Abstract
The central nervous melanocortin system is a neural network linking nutrient-sensing systems with hypothalamic, limbic and hindbrain neurons regulating behavior and metabolic homeostasis. Primary melanocortin neurons releasing melanocortin receptor ligands residing in the hypothalamic arcuate nucleus are regulated by nutrient-sensing and metabolic signals. A smaller group of primary neurons releasing melanocortin agonists in the nucleus tractus solitarius in the brainstem are also regulated by signals of metabolic state. Two melanocortin receptors regulate energy homeostasis. Melanocortin-4 receptors regulate satiety and autonomic outputs controlling peripheral metabolism. The functions of melanocortin-3 receptors (MC3R) expressed in hypothalamic and limbic structures are less clear. Here we discuss published data and preliminary observations from our laboratory suggesting that neural MC3R regulate inputs into systems governing the synchronization of rhythms in behavioral and metabolism with nutrient intake. Mice subjected to a restricted feeding protocol, where a limited number of calories are presented at a 24 h interval, rapidly exhibit bouts of increased wakefulness and activity which anticipate food presentation. The full expression of these responses is dependent on MC3R. Moreover, MC3R knockout mice are unique in exhibiting a dissociation of weight loss from improved glucose homeostasis when subject to a restricted feeding protocol. While mice lacking MC3R fed ad libitum exhibit normal to moderate hyperinsulinemia, when subjected to a restricted protocol they develop hyperglycemia, glucose intolerance, and dyslipidemia. Collectively, our data suggest that the central nervous melanocortin system is a point convergence in the control of energy balance and the expression of rhythms anticipating nutrient intake.
The paper represents an invited review by a symposium, award winner or keynote speaker at the Society for the Study of Ingestive Behavior [SSIB] Annual Meeting in Pittsburg, July 2010.
Keywords: Circadian rhythm, anticipation, food anticipatory activity, satiety, homeostasis, clock, melanocortin, hypothalamus
1. INTRODUCTION
The ability of organisms to maintain a constant physiological state by regulating their internal environment was first described by Claude Bernard in the 19th century as the milieu intérieur (the environment within); Walter Bernard Cannon later coined the now commonly used term homeostasis in the early 20th Century [1]. Homeostasis is one of the basic tenets of physiology, and many examples for the homeostatic regulation of essential physiologic processes by neuroendocrine systems have been described. Recently, an increase in the diagnosis of obesity and a concomitant increase in type 2 diabetes have raised interest in the concept of energy homeostasis, or how a stable body weight is maintained in response to variation in the availability of nutrients.
The problem faced by modern societies is that obesity increases risk for the Metabolic Syndrome. The Metabolic Syndrome is a cluster of metabolic disorders that includes impaired fasting glucose, atherogenic dyslipidemia and hypertension. These conditions represent deteriorating homeostatic control of metabolic processes due to disruption of internal sensors of metabolic state and nutrient flux [2-4]. Insulin resistance, the impaired ability of insulin to suppress hepatic glucose output and stimulate glucose disposal, is a defining feature of the Metabolic Syndrome [5]. The incidence of insulin resistance increases markedly with obesity. Insulin resistance precedes type 2 diabetes, a condition of persistent hyperglycemia due to the failure of β–cells to produce sufficient insulin [6]. This form of diabetes is one of the most significant health issues facing the United States in the 21st Century. The Centers for Disease Control and Prevention (www.cdc.gov) estimates that 1 in 3 Americans are clinically obese; that nearly 24 million people in the United States have diabetes, while a further 57 million have the pre-diabetic condition of impaired fasting glucose, and are at risk of progressing to type 2 diabetes. Obesity-related morbidities such as diabetes are projected to add significant stress to an already financially troubled health care system, and may also result in a decline in life expectancy in the 21st Century [7, 8]. Also of concern is the marked increase of these conditions in the children [9]. While the currently available pharmacopeia is effective at delaying the progression of obesity-related metabolic disorders, there remains a need for new therapies effective for treating these conditions over longer periods.
