Abstract
In normal healthy individuals, mineral formation is restricted to specialized tissues which form the skeleton and the dentition. Within these tissues, mineral formation is tightly controlled both in growth and development and in normal adult life. The mechanism of calcification in skeletal and dental tissues has been under investigation for a considerable period. One feature common to almost all of these normal mineralization mechanisms is the elaboration of matrix vesicles, small (20–200 nm) membrane particles, which bud off from the plasma membrane of mineralizing cells and are released into the pre-mineralized organic matrix. The first crystals which form on this organic matrix are seen in and around matrix vesicles. Pathologic ectopic mineralization is seen in a number of human genetic and acquired diseases, including calcification of joint cartilage resulting in osteoarthritis and mineralization of the cardiovasculature resulting in exacerbation of atherosclerosis and blockage of blood vessels. Surprisingly, increasing evidence supports the contention that the mechanisms of soft tissue calcification are similar to those seen in normal skeletal development. In particular, matrix vesicle-like membranes are observed in a number of ectopic calcifications. The purpose of this review is to describe how matrix vesicles function in normal mineral formation and review the evidence for their participation in pathologic calcification.
Introduction
The mechanisms involved in the formation of mineralized tissues have been under investigation for many years. While much progress has been made in this period, much still remains to be discovered. One surprising outcome from this research is the notion that pathological calcification in normal soft tissues may utilize mechanisms similar to those found in hard tissues. A key constituent found in both normal and ectopic calcifications are matrix vesicles (MVs) and MV-like particles. These small (20–200 nm) particles are budded off from the plasma membrane of chondrocytes, osteoblasts, and odontoblasts prior to the onset of matrix mineralization. Similar structures have been observed in soft tissue calcification. The purpose of this review is to first describe the role of MV in hard tissue mineralization and then to relate that information to pathological calcification.
The discovery of matrix vesicles
Ultrastructural studies of pre-mineralized matrices provided the first evidence that the initial crystals were formed in and around MV [1, 2]. As is common in emerging science, these initial observations were greeted with considerable skepticism. Some authorities dismissed them as preparation artifacts, which were rarely seen when the tissue was prepared anhydrously [3]. Over time, considerable evidence collected in vitro and in vivo substantiated the role of MV in calcification of bone, cartilage, and dentin [4-9]. Indeed, even some of the skeptics began to report the existence and function of MV [10]. By the mid 1980s, the study of MV in the mineralization of hard tissues blossomed. A plethora of studies were carried out to ascertain how MVs are formed and how they participated in the mineralization process. While these studies are by no means complete, a cohesive picture of MV action is emerging.
Composition of matrix vesicles
MV are budded from the plasma membranes of mineral-forming cells and oriented so that the exterior face of MV is the same as that of the parent membrane [11-13]. The composition of MV is, however, different from that of the membranes from which they originate. The principle components of MV are shown in Table 1. MVs are significantly enriched in tissue nonspecific alkaline phosphatase (TNAP) and phosphatidylserine compared to the cell plasma membrane [5, 14-18]. Other important components include the annexins, nucleotide pyrophosphatase phosphodiesterase 1 (NPP1; PC1), the type III Na+/PO4 3− cotransporters Pit1,2 and Phospho-1, a phosphatase highly specific for phosphocholine and phosphoethanolamine [19-22]. The mechanism of MV biogenesis is not fully understood, but one component of the mechanism involves the formation of cholesterol-rich lipid rafts in the parent membrane prior to MV formation [23, 24]. Each of the components in the table has been linked to key functions of MV and will be highlighted below.
Table 1.
Major components of matrix vesicles
| Enzymes |
| Alkaline phosphatase (TNAP) |
| Phospho-1 |
| Na+/K+ATPase |
| NPP1/PC-1 |
| MMP-2 |
| MMP-3 |
| MMP-13 |
| Transport proteins |
| Annexins 5, 2, 6, 11, 4, 1, 7 |
| Pit 1,2 |
| Other proteins |
| Integrins β1, β5, αV, α11, α1, α3 |
| Lipids |
| Neutrals |
| Free fatty acids |
| Phosphatidylcholine |
| Phosphatidylethanolamine |
| Phosphatidylinositol |
| Phosphatidylserine |
The biochemistry and cell biology of mineralization
The vertebrate skeleton produces five different mineralized tissues: dental enamel, dentin, cementum, calcified cartilage, and bone. The composition of these tissues is shown in Table 2. While the mineral content in each of these tissues varies, the mineralized phase is the same, a carbonate-rich, hydroxyapatite, containing small amounts of Na+, K+,Mg+2, and Cl−, termed biological apatite [25, 26]. The organic phase of these tissues is composed primarily of collagens, except in enamel. This most highly mineralized tissue found on the exterior surface of the teeth is produced by a unique mechanism, different from that of the other four tissues. Consideration of this mechanism is outside the scope of this review; further information can be found in these recent reviews [27, 28].
Table 2.
