 J/cm2 at 75 mW/cm2). Graph represents mean blood flow measurements +/− standard error of the mean (SEM) of replicate experiments (n=2) over a period of 190 min (blood flow was measured for 10 min prior to light delivery). Two source-detector distances were used of 2 and 3.5 mm, correlating to measurements at approximately 1 and 1.75 mm deep. Light delivery began at time = 0 min and was complete at 18 min.
Topical vadimezan treatment reduces vascular density in tumors but not in normal skin tissue
Vadimezan and vehicle DMSO were applied topically to skin on the upper right back of naïve BALB/c mice or animals bearing subcutaneous Colon26 tumors. Twenty four hours after treatment, mice were euthanized and skin or tumors were removed for immunohistochemical staining. An antibody against CD31 was used to stain the endothelial cells within the skin and Colon26 tumor samples. Vessel density was determined by counting the number of vessels in 5X magnification fields, at least 3 fields per sample. In Figure 2, compared to BALB/c skin treated with DMSO only, skin exposed to vadimezan did not demonstrate a significant decrease in CD31 stained vessel number (75.3 +/− 5.3 vessels for DMSO, 83.6 +/− 3.7 vessels for vadimezan, p=0.2455 using the unpaired two-tailed t-test, n=4). Furthermore, no change in vessel size or extravasation of red blood cells was observed in the blood vessels from vadimezan treated murine skin samples from BALB/c mice. This demonstrates that topically applied vadimezan does not cause damage to the normal blood vessels in the skin in this model.
Figure 2. Vadimezan effects on blood vessels in normal mouse skin.
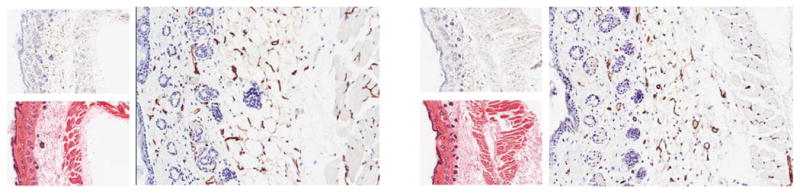
Normal BALB/c mouse skin lacking hair was untreated (left panel) or treated with topical vadimezan (right panel). Skin was harvested at 24 h, fixed in zinc fixative and processed for embedding in paraffin. Sections were stained with anti-CD31 antibody. No decrease in the number of skin blood vessels was observed with vadimezan treatment. In each panel, top left: anti-CD31, 5X magnification, bottom left: H&E, 5X magnification, right: anti-CD31, 20X magnification. Figure is representative of replicate samples (n= at least 3).
Vadimezan caused a significant decrease in the number of tumor blood vessels (85.1 +/−12.0) to the full depth of the tumor of 1.78 +/−0.17 mm (mean +/− standard deviation, n = 3) compared to untreated (158.4 +/− 32.4) or vehicle treated tumor samples harvested 24 hours after application (p=0.0175, unpaired two-tailed t-test, n = 3), Figure 3 and Table 1. It is important to note that the vascular damage caused by vadimezan was not confined to the region of tumor nearest to the epidermis, the site of topical application, but was observed throughout the tumor including at the tumor base (Figure 3B) which is well vascularized in the untreated control (Figure 3A). The enlarged and magnified images in Figure 3 show the extensive damage to the endothelial cells induced by vadimezan at the base of the Colon26 tumors in the region furthest from the skin (Figure 3B) compared to the untreated tumor (Figure 3A). Increased vascular leakiness also was observed at 24 hours post vadimezan application by the extensive extravasation of red blood cells into the tumor tissue (black arrows, Figure 3B), which was not observed in untreated samples. Also, some regions of nuclear condensation were observed at 24 h post topical vadimezan treatment, which may be indicative of apoptosis. These results provide proof of concept that vadimezan is effective at damaging blood vessels when applied topically.
Figure 3. Topical vadimezan causes damage to blood vessels throughout Colon26 tumors.
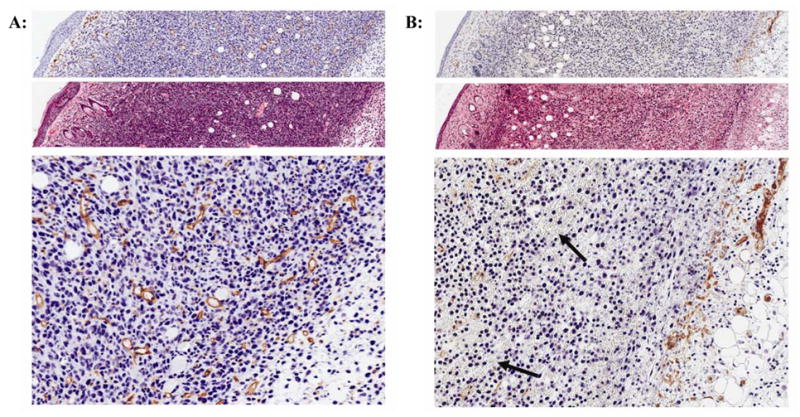
A: Untreated or B: topical vadimezan treated tumor samples from mice sacrificed at 24 h after vadimezan application. Top (anti-CD31) and middle (H&E) panels are at 20X magnification; bottom panel is an enlarged field of the region of tumor furthest from the skin. Figures are representative of replicate samples (n = at least 3). Black arrows indicate red blood cell extravasation in the vadimezan treated sample, not present in the untreated tumor samples. Note: the CD31 and H&E panels (top and middle) are composed of a sequence of adjoining images taken from the stratum corneum to the base of the tumor to show the extent of the vadimezan effect on tumor blood vessels.
Table 1. Blood vessel density in Colon26 tumors.