That an individual can maintain a relatively stable body weight while consuming millions of calories over the lifespan suggests the evolution of mechanisms for preventing obesity. This process is thought to include the active regulation of both sides of the energy balance equation through controlling satiety and energy expenditure, balancing energy intake with energy requirements and vice versa [10]. However, as shall be discussed later, recent data supports the hypothesis that the timing of food consumption in relation to the internal clocks controlling circadian rhythms in behavior and metabolic processes is also important for maintaining health [11–14]. Moreover, the clocks governing circadian rhythms are fully integrated into cellular pathways involved in the control glucose and fatty acid metabolism at a transcriptional level [15]. The concept of the importance of food anticipation, which was early considered to be mediated by a clock-like mechanism [16], as a homeostatic function is not new. The work of Stephen C. Woods beginning in the 1970’s led to the proposal that anticipation of nutrient consumptions by neuroendocrine systems governing glucose homeostasis is essential for minimizing the impact of nutrient consumption on the body’s organs and tissues [17, 18].
2. THE MELANOCORTIN SYSTEM - AN INTERFACE BETWEEN SIGNALS OF METABOLIC STATE AND NEURAL PATHWAYS GOVERNING SATIETY AND METABOLISM
The cloning of leptin and its receptor [19, 20], and the characterization of the role of the central nervous melanocortin system (reviewed in [21]), were landmark achievements in the development of the modern theoretical models of energy homeostasis. The secretion of leptin from adipocytes is a signal of nutrient sufficiency, and a decline in secretion associated with fasting instigates behavioral and metabolic adaptation to nutrient scarcity [22]. While functions for leptin in the periphery have been reported (see [23–25] for examples), restoring leptin signaling in the brain is sufficient to rescue the obese metabolic phenotype observed with mutations in the leptin receptor [26–28]. Melanocortin neurons were one of the first targets of leptin identified in the brain, and are important mediators of leptin action [29–31]. Leptin receptors expressed on melanocortin neurons are particularly important for mediating the actions of leptin in maintaining glucose homeostasis, with a more modest contribution to maintaining body [32, 33].
The melanocortin neurons governing satiety and ‘facultative’ thermogenesis (the ability to “burn off” excess calories using oxidative metabolism) during times of plenty [34–39], respond to sensors of metabolic state and nutrient intake/flux in the gut and adipose tissue via endocrine and vagal afferents. The contribution of facultative thermogenesis to the energy homeostasis equation has been debated [34, 36, 40, 41]. However, investigating the mechanisms involved in maintaining body weight and glucose homeostasis by the leptin-melanocortin pathway is important, with the potential to provide leads in the development of drugs to treat obesity and diseases of the metabolic syndrome. In rodents, the leptin-melanocortin system exerts regulatory control over glucose and lipid metabolism independently of effects on ingestive behaviors and body weight [26, 42–46]. Mutations affecting the activity of the leptin-melanocortin system are associated with childhood obesity [47, 48], indicating similar functions in humans.
The central nervous melanocortin system comprises neurons expressing the endogenous ligands and the two melanocortin receptors expressed in the brain (MC3R, MC4R) (see [49] and [50] for recent reviews). Melanocortin receptor agonists are released by neurons expressing proopiomelanocortin (POMC). POMC is a prohormone modified posttranslationally by prohormone convertases to produce the endogenous melanocortin receptor agonists melanocyte stimulating hormones (α/β/γ–MSH) and adrenocorticotropic hormone (ACTH), and other peptides such as β–endorphin and lipotropin (LPH). The largest population of POMC neurons is located in the arcuate nucleus of the hypothalamus (ARC); a second smaller population is found in the nucleus tractus solitarius (NTS) in the brainstem. MSH exhibit agonist activity at the melanocortin receptors, increasing cAMP accumulation through Gs activation of adenylyl cyclase [21]. Central administration of MSH peptides is associated with a reduction of food intake [51, 52]. Release of MSH from POMC neurons also supports energy expenditure during periods of caloric sufficiency, and has a stimulatory effect on the autonomic nervous system and thyroid function [53]. A second population of ARC neurons release agouti-related peptide (AgRP); AgRP was first shown to act as a competitive antagonist at the MC3R and MC4R [54, 55]. Subsequent studies suggested inverse agonist functions [56] and actions as a biased agonist, with AgRP promoting signaling through the Gi [57]. Central administration of AgRP increases food intake and reduces energy expenditure through suppression of autonomic output and inhibition of the thyroid.