Mineralized tissue composition
| Tissue | Mineral (%) | Collagen | Major non-collagen proteins |
|---|---|---|---|
| Enamel | 95–98% | None | Amelogein Enamelin |
| Dentin | 80–52% | Type I | Dentin phosphoprotein Dentin sialoprotein Dentin matrix protein 1 |
| Cementum | 50–70% | Type I | Sibling proteinsa Osteocalcin |
| Bone | 50–90% | Type I, III | Sibling proteinsa Osteocalcin |
| Calcified cartilage | 35–50% | Type II, IX, X | Proteoglycan (aggrecan) |
Sibling proteins are small integrin-binding ligand, n-linked glycoproteins and include osteopontin, bone sialoprotein, dentin matrix protein 1, dentinsialophosphoprotein, enamelin, and matrix extracellular phosphoglycoprotein
Of the collagenous mineralized tissues, three are composed of mainly type I collagen, the exception being calcified cartilage which is built on a type II collagen matrix. All of the collagenous mineralized tissue matrices also contain non-collagenous proteins, whose function in mineralization and tissue function is a major research area in hard tissue biology. Some of the important non-collagenous proteins in mineralized tissues are listed in Table 2. The overall mechanism of mineralization of the four collagenous tissues is the same, cells secrete an unmineralized organic matrix and the mineral phase then forms in the space between the cells and the mineralization front (see Fig. 1). In the figure, OB labels an osteoblast, MF shows the mineralization front, C labels collagen fibrils, and MV points to a matrix vesicle with crystals forming in/around it. As can be seen in this image, there is a space between the osteoblasts and the mineralization front, which is composed of the organic matrix. In addition to synthesizing and secreting the organic matrix, the cells maintain the ion composition of the extracellular space through the action of Ca2+ and PO4 3− ion pumps [29, 30].
Fig. 1.
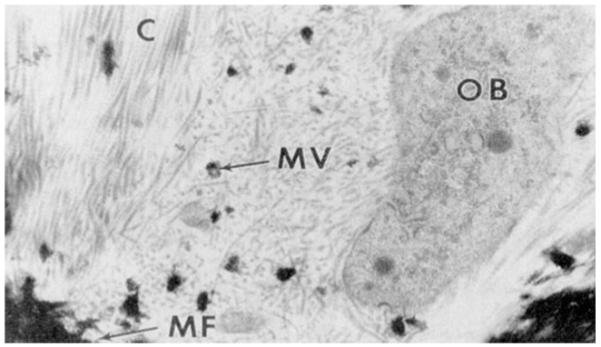
Transmission electron micrograph of the mineralization front (MF) in forming bone. An osteoblast (OB) is secreting matrix including collagen (C). A matrix vesicle (MV) with mineral is seen in the matrix
The activity of these ions in the extracellular space is modulated by non-collagenous matrix proteins and the presence of inorganic pyrophosphate (POP). The latter compound, at low concentrations, is a potent inhibitor of mineral formation. Non-collagenous proteins have been shown to enhance mineral formation, inhibit mineral formation, or do both [31-39] Thus, in the pre-mineralized matrix, conditions are poised for mineral to form, but the process is blocked by the inhibitors. It is currently believed that mineralization is triggered by alteration of the PO4 3−/POP ratio [40]. This formulation is schematized in Fig. 2.
Fig. 2.
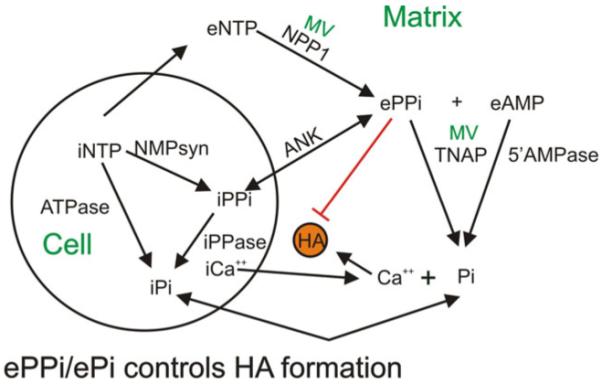
A schematic representation of the regulation of Ca2+ and Pi concentration in the extracellular matrix by cells and MV. Intracellular nucleotide triphosphates (iNTP) contribute to the intracellular Pi pool through enzymatic hydrolysis (ATPase) and the intracellular pyrophosphate pool (iPPi) by enzymes which produce nucleotide monophosphates (NMPsyn). iPPi also feeds the Pi pool by hydrolysis by intracellular pyrophosphatase (iPPase). iPPi is transported into the matrix via the progressive ankylosis protein (ANK). iNTPs also are secreted into the matrix to form a pool of extracellular NTPs (eNTP). eNTPs are converted by MV enzyme nucleotide pyrophosphohydrolase (NPP1) to ePPi, and ePPI is broken down to Pi by MV enzyme tissue nonspecific alkaline phosphatase (TNAP). In the matrix, Ca2+ and PI combine to form hydroxyapatite (HA)
The figure indicates the interactions of the cell (osteoblast, odonotoblast, or chondrocyte) and MV in regulating the extracellular concentrations of Ca2+ and PO4 3−. As shown in the figure, cells pump Ca2+ out into the extracellular space. The cytoplasmic calcium concentration is approximately 0.1 μM. The origin of the matrix Ca2+ is from the blood, which is regulated to a fixed high level (2.5 mM total, ~1 mM ionic) by the calcium homeostasis system [41]. The ionic concentration of calcium in the extracellular space is modulated by the presence of non-collagenous matrix proteins [42, 43]. In contrast, cellular PO4 3− levels are in the range of 5 mM as PO4 3− is required for many metabolic reactions. Keeping the cytoplasmic Ca2+ concentration low allows the cytoplasmic concentration of PO4 3− to be high without causing mineralization to occur intracellularly. The intracellular phosphate pool is fed by PO4 3− uptake from the blood and adenosine triphosphate (ATP) and AMP hydrolysis. POP is formed in a number of ATP requiring reactions, and some of these POPs are hydrolyzed to PO4 3− by the enzyme pyrophosphatase. Some of the POPs, however, are transported into the matrix via the progressive ankylosis protein (ANK) [44-46]. The importance of this protein in regulating mineralization became evident when it was discovered that loss of function mutations in ANK resulted in hypermineralization [44-47]. MVs play a key role in the maintenance of the PO4 3−/POP ratio in the matrix because they contain two key enzymes which regulate this ratio: NPP1 which hydrolyzes extracellular nucleoside triphosphates to increase extracellular POP concentration and TNAP, which decreases POP and increases PO4 3− [44-48]. Upregulation of TNAP is a key developmental event in mineralization, and loss of function mutations in TNAP result in hypophosphatasia, characterized by undermineralization [49-54]. Systematic analysis of transgenic mice with deletions of NPP1, TNAP, and ANK has resulted in our current understanding of the regulatory role of the PO4 3−/POP ratio in controlling the onset of mineral formation [40, 55, 56].
