Abstract
Background
While aberrant fibrinolysis and plasminogen activator inhibitor-1 (PAI-1) are implicated in ALI, the role of this serpin in the pathogenesis of wood bark smoke (WBS) induced acute lung injury (SIALI) and its regulation in resident lung cells after exposure to smoke is unclear.
Methods
A total of 22 mechanically ventilated pigs were included in this study. Immunohistochemical analyses were used to assess fibrin and PAI-1 in the lungs of pigs with SIALI in situ. PAI-1 was measured in bronchoalveolar lavage fluids by Western blotting. Induction of PAI-1 was determined at the protein and mRNA levels by Western and PCR analyses in primary porcine alveolar type II (ATII) cells, fibroblasts and pleural mesothelial cells (PMCs). PAI-1 mRNA stability was determined by transcription chase studies. Gel shift analyses were used to characterize the mechanism regulating PAI-1 mRNA stability.
Results
SIALI induced PAI-1, with prominent extravascular fibrin deposition in large and small airways as well as alveolar and subpleural compartments. In PMCs, lung fibroblasts and ATIIs, PAI-1 mRNA was stabilized by WBS extract and contributed to induction of PAI-1. The mechanism involves dissociation of a novel 6-Phospho-D-Gluconate-NADP oxidoreductase -like PAI-1 mRNA binding protein from PAI-1 mRNA.
Conclusions
Exposure to WBS induces prominent airway and mesothelial expression of PAI-1, associated with florid distribution of fibrin in SIALI in vivo. WBS components induce PAI-1 in vitro in part by stabilization of PAI-1 mRNA, a newly recognized pathway that may promote extravascular fibrin deposition and lung dysfunction in SIALI.
Keywords: Acute lung injury, fibrin, fibrinolysis, serpin, smoke inhalation
Introduction
Smoke inhalation injury occurs in 5-15% of patients currently admitted to U.S. burn centers, and greatly increases post-burn morbidity and mortality (1). The pathogenesis of smoke-inhalation-induced acute lung injury (SIALI) involves activation of a number of proinflammatory cascades, leading to disordered coagulation and perturbed fibrinolysis. In particular, derangements of these pathways have been strongly implicated in abnormal extravascular fibrin turnover (1;2). Fibrin deposition in the small airways, in combination with airway hyperreactivity, has been associated with respiratory dysfunction and obstruction in SIALI (2). These observations provide a strong rationale for therapy targeting disordered fibrin turnover in this setting, and inhalational anticoagulant and fibrinolytic interventions have been successfully used to ameliorate lung dysfunction in SIALI in animal models (3-5).
Disordered fibrin turnover in the lung in patients with ALI and acute respiratory distress syndrome (ARDS) is consistently associated with a profound local defect in fibrinolytic activity. As measured in bronchoalveolar lavage (BAL) fluid, this defect in fibrinolysis is mainly attributable to over-expression of plasminogen activator inhibitor-1 (PAI-1) (6;7). In the lung, PAI-1 not only regulates alveolar proteolysis, but also participates in cellular signaling and regulates airway and alveolar injury and repair (8). Increased expression of PAI-1 (or homozygosity for the PAI-1 4G allele) is associated with poor outcomes in patients with ALI (9-11). Elevated plasma PAI-1 levels have also been reported in 15 patients with burn and inhalation injury (12). However, understanding of the role and regulation of PAI-1 in the progression of SIALI has been limited. This represents an important gap in our current understanding of the pathogenesis of SIALI. In vivo and in vitro approaches were applied to address this gap.
We used a porcine model of wood-bark-smoke (WBS)-induced ALI to determine whether robust expression of PAI-1 is induced in the lungs, and whether its distribution occurs in proximity to extravascular fibrin in situ. We also sought to determine whether wood bark smoke (WBS) components induce expression of PAI-1 by resident lung cells in vitro. In these analyses, we first tested the ability of WBS extracts to induce PAI-1 in early primary cultures of porcine lung alveolar type II cells (ATIIs), lung fibroblasts and pleural mesothelial cells (PMCs). Next, we sought to shed light on the mechanism by which PAI-1 is induced in these cells by WBS. We specifically sought to determine whether the mechanism involves stabilization of PAI-1 mRNA, as it is well-established that PAI-1 is regulated at the transcriptional level by mediators likely to be operative in SIALI (8;13). Understanding of the role of posttranscriptional regulation of PAI-1 in this context or in any other form of ALI, has not, to our knowledge, previously been studied and is addressed here.
Materials and Methods
Animals
This study was approved by the US Army Institute of Surgical Research Animal Care and Use Committee, and was carried out in accordance with the guidelines set forth by the Animal Welfare Act and other federal statutes and regulations relating to animals and studies involving animals and by the 1996 Guide for the Care of Laboratory Animals from the National Research Council. For this study we enrolled a randomly selected subset of swine which were part of a larger study evaluating the efficacy of conventional mechanical ventilation vs. airway-pressure-release ventilation for treatment of SIALI. A total of 22 female, non-pregnant, Yorkshire pigs (35–45 kg) were used in this study. Lung tissues from 8 of animals not subjected to BAL were used for histologic and immunohistochemical analyses exclusively. Of these 8 animals, 5 were euthanized at 48 h after induction of SIALI and 2 at 24 h after SIALI, while 1 served as a mechanically ventilated time control (over 48h). Fourteen additional animals were subjected to BAL and tissues were obtained for histologic analyses. Of these 14 animals, 12 underwent WBS challenge, while 2 served as mechanically ventilated time controls. Three of these 12 WBS-challenged animals did not develop ARDS while the other 9 pigs developed SIALI over 48h. The pigs were premedicated, anesthetized, intubated and mechanically ventilated using a Dräger Evita XL™ ventilator (Dräger Medical, Lübeck, Germany) in a volume-controlled mode with a baseline setting of tidal volume (TV) of 10 ml/kg, a positive-end expiratory pressure (PEEP) of 5 cm H2O, an inspiratory-to-expiratory ratio of 1:3, and fraction of inspired oxygen (FiO2) of 21%. At baseline (before injury), the partial pressure of carbon dioxide (PaCO2) was maintained between 35 and 45 mmHg by adjusting the respiratory rate. Arterial, venous, pulmonary artery and urinary bladder catheters were placed for physiologic monitoring. A tracheostomy was performed, to maintain a safe airway during smoke inhalation and subsequent ICU care.
Physiologic monitoring
Pigs were monitored for 48h. The following variables were measured: number of smoke breaths received; volume of smoke received; peak carboxyhemoglobin level after smoke injury; peak inspiratory pressure (PIP); partial pressure of O2 in arterial blood (PaO2); partial pressure of CO2 in arterial blood (PaCO2); fraction of inspired oxygen (FiO2); and PaO2-to-FiO2 ratio (PFR). Data were recorded at baseline (before injury) and every 6 h thereafter for 48 h.
WBS inhalation
After a 45-90 minute stabilization period baseline (pre-injury) physiologic values were obtained, after which the animals were taken to a room for administration of WBS(14). Briefly, 100 g of pine wood bark chips were burned in a custom combustion chamber. Room air was blown through the chamber and the smoke was vented into a mixing box for cooling to avoid thermal injury. Next, the smoke/air/oxygen mixture was drawn into a volume-controlled hand-operated piston and delivered to the animals via a tracheostomy. Tidal volume (TV) per smoke breath was set at 30 mL/kg and smoke was delivered as described in Table 1. Smoke injury was terminated if the pig developed hemodynamic instability, manifested by severe hypotension or desaturation during the injury.
Table 1. Smoke Inhalation Data.
| Mode | Weight (kg) | Smoke breaths (n) | Smoke (L) | Peak COHb (%) |
|---|---|---|---|---|
| CMV (n=9) | 42.31±1.55 | 22.44±1.67 | 27.44±3.59 | 80.88±2.10 |
| APRV (n=7) | 41.51±1.36 | 23.29±1.63 | 28.58±2.31 | 86.70±1.63 |
| p value | 0.31 | 0.73 | 0.81 | 0.05 |
Legend: Inhalation injury data in the conventional mechanical ventilation (CMV) and airway-pressure-release ventilation (APRV) groups with SIALI, indicating injury equivalency. Peak COHb (%): peak carboxyhemoglobin concentration at end of smoke injury by arterial blood gas analysis. Values are means ± SEMs. Significance by two-tailed student's t-test or Wilcoxon two-sample test.