POMC and AgRP neurons are regulated by systemic and neural signals of metabolic state [50]. For example, POMC neurons express receptors for leptin and insulin (although the receptors are expressed by two distinct populations of POMC neurons [58]), and also exhibit a response to glucose [59, 60] . AgRP neurons also respond to leptin and insulin, and express receptors for the stomach hormone ghrelin [61]. Both neuronal populations express enzymes which sense nutrient flux, including AMP kinase [60] and Sirt1 [62, 63]. The actions of the endogenous melanocortin ligands produced in the central nervous system to affect energy homeostasis involve two melanocortin receptors. MC4R are widely expressed throughout the brain [64–66], and are necessary for the regulation of satiety, autonomic function and the thyroid by melanocortin agonists [67–72]. Loss of function mutations in the MC4R gene causes a hyperphagic obesity syndrome in mice [73]. MC4R haploinsufficiency remains the most frequently observed single-gene mutation associated with childhood obesity [47].
3. THE FUNCTIONS OF MELANOCORTIN-3 RECEPTOR ARE SUBTLE BUT NECESSARY FOR ENERGY HOMEOSTASIS
When compared to MC4R, the distribution of MC3R expression in the brain is more limited, with expression limited to the hypothalamic and limbic regions [74]. Targeted deletion of the mouse Mc3r gene also compromises energy homeostasis, and is associated with increased accumulation of fat mass [75, 76]. However, in chow-fed conditions the obese phenotype is far less severe than that observed in Mc4r knockout (−/−) mice. When the effects of centrally administered MSH on satiety, sympathetic activity and thyroid function were assessed using various melanocortin receptor knockout strains, it is evident that MC3R cannot compensate for loss of MC4R [67, 69, 70, 72, 76, 77]. Moreover, obesity observed in Mc3r−/− mice is not due to hyperphagia. This raises the question as to what, and whether, ‘homeostatic’ processes are regulated by MC3R. MC3R expressed in the limbic regions could regulate “non-homeostatic” ingestive behaviors related to food seeking and reward. However, the accelerated weight gain and accumulation of extra fat mass relative to controls observed when Mc3r−/− mice on high fat diets independently of hyperphagia suggests an important role in regulating peripheral metabolism [76, 78-80].
Our laboratory has focused on a role for MC3R in the expression of circadian rhythms in anticipatory behavior [81, 82]. Recently, this research was extended to investigate whether neural MC3R regulate rhythms in metabolic processes in the periphery [83]. The over arching hypothesis is that MC3R transmit signals of metabolic status of nutrient intake into the systems governing the expression of rhythms anticipating nutrient intake. This hypothesis was based on earlier studies indicating that lesions in the ventromedial hypothalamic area (VMH), which exhibits dense MC3R expression [74], result in the loss of food anticipatory activity in rats [84]. It is important to note that, since these studies started, results from other laboratories suggest that the dorsomedial hypothalamus may have functions related to the entrainment of anticipatory rhythms to food intake [85, 86]. More recently, the results from other laboratories have led to a debate whether hypothalamic neurons have any role in the expression of rhythms anticipating food intake [87, 88].
The results from our studies examining whether MC3R regulate inputs into systems governing the entrainment of behavior to food presentation suggest functions that could be considered as “non-homeostatic”. The ability to synchronize rhythms of increased vigilance and food seeking behaviors that anticipate nutrient consumption require functional MC3R [81]. However, our studies suggest a novel homeostatic function for MC3R in regulating glucose and fatty acid metabolism during periods of nutrient scarcity [83]. This role may be similar to that posulated by Stephen C. Woods, that the ability of the organism to anticipate nutrient intake is an important homeostatic function which facilitates “food tolerance”, readying organisms nutrient-processing pathways for the ingestion of large meals [17].
4. THE LINK BETWEEN CIRCADIAN RHYTHMS AND ENERGY HOMEOSTASIS
The basic premise for energy homeostasis is that the neural systems governing behavior and metabolism respond to signals of nutrient intake and energy balance; this premise is intuitive and based on simple thermodynamic laws (i.e., energy intake must equal energy expended). However, as stated above balancing intake and expenditure is not the only component of the energy homeostasis equation.