Role of collagens in mineralization
The endpoint for matrix mineralization is the deposition of small crystallites into collagen fibrils probably at the hole zones in the collagen structure [57, 58]. It has been shown that the classical Hodge–Petruska quarter staggered array of collagen molecules can be arranged so that the hole zones are aligned to form channels large enough to accommodate nanocrystals [58, 59]. In early mineralization, apatite platelets become oriented so that their c-axes are parallel to the fiber axis; ultimately, all of the intrafibrillar spaces are filled with mineral, resulting in a flexing of collagen molecules away from the fiber axis [60, 61]. Additional observations using atomic force microscopy have provided evidence consistent with these models. For example, Tong, et al. have shown that the mineral in young bovine bone consists mainly of small apatite platelets (9×6×2 nm) which can be fit into aligned hole zones in the fibrillar structure as postulated by Katz and co-workers [57, 62, 63]. The conclusion from these studies is that small crystals can enter the fibril, probably via the hole zones and fibrillar pores, and that they further propagate within the fibril to fill all available spaces.
Mineral nucleation
The exact mechanism through which hydroxyapatite crystals form in vertebrate hard tissues has been widely debated [64-69]. The most likely mechanism proposed for bone and cartilage mineralization is based on the concept of heterogeneous nucleation [70]. This mechanism relies on organic or inorganic precursor seeds to direct the formation of apatite from soluble inorganic ions. Substantial differences exist among authorities as to where this nucleation occurs and the exact molecular nature of the nucleator. One group of investigators propose that matrix vesicles are the site of initial or primary nucleation, as a prerequisite to subsequent secondary mineralization of the extracellular matrix [8, 71-73]. In this scheme, Ca2+ and PO4 3− in the extracellular matrix are transported into the interior of MV via MV transporters, annexins for Ca2+ and Pit-1,2 for PO4 3− [74, 75]. Within MV, the ions interact with phospholipids to form a nucleational core complex, resulting in the formation of nanocrystals [76-79]. How these nanocrystals exit the vesicle and reach the collagen fibrils is a matter of some debate. Propagation of the initial crystal seeds is thought to proceed by purely physical chemical means, based on the metastable nature of the extracellular fluid [38, 80-82].
An alternative view questions the feasibility of this approach on physical chemical grounds, and proposes instead direct nucleation of apatite by matrix macromolecules, principally collagen, but possibly also involving phosphoproteins, phospholipids, and proteolipids [68, 83-87]. Further, studies of the behavior of phosphoproteins in vitro are consistent with their role as nucleators or facilitators of nucleation [34, 87]. While each of the major hypothesis for the initiation of mineralization is plausible and backed by a substantial body of evidence, neither hypothesis has been able to fully explain all of the known features of mineralized tissue calcification.
Summary of the role of MV in normal calcification
At present, MVs have two well-established roles in initiating matrix mineralization. The best and most accepted role for these membranes is their function in regulating the PO4 3−/POP ratio in the pre-mineralized extracellular space. The data supporting this view have been discussed above, and while some questions remain as to the exact participation of each of the MV enzymes (NPP1, TNAP, and Phospho1) in the process, it seems clear that these enzymes are key players in the mineralization process. The second putative role for MV is to nucleate mineral crystals and thus begin the mineralization cascade which results in massive mineralization of the matrix. Evidence has been reviewed showing that MV lipids, and particularly, an MV complex, proposed by Wuthier and colleagues as well as Boskey and collaborators and Boyan and co-workers, are the nucleation sites in bone and calcifying cartilage [68, 83, 88-92]. As the initial crystals observed in mineralizing tissues have frequently been seen in and around MV, the role of membranes and membrane constituents in mineral formation cannot be ignored and will be seen to be a consistent feature of pathological calcifications.
Pathological ectopic calcification
The inappropriate formation of biological mineral in soft tissues is called ectopic calcification and is considered pathologic. In some instances of ectopic calcification, the mineral formed is hydroxyapatite, although other calcium salts (e.g., oxalate or octacalcium phosphate) are formed in kidney stones, for example. Calcification in joints results in osteoarthritis, and mineralization in the cardiovascular system (as well as in cardiovascular prostheses) is especially troubling, resulting in increased morbidity and mortality [93]. Almost as soon as the existence of MV in mineralizing tissues was determined, and their putative role in normal calcification proposed, reports of MV and MV-like particles in abnormal calcification began to appear [94]. Subsequent studies of vascular calcification have resulted in the surprising finding that the molecular events associated with calcification of the vasculature resemble endochondral ossification of bone [93, 95]. The underlying mechanisms resulting in cardiovascular calcification derive from observations that vessel cells, including periocytes, vascular endothelial cells, and myofibroblasts can undergo aspects of osteoblastic differentiation. In particular, upregulation of TNAP and the production of MV-like particles have been observed [96, 97]. While the progression of vascular calcification appears to follow the well-known biomineralization pathway, the signals which initiate this process are less well understood. Genetic diseases which result in ectopic mineralization have been shown to derive from either the absence of a circulating mineralization inhibitor, such as matrix gla protein, or from altered signaling, as is the case in fibrodysplasia ossificans progressiva [98-102]. Why mineralization begins in the absence of such mutations is less clear. A number of factors which have been proposed for the stimulation of vascular calcification include oxidative stress, hyperlipidemia, and inflammation [93, 103]. Whatever initiates the process, MV or MV-like particles appear to play a role in vascular calcification, as they do in normal hard tissue biogenesis.