ICU care
After injury, pigs were transported to the animal intensive care unit (ICU), where they remained under round-the-clock clinical monitoring for 48 hours under total intravenous anesthesia with ketamine hydrochloride (20–30 mg/kg/hr), midazolam hydrochloride (1.0–1.5 mg/kg/hr), and propofol (50–100 mcg/kg/min). Analgesia was provided with buprenorphine (0.05 mg/kg IM) every 6 h. For 2 h after injury, a FiO2 of 100% was used to accelerate carbon monoxide clearance. After 2 h, the FiO2 was tapered to 21%, and titrated to an arterial oxygen saturation > 92%. Suctioning was performed as needed. Fiberoptic bronchoscopy was performed at baseline, immediately after injury, and every 6 h during the first 24 h; then at 24 and 48 h. This served to remove any major obstructing casts, and to carry out bronchoalveolar lavage as described below.
Mechanical ventilation protocols
Prior to injury, pigs exposed to WBS were randomized to receive either conventional mechanical ventilation using volume assist-control (CMV, n = 12) or airway-pressure-release ventilation (APRV; n = 7). Three of the WBS-challenged animals ventilated with CMV did not develop severe hypoxemia within 2h, while all other pigs required 100 percent oxygen supplementation, consistent with the development of SIALI. For 2 h after injury, FiO2 of 100% was used to accelerate carbon monoxide clearance; other ventilator settings continued as at baseline with a TV = 10 mL/kg, respiratory rate (RR) = 12 breaths per minute, and positive end-expiratory pressure (PEEP) = 5 cm H2O. After 2 h, FiO2 was tapered down to 21%, and was subsequently titrated to achieve an arterial oxygen saturation > 92%. At that time, pigs were either continued on CMV or transitioned to APRV. A plateau pressure < 35 mmHg was sought in both the CMV and APRV arms. In the CMV arm, the respiratory rate (up to a rate of 30), followed by the TV, were adjusted in order to achieve 7.5 > pHa > 7.2. In the APRV arm, the Phigh and Tlow were adjusted to maintain 7.5 > pHa > 7.2. Uninjured time-control pigs (n=3) were maintained on CMV at TV = 10 ml/kg, PEEP = 5 cm H2O, and FiO2 = 21%. Pigs in all 3 groups received humidification in the ventilator circuit.
BAL Protocol
Bronchoalveolar lavage (BAL) was performed at baseline, and at 24 and 48 h after injury. Control BAL fluid (BALF) was obtained at the same intervals in mechanically ventilated pigs not challenged with WBS. The bronchoscope was gently wedged in a subsegmental bronchus (in the accessory lobe on the left) and 20 ml of normal saline solution (NSS) was instilled and gently aspirated. The procedure was repeated 3 times, recording the yield after each instillation. The cells were removed by centrifugation at 400 g for 10 min, after which cell-free fluids were frozen at -70°C until analyzed.
Experimental termination, necropsy and specimen collection
All animals that survived 48 h after injury received a euthanasia solution (Fatal-Plus®, Med-Vet International, Mettawa, IL) in accordance with the AVMA Guidelines on Euthanasia, June 2007.
Collection and propagation of primary cultures of ATII cells, lung fibroblasts and PMCs
Porcine ATII epithelial cells were harvested at necropsy and isolated by modifications of a previously described protocol (15). Excised porcine lung tissue was cut in 1-mm pieces with the aid of a tissue chopper and fine scissors. Each isolation involved use of 20-25 g of tissue. Chopped tissue was shaken vigorously at 250 rpm in 100 mL of Ca++, Mg++-free HBSS and at 37°C for 5 min. Tissue fragments were then filtered three times over a 100 μm nylon mesh to remove red blood cells. Washed tissue was with 15 mL of an enzymatic solution containing 50 U/mL Dispase I (BD Pharmingen cat no 354235) supplemented with 2000 U/mL DNAse I (Sigma cat # D5025). The tissue was then digested for 45 min at 37°C in a water bath shaker at 125 rpm. The enzymatic reaction was stopped with 20 mL of DMEM/10% FBS. Undigested tissue was then removed by sequential filtration over 100 μm and 40 μm cell strainers (Fisher Scientific). Filtrates were then centrifuged at 250 × g, 10°C for 20 min. Cell pellets were re-suspended in erythrocyte lysis buffer (0.5 mL/gr tissue digested) and incubated at room temperature for 4 min. An equal volume of DMEM/10% FBS was then added and cells recovered by centrifugation at 250 × g, 10°C for 20 min. The cells were washed in 20 mL of Ca++, Mg++-free HBSS re-suspended in alveolar epithelial cell medium (ScienCell cat no 3201) supplemented with 10 U/mL DNAase I and cultured in a 75 cm2 tissue culture plate for 1 hr at 37°C in a humidified 95% air / 5% CO2 incubator to adhere macrophages. Non-adherent cells were removed, centrifuged as above, and re-suspended in alveolar epithelial cell medium. Epithelial cells were isolated by Percoll density gradient centrifugation as described previously (15). The Percoll density gradient consisted of two layers; a light upper layer at Percoll density of 1.040 g/mL and a lower heavy layer at a density of 1.089 g/mL in Ca++, Mg++-free HBSS supplemented with 0.05% FBS. Epithelial cells were harvested from the interface using after centrifugation at 250 × g for 30 min at 10°C, diluted in 20 mL of Ca++, Mg++-replete HBSS, washed twice by centrifugation, and cultured in 25 cm2 tissue culture plates for 48 hrs. The cells were harvested using 0.05% trypsin/0.5 mM EDTA (Invitrogen), washed, and stored frozen at -80°C in cell freezing medium (ScienCell cat # 0133) until use. PMCs were cultured from citrated (3.8 per cent sodium citrate in a 1:9 vol:vol ratio) pleural fluids from the injured pigs, placed on ice, and PMCs and lung fibroblasts were harvested and characterized with minor modifications of the techniques we previously reported (16). ATII cells and PMCs used in these studies were successfully harvested from the lungs (n=3 animals) and pleural fluid (n=1/18 cultures attempted) from pigs at 48h after SIALI. Lung fibroblasts were harvested from lungs of ventilated control pigs (n=2) and from animals or 48h after SIALI (n=8).
Preparation of WBS extract
Pine bark wood chips were combusted in a conical flask. The smoke generated was collected and passed into PBS through a peristaltic pump attached. An optical density of 1 at 230 nm was considered 100% and fresh WBS extract (WBSE) was made 30 min prior to each use. The WBSE was sterilized through a 0.2-μm filter and diluted with appropriate media to the desired concentration before application to primary cells in culture.
Western and PCR analyses
Western blotting of BAL and conditioned media was done as we previously described (17). PAI-1 was detected using rabbit polyclonal PAI-1 AB (Abcam # 28207). The PAI-1 standard was from American Diagnostica, Greenwich, CT # 1092. Total RNA was isolated from cultured porcine primary cells by using Tri-reagent. 5μg of total RNA was reverse transcribed for 45min at 42°C using RT-PCR kit from Promega. PAI-1 and β-actin mRNA was amplified for over 30 cycles using porcine specific primers along with P[32]-dCTP. The forward porcine PAI-1 primer was 5′-ATT GCT CCT CTC TTT TCC TTG-3′. The reverse primer was 5′-TCA TTT TCT CTC CCT CTC TCA C-3′. The PCR product was separated using a 5% urea gel, autoradiographed over night and normalized to β-actin for quantification of PAI-1 mRNA.
Fibrin plate radioassay, fibrin enzymography and reverse fibrin enzymography
The fibrinolytic capacity of BAL fluids was determined in a fibrin plate assay in which the release of radiolabeled fibrin was measured, as we previously reported (7). The fibrinolytic activity of BAL was further characterized by fibrin enzymography, a technique in which BAL samples were subjected to SDS-polyacrylamide gel electrophoresis after which zones of fibrinolytic activity were detected on a fibrin gel underlay, as we previously reported (7). Reverse enzymography was used to identify zones of plasminogen activator inhibitor activity, as previously reported (7).
Proteins and Reagents
Plasminogen, plasmin and fluorogenic plasmin substrate were purchased at Haematologic Technologies Inc. (HTI, Essex Junction, VT). Specific synthetic fluorogenic urokinase plasminogen activator (uPA) and chromogenic plasmin substrates were from Centerchem (Switzerland). Concentrations of proteins were calculated with a BCA protein assay kit (Pierce).