Most organisms, including primitive single celled bacteria to complex metazoans, exhibit an ability to express a circadian rhythm. At a systems level, the ability of an organism to develop internal models allowing for the prediction of cyclical changes in the environmental milieu, such as the flux of nutrient-bearing waters into the tidal zone or the presentation of nectar by flowering plants, would have clear selective advantages. The ability to predict nutrient intake may also be an important homeostatic function, both in terms of competition with other organism for limited resources and for maintaining metabolic homeostasis [17, 18].
In most vertebrate species, photoperiod serves as the dominant zeitgeber (“time giver”). The initial investigation of how rhythms are maintained identified a master clock residing in the suprachiasmatic nucleus of the hypothalamus (SCN) which senses signals of photoperiod via the retinohypothalamic tract and, in the absence of daylight, is responsible for maintaining the circadian rhythm [89]. However, the laboratory rodent exhibits an ability to entrain their circadian rhythm to food presentation; an ability that is retained when the SCN is destroyed. The existence of a second independent “food entrainable oscillator” (FEO) was suggested soon after the discovery of the SCN [16].
Caloric intake and signals of metabolic state are important inputs into, and are regulated by, the molecular clocks responsible for maintaining a rhythm [15, 90–93]. The finding that some clock mutants still express food anticipatory behaviors has, however, cast some doubts on the importance of the known clock in food entrainment [94]. However, inconsistencies in the literature exist, with others showing that critical clock genes are needed for the expression of food anticipatory rhythms [95]. The reason for the inability of various laboratories to replicate the results of others is problematic, but is not new in the field of behavioral research [96, 97]. What is clear is that the timing of food intake in relation to the internal clock has a significant effect on metabolic homeostasis. Forced desynchronization of rhythms in food intake and activity away from the normal 24 h rhythm entrained to photoperiod results in a metabolic syndrome [11, 12].
The use of forward genetic screens and molecular biology led to the identification of genes encoding transcription factors and repressors which are components of the clock. Transcription factors include the members of retinoic orphan receptor family (ROR-alpha/beta/gamma), the aryl hydrocarbon receptor nuclear translocator-like family (BMAL1/2), circadian locomoter output cycles kaput (CLOCK). Members of Period gene family (PER1/2/3) and Cryptochrome (CRY1/2) family, and members of the Reverb family (NR1D1) have complex roles as both repressors of the transcription factors, and are also regulate other outputs controlled by the clock (reviewed in [98, 99]). At the same time, it became apparent that the molecular components of circadian clocks are essentially ubiquitous, and possess functions beyond being simple time keepers. Clock genes possess “housekeeping” functions, and regulate basic cellular processes in most cells, including for example the regulation of the cell cycle, regulation of gene transcription and the regulation of glucose and fatty acid metabolism [15, 91, 100].
Many groups have attempted to locate the FEO, and some of the published data suggests that hypothalamic (and perhaps extra-hypothalamic) structures outside of the SCN regulate inputs into the FEO [85, 86, 101–103]. Calorie intake is the dominant zeitgeber for clocks residing outside the SCN in the brain, and for clocks residing in the periphery [104]. Indeed, it has been proposed that that the FEO does not reside in a single definable structure within the brain, and that entrainment to feeding involves the response of clocks dispersed throughout the central nervous system and periphery to autonomous signals of metabolic state [88, 102, 105]. Cultured cells exhibit an ability to respond autonomously to nutrient flux via sensors of redox state, suggesting a cell autonomous response to altered nutrient flux [15]. Non-caloric signals may also be involved. In the whole animal, oscillators in the periphery can entrain to modest fluctuations in body temperature [106].
5. MELANOCORTIN-3 RECEPTORS ARE INVOLVED IN THE EXPRESSION OF RHYTHMS ANTICIPATING NUTRIENT INTAKE
The basic thesis investigated by our laboratory is that the hypothalamic melanocortin system forms a nexus between systems governing energy balance and the expression of rhythms anticipating food presentation. The data supporting this conclusion was discussed in our earlier articles [81, 82]. Here, we extend the discussion to include recently published data further integrating pathways linked to energy homeostasis and circadian function, and include a discussion on our recently published data suggesting a role for MC3R in maintaining normal rhythms in the expression of clock genes in the liver [83].