Evidence for the occurrence of MV in ectopic calcification
As indicated above, MV-like particles were seen by ultrastructural analyses in arthritic cartilage [94, 104, 105]. Further studies in vitro have substantiated the hypothesis that these membraneous bodies are very similar or identical to MV [105-107]. It is also well-known that chondrocyte apoptosis is seen in arthritic cartilage and that chondrocyte apoptosis is a prominent feature of calcifying cartilage [108, 109]. One question which might be asked is whether the membraneous structures seen in ectopic calcification arise either from necrotic membrane fragmentation or are the results of chondrocyte apoptosis. To answer this question, we cultured chondrocytes from embryonic chick tibia and measured the release of MV from the cells. We also measured the release of lactate dehydrogenase as a measure of cell fragmentation [110]. Under these conditions, MV release was accompanied by minimal release of cell contents. In contrast, cytotoxic agents resulted in massive cell fragmentation. The membranes released in this process could be distinguished from authentic MV by the presence of marker enzymes not associated with MV. Subsequently, Kirsch has clearly shown that in growth plate chondrocytes, MVs are not apoptotic bodies [111]. Although similar studies have not yet been done with cells from soft tissue calcification sites, MV-like particles have also been observed in vascular calcification [97, 112, 113]. In addition, cultured blood vessel smooth muscle cells (BVSMC) have been demonstrated to produce vesicles in culture, which are very similar in content to those produced by chondrocytes [97, 114]. The similarity of BVSMC vesicle composition and vesicles seen in arthritic and arthritic calcification to the composition of skeletal MV suggests that they act via similar mechanisms to those seen in hard tissues: they modulate the local PO4 3−/POP ratio and they provide nucleation sites for the initial crystals. For example, Millan and co-workers have shown that TNAP inhibitors block calcification of vascular smooth muscle cells, which suggests parallels to the role of this enzyme in skeletal mineralization [115].
What can be learned about ectopic calcification from consideration of normal mineralization mechanisms?
Most structures in the vertebrate body are in ionic equilibrium with the blood, and the blood is a metastable solution of Ca2+ and PO4 3− [82]. Mineral doesn’t form all over the body because there are mineralization inhibitors in the blood and elsewhere which stabilize the metastable solution. In normal skeletal development, MV functions to remove one potent inhibitor of calcification, POP. Therefore, once cells begin to produce MVs, they can promote mineral formation. What causes articular chondrocytes or vascular cells to begin this process? Pathogenic signals in the form of inflammation, hyperlipidemia, and stress are the likely culprits, and it appears that they act by stimulating a signaling cascade not unlike that seen in the skeleton. Local release of pro-calcification molecules like BMPs reprogram cells of mesenchymal origin to begin recapitulating endochondral ossification. One consequence of this mechanism is the elaboration of MV. Thus, by better understanding the role of MVs in normal mineralization, we can better deal with them in pathologic calcification.
Acknowledgement
This work was supported by a grant from the National Institute for Dental and Craniofacial Research, NIH, DE017323.
References
- 1.Anderson HC. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol. 1967;35:81–101. doi: 10.1083/jcb.35.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20:33–50. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- 3.Landis WJ, Paine M, Glimcher MJ. Electron microscopic observations of bone tissue prepared anhydrously in organic solvents. J Utrastruct Res. 1977;59:1–30. doi: 10.1016/s0022-5320(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 4.Majeska RJ, Wuthier RE. Studies on matrix vesicles isolated from chick epiphyseal cartilage. Association of pyrophosphatase and ATPase activities with alkaline phosphatase. Biochim Biophys Acta. 1975;391:51–60. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- 5.Ali SY. Analysis of matrix vesicles and their role in the calcification of epiphyseal cartilage. Fed Proc. 1976;35:135–142. [PubMed] [Google Scholar]
- 6.Howell DS, Pita JC, Alvarez J. Possible role of extracellular matrix vesicles in initial calcification of healing rachitic cartilage. Fed Proc. 1976;35:122–126. [PubMed] [Google Scholar]
- 7.Wuthier RE. A review of the primary mechanism of endochondral calcification wilh special emphasis on the role of cells, mitochondria and matrix vesicles. Clin Orthop Relat Res. 1982;169:219–242. [PubMed] [Google Scholar]
- 8.Anderson HC. Mineralization by matrix vesicles. Scan Electron Microsc. 1984;2:953–964. [PubMed] [Google Scholar]
- 9.Hayashi Y. Ultrastructural characterization of extracellular matrix vesicles in the mineralizing fronts of apical cementum in cats. Arch Oral Biol. 1985;30:445–449. doi: 10.1016/0003-9969(85)90074-3. [DOI] [PubMed] [Google Scholar]