Measurement of urokinase (uPA) and plasmin (PL) amidolytic activity
Amidolytic uPA and PL activity was determined from time traces of changes in the fluorescence emission at 440 and 470 nm, respectively (excitation 344 and 352 nm, respectively) of fluorogenic uPA (Pefafluor uPA; Centerchem Inc; Norwalk, CT) or fluorogenic PL (HTI, Essex Junction, VT) substrates, respectively in 0.1 M HEPES buffer (pH 7.4, 20 mM NaCl). Aliquots (5-20 μl) of samples were mixed with buffer (total volume 50 μl) in 96-well white flat bottom Costar plates (Corning Inc, NY). Equal volumes of 0.1 mM Pefafluor uPA or 0.2 mM PL substrates in the same buffer were added and mixed. Increases in fluorescence emission were detected using a Varian Cary Eclipse fluorescence spectrophotometer equipped with 96-well plate reader accessory (Varian Inc, IL). A linear equation was fit to the results using Varian software. Enzymatic activities were reported as arbitrary units (AU) calculated based upon tcuPA (American Diagnostica, Stamford, CT), and PL (HTI, Essex Junction, VT) standards. Plasminogen (PGN) activation was measured as a difference between endogenous PL activity of the BAL sample and increasing PL activity, which was generated due to activation of exogenous human PGN (0.2-1 μM) added to the sample. PGN was purchased from (HTI, Essex Junction, VT). Amidolytic uPA, PL and PGN activation activity was analyzed employing Varian Eclipse Kinetic Software (Varian Inc, IL). The quality of the fit was estimated by visual analysis of plots of the residuals (deviation of the fitted function from the actual data). Statistical significance, as well as medians and values at 75 and 25% intervals were determined using the Mann-Whitney Rank Sum Test as described previously (18). P<0.05 was considered statistically significant. Box plots of the results of measuring of uPA, PL, PGN activating and fibrinolytic activity were generated as previously described (18) using SigmaPlot 11.0 and SigmaStat 3.5 software (SPSS Inc., CA).
Assessment of apoptosis
Apoptotic cells were detected in lung tissues of ventilated control pigs and those with SIALI by TUNEL staining, as we previously reported (19).
Transcription chase analyses
The determination of the effects of WBSE on PAI-1 mRNA stability were performed as we previously reported (19). In these analyses, ongoing transcription was stopped after which the stability of the PCR-amplified transcripts of cells exposed to WBSE was compared with that of control vehicle-exposed cells.
Gel shift and UV-cross-linking analyses
The interaction of a newly characterized PAI-1 mRNA binding protein with its cognate 33 nucleotide (nt) 3′UTR binding sequence on PAI-1 mRNA was tested as we recently reported (20). The fibroblasts used in these analyses were derived from control ventilated pigs to maximize the opportunity to visualize differences in the binding interaction in control versus WBSE or TGF-β.
Histologic, immunohistochemical and morphometric analyses
Formalin-fixed right lower lobe tissue was used for histologic and immunohistochemical analyses. All 24 lung tissues from pigs surviving 24 or 48 h after treatment and lungs from control untreated, ventilated pigs were processed and examined. Pig lungs were sectioned grossly and sections containing pleura, lung parenchyma, small airways, and conducting airways were placed into paraffin blocks. Five-μ sections from each pig lung block were placed on glass slides, stained with hematoxylin and eosin (H&E), and cover-slipped H&E scores were interpreted as previously described (17). One hundred high-power fields per slide were examined in morphometric analyses (400×, except for large and small airway fibrin at 100×). Lung injury score was calculated on the H&E slides as follows: Atelectasis or Edema—0: none; 1: mild airspace atelectasis or edema (<25% of airspaces affected); 2: moderate airspace atelectasis or edema (25-75% of airspaces affected); and 3: severe airspace atelectasis or edema (>75% of airspaces affected); inflammation—0: no alveolar/nterstitial inflammatory infiltrate; 1: mild alveolar/interstitial inflammatory cell infiltrate (no or minimal septal thickening, involving <25% of alveoli and interstitium); 2:moderate interstitial chronic inflammatory cell infiltrate (minimal septal thickening involving 25-75% of the alveoli and interstitium; 3:severe interstitial inflammatory cell infiltrate including septal thickening involving >75% of the alveoli and interstitium. Fibrin immunostained lung sections from control, 24 or 48 h post-SIALI pigs were scored as follows: Fibrin—0: no fibrin; 1: fibrin involving <25% of large, small or alveolar spaces; 2: fibrin involving 25-75% of the same airspaces; 3: fibrin involving >75% of airspaces. Immunohistochemical analyses for detection of fibrin and PAI-1 was performed using antibodies directed against the human proteins, as we previously described (17). In immunohistochemical analyses for p53, antibody sc-98 (Santa Cruz Biotechnology, Santa Cruz, CA) was used as the primary antibody.
Statistical Analysis
For physiologic analyses, all values expressed are means ± standard error of the mean. Statistical analyses performed using SAS® 9.1 software: two-way analysis of variance (ANOVA) with repeated measures and Tukey-Kramer adjustment for multiple comparisons, when appropriate as well as two-tailed student's t-test or Wilcoxon two-sample test. Significance was accepted at p < 0.05. Non-normally distributed data was log-transformed before analysis.
Results
WBS exposure and physiologic derangements confirming induction of ALI
All animals with SIALI, whether subjected to CMV or APRV, received comparable smoke exposure as indicated by the number of breaths, the volume of smoke, and the peak carboxyhemoglobin concentration (Table 1). By 24h after injury, peak inspiratory pressure was significantly increased in animals with SIALI (36 cm H2O) versus time controls (18 cm H2O; p<0.05). From 30-48h after injury, the paO2 to FiO2 ratio (PFR) was significantly lower in injured animals (PFR 139) than in controls (PFR 440; p<0.05).
Histologic and morphometric assessment of SIALI
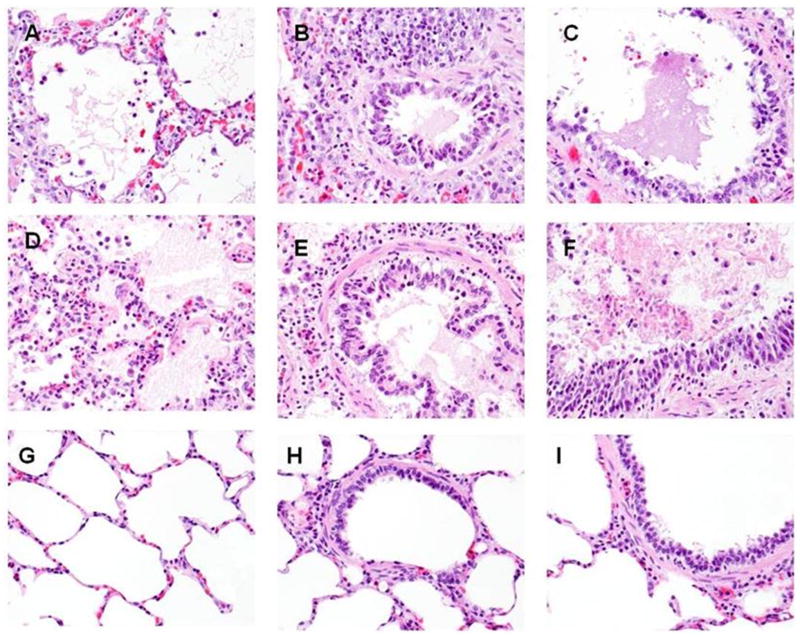
We initially characterized the extent and distribution of acute inflammation, alveolar edema and extravascular fibrin deposition in pigs with evolving SIALI. The histologic appearance of SIALI did not differ between animals that succumbed early at 24h (n=2; 1 ventilated with CMV, the other with APRV) versus those that survived for 48h (n=14), nor in pigs maintained on CMV versus APRV, with the exception that animals ventilated with CMV demonstrated significantly less focal atelectasis versus those ventilated with APRV (Table 2). All animals exposed to WBS demonstrated histologic evidence of ALI except for 3 animals ventilated with CMV which did not develop gas exchange abnormalities indicative of ARDS. These 3 animals uniformly demonstrated grade 1 fibrin deposition but otherwise did not exhibit histologic changes that differed from mechanically ventilated controls (n=3). At 24 h after WBS exposure, alveolar edema was prominent with irregular foci of alveolitis. At 48h after induction of ALI by WBS, the histologic appearance of SIALI was characterized by florid alveolar edema which appeared to contain fibrinous debris, interstitial edema, hyaline membrane formation and alveolitis which involved a prominent neutrophilic component (Figure 1B, C and D) and fibrinous airway casts (Figure 1E). We found no evidence of lung edema or acute inflammation in the lungs of the control animals (n=3), while patchy areas of atelectasis were observed in all of these cases. There was no evidence of dense neutrophilic infiltrates characteristic of bronchopneumonia in any of the pigs (n=22).