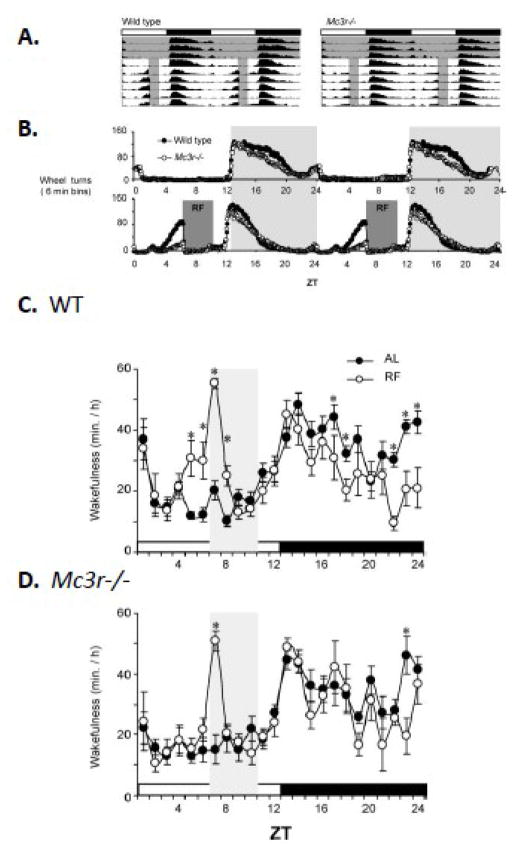
The expression of food anticipatory activity (FAA) in the C57BL/6J (B6) mouse can be induced by a restricted feeding protocol (RF). In our laboratory, this has involved a 30–40% reduction in the amount of calories presented as a single meal at 1300 h in mice housed in a 12 h light:dark setting (lights on 0600–1800). Male B6 mice subjected to the RF protocol exhibit FAA after 2–3 days, and will continue to express the behavior for as long as the protocol is maintained (Fig. 1A, B). We also measured FAA using a non-food seeking behavior (sleep), with wild type (WT) and Mc3r−/− B6 mice sent to a collaborator Dr Jidong Fang at the Department of Psychiatry, Pennsylvania State College of Medicine. Within 3 days of RF, it is evident that WT mice exhibit an increase in wakefulness 2 h prior to food presentation (Fig. 1C). The increased wakefulness anticipating food presentation is not observed in Mc3r−/− mice (Fig. 1D). Mc3r−/− mice do exhibit the marked increase in wakefulness in the hour after food presentation (Fig. 1C, D). This period coincides with gorging, as we and others have observed that mice subject to protocols involving restricted access to food will rapidly consume all of the food presented to them [107].
Figure 1.
MC3R are required for food anticipatory activity. (A) Actograms of averaged wheel running for WT and Mc3r−/− mice. The lights-on and dark period are indicated by the white and black rectangles; the period of food access is indicated by the grey shading. (B) Wheel running data showing activity of ad libitum fed mice, and during restricted feeding (RF; 60% of normal calories provided at 1300 h / ZT7). In both panels, it is clear that the expression of anticipatory activity is severely attenuated in Mc3r−/− mice. (C, D) Increased wakefulness anticipating food presentation is not observed in Mc3r−/− mice. 24h patterns of wakefulness are shown for WT (C, n=9) and Mc3r−/− (D, n=6) mice during ad libitum (AL, solid circles in both panels) or after 3d of restricted feeding (open circles). RF was associated with increased wakefulness in WT mice in the 2h period preceding food presentation and reduced wakefulness in the lights-off phase. In the lights on phase, Mc3r−/− mice exhibited increased wakefulness restricted to the 1h after food presentation. * p<0.05 AL vs. RF. [Adapted from Sutton et al. J. Neuroscience copyright 2008 with permission from The Society for Neuroscience]
The food anticipatory phenotype of Mc3r−/− mice is not sensitive to genetic background, and has been reproduced in three separate laboratories. The Mc3r−/− mice used for the studies shown here were back crossed onto the B6 background. However, a similar phenotype was observed in Dr Roger Cone’s laboratory when the mice were on the outbred on the 129/Sv;B6 backgrounds. Moreover, in unpublished studies we have observed this behavior in Mc3r deficient mice on a mixed FVB/NJ;B6 background.