- 10.Landis WJ, Paine MC, Hodgens KJ, Glimcher MJ. Matrix vesicles in embryonic chick bone: considerations of their identification, number, distribution, and possible effects on calcification of extracellular matrices. J Ultrastruct Mol Struct Res. 1986;95:142–163. doi: 10.1016/0889-1605(86)90037-6. [DOI] [PubMed] [Google Scholar]
- 11.Anderson HC, Stechschulte DJ, Jr, Collins DE, Jacobs DH, Morris DC, Hsu HH, Redford PA, Zeiger S. Matrix vesicle biogenesis in vitro by rachitic and normal rat chondrocytes. Am J Pathol. 1990;136:391–398. [PMC free article] [PubMed] [Google Scholar]
- 12.Iannotti JP, Naidu S, Noguchi Y, Hunt RM, Brighton CT. Growth plate matrix vesicle biogenesis. The role of intracellular calcium. Clin Orthop Relat Res. 1994;306:222–229. [PubMed] [Google Scholar]
- 13.Rabinovitch AL, Anderson HC. Biogenesis of matrix vesicles in cartilage growth plates. Fed Proc. 1976;35:112–116. [PubMed] [Google Scholar]
- 14.Ali SY. Constitutive enzymes of matrix vesicles. Bone Miner. 1992;17:168–171. doi: 10.1016/0169-6009(92)90730-2. [DOI] [PubMed] [Google Scholar]
- 15.Balcerzak M, Malinowska A, Thouverey C, Sekrecka A, Dadlez M, Buchet R, Pikula S. Proteome analysis of matrix vesicles isolated from femurs of chicken embryo. Proteomics. 2008;8:192–205. doi: 10.1002/pmic.200700612. [DOI] [PubMed] [Google Scholar]
- 16.Dean DD, Schwartz Z, Muniz OE, Gomez R, Swain LD, Howell DS, Boyan BD. Matrix vesicles are enriched in metalloproteinases that degrade proteoglycans. Calcif Tissue Int. 1992;50:342–349. doi: 10.1007/BF00301632. [DOI] [PubMed] [Google Scholar]
- 17.Majeska RJ, Holwerda DL, Wuthier RE. Localization of phosphatidylserine in isolated chick epiphyseal cartilage matrix vesicles with trinitrobenzenesulfonate. Calcif Tissue Int. 1979;27:41–46. doi: 10.1007/BF02441159. [DOI] [PubMed] [Google Scholar]
- 18.Wuthier RE. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim Biophys Acta. 1975;409:128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]
- 19.Houston B, Stewart AJ, Farquharson C. PHOSPHO1—a novel phosphatase specifically expressed at sites of mineralisation in bone and cartilage. Bone. 2004;34:629–637. doi: 10.1016/j.bone.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Roberts SJ, Stewart AJ, Sadler PJ, Farquharson C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem J. 2004;382:59–65. doi: 10.1042/BJ20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen LB, Pedersen FS, Pedersen L. Expression of type III sodium-dependent phosphate transporters/retroviral receptors mRNAs during osteoblast differentiation. Bone. 2001;28:160–166. doi: 10.1016/s8756-3282(00)00418-x. [DOI] [PubMed] [Google Scholar]
- 22.Anderson HC, Harmey D, Camacho NP, Garimella R, Sipe JB, Tague S, Bi X, Johnson K, Terkeltaub R, Millan JL. Sustained osteomalacia of long bones despite major improvement in other hypophosphatasia-related mineral deficits in tissue nonspecific alkaline phosphatase/nucleotide pyrophosphatase phosphodiesterase 1 double-deficient mice. Am J Pathol. 2005;166:1711–1720. doi: 10.1016/S0002-9440(10)62481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damek-Poprawa M, Golub E, Otis L, Harrison G, Phillips C, Boesze-Battaglia K. Chondrocytes utilize a cholesterol-dependent lipid translocator to externalize phosphatidylserine. Biochemistry. 2006;45:3325–3336. doi: 10.1021/bi0515927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro IM, Burke A, Schattschneider S, Golub EE. Proceedings of the third international conference on matrix vesicles; Wichtig Editore, Milan. 1981. [Google Scholar]
- 25.Termine JD. Mineral chemistry and skeletal biology. Clin Orthop Relat Res. 1972;85:207–239. doi: 10.1097/00003086-197206000-00036. [DOI] [PubMed] [Google Scholar]
- 26.Trautz OR. X-ray diffraction of biological and synthetic apatites. Ann NY Acad Sci. 1955;60:696–712. doi: 10.1111/j.1749-6632.1955.tb40060.x. [DOI] [PubMed] [Google Scholar]
- 27.Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, Hu JCC. Regulation of dental enamel shape and hardness. J Dent Res. 2010;89:1024–1038. doi: 10.1177/0022034510375829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bei M. Molecular genetics of ameloblast cell lineage. J Exp Zool. 2009;312B:437–444. doi: 10.1002/jez.b.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosnoski DM, Gay CV. NCX3 is a major functional isoform of the sodium-calcium exchanger in osteoblasts. J Cell Biochem. 2008;103:1101–1110. doi: 10.1002/jcb.21483. [DOI] [PubMed] [Google Scholar]
- 30.Palmer G, Manen D, Bonjour JP, Caverzasio J. Species-specific mechanisms control the activity of the Pit1/PIT1 phosphate transporter gene promoter in mouse and human. Gene. 2001;279:49–62. doi: 10.1016/s0378-1119(01)00747-8. [DOI] [PubMed] [Google Scholar]
- 31.Boskey A, Maresca M, Appel J. The effects of non-collagenous matrix proteins on hydroxyapatite formation and proliferation in a collagen gel system. Connect Tissue Res. 1989;21:171–176. doi: 10.3109/03008208909050007. [DOI] [PubMed] [Google Scholar]