Table 2. Acute Lung Injury Scoring.
| Mode | Atelectasis | Fibrin | Alveolar Septal Inflammatory Cell Infiltrate | Alveolar Edema | ||
|---|---|---|---|---|---|---|
| ALV | SA | LA | ||||
| CMV (n=9) | 0.89 ± 0.26* | 2.67 ± 0.17+ | 2.22 ± 0.15+ | 1.78 ± 0.28+ | 2.56 ± 0.29+ | 1.56 ± 0.29 |
| APRV (n=7) | 1.86 ± 0.14++ | 3.00 ± 0++ | 2.71 ± 0.18++ | 2.00 ± 0.22++ | 2.86 ± 0.14++ | 2.14 ± 0.40 |
| CTRL (n=3) | 0.67 ± 0.33 | 0 | 0 | 0 | 0 | 0.67 ± 0.33 |
Legend. Ventilatory modes are as defoned in the Materials and Methods. CTRL: control mechanically ventilated pigs. ALV: alveolar; SA: small airway. LA: Large airway Scoring for each parameter: 0: none present; 1: involvement <25 percent of the field; 2: 25-50 percent involvement; 3>75 percent involvement. Hematoxylin and eosin (H+E)-stained sections of lung (n=22) were examined by a pathologist with specialized training in lung pathology (TCA). 100 high power (400×) were examined in each to assess alveolar fibrin deposition and inflammation in each case and small and large airway involvement was assessed by scanning the sections at medium (100×) power. Means ± standard errors are shown.
P<0.05 CMV versus APRV.
P<0.05 CMV versus CTRL.
P<0.05 APRV versus CTRL.
Figure 1.
Lung sections were fixed as described in the Methods and stained with hematoxylin and eosin. Images representative of the findings in the lungs of control ventilated pigs or those with SIALI are illustrated at 400× magnification. Comparison of alveoli, small airways, and large airways in representative APRV and CMV ventilated animals (48h), and in ventilated controls (48h), shows similar fibrin deposition in alveoli (arrows) and alveolar septal chronic inflammation in the APRV and CMV ventilated animals (A and D, respectively), and essentially normal alveoli in the ventilated control animal (G). Small airways and large airways in the APRV and CMV ventilated animals (B and E, and C and F, respectively) show fibrin deposition within airways, whereas the small airways and large airways in the ventilated control animal (H and I, respectively) demonstrate no fibrin deposition.
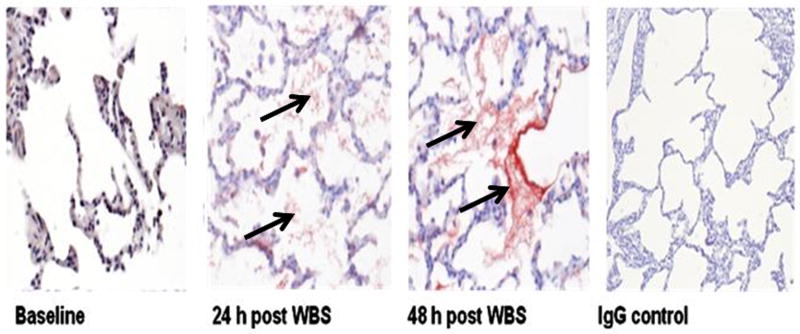
We next used immunohistochemistry to confirm the presence of airway fibrin in animals with established SIALI. Extravascular fibrin deposition was not detectable in the lung parenchyma of time-control pigs over 48 h (Figure 2, baseline left panel). As anticipated, there was florid alveolar fibrin deposition at 24 and 48 h after injury (Figure 2A). The small airways were also involved. As described in the Methods, large airway casts were removed by bronchoscopy, but fibrin was identified within some conducting airways in 48-h animals (Figure 2B). Using two independent analyses in which we used either a monoclonal antibody against human fibrinogen or a rabbit polyclonal antibody to murine fibrinogen, we confirmed that fragments of large airway casts exhibited diffuse reactivity and therefore contained fibrin (Figure 2E). No reactivity was demonstrated in controls exposed to the respective IgG primary antibodies (Figure 2A, rightmost panel). By morphometric analyses performed in all controls and randomly selected 24h pigs (n=3/group) and 3 randomly selected 48-h SIALI animals, fibrin was demonstrated in the alveoli and small airways of the lungs of pigs with SIALI. Scores for alveoli, small airways, and conducting airways were, respectively, 0/0/0 for baseline animals. In the alveoli, the scores alveolar and small airway fibrin deposition were uniformly 3 and 2 for 24- and 48-h pigs (n=3/group), while conducting airway fibrin was less frequent with a morphometric score of 1 in 1 24h animal and 2 48h animals and 0 in the others.
Figure 2.
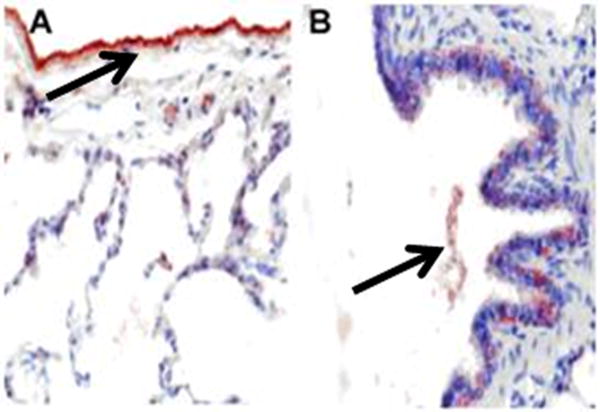
A. Fibrin deposition (arrows) in the lungs of control-ventilated and pigs with SIALI (48 h) is illustrated by fibrin immunostain (red chromogen) with representative images shown, all at 400× magnification. The baseline image shows no fibrin deposition within normal-appearing lung parenchyma. The 24-h and 48-h SIALI images show similar amounts of fibrin lying within alveolar spaces. The IgG control image shows thickened alveolar septa within the treated animal lung, with no fibrin in the representative control slide. B. Images at 400× magnification of fibrin immunostaining (arrows) of 48-h animals show (A) mesothelial staining and subpleural alveolar fibrin, and (B) fibrin within a conducting airway.
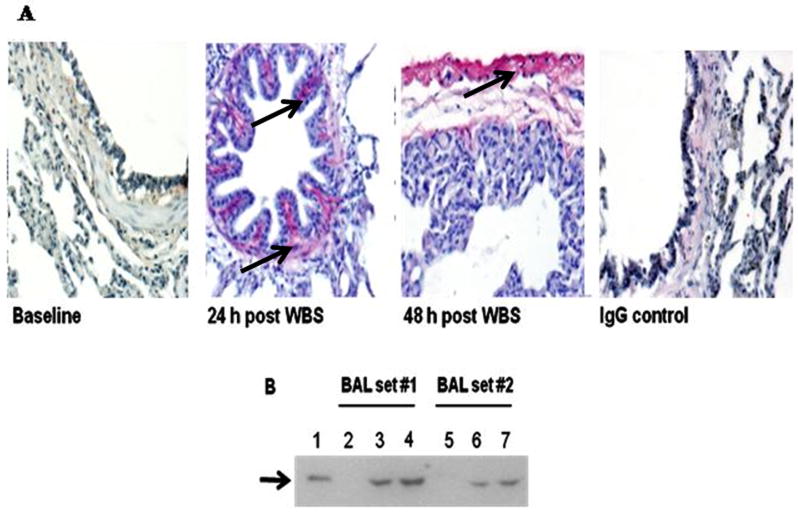
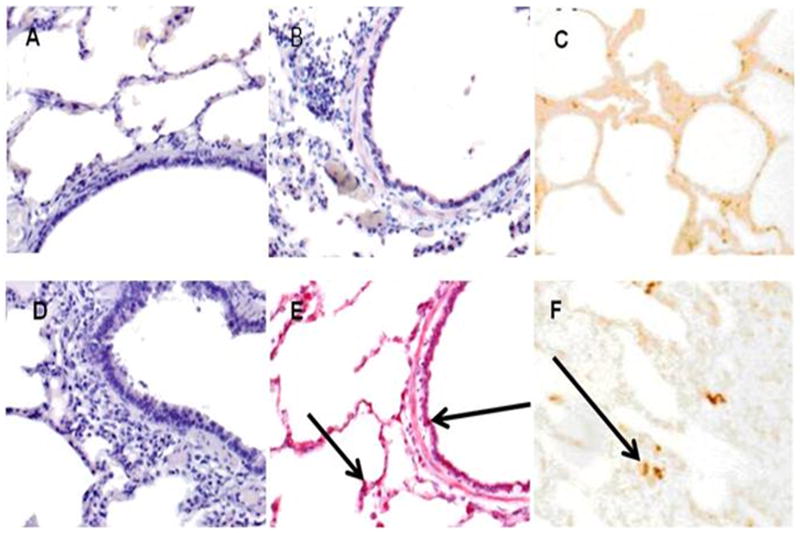
As PAI-1 has been strongly implicated in extravascular fibrin maintenance and in the progression of other forms of ALI (6), we next sought to establish that this inhibitor was likewise expressed proximate to areas of fibrin deposition in SIALI. Focal mucosal and submucosal PAI-1 expression was noted in the large and small airways of pigs with SIALI at 24 and 48 h (Figure 3A). The same animals were used for morphometric assessment of the distribution of PAI-1. Faint PAI-1 was detectable in the alveolar walls of all animals (n=3/group), while strong staining for PAI-1 was demonstrable in the submucosal areas of the airways of pigs with SIALI (Figure 3A). PAI-1 expression was readily detected in BAL of pigs with SIALI (Figure 3B), likely because PAI-1 is secreted by lung epithelial cells. Strong staining for PAI-1 was observed in the mesothelium and subpleural connective tissues (Figure 3A
Figure 3.