The metabolic response of Mc3r−/− mice to restricted feeding was also of interest. Previous studies of the insulin resistant phenotype of this model yielded unremarkable results, with a modest hyperinsulinemia considered to be commensurate with the mild increase in fat mass [75, 76, 79, 83]. It has been suggested that Mc3r−/− mice fed a high fat diet are a model of obesity without, or at worst very mild, increase in insulin resistance relative to controls [79, 80, 108]. A delay in the onset of inflammation associated with the obese state may contribute to this mild phenotype [80, 108]. However, the significance of this observation is unclear, as MC3R expressed in the immune system are generally considered to be pro-inflammatory [109]. Moreover, with prolonged exposure to high fat diets indicators of the inflammatory state are similar in Mc3r−/− and Mc4r−/− mice of comparable adiposity [108].
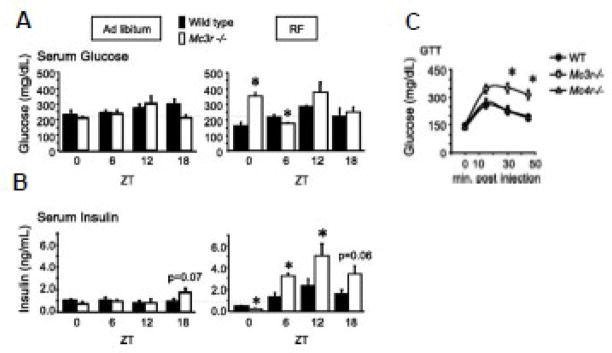
We also assessed the activity of clocks in the forebrain and liver using the expression of clock genes as readouts of oscillator activity. For studies examining the response of Mc3r−/− mice to restricted feeding, we collected samples at 6 h intervals. The mice were not fed on the last day allowing for an analysis of clock-driven processes (as opposed to the gene regulation by nutrients). We observed that while Mc3r−/− mice fed ad libitum exhibited relatively normal insulin and serum glucose levels, restricted feeding in Mc3r−/− mice induces a diabetic-like condition. Despite weight loss due to reduced calories intake, Mc3r−/− mice subjected to the restricted feeding protocol exhibit hyperglycemia and hyperinsulinemia (Fig. 2A, B), and are glucose intolerant (Fig. 2C). The response of mice where obesity is induced by impaired MC4R signaling (Mc4r−/−, Ay/a) is that predicted for weight loss, with an improvement in glucose tolerance (Fig. 2C and unpublished data).
Figure 2.
Mc3r−/− mice develop insulin resistance when subjected to the restricted feeding protocol. (A, B) Serum glucose (A) and insulin (B) levels in WT and Mc3r−/− mice are similar with ad libitum feeding (left panel). However Mc3r−/− mice exhibit abnormal glucose levels and altered insulinemia when subjected to restricted feeding (right panel). The altered insulin observed in Mc3r−/− mice exhibits a circadian pattern; hypoinsulinemia is observed at ZT0, while hyperinsulinemia is observed at ZT6 and ZT12. (C) Mc3r−/− mice are glucose intolerant compared to Mc4r−/− and WT mice; all groups were subjected to the restricted feeding for 2 weeks. * p<0.05 vs. WT within ZT [Adapted from Sutton et al. FASEB J. copyright 2010 with permission from the Federation of American Societies for Experimental Biology]
Inspection of the serum glucose and insulin data in the restricted feeding group shown in Fig. 2A and 2B suggest the involvement of a circadian mechanism. Mc3r−/− mice were hypoinsulinemic at ZT0 (6 AM, the onset of the lights-on period); at ZT6, 12 and 18 they were hyperinsulinemic. The fasting hyperglycemia observed at ZT0 in Mc3r−/− mice suggests that the β–cells are not producing sufficient insulin; the hyperinsulinemia observed at later time points is sufficient to reduce serum glucose to normal levels. This suggests that the combination of hyperglycemia and hypoinsulinemia at ZT0 is not a result of β–cell failure. This data can be interpreted as suggesting desensitization of β–cells to high glucose, inhibition of insulin release via autonomic inputs, or a programmed absence of insulin output at this period owing to clock activity in the β–cell being “out of phase” with the timing of food intake. Using a sensitive technique combining PCR with Southern blotting, Gantz and colleagues observed that the Mc3r transcript might be expressed in the pancreas [110]. However, little is known about whether melanocortins have direct actions on β–cells in the pancreas. Therefore, it is not possible to state at this time whether the phenotype is due to altered melanocortin signaling in the pancreas, or indicates altered autonomic control of β–cell function by MC3R expressed in the central nervous system.