CD31 stained blood vessels were counted in untreated or treated (ALA-PDT, topical vadimezan or the combination) Colon26 tumors removed 24 h after treatment. At least 8 fields (5X magnification) per group of 3 mice were counted. Values are the mean +/− standard error of the mean (SEM).
| Avg. # vessels per 5X field | S.E.M. | |
|---|---|---|
| Untreated control | 158.4 | 32.4 |
| Vadimezan | 85.1 | 12.0 |
| ALA-PDT | 216.4 | 21.9 |
| ALA-PDT + Vadimezan | 159.8 | 33.0 |
Topically applied vadimezan induced TNF-α production in Colon26 tumors
The hemorrhagic necrosis observed in murine tumors in response to vadimezan treatment has been reported to be a response to tumor cell production of cytokines, largely TNF-α (35,54,55). To determine if topically applied vadimezan induced TNF-α within treated tumors, the cytokine levels in tumor lysates prepared at different time points after topical vadimezan treatment were measured by ELISA. Control lysates were from tumors treated with DMSO only. Topical application of vadimezan led to a significant and maximal increase in tumor TNF-α levels by 3 hours post application compared to vehicle treated tumors (Figure 4, * p=0.0338, unpaired two-tailed t-test), demonstrating a probable role of this cytokine in vascular damage with topical use of vadimezan.
Figure 4. Topical vadimezan leads to increased TNF-α.
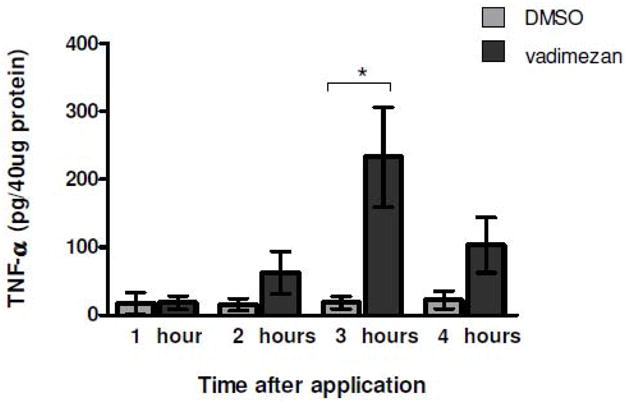
Topical application of vadimezan induces TNF-α, reaching maximal levels at 3 h as determined by ELISA. Tumor lysates were prepared at various times after topical application. The values reported are the mean +/− SEM of 4–6 mice per group (1 h DMSO, n= 2).
The combination treatment of ALA-PDT and topical vadimezan caused a rapid decrease in blood flow
In a previous section we showed that ALA-PDT alone caused an immediate decrease in tumor blood flow during irradiation that did not recover during the 3 hours of monitoring. Previous data showed that topical vadimezan induced a rapid and steady drop in tumor blood flow to about 25% of baseline over 4 h, and that the optimal application time to maintain tumor perfusion during ALA-PDT was probably 1 hour prior to illumination (12). Next, we monitored the effect of the combined treatments on tumor blood flow (n=2), with the vadimezan applied 1 hour before light delivery. In the combination study, blood flow was monitored at one depth (approximately 1 mm) within the tumor, because there was no apparent difference in the blood flow changes at the two depths. When vadimezan was applied topically, a net decrease in blood flow of approximately 20% was observed by 1 hour when light delivery began (Figure 5A). Blood flow then steadily decreased over time to reach approximately 55 to 60% of baseline at 2 hours post topical application, when monitoring ceased (Figure 5A). This was similar to that observed at 2 hours post topical treatment of vadimezan as a single therapy (12) and greater than at any time after light delivery with the ALA-PDT single therapy (Figure 1). The combination therapy therefore decreased tumor blood flow consistent with the effect contributed by the vadimezan, but with no additional contribution from the ALA-PDT. It is possible that the vadimezan induced fluctuations in tumor blood flow and damage to tumor endothelial cells might interfere with the ALA-PDT vascular response. Topical vadimezan alone eventually decreased blood flow to 30–35% of baseline by 3 hours post application (12) and it is possible that extended monitoring of the combination might eventually show a greater decrease in blood flow than the 40% achieved after 2.5 hours of monitoring the combination treatment in Figure 5A.
Figure 5.
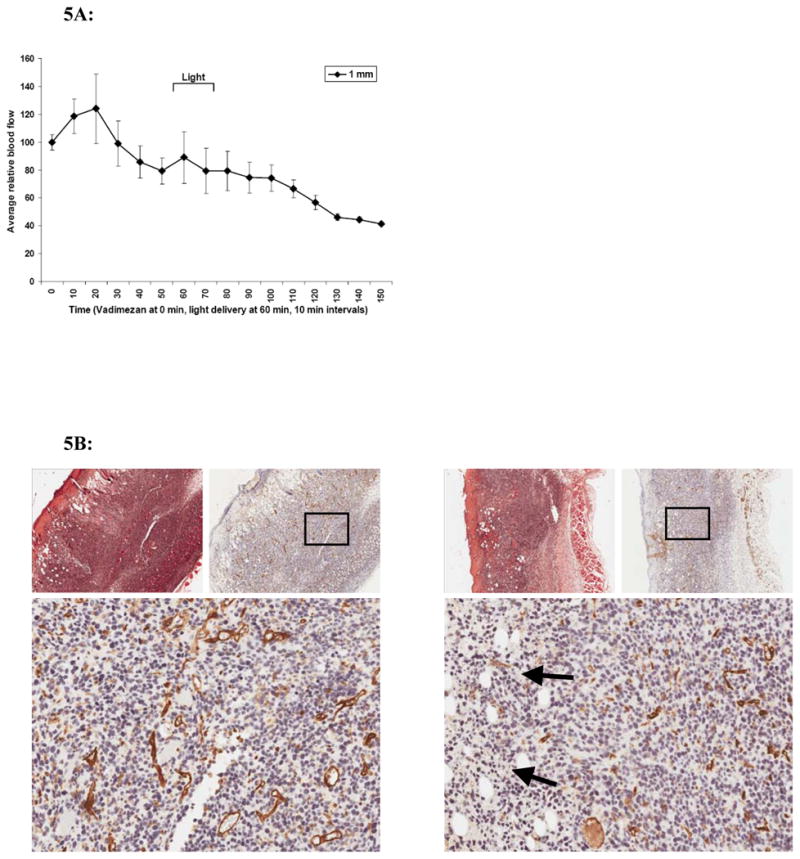
A: ALA-PDT plus topical vadimezan. DCS was used to monitor blood flow in response to combination treatment. Vadimezan was applied 1 h prior to light delivery (2 h post ALA application). The graph is representative of the average tumor blood flow +/− SEM at 1 mm depth within the tumor (n=2). B: IHC stain of endothelial cells in response to ALA-PDT with or without topical vadimezan. 24 h after ALA-PDT alone (left panel) or in combination with vadimezan treatment (right panel), mice were sacrificed and tumors excised. Within each panel: Left-top: H&E, right top, anti-CD31: both at 5X magnification. Bottom: anti-CD31 at 20X magnification. Figures are representative of replicate tumor samples (n = at least 3). Black arrows indicate red blood cell extravasation.