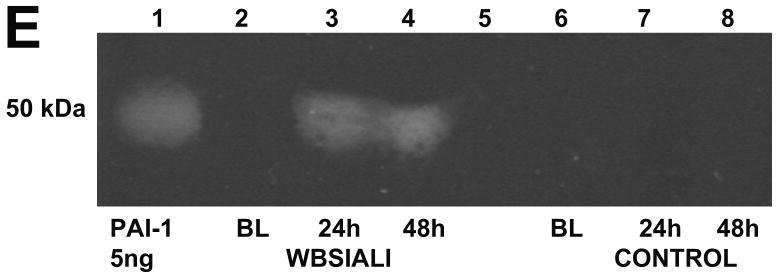
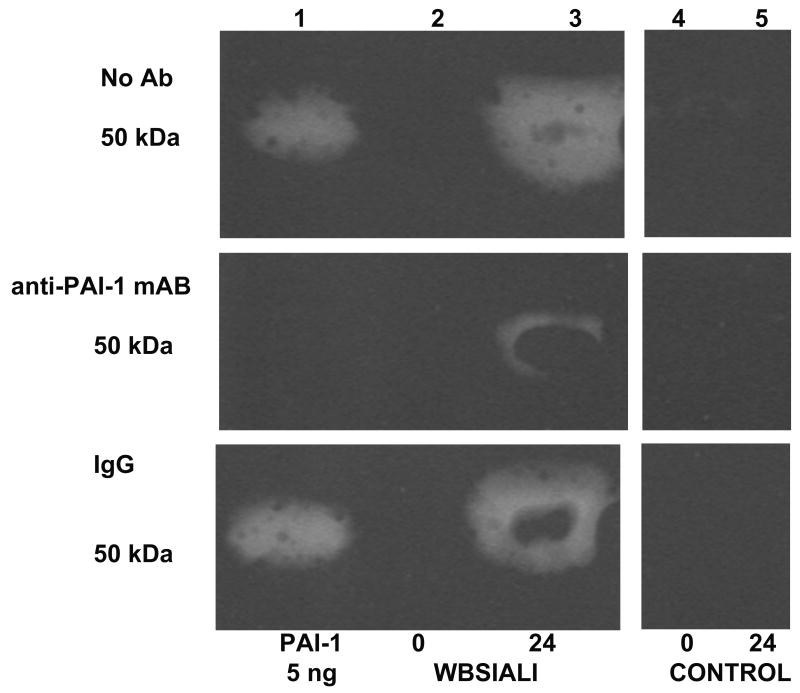
A. PAI-1 staining was not detectable in the lungs of control ventilated animals. Immunopositivity for PAI-1 antigen was detectable in the peribronchial tissues at 24 h after injury. Strong immunopositivity for PAI-1 antigen was detectable in the mesothelium and in subpleural connective tissues at 48 h after injury. Arrows indicate areas of immunoreactivity. IgG represents negative control to show the typical extent of background staining (400×) (data representative of sets from n=3 animals)). B. Western blot analysis of porcine BAL for PAI-1 expression. Lane 1: recombinant PAI-1 standard (10 ng) is illustrated. Lanes 2 and 5: BAL from before injury. Lanes 3 and 6: BAL from animals harvested at 24 h after injury. Lanes 4 and 7: BAL 48 h after injury. Data illustrated are representative of those analyzed from serially obtained BALs (n= 6 animals).
In further analyses, we found that SIALI was further characterized by prominent interstitial apoptosis (Figure 4). Apoptotic cells were identified predominantly in alveolar septae. In selected instances, the apoptotic cells appeared to be alveolar and airway epithelial cells. As p53 has been strongly implicated in apoptosis of resident lung cells, we also sought to determine the distribution of this tumor suppressor in the lungs of pigs with SIALI exposed to wood bark smoke for 24 or 48h and control pigs (n=3/group). In lung tissues at 24 or 48 h after induction of SIALI, p53 expression was prominent in the airway epithelium, in submucosal tissues of the airways, vascular smooth muscle and in the alveoli (not shown).
Figure 4.
Immunohistichemical staining of representative lung tissue images from control lungs (Panels A, B and C and those from pigs with SIALI at 48h (Panels D, E and F, n=3 animals/group) for p53. Strong p53 staining was seen in the airway epithelium and alveolar interstitium (arrows) at 48h after SIALI (E) versus control lung tissue (B). No staining was detectable with control IgG in each of the tissues (Panels A and D). Histologic examination of TUNEL-stained slides of porcine lungs revealed negative immunonegative staining in the control animals (Panel C) and strong nuclear positivity indicative of apoptotic change in the interstitium and lung parenchyma of animals with SIALI at 48h (n=3/group, Panel F).. Images are shown at 400× magnification.
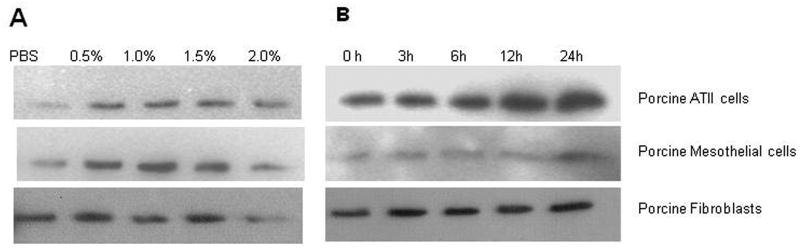
Based on the immunohistochemical demonstration of PAI-1 induction in evolving SIALI, we next sought to determine if exposure of primary resident porcine lung cells to components of wood bark smoke was capable of inducing PAI-1. To this end, we isolated primary lung ATIIs, lung fibroblasts and PMCs from porcine lung tissue or pleural fluids (PFs) and exposed the cells to WBSE after which PAI-1, a secreted protein, was measured in the conditioned media. Cells exposed to the extract vehicle served as negative controls. In initial dose-response analyses (Figure 5A), we found that exposure of each of these cell types to WBSE for 24 h induced PAI-1, with maximal induction occurring at a concentration of 0.5-1.5%. In time-course analyses (Figure 5B), exposure of ATIIs, lung fibroblasts and pleural mesothelial cells to 1% WBSE was found to induce maximal expression of PAI-1 in the conditioned media after 24 h of exposure. The responses of fibroblasts cultured from control ventilated pigs and those at 48h after SIALI were comparable in these analyses (not shown). These experiments confirm that cultured porcine ATIIs, lung fibroblasts and PMCs uniformly respond to WBSE with induction of PAI-1.
Figure 5. Induction of PAI-1 antigen in primary porcine PMCs, ATIIs and lung fibroblasts exposed to WBS extract in vitro.
A. Dose responses: Conditioned media from porcine primary cells exposed to different doses of wood bark smoke extract (WBSE) for 24h and resolved by 10% SDS-PAGE gel. The separated proteins were then transferred to a nitrocellulose membrane followed by PAI-1 antigen detection using rabbit polyclonal PAI-1. B. Time course experiments: 50 μl of conditioned media from porcine cells exposed to 1.0% WBSE in a time-dependent manner was analyzed for PAI-1 antigen by Western blotting. The blots in each case are representative of the findings in duplicate independent experiments.