- 32.Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41:462–473. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- 33.Hao J, Zou B, Narayanan K, George A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 2004;34:921–932. doi: 10.1016/j.bone.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 34.He G, George A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem. 2004;279:11649–11656. doi: 10.1074/jbc.M309296200. [DOI] [PubMed] [Google Scholar]
- 35.Ito S, Saito T, Amano K. In vitro apatite induction by osteopontin: interfacial energy for hydroxyapatite nucleation on osteopontin. J Biomed Mater Res A. 2004;69:11–16. doi: 10.1002/jbm.a.20066. [DOI] [PubMed] [Google Scholar]
- 36.Mckee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. Ultrastructural immunolocalization of non-collagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J Bone Miner Res. 1993;8:485–496. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]
- 37.Mckee MD, Zalzal S, Nanci A. Extracellular matrix in tooth cementum and mantle dentin: localization of osteopontin and other noncollagenous proteins, plasma proteins, and glycoconjugates by electron microscopy. Anat Rec. 1996;245:293–312. doi: 10.1002/(SICI)1097-0185(199606)245:2<293::AID-AR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 38.Murshed M, Mckee MD. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr Opin Nephrol Hypertens. 2010;19:359–365. doi: 10.1097/MNH.0b013e3283393a2b. [DOI] [PubMed] [Google Scholar]
- 39.Young MF, Kerr JM, Ibaraki K, Heegaard AM, Robey PG. Structure, expression, and regulation of the major non-collagenous matrix proteins of bone. Clin Orthop Relat Res. 1992;281:275–294. [PubMed] [Google Scholar]
- 40.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bronner F, Stein WD. Calcium homeostasis—an old problem revisited. J Nutr. 1995;125:1987S–1995S. doi: 10.1093/jn/125.suppl_7.1987S. [DOI] [PubMed] [Google Scholar]
- 42.Talmage DW, Talmage RV. Calcium homeostasis: how bone solubility relates to all aspects of bone physiology. J Musculoskelet Neuronal Interact. 2007;7:108–112. [PubMed] [Google Scholar]
- 43.Talmage RV, Talmage DW. Calcium homeostasis: solving the solubility problem. J Musculoskelet Neuronal Interact. 2006;6:402–407. [PubMed] [Google Scholar]
- 44.Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79:1017–1029. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 46.Kim HJ, Minashima T, McCarthy EF, Winkles JA, Kirsch T. Progressive ankylosis protein (ANK) in osteoblasts and osteoclasts controls bone formation and bone remodeling. J Bone Miner Res. 2010;25:1771–1783. doi: 10.1002/jbmr.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 48.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp 1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alini M, Carey D, Hirata S, Grynpas MD, Pidoux I, Poole AR. Cellular and matrix changes before and at the time of calcification in the growth plate studied in vitro: arrest of type X collagen synthesis and net loss of collagen when calcification is initiated. J Bone Miner Res. 1994;9:1077–1087. doi: 10.1002/jbmr.5650090716. [DOI] [PubMed] [Google Scholar]
- 50.Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- 51.Collin P, Nefussi JR, Wetterwald A, Nicolas V, Boy-Lefevre ML, Fleisch H, Forest N. Expression of collagen, osteocalcin, and bone alkaline phosphatase in a mineralizing rat osteoblastic cell culture. Calcif Tissue Int. 1992;50:175–183. doi: 10.1007/BF00298797. [DOI] [PubMed] [Google Scholar]
- 52.Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18:444–448. [Google Scholar]
- 53.Weiss MJ, Cole DE, Ray K, Whyte MP, Lafferty MA, Mulivor R, Harris H. First identification of a gene defect for hypophosphatasia: evidence that alkaline phosphatase acts in skeletal mineralization. Connect Tissue Res. 1989;21:99–104. doi: 10.3109/03008208909050000. [DOI] [PubMed] [Google Scholar]
- 54.Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. [Review] Endocr Rev. 1994;15:439–461. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- 55.Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, Waymire K, Narisawa S, Millan JL, Macgregor GR, Whyte MP. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson K, Pritzker K, Goding J, Terkeltaub R. The nucleoside triphosphate pyrophosphohydrolase isozyme PC-1 directly promotes cartilage calcification through chondrocyte apoptosis and increased calcium precipitation by mineralizing vesicles. J Rheumatol. 2001;28:2681–2691. [PubMed] [Google Scholar]
- 57.Katz EP, Wachtel E, Yamauchi M, Mechanic GL. The structure of mineralized collagen fibrils. Connect Tissue Res. 1989;21:149–158. doi: 10.3109/03008208909050005. [DOI] [PubMed] [Google Scholar]
- 58.Landis WJ. An overview of vertebrate mineralization with emphasis on collagen–mineral interaction. Gravit Space Biol Bull. 1999;12:15–26. [PubMed] [Google Scholar]
- 59.Landis WJ. Tomographic imaging of collagen–mineral interaction: implications for osteogenesis imperfecta. Connect Tissue Res. 1995;31:287–290. doi: 10.3109/03008209509010825. [DOI] [PubMed] [Google Scholar]