The tumor samples treated with ALA-PDT alone (Figure 5B, left) show a slight increase in vessel diameter as well as blood vessel number (216.4 +/− 21.9) compared to the untreated tumors (158.4 +/− 32.4) at 24 hours post-treatment, Table 1. Destruction of tumor vasculature, extravasation of red blood cells into the tumor stroma and some nuclear condensation was observed in tumor samples exposed to the combined treatment (Figure 5B, right, black arrows, extravasated red blood cells), although the number of vessels remaining intact at 24 hours (159.8 +/− 33.0) was higher than in tumors treated with vadimezan alone (85.1 +/− 12.0).
Combination treatment with vadimezan and ALA-PDT decreases tumor weight
Compared to ALA-PDT alone, the combination of ALA-PDT and vadimezan caused a substantial decrease in tumor blood flow up to 2 hours following light delivery. We hypothesized that the combined treatment of ALA-PDT direct tumor cell destruction with the vascular disrupting agent vadimezan would enhance treatment outcome. Colon26 tumors were treated with ALA-PDT (80 J/cm2 delivered at 75 mW/cm2) alone or in combination with topical vadimezan applied 1 hour prior to PDT light delivery. At the endpoint (tumor growth of controls to 400 mm3), mice were sacrificed and all tumors were resected and weighed. In Figure 6, it is shown that the combination led to a significant decrease in tumor weight compared to ALA-PDT treatment (***, p=0.0006) or vadimezan treatment alone (p=<0.0001), unpaired two-tailed t-test). No significant decrease was observed when DMSO was applied one hour prior to ALA-PDT (p=0.3261, unpaired two-tailed t-test).
Figure 6. Tumor response to combination therapy.
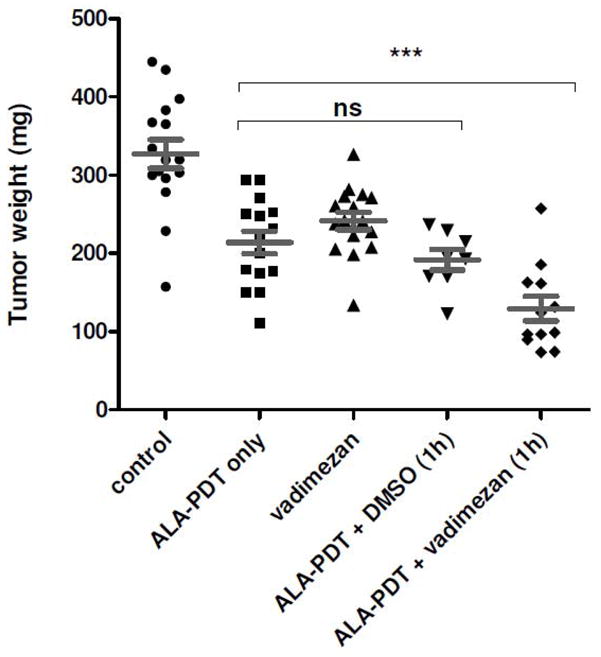
Tumor weights were measured post in vivo ALA-PDT, vadimezan, DMSO or combination therapy. Vehicle DMSO and vadimezan were applied 1 h prior to light delivery. The combination of ALA-PDT and vadimezan caused a significant decrease in tumor weight (p<0.001) compared to ALA-PDT alone. No significant change in tumor weight was observed in the ALA-PDT alone, vadimezan alone or ALA-PDT plus DMSO treated groups (p>0.05). Groups contained 8 to 12 mice.
DISCUSSION
While topical ALA-PDT has been useful for the treatment of precancerous actinic keratosis lesions and superficial BCC lesions, its use for treatment of nodular BCC skin cancers has to date been less successful (8,23,24). Therefore, approaches to enhance ALA-PDT efficacy would be beneficial for treatment of nodular lesions. It is understood that the choice of photosensitizer used can influence the mechanism of cell death induced by PDT. While some photosensitizers rely on vascular damage (56–58), ALA-PDT causes minimal damage to the vasculature and relies mostly on direct cytotoxicity to tumor cells (8,59). Peng et al applied a dual photosensitizer approach with non-vascular ALA-PDT plus low dose vascular destructive Photofrin-PDT, resulting in a significant decrease in tumor growth due to the lethal combination of microcirculatory shut down and direct cell toxicity (27). In the present study, a similar approach was employed by combining the small molecule vascular destructing agent vadimezan in place of anti-vascular PDT with the cytotoxic ALA-PDT treatment.