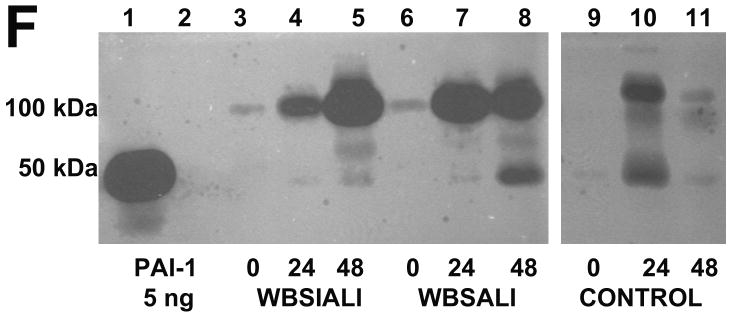
We next used amidolytic, fibrin-plate and enzymographic analyses to confirm that PAI-1 activity is expressed in BAL and, in particular, by PMCs (Figure 6), which exhibited robust expression of PAI-1 (Figure 3A). We found that BAL PGN activator (PA) activity and ability to lyse fibrin were both significantly decreased by 24 or 48 h after induction of SIALI (Figures 6A and B). Interestingly, relatively low levels of BAL PL and uPA activity were detectable at 24 and 48h (Figure 6 C and D). There were no significant differences for any of the parameters illustrated in Figure 6A, B, C and D between the CMV and APRV groups. While BAL from only two ventilated controls were available, a trend of preserved PA and fibrinolytic activity in BAL was observed over 48h. Depressed BAL fibrinolytic activity at 24 and 48 h appeared to be, at least in part, suppressed by increased PAI-1. This was shown by reverse fibrin enzymography (Figure 6E), showing increased formation of PA-PAI-1 complexes (Figure 6F). Zones of resistance to PA activity were inhibited by an antibody to PAI-1 impregnated in the indicator gel, indicating that the PAI we identified was, in fact, PAI-1 (Figure 6G).
Figure 6.
A. A box plot of changes in plasminogen (PGN) activator activity with time. PGN activity (expressed in arbitrary units (AU) was determined in BAL of animals before (0 h) and after (24 h and 48 h) induction of SIALI (n=9, including 4 pigs ventilated with CMV and 5 with APRV); each value shown as a circle, ventilated controls (n=2) are shown in dark circles). Statistically significant differences (P<0.05) are indicated with asterisks, all samples done in duplicate analyses). B. A box plot of changes in the fibrinolytic activity as determined by release of radiolabeled fibrin in the same samples. Fibrinolytic activity was expressed in international units (IU) per ml of BAL. C and D. Changes in plasmin and urokinase amidolytic activities (expressed in arbitrary units (AU) determined in the same BAL samples. E. Reverse fibrin enzymography representative of the findings in BAL samples of all 9 48h SIALI and 2 control uninjured, mechanically ventilated pigs, showing the appearance of zones of resistance to fibrinolytic activity in BAL after ALI and absence of these bands in the BAL of timed-control pigs. F. Enzymographic analysis representative of the BAL findings in 48h SIALI pigs (n=9) and controls (n=2), showing increased PA-PAI-1 complexes at 100 kDa after injury. Zones of free uPA activity were also detectable in control and injured animals. G. Neutralization of zones of resistance to fibrinolysis in BAL of animals with SIALI by impregnation of the indicator gel with an antibody to human PAI-1 in reverse enzymographic analyses.
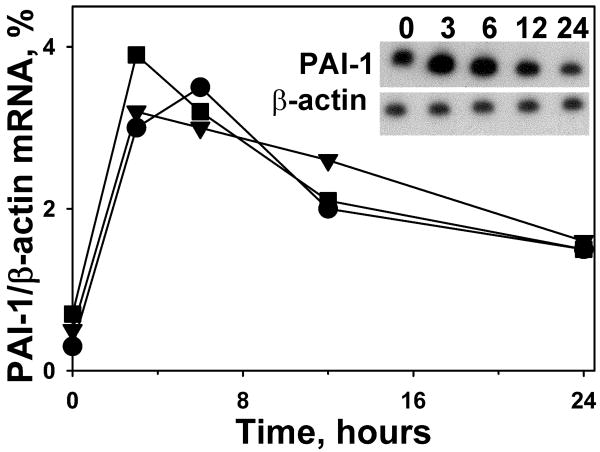
In complementary studies, we assessed the time course of induction of PAI-1 mRNA in these cells by PCR analyses (Figure 7, panels A-C). As shown in the densitometric plots of PAI-1 mRNA normalized to the expression of β-actin mRNA (Figure 7D), PAI-1 mRNA was induced in ATIIs, lung fibroblasts and pleural mesothelial cells at 3-6 h after exposure to 1% WBSE for 24h. These analyses demonstrate that the induction of PAI-1 mRNA precedes that of the induction of the protein in resident porcine lung cells.
Figure 7.
Total RNA was isolated by phenol chloroform extraction after the indicated time intervals. 5 μg of total RNA was subjected to reverse transcription PCR for 45 min at 42°C. The reverse-transcribed product was PCR-amplified for 30 cycles using porcine PAI-1 and β-actin primers followed by separation of the PCR product on 5.0% urea gel. The gel was later dried for 2 h and autoradiographed overnight at -80°C. The densitometric ratios of PAI-1/β-actin mRNA were plotted over time as illustrated in the line graph (●epithelial cells, ▼mesothelial cells, ■ fibroblasts). The gel image represents the maximal PAI-1 mRNA induction at 3 h in PMCs. The plotted densitometric data is representative of 3 independent experiments in PMCs, ATIIs and lung fibroblasts. Symbols:
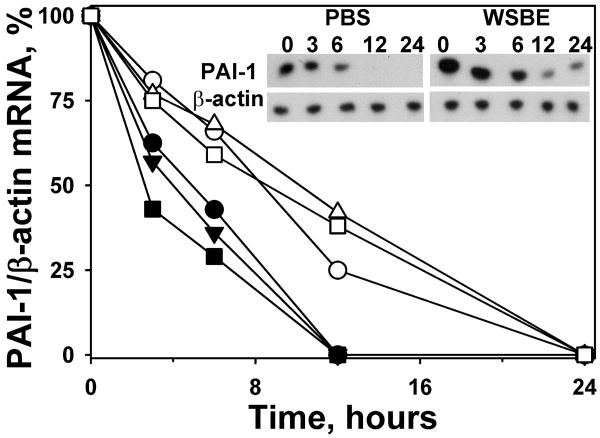
While PAI-1 expression is well-known to be regulated at the transcriptional level, we recently reported that it was controlled at the level of PAI-1 mRNA stability in pleural mesothelial cells (20). We next blocked ongoing transcription and found that PAI-1 mRNA was stabilized in primary porcine ATIIs, lung fibroblasts and PMCs exposed to 1% WBSE for 24h compared with PBS treated cells (Figure 8A-C). This is visualized in a densitometric plot of PAI-1 mRNA normalized to β-actin mRNA (fig 8D).These analyses confirm that these cell types respond to WBSE exposure with stabilization of PAI-1 mRNA. These studies also indicate that increased stability of PAI-1 mRNA contributes to the induction of PAI-1 in each of these cell types and strongly suggest that the process could be operative in SIALI in vivo.
Figure 8.
Porcine primary cells were exposed to phosphate-buffered saline (PBS, filled symbols) or 1.0% wood-bark-smoke extract (WBSE, empty symbols) for 3 h, followed by blocking ongoing transcription with DRB (20μg/ml) and actinomycin (5μg/ml) at the indicated time intervals. Total RNA was isolated and reverse transcribed as described earlier. The densitometric ratios of PAI-1/β-actin mRNA stabilized after DRB treatment were plotted over time as shown in the line graph (circles-epithelial cells, triangles-mesothelial cells, squares-fibroblasts):. The gel image is representative of PAI-1 mRNA stabilization after DRB treatment in primary porcine PMCs.
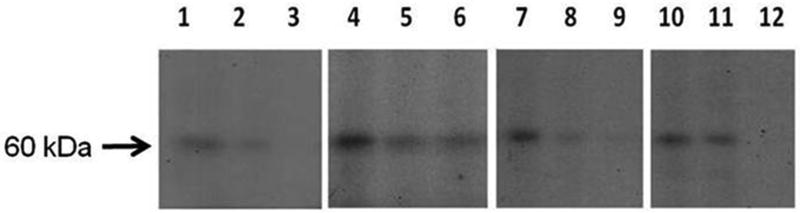
Lastly, we sought to further elucidate the mechanism by which PAI-1 mRNA was regulated in porcine lung cells. Given the limited availability of ATII, lung fibroblasts and PMCs, analyses were by necessity restricted to elucidation of the posttranscriptional control of PAI-1 expression. Using gel-shift and UV-cross-linking analyses, we were able to detect an interaction between PAI-1 mRNA binding protein and PAI-1 mRNA in porcine PMCs, lung fibroblasts and ATIIs (Figure 9). The interaction involves a PAI-1 mRNA binding protein that, like human 6-phospho-D-gluconate-NADP oxidoreductase (6PGD), migrates at about 60kDa (20). Exposure of each of these cell types to 1% WBSE for 24 h disrupted the binding protein–PAI-1 mRNA interaction (Figure 9, lanes 2, 5,8 and 11), as did treatment of the cells with TGF-β(lanes 3, 6, 9 and 12. The response to TGF-β recapitulates the responses of human pleural mesothelial and lung epithelial cells, as we recently reported (20) and show that MeT5A cells (Lanes 1-3) respond to WBSE with dissociation of the regulatory complex as they do to TGF-β. Because we assessed the binding of putative porcine PAI-1 mRNA binding interactions using a 33 nt transcript known to bind the PAI-1 mRNA destabilizing protein 6 PGD, we can conclude that the binding interaction we observed here involves a porcine 6PGD-like protein.