- 60.Fratzl P, Groschner M, Vogl G, Plenk H, Jr, Eschberger J, Fratzl-Zelman N, Koller K, Klaushofer K. Mineral crystals in calcified tissues: a comparative study by SAXS. J Bone Miner Res. 1992;7:329–334. doi: 10.1002/jbmr.5650070313. [DOI] [PubMed] [Google Scholar]
- 61.Tesch W, Vandenbos T, Roschgr P, Fratzl-Zelman N, Klaushofer K, Beertsen W, Fratzl P. Orientation of mineral crystallites and mineral density during skeletal development in mice deficient in tissue nonspecific alkaline phosphatase. J Bone Miner Res. 2003;18:117–125. doi: 10.1359/jbmr.2003.18.1.117. [DOI] [PubMed] [Google Scholar]
- 62.Tong W, Glimcher MJ, Katz JL, Kuhn L, Eppell SJ. Size and shape of mineralites in young bovine bone measured by atomic force microscopy. Calcif Tissue Int. 2003;72:592–598. doi: 10.1007/s00223-002-1077-7. [DOI] [PubMed] [Google Scholar]
- 63.Yamauchi M, Katz EP. The post-translational chemistry and molecular packing of mineralizing tendon collagens. Connect Tissue Res. 1993;29:81–98. doi: 10.3109/03008209309014236. [DOI] [PubMed] [Google Scholar]
- 64.Glimcher MJ, Krane SM. The organization and structure of bone and the mechanism of calcification. In: Ramachandran GN, Gould BS, editors. Treatise on collagen. 7B. Academic; New York: 1968. pp. 68–251. [Google Scholar]
- 65.Glimcher MJ. The nature of the mineral component of bone and the mechanism of calcification. Instr Course Lect. 1987;36:49–69. [PubMed] [Google Scholar]
- 66.Moradian-Oldak J, Frolow F, Addadi L, Weiner S. Interactions between acidic matrix macromolecules and calcium phosphate ester crystals: relevance to carbonate apatite formation in biomineralization. Proc Biol Sci. 1992;247:47–55. doi: 10.1098/rspb.1992.0008. [DOI] [PubMed] [Google Scholar]
- 67.Posner AS, Blumenthal NC, Boskey AL. Calcified tissue. Facta Publications; Vienna: 1973. [Google Scholar]
- 68.Raggio CL, Boyan BD, Boskey AL. In vivo hydroxyapatite formation induced by lipids. J Bone Miner Res. 1986;1:409–415. doi: 10.1002/jbmr.5650010505. [DOI] [PubMed] [Google Scholar]
- 69.Wuthier RE. Involvement of cellular metabolism of calcium and phosphate in calcification of avian growth plate cartilage. J Nutr. 1993;123:301–309. doi: 10.1093/jn/123.suppl_2.301. [DOI] [PubMed] [Google Scholar]
- 70.Glimcher MJ. The chemistry and biology of mineralized connective tissues. Elsevier; New York: 1981. [Google Scholar]
- 71.Boskey AL. Mineral–matrix interactions in bone and cartilage. Clin Orthop Relat Res. 1992;281:244–274. [PubMed] [Google Scholar]
- 72.Boyan BD, Schwartz Z, Swain LD. Matrix vesicles as a marker of endochondral ossification. Connect Tissue Res. 1990;24:67–75. doi: 10.3109/03008209009152423. [DOI] [PubMed] [Google Scholar]
- 73.Wuthier RE. Mechanism of de novo mineral formation by matrix vesicles. Connect Tissue Res. 1989;22:27–33. doi: 10.3109/03008208909114117. [DOI] [PubMed] [Google Scholar]
- 74.Kirsch T, Nah HD, Demuth DR, Harrison G, Golub EE, Adams SL, Pacifici M. Annexin V-mediated calcium flux across membranes is dependent on the lipid composition: implications for cartilage mineralization. Biochemistry. 1997;36:3359–3367. doi: 10.1021/bi9626867. [DOI] [PubMed] [Google Scholar]
- 75.Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci. 2005;10:822–837. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 76.Wu LN, Yoshimori T, Genge BR, Sauer GR, Kirsch T, Ishikawa Y, Wuthier RE. Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J Biol Chem. 1993;268:25084–25094. [PubMed] [Google Scholar]
- 77.Wu LN, Genge BR, Dunkelberger DG, LeGeros RZ, Concannon B, Wuthier RE. Physicochemical characterization of the nucleational core of matrix vesicles. J Biol Chem. 1997;272:4404–4411. doi: 10.1074/jbc.272.7.4404. [DOI] [PubMed] [Google Scholar]
- 78.Wuthier RE, Wu LN, Sauer GR, Genge BR, Yoshimori T, Ishikawa Y. Mechanism of matrix vesicle calcification: characterization of ion channels and the nucleational core of growth plate vesicles. Bone Miner. 1992;17:290–295. doi: 10.1016/0169-6009(92)90753-z. [DOI] [PubMed] [Google Scholar]
- 79.Wiesmann HP, Meyer U, Plate U, Hohling HJ. Aspects of collagen mineralization in hard tissue formation. Int Rev Cytol. 2005;242:121–156. doi: 10.1016/S0074-7696(04)42003-8. [DOI] [PubMed] [Google Scholar]
- 80.Tenenbaum HC. Levamisole and inorganic pyrophosphate inhibit beta-glycerophosphate induced mineralization of bone formed in vitro. Bone Miner. 1987;3:13–26. [PubMed] [Google Scholar]
- 81.Nancollas GH, Zawacki SJ. Calcium phosphate mineralization. Connect Tissue Res. 1989;21:239–244. doi: 10.3109/03008208909050013. [DOI] [PubMed] [Google Scholar]
- 82.Neuman WF, Neuman MW, Diamond AG, Menanteau J, Gibbons WS. Blood:bone disequilibrium. VI. Studies of the solubility characteristics of brushite: apatite mixtures and their stabilization by noncollagenous proteins of bone. Calcif Tissue Int. 1982;34:149–157. doi: 10.1007/BF02411226. [DOI] [PubMed] [Google Scholar]
- 83.Boyan BD, Boskey AL. Co-isolation of proteolipids and calcium–phospholipid–phosphate complexes. Calcif Tissue Int. 1984;36:214–218. doi: 10.1007/BF02405320. [DOI] [PubMed] [Google Scholar]