Unlike antiangiogenics which target neovascular growth, vadimezan leads to the selective destruction of tumor blood vessels (60). The specific tumor vascular collapse results in tumor hypoxia, hemorrhagic necrosis and ultimately tumor cell death (60–62). The selectivity of vadimezan is in part due to the direct and rapid induction of apoptosis of the tumor endothelial cells and induction of cytokines including TNF-α (55,63). In our studies, tumor samples removed at 24 hours after topical application of vadimezan demonstrated a significant decrease in the number of tumor blood vessels and a substantial decrease in CD31 endothelial cell staining, similar to the response observed by Seshadri et al with i.p. delivered vadimezan (32). DCS monitoring demonstrated a decrease in blood flow early after the application of vadimezan, which continued to decline over the monitored period and we believe that the early changes in blood flow are ultimately reflected in the blood vessel damage, which is seen in the histology of samples removed at 24 hours after treatment. A potential benefit to the combination treatment is that vadimezan and ALA-PDT would act in a complementary manner if timed correctly, targeting tumor tissue with non-overlapping toxicity by different mechanisms of action. While vadimezan selectively damages tumor vessels, it is less effective in targeting tumor cells of the periphery which are supplied by the normal tissue vasculature. This is often observed after single treatment among many vascular disrupting agents as a viable rim of tumor which persists at the periphery (64,65). To the contrary, PDT is highly effective in treatment of the outer, well perfused rim of tumor but often the inner, hypoxic and less-perfused central region of tumor has increased resistance to PDT (66).
The order of drug application is critical for a response to combination therapy. With PDT, the best tumor response was observed when cellular damage occurs first followed by vascular damage (66). As oxygen is critical for the photodynamic reaction during light irradiation, vadimezan needs to be administered such that blood vessels remain functional during light delivery to maintain blood flow and oxygen delivery. Previously we found that blood flow decreased gradually to 50–55% of baseline levels 2 hours after topical application of vadimezan (12) similar to that observed with ALA-PDT (Figure 1). We considered that a later application of vadimezan one hour prior to light delivery in ALA-PDT would not interfere with blood flow during light delivery as the vascular damage is a more gradual response and will occur after light delivery is complete. A previous report of the vascular damage induced by vadimezan using MRI showed that at 24 hours after treatment a significant extravasation of the contrast agent was observed in the tumors, indicative of vascular leakiness and damage (67). This corresponds to our studies of CD31 immunostained tumors, which showed red blood cell extravasation and a marked decrease in endothelial cell staining 24 hours after topical vadimezan application (Figure 3B). Previously by using DCS, we were able to monitor tumor blood flow in real time immediately after application of topical vadimezan (12). This technique was beneficial in this study as it permitted simultaneous monitoring of the tumor perfusion response without interrupting the laser irradiation and provided significant information about the rapid response to vadimezan and ALA-PDT of decreasing tumor blood flow at the monitored depths within the tumor tissue. Previous observations suggested that the optimal time for topical vadimezan application to tumors should be approximately 1 to 2 hours prior to PDT treatment such that decreased blood flow and vascular damage would follow the PDT induced cellular damage (12). This timeframe matched our previous report in which the combination of systemic vadimezan and Photofrin-PDT had maximal efficacy when vadimezan was administered 1–3 hours prior to light delivery, presumably because some time was required before the vadimezan effects occurred (34). Here, we found a successful decrease in tumor weight when topical vadimezan was applied 1 hour prior to ALA-PDT treatment. However, blood vessel numbers with the combination were similar to untreated controls at 24 h post treatment, which is of some concern despite the delay in tumor growth found, so 1 hour prior to light delivery still may not be the optimal application time for topical vadimezan and further studies are required to determine this.
The immunohistochemical staining of tumor sections and DCS monitoring of blood flow at two tissue depths show that topically applied vadimezan affects blood vessels throughout the tumor and is not limited to the surface region. While we have used Colon26 tumors as a reproducible model of a nodular subcutaneous lesion, topical vadimezan applied to the spontaneous tumors of the K5-Gli-2 mouse model, which are a closer histological and morphological representation of human BCC, also caused extensive vascular destruction, red blood cell extravasation and tumor damage (12). While further studies need to be carried out to determine the usefulness of topical vadimezan in clinical treatment of cutaneous disease, especially nodular BCC, the positive response in the K5-Gli-2 model supports further investigation. Interestingly, it was observed that ALA-PDT caused a slight increase in vessel diameter and vessel numbers at 24 hours post-treatment as observed in Figure 5B. Previously, it was reported that ALA-PDT increased the number of blood vessels in the capillary beds of psoriasis plaques during and after ALA-PDT treatment (68). Increased expression of VEGF after ALA-PDT was also reported (69). It has been proposed that the post-PDT induction of VEGF may be responsible for the tumor recurrence observed after sub-curative PDT treatments as it provides a survival stimulus for the tumor cells (69,70). It is also possible that indirect effects of ALA-PDT, including inactivation of cytokines and hypoxia (due to the photochemical consumption of oxygen during the photochemical reaction) may stimulate endothelial cell proliferation (71–73). The intact blood vessels observed 24 h after treatment both in the ALA-PDT and combination treatments may help with re-oxygenation of the treated area and cause reperfusion injury which may contribute to ALA-PDT mediated damage, which has been shown in normal colon (74). However, there is a balance between pro-and anti-tumor effects and we currently do not know whether the blood vessels in the combination treatment would decrease at later time points, before recovering and sustaining tumor growth. This scenario could support a later, and/or additional application of vadimezan to inhibit the regrowth of blood vessels as tumor growth recovers.