Figure 9.
100 μg of total cellular protein from porcine primary cells treated with 1.0% WBSE for 24 h was hybridized with 32P d[UTP] 33′ nucleotide PAI-1 mRNA transcript for 30 min. The sample was then incubated with RNAse for 30 min at 37°C and heparin for 10 min at room temperature. The samples were then UV cross-linked at 2500 J for 45 min on ice followed by separation of the mixture on 10% SDS-PAGE at 35V. The gels were later dried and autoradiographed overnight at -80°C to detect the PAI-1 mRNA binding protein. Arrow indicates the binding interaction (representing the 60kDa 6PGD-like PAI-1mRNA binding protein (PAI-1 mRNABp). Lanes 1-3: PAI-1 mRNA – PAI-1 mRNA BP interaction in MeT5A human PMCs exposed to buffer, 1.0% WBSE (Lane 2) or 2 ng/ml TGF-β (Lane 3) over 24 h. Lanes 4-6: Porcine PMCs; Lanes7-9: Porcine ATIIs; Lanes 10-12: Porcine fibroblasts (same format; n=1-3 independent experiments per cell type). Free probe is not shown.
Discussion
We found that extravascular fibrin is florid in this porcine model of SIALI and that it is distributed in the conducting airways as well as in the small airways and alveolar space. While clinical inhalation injury involves variable exposure to a complex mixture of particulates and toxic gases, this model reliably generates inhalationally-induced ALI that exhibits key features of the clinical disease. Airway casts that form within 24 h after induction of SIALI were confirmed to be fibrinous by immunohistochemical analyses. The prominent fibrinous casts in the conducting airways likely contribute to the increased airway resistance often observed in SIALI Induction of bronchospasm initiated by coagulation in the airways has previously been reported in SIALI. This may be attributable to intraluminal deposition of airway fibrin (21). In our model, bronchoscopic removal of these casts was mandatory for survival of animals challenged with SIALI, which may have limited their detection in conducting airways of tissues harvested at necropsy. Prominent alveolar fibrin deposition likely contributes to the surfactant dysfunction, atelectasis and impaired gas exchange that characterize this model and that are known to occur in clinical SIALI.
Our data indicate that PAI-1 is induced in the lungs in SIALI, and that increased PAI-1 is temporally associated with and proximate to sites of extravascular fibrin deposition. PAI-1 was found to be induced in the epithelium and connective tissues of the airways and in the subpleural connective tissues and mesothelium within 24-48 h after injury. In addition, PAI-1 expression was increased in BAL of pigs during the progression of SIALI over this same time frame, indicating that small airway and alveolar lining fluids were enriched. By fibrin plate analyses, fibrinolytic activity was detectable in the BAL of control animals and relatively decreased in that of pigs with SIALI, indicating that induction of PAI-1 was at least in part responsible for the defect in local fibrinolytic activity in BAL. Despite evidence of relatively low levels of uPA and PL amidolytic activities in BAL, the presence of concurrently increased PAI-1 was demonstrated by reverse enzymography and likely accounts for much of the decrement of PA activity in amidolytic analyses. These results could indicate formation of “moleculer cage” type complexes between macroglobulins and uPA and PL, which we detected in PFs of animals with pleural injury (18). Collectively, these data confirm that PAI-1 contributes to the decreased fibrinolytic activity observed in BAL of pigs with SIALI, promotes persistence of airway and alveolar fibrin deposition, and thereby contributes to abnormal gas exchange observed in this model. We found that removal of fibrinous large airway casts by bronchoscopy improved gas exchange and decreased peak airway pressure, suggesting that this approach may likewise be of lifesaving value in patients with SIALI, especially those with evidence of large airway obstruction and atelectasis.
Since parenchymal lung expression of PAI-1 was robust in SIALI, as shown by immunohistochemical analyses, we inferred that resident lung cells would substantively contribute to PAI-1 expression if challenged with components of wood bark smoke. To address this possibility, we isolated and tested the ability of WBSE to induce PAI-1 in porcine ATII, lung fibroblasts and PMCs by Western and PCR analyses. Interestingly, we found that WBSE induced PAI-1 secretion by all of these resident lung cells and that the magnitude of the response was comparable. As PAI-1 is a secreted protein, ATIIs and lung fibroblasts may contribute to the levels of PAI-1 seen in BAL after induction of SIALI, and PAI-1 is likewise induced in PMCs. While it is likely that the cells harvested from the lungs of the SIALI animals were activated, our data clearly show that they retain the ability to increase PAI-1 in response to WBSE exposure.
We next sought to test the mechanism by which WBSE induced PAI-1. It is well-known that PAI-1 gene expression is regulated at the level of transcriptional activation by mediators that like TGF-β are present in the setting of high-grade inflammation (13). To our knowledge, however, there have been no prior studies that addressed the possibility that PAI-1 is regulated at the level of posttranscriptional control of mRNA stability in this context. We recently reported that lung epithelial cells and PMCs regulate PAI-1 at the posttranscriptional level (20), providing us with a strong rationale to investigate whether this pathway is operative in SIALI. In that study, we reported a novel PAI-1 mRNA destabilizing interaction involving the interaction of PAI-1 mRNA with a protein we characterized as 6-PGD. TGF-β–mediated induction of PAI-1 was found to dissociate 6PGD from its specific 33 nt binding sequence within the 3′ untranslated region (3′UTR) of PAI-1 mRNA. In the present study, we report, for the first time, that a similar mechanism is operative when primary porcine PMCs, lung fibroblasts or ATIIs are stimulated with WBSE or TGF-β. Our group previously showed that PAI-1 may be induced by an alternate mechanism in which induction of PAI-1 in lung epithelial cells of mice with bleomycin-induced ALI or human ATII cells exposed to bleomycin or cigarette smoke extract occurred through interaction of p53 with PAI-1 mRNA (8). While we found immunohistochemical evidence of induction of p53 and interstitial apoptosis of the lungs of animals with SIALI, we found no evidence of the interaction of p53 with PAI-1 mRNA by gel shift analysis. It is possible that this finding relates to differences in the interaction of the human PAI-1 mRNA binding sequence with porcine p53. Our findings are consistent with the immunohistochemical findings indicating that PAI-1 is induced in these cell types in SIALI and suggest the likely possibility that the process could contribute to PAI-1 expression in the airways, alveoli and mesothelium in pigs with SIALI.
Our observations confirm that SIALI exhibits profound impairment of the pulmonary fibrinolytic system and that PAI-1 over-expression contributes to the effect. These changes are characteristic of ALI (8) but their contribution to the pathogenesis of ALI appears to be complex. For example, lung function is not altered in PAI-1 or fibrinogen knockout mice with ALI induced by hydrochloric acid (HCl) (22), but the authors acknowledge that the effects of HCl on surfactant and lung dysfunction do not obviate the potentially important effects of locally increased PAI-1 and fibrin deposition in ALI on altered microvascular permeability, inflammatory cell recruitment, lung remodeling or subsequent fibrotic repair. These derangements may have special relevance to clinical SIALI, since fibrinous airway casts are a prominent feature not usually seen in other forms of ALI. It is of interest that fibrinolytic therapy has successfully been used to clear the intravascular clots and fibrinous transitional matrix, and to reverse respiratory dysfunction in various forms of ALI, in swine and in patients with ARDS (23;24). To mitigate the risk of systemic bleeding, nebulized therapy with tissue plasminogen activator has more recently been used and was found to reverse pulmonary dysfunction when instituted early (within 4h) in SIALI (3). Aerosolized anticoagulants were also shown to be beneficial in SIALI but were initiated even earlier (within 2 h of inhalation injury) in sheep (4). These studies provide proof of principle that localized intervention that targets airway and alveolar fibrin is salutary. These studies raise the possibility that this or other relatively PAI-1 resistant agents such as single-chain urokinase (scuPA) (17) could be of advantage when delivered by inhalation in SIALI. We found that activity of scuPA is protected from inactivation by PAI-1 in BAL fluids of pigs with SIALI (not shown), which suggests that the mechanism of protection recapitulates that which occurs in pleural fluids--where formation of scuPA or two chain uPA complexes with α-2 macroglobulin (18).