- 84.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003;2:552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 85.McEwen BF, Song MJ, Landis WJ. Quantitative determination of the mineral distribution in different collagen zones of calcifying tendon using high voltage electron microscopic tomography. J Comput Assist Microsc. 1991;3:201–210. [PubMed] [Google Scholar]
- 86.Gajjeraman S, He G, Narayanan K, George A. Biological assemblies provide novel templates for the synthesis of hierarchical structures and facilitate cell adhesion. Adv Funct Mater. 2008;18:3972–3980. doi: 10.1002/adfm.200801215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He G, Ramachandran A, Dahl T, George S, Schultz D, Cookson D, Veis A, George A. Phosphorylation of phosphophoryn is crucial for its function as a mediator of biomineralization. J Biol Chem. 2005;280:33109–33114. doi: 10.1074/jbc.M500159200. [DOI] [PubMed] [Google Scholar]
- 88.Boskey AL, Posner AS. The role of synthetic and bone extracted calcium–phospholipid–phosphate complexes in hydroxyapatite formation. Calcif Tissue Res. 1977;23:251–258. doi: 10.1007/BF02012794. [DOI] [PubMed] [Google Scholar]
- 89.Boskey AL, Dickson IR. Influence of vitamin D status on the content of complexed acidic phospholipids in chick diaphyseal bone. Bone Miner. 1988;4:365–371. [PubMed] [Google Scholar]
- 90.Boyan BD, Schwartz Z, Swain LD, Khare A. Role of lipids in calcification of cartilage. Anat Rec. 1989;224:211–219. doi: 10.1002/ar.1092240210. [DOI] [PubMed] [Google Scholar]
- 91.Genge BR, Wu LN, Wuthier RE. Identification of phospholipid-dependent calcium-binding proteins as constituents of matrix vesicles. J Biol Chem. 1989;264:10917–10921. [PubMed] [Google Scholar]
- 92.Wuthier RE. Lipids of matrix vesicles. Fed Proc. 1976;35:117–121. [PubMed] [Google Scholar]
- 93.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ali SY, Wisby A. Apatite crystal nodules in arthritic cartilage. Eur J Rheumatol Inflamm. 1978;1:115–119. [Google Scholar]
- 95.Kirsch T. Determinants of pathological mineralization. Curr Opin Rheumatol. 2006;18:174–180. doi: 10.1097/01.bor.0000209431.59226.46. [DOI] [PubMed] [Google Scholar]
- 96.Tanimura A, McGregor DH, Anderson HC. Matrix vesicles in atherosclerotic calcification. Proc Soc Exp Biol Med. 1983;172:173–177. doi: 10.3181/00379727-172-41542. [DOI] [PubMed] [Google Scholar]
- 97.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 98.Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284:7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koos R, Krueger T, Westenfeld R, Kuhl HP, Brandenburg V, Mahnken AH, Stanzel S, Vermeer C, Cranenburg EC, Floege J, Kelm M, Schurgers LJ. Relation of circulating matrix Gla-protein and anticoagulation status in patients with aortic valve calcification. Thromb Haemost. 2009;101:706–713. [PubMed] [Google Scholar]
- 101.Krueger T, Westenfeld R, Schurgers L, Brandenburg V. Coagulation meets calcification: the vitamin K system. Int J Artif Organs. 2009;32:67–74. doi: 10.1177/039139880903200202. [DOI] [PubMed] [Google Scholar]
- 102.Munroe PB, Olgunturk RO, Fryns JP, Van Maldergem L, Ziereisen F, Yuksel B, Gardiner RM, Chung E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet. 1999;21:142–144. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- 103.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Einhorn TA, Gordon SL, Siegel SA, Hummel CF, Avitable MJ, Carty RP. Matrix vesicle enzymes in human osteoarthritis. J Orthop Res. 1985;3:160–169. doi: 10.1002/jor.1100030205. [DOI] [PubMed] [Google Scholar]
- 105.Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- 106.Jubeck B, Gohr C, Fahey M, Muth E, Matthews M, Mattson E, Hirschmugl C, Rosenthal AK. Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis Rheum. 2008;58:2809–2817. doi: 10.1002/art.23762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karpouzas GA, Terkeltaub RA. New developments in the pathogenesis of articular cartilage calcification. Curr Rheumatol Rep. 1999;1:121–127. doi: 10.1007/s11926-999-0008-2. [DOI] [PubMed] [Google Scholar]
- 108.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 109.Gibson G. Active role of chondrocyte apoptosis in endochondral ossification. Microsc Res Tech. 1998;43:191–204. doi: 10.1002/(SICI)1097-0029(19981015)43:2<191::AID-JEMT10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 110.Golub EE, Schattschneider SC, Berthold P, Burke A, Shapiro IM. Induction of chondrocyte vesiculation in vitro. J Biol Chem. 1983;258:616–621. [PubMed] [Google Scholar]
- 111.Kirsch T, Wang W, Pfander D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res. 2003;18:1872–1881. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- 112.Schlieper G, Aretz A, Verberckmoes SC, Kruger T, Behets GJ, Ghadimi R, Weirich TE, Rohrmann D, Langer S, Tordoir JH, Amann K, Westenfeld R, Brandenburg VM, D’Haese PC, Mayer J, Ketteler M, Mckee MD, Floege J. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–696. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 114.Chen NX, OΓÇÖNeill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Narisawa S, Harmey D, Yadav MC, O’Neill WC, Hoylaerts MF, Millan JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res. 2007;22:1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]