Attempts to increase ALA-PDT efficacy with penetration enhancers such as dimethyl sulfoxide (DMSO) and iron chelators such as ethylenediamine tetraacetic acid (EDTA) in the application vehicle to increase the delivery of topically applied ALA to deeper sections of the tumor improved the complete response rates of nodular BCCs (20). In this report, the vehicle used for topical application of vadimezan was DMSO. However, DCS monitoring showed that DMSO alone did not affect tumor blood flow after topical application. Additionally, topical DMSO applied one hour prior to ALA-PDT did not significantly decrease tumor growth compared to ALA-PDT alone. Therefore the increased efficacy of the combination treatment is not due to any increased penetration of ALA that may occur with a topical DMSO application, but rather is due to the vascular damage caused by vadimezan.
In summary, this is a preliminary study exploring the use of vadimezan as a topical vascular disrupting agent, and whether this approach would be feasible combined with topical ALA-PDT. The experiments demonstrated a clear enhancement of the PDT effect with the addition of topical vadimezan, inducing a significant decrease in tumor weight compared to the single therapies alone and suggest that the combined approach deserves further investigation. Based on previous studies with topical and systemic vadimezan we considered that application of vadimezan 1 hour before light delivery for PDT would be optimal. However, taking into account the topical vadimezan mediated decreased blood flow that occurs before light delivery, application closer to or even after the light delivery might enhance the effects further. We were concerned that the damage caused by ALA-PDT to the tumor, including edema, swelling, dilation of tumor vessels and altered microenvironment responses might impair the vadimezan effect if it were applied topically immediately following illumination. However, future studies exploring the temporal importance of single or multiple applications of vadimezan with PDT, should address these possibilities. Also, using a more representative model for nodular BCC, in which the vascular supply is confined to the periphery of the nodules rather than throughout the tumor (as occurs in the subcutaneous Colon26 model), may further increase the clinical applicability of these studies.
Acknowledgments
This work has been supported in part by grant numbers P01CA55791, RO1CA89656 and F31CA126490 from the National Cancer Institute. The content was solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We would like to thank Dr. Allan Oseroff for his excellent advice and input to this project, Mary Vaughan for assistance with the immunohistochemical studies and the Roswell Park Cancer Institute shared resources, Cancer Center Support Grant CA16056. We also thank Dr. Barbara Henderson and Dr. Mukund Seshadri for critical review of the manuscript.
References
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moriwaki SI, Misawa J, Yoshinari Y, Yamada I, Takigawa M, Tokura Y. Analysis of photosensitivity in Japanese cancer-bearing patients receiving photodynamic therapy with porfimer sodium (Photofrin) Photodermatol Photoimmunol Photomed. 2001;17:241–243. doi: 10.1034/j.1600-0781.2001.170507.x. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990;6:143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 4.Bellnier DA, Greco WR, Loewen GM, Nava H, Oseroff AR, Dougherty TJ. Clinical pharmacokinetics of the PDT photosensitizers porfimer sodium (Photofrin), 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (Photochlor) and 5-ALA-induced protoporphyrin IX. Lasers Surg Med. 2006;38:439–444. doi: 10.1002/lsm.20340. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B, He YY. Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Rev Anticancer Ther. 2010;10:1797–1809. doi: 10.1586/era.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf P, Kerl H. Photodynamic therapy with 5-aminolevulinic acid: a promising concept for the treatment of cutaneous tumors. Dermatology. 1995;190:183–185. doi: 10.1159/000246681. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa FS, Bentley MV. Photodynamic therapy of skin cancers: sensitizers, clinical studies and future directives. Pharm Res. 2000;17:1447–1455. doi: 10.1023/a:1007612905378. [DOI] [PubMed] [Google Scholar]
- 8.Wang I, Bendsoe N, Klinteberg CA, Enejder AM, Andersson-Engels S, Svanberg S, Svanberg K. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol. 2001;144:832–840. doi: 10.1046/j.1365-2133.2001.04141.x. [DOI] [PubMed] [Google Scholar]
- 9.Ibbotson SH. An overview of topical photodynamic therapy in dermatology. Photodiagnosis Photodyn Ther. 2010;7:16–23. doi: 10.1016/j.pdpdt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Sidoroff A, Thaler P. Taking treatment decisions in non-melanoma skin cancer--the place for topical photodynamic therapy (PDT) Photodiagnosis Photodyn Ther. 2010;7:24–32. doi: 10.1016/j.pdpdt.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Henderson BW, Vaughan L, Bellnier DA, Van Leengoed H, Johnson PG, Oseroff AR. Photosensitization of murine tumor, vasculature and skin by 5-aminolevulinic acid-induced porphyrin. Photochemistry and Photobiology. 1995;62:780–789. doi: 10.1111/j.1751-1097.1995.tb08730.x. [DOI] [PubMed] [Google Scholar]