In conclusion, PAI-1 is induced in the lungs of swine with wood-bark-smoke-induced acute lung injury. The increment of PAI-1 in the airways, mesothelium and alveoli is concurrently associated with decreased BAL fibrinolytic activity, and promotes extravascular fibrin in these locations. Over-expression of PAI-1 in the airways likely sustains intrabronchial fibrin casts, which are amenable to clearance by inhalational interventions that target fibrin deposition. WBSE induces PAI-1 expression in primary ATIIs, lung fibroblasts and pleural mesothelial cells in vitro. This strongly suggests that these cells may all contribute to PAI-1 expression in this model. The induction mechanism involves stabilization of PAI-1 mRNA in ATIIs and PMCs. This study represents the first demonstration of this mode of regulation of PAI-1 in this or any other form of ALI.
Acknowledgments
The technical and administrative support of the following individuals is gratefully acknowledged: Bryan Jordan MSN, Michael Lucas, Corina Necsoiu M.D., and Ruth Nguyen M.D.
This study was supported by National Institute of Health (NHLBI PPG PO1 HL076406, R-21-HL093547, FAMRI-ID-082380) the Texas Lung Injury Institute; and the Combat Critical Care Engineering task area, Combat Casualty Care Research Program, U.S. Army Medical Research and Materiel Command.
Abbreviations
- 3′UTR
3′ Untranslated region
- 6PGD
6Phospho-D-Gluconate-NADP oxidoreductase
- ARDS
Acute respiratory distress syndrome
- APRV
Airway-pressure-release ventilation
- ATII
Alveolar type II cells
- AU
Arbitrary fluorescence emission units
- BAL
Bronchoalveolar lavage
- BALF
BAL fluid
- CMV
Conventional mechanical ventilation
- FiO2
Fraction of inspired oxygen
- HBSS
Hanks balanced salt solution
- ICU
Intensive care unit
- NSS
Normal saline solution
- PBS
phosphate-buffered saline
- PL
Plasmin
- PGN
Plasminogen
- PA
PGN activator
- PAI-1
Plasminogen activator inhibitor-1
- PMCs
Pleural mesothelial cells
- PEEP
Positive-end expiratory pressure
- PaCO2
Partial pressure of carbon dioxide in arterial blood
- PaO2
Partial pressure of oxygen in arterial blood
- PIP
Peak inspiratory pressure
- PFs
Pleural fluids
- PFR
PaO2-to-FiO2 ratio
- scuPA
Single-chain urokinase
- SIALI
Smoke-induced acute lung injury
- TV
tidal volume
- uPA
Urokinase-type plasminogen activator
- WBSE
Wood bark smoke extract
- WBSIALI
wood-bark-smoke-induced acute lung injury
Footnotes
The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
References
- 1.Enkhbaatar P, Traber DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci(Lond) 2004;107(2):137–143. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- 2.Demling RH. Smoke inhalation lung injury: an update. Eplasty. 2008;8:e27–e27. [PMC free article] [PubMed] [Google Scholar]
- 3.Enkhbaatar P, Murakami K, Cox R, Westphal M, Morita N, Brantley K, Burke A, Hawkins H, Schmalstieg F, Traber L, Herndon D, Traber D. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock. 2004;22(1):70–75. doi: 10.1097/01.shk.0000129201.38588.85. [DOI] [PubMed] [Google Scholar]
- 4.Enkhbaatar P, Cox RA, Traber LD, Westphal M, Aimalohi E, Morita N, Prough DS, Herndon DN, Traber DL. Aerosolized anticoagulants ameliorate acute lung injury in sheep after exposure to burn and smoke inhalation. Crit Care Med. 2007;35(12):2805–2810. doi: 10.1097/01.ccm.0000291647.18329.83. [DOI] [PubMed] [Google Scholar]
- 5.Murakami K, McGuire R, Cox RA, Jodoin JM, Bjertnaes LJ, Katahira J, Traber LD, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock. 2002;18(3):236–241. doi: 10.1097/00024382-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Idell S. Coagulation, Fibrinolysis, and Fibrin Deposition in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 7.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. Journal of Clinical Investigation. 1989;84(2):695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty S, Padijnayayveetil J, Tucker T, Stankowska D, Idell S. The fibrinolytic system and the regulation of lung epithelial cell proteolysis, signaling, and cellular viability. Am J Physiol Lung Cell Mol Physiol. 2008;295(6):L967–L975. doi: 10.1152/ajplung.90349.2008. [DOI] [PubMed] [Google Scholar]
- 9.Sapru A, Curley MA, Brady S, Matthay MA, Flori H. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med. 2010;36(1):157–163. doi: 10.1007/s00134-009-1690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsangaris I, Tsantes A, Bonovas S, Lignos M, Kopterides P, Gialeraki A, Rapti E, Orfanos S, Dimopoulou I, Travlou A, Armaganidis A. The impact of the PAI-1 4G/5G polymorphism on the outcome of patients with ALI/ARDS. Thromb Res. 2009;123(6):832–836. doi: 10.1016/j.thromres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physio. 2003;285(1):L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 12.Aoki K, Aikawa N, Sekine K, Yamazaki M, Mimura T, Urano T, Takada A. Elevation of plasma free PAI-1 levels as an integrated endothelial response to severe burns. Burns. 2001;27(6):569–575. doi: 10.1016/s0305-4179(01)00011-0. [DOI] [PubMed] [Google Scholar]
- 13.Dimova EY, Kietzmann T. Metabolic, Hormonal and environmental regulation of plasminogen activatorinhibitor-1 (PAI-1) expression: Lessons from the liver. Thromb Haemost. 2008;100(6):992–1006. [PubMed] [Google Scholar]
- 14.Park MS, Cancio LC, Batchinsky AI, McCarthy MJ, Jordan BS, Brinkley WW, Dubick MA, Goodwin CW. Assessment of severity of ovine smoke inhalation injury by analysis of computed tomographic scans. J Trauma. 2003;55(3):417–427. doi: 10.1097/01.TA.0000083609.24440.7F. [DOI] [PubMed] [Google Scholar]
- 15.Murphy SA, Dinsdale D, Hoet P, Nemery B, Richards RJ. A Comparative study of the isolation of type II epithelial cells from bat, hamster, pig and human lung tissue. Methods Cell Sci. 1999;21(1):31–38. doi: 10.1023/a:1009825008023. [DOI] [PubMed] [Google Scholar]
- 16.Shetty S, Idell S. A Urokinase Receptor MRNA binding protein from rabbit lung fibroblasts and mesothelial cells. American Journal of Physiology. 1998;274(6 Pt 1):L871–L882. doi: 10.1152/ajplung.1998.274.6.L871. [DOI] [PubMed] [Google Scholar]
- 17.Idell S, Allen T, Chen S, Koenig K, Mazar A, Azghani A. Intrapleural activation, processing, efficacy, and duration of protection of single-chain urokinase in evolving tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L25–L32. doi: 10.1152/ajplung.00118.2006. [DOI] [PubMed] [Google Scholar]
- 18.Komissarov AA, Mazar AP, Koenig K, Kurdowska AK, Idell S. Regulation of intrapleural fibrinolysis by urokinase-alpha-macroglobulin complexes in tetracycline-induced pleural injury in rabbits. AM J Physiol Lung Cell Mol Physiol. 2009;297(4):L568–L577. doi: 10.1152/ajplung.00066.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein P53. J Biol Chem. 2008;283(28):19570–19580. doi: 10.1074/jbc.M710268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shetty S, Velusamy T, Shetty RS, Marudamuthu AS, Shetty SK, Florova G, Tucker T, Koenig K, Shetty P, Bhandary YP, Idell S. Posttranscriptional regulation of plasminogen activator inhibitor-1 expression in human pleural mesothelial cells. Am J Respir Cell Mol Biol. 2009;43(3):358–367. doi: 10.1165/rcmb.2009-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest. 2004;114(1):104–111. doi: 10.1172/JCI19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen GB, Cloutier ME, Larrabee YC, Tetenev K, Smiley ST, Bates JH. Neither fibrin nor plasminogen activator inhibitor-1 deficiency protects lung function in a mouse model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;296(3):L277–L285. doi: 10.1152/ajplung.90475.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardaway RM, Williams CH, Marvasti M, Farias M, Tseng A, Pinon I, Yanez D, Martinez M, Navar J. Prevention of adult respiratory distress syndrome with plasminogen activator in pigs. Crit Care Med. 1990;18(12):1413–1428. doi: 10.1097/00003246-199012000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Hardaway RM, Harke H, Williams CH. Fibrinolytic agents: a new approach to the treatment of adult respiratory distress syndrome. Adv Ther. 1994;11(2):43–51. [PubMed] [Google Scholar]