- 12.Marrero A, Sunar U, Sands T, Oseroff A, Bellnier D. SPIE Digital Library. Jul 13, 2009. Potentiation of ALA-PDT antitumor activity in mice using topical DMXAA. Kessel, D. Photodynamic Therapy: Back to the Future 7380. Ref Type: Conference Proceeding. [Google Scholar]
- 13.Sands T, Sunar U, Foster TH, Oseroff A. SPIE Digital Library. Jul 13, 2009. Monitoring blood flow and photobleaching during topical ALA PDT treatment SPIE 7164 (2009). Kessel, D. International Photodynamic Therapy Association. Photodynamic Therapy: Back to the Future 7380, 7164. Ref Type: Conference Proceeding. [Google Scholar]
- 14.Cottrell WJ, Paquette AD, Keymel KR, Foster TH, Oseroff AR. Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas. Clin Cancer Res. 2008;14:4475–4483. doi: 10.1158/1078-0432.CCR-07-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker TL, Paquette AD, Keymel KR, Henderson BW, Sunar U. Monitoring blood flow responses during topical ALA-PDT. Biomedical Optics Express. 2011;2:123–130. doi: 10.1364/BOE.2.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuijpers DI, Thissen MR, Thissen CA, Neumann MH. Similar effectiveness of methyl aminolevulinate and 5-aminolevulinate in topical photodynamic therapy for nodular basal cell carcinoma. J Drugs Dermatol. 2006;5:642–645. [PubMed] [Google Scholar]
- 17.de Bruijn HS, Meijers C, van der Ploeg-van den Heuvel, Sterenborg HJ, Robinson DJ. Microscopic localisation of protoporphyrin IX in normal mouse skin after topical application of 5-aminolevulinic acid or methyl 5-aminolevulinate. J Photochem Photobiol B. 2008;92:91–97. doi: 10.1016/j.jphotobiol.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg C, Halldin CB, Ericson MB, Larko O, Krogstad AL, Wennberg AM. Bioavailability of aminolaevulinic acid and methylaminolaevulinate in basal cell carcinomas: a perfusion study using microdialysis in vivo. Br J Dermatol. 2008;159:1170–1176. doi: 10.1111/j.1365-2133.2008.08795.x. [DOI] [PubMed] [Google Scholar]
- 19.Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245–1266. doi: 10.1111/j.1365-2133.2008.08882.x. [DOI] [PubMed] [Google Scholar]
- 20.Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, Nesland JM. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79:2282–2308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Piacquadio DJ, Chen DM, Farber HF, Fowler JF, Jr, Glazer SD, Goodman JJ, Hruza LL, Jeffes EW, Ling MR, Phillips TJ, Rallis TM, Scher RK, Taylor CR, Weinstein GD. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140:41–46. doi: 10.1001/archderm.140.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Clark C, Bryden A, Dawe R, Moseley H, Ferguson J, Ibbotson SH. Topical 5-aminolaevulinic acid photodynamic therapy for cutaneous lesions: outcome and comparison of light sources. Photodermatol Photoimmunol Photomed. 2003;19:134–141. doi: 10.1034/j.1600-0781.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 23.Berroeta L, Clark C, Dawe RS, Ibbotson SH, Fleming CJ. A randomized study of minimal curettage followed by topical photodynamic therapy compared with surgical excision for low-risk nodular basal cell carcinoma. Br J Dermatol. 2007;157:401–403. doi: 10.1111/j.1365-2133.2007.07996.x. [DOI] [PubMed] [Google Scholar]
- 24.Mosterd K, Thissen MR, Nelemans P, Kelleners-Smeets NW, Janssen RL, Broekhof KG, Neumann HA, Steijlen PM, Kuijpers DI. Fractionated 5-aminolaevulinic acid-photodynamic therapy vs. surgical excision in the treatment of nodular basal cell carcinoma: results of a randomized controlled trial. Br J Dermatol. 2008;159:864–870. doi: 10.1111/j.1365-2133.2008.08787.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Xu Y, Roskams T, Delaey E, Agostinis P, Vandenheede JR, de WP. Efficacy of antitumoral photodynamic therapy with hypericin: relationship between biodistribution and photodynamic effects in the RIF-1 mouse tumor model. Int J Cancer. 2001;93:275–282. doi: 10.1002/ijc.1324. [DOI] [PubMed] [Google Scholar]
- 26.Fingar VH, Kik PK, Haydon PS, Cerrito PB, Tseng M, Abang E, Wieman TJ. Analysis of acute vascular damage after photodynamic therapy using benzoporphyrin derivative (BPD) Br J Cancer. 1999;79:1702–1708. doi: 10.1038/sj.bjc.6690271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Q, Warloe T, Moan J, Godal A, Apricena F, Giercksky KE, Nesland JM. Antitumor effect of 5-aminolevulinic acid-mediated photodynamic therapy can be enhanced by the use of a low dose of photofrin in human tumor xenografts. Cancer Res. 2001;61:5824–5832. [PubMed] [Google Scholar]
- 28.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- 29.McKeage MJ, Reck M, Jameson MB, Rosenthal MA, Gibbs D, Mainwaring PN, Freitag L, Sullivan R, Von Pawel J. Phase II study of ASA404 (vadimezan, 5,6-dimethylxanthenone-4-acetic acid/DMXAA) 1800 mg/m<sup>2</sup> combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Lung Cancer. 2009;65:192–197. doi: 10.1016/j.lungcan.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 30.McKeage MJ. The potential of DMXAA (ASA404) in combination with docetaxel in advanced prostate cancer. Expert Opinion on Investigational Drugs. 2008;17:23–29. doi: 10.1517/13543784.17.1.23. [DOI] [PubMed] [Google Scholar]
- 31.Pili R, Rosenthal MA, Mainwaring PN, Van Hazel G, Srinivas S, Dreicer R, Goel S, Leach J, Wong S, Clingan P. Phase II study on the addition of ASA404 (vadimezan; 5,6- dimethylxanthenone-4-acetic acid) to docetaxel in CRMPC. Clinical Cancer Research. 2010;16:2906–2914. doi: 10.1158/1078-0432.CCR-09-3026. [DOI] [PubMed] [Google Scholar]
- 32.Seshadri M, Spernyak JA, Maiery PG, Cheney RT, Mazurchuk R, Bellnier DA. Visualizing the acute effects of vascular-targeted therapy in vivo using intravital microscopy and magnetic resonance imaging: correlation with endothelial apoptosis, cytokine induction, and treatment outcome. Neoplasia. 2007;9:128–135. doi: 10.1593/neo.06748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Olivo M, Lye KY, Moore S, Sharma A, Chowbay B. Enhancing the therapeutic responsiveness of photodynamic therapy with the antiangiogenic agents SU5416 and SU6668 in murine nasopharyngeal carcinoma models. Cancer Chemother Pharmacol. 2005;56:569–577. doi: 10.1007/s00280-005-1017-0. [DOI] [PubMed] [Google Scholar]
- 34.Bellnier DA, Gollnick SO, Camacho SH, Greco WR, Cheney RT. Treatment with the tumor necrosis factor-alpha-inducing drug 5,6-dimethylxanthenone-4-acetic acid enhances the antitumor activity of the photodynamic therapy of RIF-1 mouse tumors. Cancer Res. 2003;63:7584–7590. [PubMed] [Google Scholar]
- 35.Browne WL, Wilson WR, Baguley BC, Ching LM. Suppression of serum tumour necrosis factor-alpha by thalidomide does not lead to reversal of tumour vascular collapse and anti-tumour activity of 5,6-dimethylxanthenone-4-acetic acid. Anticancer Res. 1998;18:4409–4413. [PubMed] [Google Scholar]
- 36.Seshadri M, Spernyak JA, Mazurchuk R, Camacho SH, Oseroff AR, Cheney RT, Bellnier DA. Tumor vascular response to photodynamic therapy and the antivascular agent 5,6-dimethylxanthenone-4-acetic acid: Implications for combination therapy. Clinical Cancer Research. 2005;11:4241–4250. doi: 10.1158/1078-0432.CCR-04-2703. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadi S, McCarron PA, Donnelly RF, Woolfson AD, McKenna K. Evaluation of the penetration of 5-aminolevulinic acid through basal cell carcinoma: a pilot study. Exp Dermatol. 2004;13:445–451. doi: 10.1111/j.0906-6705.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 38.Ericson MB, Sandberg C, Gudmundson F, Rosen A, Larko O, Wennberg AM. Fluorescence contrast and threshold limit: implications for photodynamic diagnosis of basal cell carcinoma. J Photochem Photobiol B. 2003;69:121–127. doi: 10.1016/s1011-1344(02)00413-x. [DOI] [PubMed] [Google Scholar]
- 39.Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, Cheney RT, Morgan J. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–2126. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 40.Sunar U, Quon H, Durduran T, Zhang J, Du J, Zhou C, Yu G, Choe R, Kilger A, Lustig R, Loevner L, Nioka S, Chance B, Yodh AG. Noninvasive diffuse optical measurement of blood flow and blood oxygenation for monitoring radiation therapy in patients with head and neck tumors: a pilot study. J Biomed Opt. 2006;11:064021. doi: 10.1117/1.2397548. [DOI] [PubMed] [Google Scholar]
- 41.Sunar U, Makonnen S, Zhou C, Durduran T, Yu G, Wang HW, Lee WMF, Yodh AG. Hemodynamic responses to antivascular therapy and ionizing radiation assessed by diffuse optical spectroscopies. Opt Express. 2007;15:15507–15516. doi: 10.1364/oe.15.015507. [DOI] [PubMed] [Google Scholar]
- 42.Sunar U, Rohrbach D, Rigual N, Tracy E, Keymel K, Cooper MT, Baumann H, Henderson BH. Monitoring photobleaching and hemodynamic responses to HPPH-mediated photodynamic therapy of head and neck cancer: a case report. Opt Express. 2010;18:14969–14978. doi: 10.1364/OE.18.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berne BJ, Pecora R. Dynamic Light Scattering: With Applications to Chemistry, Biology and Physics. John Wiley & Sons Inc; New York: 1976. [Google Scholar]
- 44.Pine DJ, Weitz DA, Chaikin PM, Herbolzheimer E. Diffusing wave spectroscopy. Phys Rev Lett. 1988;60:1134–1137. doi: 10.1103/PhysRevLett.60.1134. [DOI] [PubMed] [Google Scholar]
- 45.Maret G, Wolf PE. Multiple light scattering from disordered media. The effect of brownian motion of scatterers. Zeitschrift f++r Physik B Condensed Matter. 1987;65:409–413. [Google Scholar]
- 46.Boas DA, Campbell LE, Yodh AG. Scattering and Imaging with Diffusing Temporal Field Correlations. Phys Rev Lett. 1995;75:1855–1858. doi: 10.1103/PhysRevLett.75.1855. [DOI] [PubMed] [Google Scholar]
- 47.Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. Journal of the Optical Society of America A: Optics and Image Science, and Vision. 1997;14:192–215. [Google Scholar]
- 48.Heckmeier M, Skipetrov SE, Maret G, Maynard R. Imaging of dynamic heterogeneities in multiple-scattering media. Journal of the Optical Society of America A: Optics and Image Science, and Vision. 1997;14:185–191. [Google Scholar]
- 49.Cheung C, Culver JP, Takahashi K, Greenberg JH, Yodh AG. In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys Med Biol. 2001;46:2053–2065. doi: 10.1088/0031-9155/46/8/302. [DOI] [PubMed] [Google Scholar]
- 50.Yu G, Durduran T, Zhou C, Wang HW, Putt ME, Saunders HM, Sehgal CM, Glatstein E, Yodh AG, Busch TM. Noninvasive monitoring of murine tumor blood flow during and after photodynamic therapy provides early assessment of therapeutic efficacy. Clin Cancer Res. 2005;11:3543–3552. doi: 10.1158/1078-0432.CCR-04-2582. [DOI] [PubMed] [Google Scholar]
- 51.Leveckis J, Brown NJ, Reed MW. The effect of aminolaevulinic acid-induced, protoporphyrin IX-mediated photodynamic therapy on the cremaster muscle microcirculation in vivo. Br J Cancer. 1995;72:1113–1119. doi: 10.1038/bjc.1995.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enejder AM, af KC, Wang I, ndersson-Engels S, Bendsoe N, Svanberg S, Svanberg K. Blood perfusion studies on basal cell carcinomas in conjunction with photodynamic therapy and cryotherapy employing laser-Doppler perfusion imaging. Acta Derm Venereol. 2000;80:19–23. doi: 10.1080/000155500750012441. [DOI] [PubMed] [Google Scholar]
- 53.Wang KK, Cottrell WJ, Mitra S, Oseroff AR, Foster TH. Simulations of measured photobleaching kinetics in human basal cell carcinomas suggest blood flow reductions during ALA-PDT. Lasers Surg Med. 2009;41:686–696. doi: 10.1002/lsm.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ching LM, Zwain S, Baguley BC. Relationship between tumour endothelial cell apoptosis and tumour blood flow shutdown following treatment with the antivascular agent DMXAA in mice. Br J Cancer. 2004;90:906–910. doi: 10.1038/sj.bjc.6601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao L, Ching LM, Kestell P, Baguley BC. The antitumour activity of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice. Br J Cancer. 2002;87:465–470. doi: 10.1038/sj.bjc.6600479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henderson BW, Waldow SM, Mang TS, Potter WR, Malone PB, Dougherty TJ. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985;45:572–576. [PubMed] [Google Scholar]
- 57.Moan J, Peng Q, Sorensen R, Iani V, Nesland JM. The biophysical foundations of photodynamic therapy. Endoscopy. 1998;30:387–391. doi: 10.1055/s-2007-1001288. [DOI] [PubMed] [Google Scholar]
- 58.vanGeel I, Oppelaar H, Oussoren YG, Stewart FA. Changes in perfusion of mouse tumours after photodynamic therapy. Int J Cancer. 1994;56:224–228. doi: 10.1002/ijc.2910560214. [DOI] [PubMed] [Google Scholar]
- 59.Herman MA, Fromm D, Kessel D. Tumor blood-flow changes following protoporphyrin IX-based photodynamic therapy in mice and humans. J Photochem Photobiol B. 1999;52:99–104. doi: 10.1016/s1011-1344(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 60.Zwi LJ, Baguley BC, Gavin JB, Wilson WR. The morphological effects of the anti-tumor agents flavone acetic acid and 5,6-dimethyl xanthenone acetic acid on the colon 38 mouse tumor. Pathology. 1994;26:161–169. doi: 10.1080/00313029400169411. [DOI] [PubMed] [Google Scholar]
- 61.Baguley BC. Antivascular therapy of cancer: DMXAA. Lancet Oncology. 2003;4:141–148. doi: 10.1016/s1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- 62.Siim BG, Laux WT, Rutland MD, Palmer BN, Wilson WR. Scintigraphic imaging of the hypoxia marker (99m)technetium-labeled 2,2′-(1,4-diaminobutane)bis(2-methyl-3-butanone) dioxime (99mTc-labeled HL-91; prognox): noninvasive detection of tumor response to the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Cancer Res. 2000;60:4582–4588. [PubMed] [Google Scholar]
- 63.Ching LM, Cao Z, Kieda C, Zwain S, Jameson MB, Baguley BC. Induction of endothelial cell apoptosis by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. British Journal of Cancer. 2002;86:1937–1942. doi: 10.1038/sj.bjc.6600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491–2499. doi: 10.1002/cncr.20299. [DOI] [PubMed] [Google Scholar]
- 65.Hill SA, Chaplin DJ, Lewis G, Tozer GM. Schedule dependence of combretastatin A4 phosphate in transplanted and spontaneous tumour models. Int J Cancer. 2002;102:70–74. doi: 10.1002/ijc.10655. [DOI] [PubMed] [Google Scholar]
- 66.Chen B, Pogue BW, Hoopes PJ, Hasan T. Combining vascular and cellular targeting regimens enhances the efficacy of photodynamic therapy. International Journal of Radiation Oncology Biology Physics. 2005;61:1216–1226. doi: 10.1016/j.ijrobp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Seshadri M, Bellnier DA. The vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid improves the antitumor efficacy and shortens treatment time associated with photochlor-sensitized photodynamic therapy in vivo. Photochemistry and Photobiology. 2009;85:50–56. doi: 10.1111/j.1751-1097.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fransson J, Ros AM. Clinical and immunohistochemical evaluation of psoriatic plaques treated with topical 5-aminolaevulinic acid photodynamic therapy. Photodermatol Photoimmunol Photomed. 2005;21:326–332. doi: 10.1111/j.1600-0781.2005.00182.x. [DOI] [PubMed] [Google Scholar]
- 69.Xie Y, Wei ZB, Zhang Z, Wen W, Huang GW. Effect of 5-ALA-PDT on VEGF and PCNA expression in human NPC-bearing nude mice. Oncol Rep. 2009;22:1365–1371. doi: 10.3892/or_00000576. [DOI] [PubMed] [Google Scholar]
- 70.Solban N, Selbo PK, Sinha AK, Chang SK, Hasan T. Mechanistic investigation and implications of photodynamic therapy induction of vascular endothelial growth factor in prostate cancer. Cancer Res. 2006;66:5633–5640. doi: 10.1158/0008-5472.CAN-06-0604. [DOI] [PubMed] [Google Scholar]
- 71.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 72.Statius van Eps RG, Adili F, Watkins MT, Anderson RR, LaMuraglia GM. Photodynamic therapy of extracellular matrix stimulates endothelial cell growth by inactivation of matrix-associated transforming growth factor-beta. Lab Invest. 1997;76:257–266. [PubMed] [Google Scholar]
- 73.Ferrario A, von Tiehl KF, Rucker N, Schwarz MA, Gill PS, Gomer CJ. Antiangiogenic treatment enhances photodynamic therapy responsiveness in a mouse mammary carcinoma. Cancer Res. 2000;60:4066–4069. [PubMed] [Google Scholar]
- 74.Curnow A, Bown SG. The role of reperfusion injury in photodynamic therapy with 5-aminolaevulinic acid – a study on normal rat colon. Br J Dermatol. 2004;86:989–992. doi: 10.1038/sj.bjc.6600178. [DOI] [PMC free article] [PubMed] [Google Scholar]