While the mechanisms are unclear, it is nevertheless apparent that the inability of Mc3r−/− mice to develop an anticipatory response to food presentation has a metabolic consequence which be interpreted as a form of “food intolerance” [17]. An analysis of the response of liver genes involved in metabolic processes related to digestion and nutrient processing provides further evidence of metabolic distress. These studies used a high carbohydrate meal (Research Diets 12450B); an assessment of the expression of lipogenic enzymes and serum triglyceride levels indicates that Mc3r−/− mice are converting glucose into fatty acids, with an increase in serum ketones indicating oxidation. This suggests a “futile cycle” of sorts, with the mice using energy to convert glucose to fatty acid for oxidation despite being in a situation where the amount of energy available is limited. These data also suggests that the insulin resistance observed in Mc3r−/− mice is restricted to certain tissues. The stimulation of the expression of lipogenic enzymes, and the suppression of gluconeogenic genes, is preserved in the liver of Mc3r−/− mice. The glucose intolerance and hyperinsulinemia observed in Mc3r−/− mice subject to the restricted feeding protocol is therefore probably due to insulin resistance in skeletal muscle.
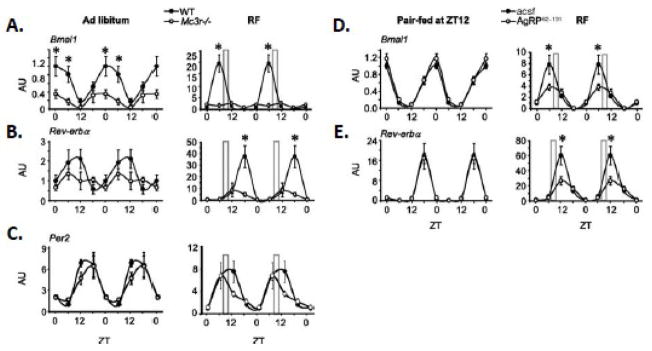
The rhythms in the expression of clock genes in the liver which are known to regulate gluconeogenic and lipogenic enzymes [111, 112] was also shown to be abnormal (Fig. 3A-C). The amplitude in the rhythms in the expression of Bmal1 and Reverba was reduced in Mc3r−/− mice, particularly during restricted feeding (Fig. 2A, B). Not all oscillators were altered, however, as rhythms in the expression of Per2 were still observed (Fig. 2C). Importantly, we observed that central infusion of AgRP, which inhibits activity of the MC3R and MC4R, also reduced rhythmicity in the expression of Bmal1 and Reverba (Fig. 3D, E). Signaling through MC3R thus appears to be required for the normal regulation of the clock in the liver, particularly during restricted feeding. Whether this regulation is direct via autonomic innervation of the liver [113], or is an indirect consequence from changes in the metabolic state of hepatocytes owing to external signals, is not clear at this time.
Figure 3.
Mc3r signaling is required for normal liver clock activity during RF. (A–C) Double-plotted expression pattern of Bmal1 (A), Reverbα (B), and Per2 (C) in liver of WT and Mc3r−/− mice fed ad libitum or subjected to restricted feeding (RF, indicated by the grey bar). (D,E) Double-plotted expression pattern of Bmal1 (D) and Rev-erbα (E) in liver of mice treated i.c.v. with AgRP82–131 or acsf pair-fed a nonlimiting amount of food (4.5 g) at ZT12 (6:00 PM, onset of lights off; left panels) or subjected to RF (right panels). *p< 0.05 vs. corresponding WT. AU, arbitrary units. [Adapted from Sutton et al. FASEB J. copyright 2010 with permission from the Federation of American Societies for Experimental Biology]
6. DO MELANOCORTIN-3 RECEPTORS REGULATE INPUTS INTO “FOOD ENTRAINABLE OSCILLATORS”?
The published data from our investigation of the role of MC3R in entrainment to nutrient intake in our laboratory used mice housed in the 12 h light:dark setting. Such studies provide information about the expression of anticipatory behavior, but do not provide an indication of the importance of this receptor in the expression of circadian rhythms per se. Determining whether MC3R regulate inputs into clocks capable of governing an actual circadian rhythm requires assessing the activity of mice housed in constant dark. In this setting, rhythms are maintained by the activity of the master clock residing in the SCN.
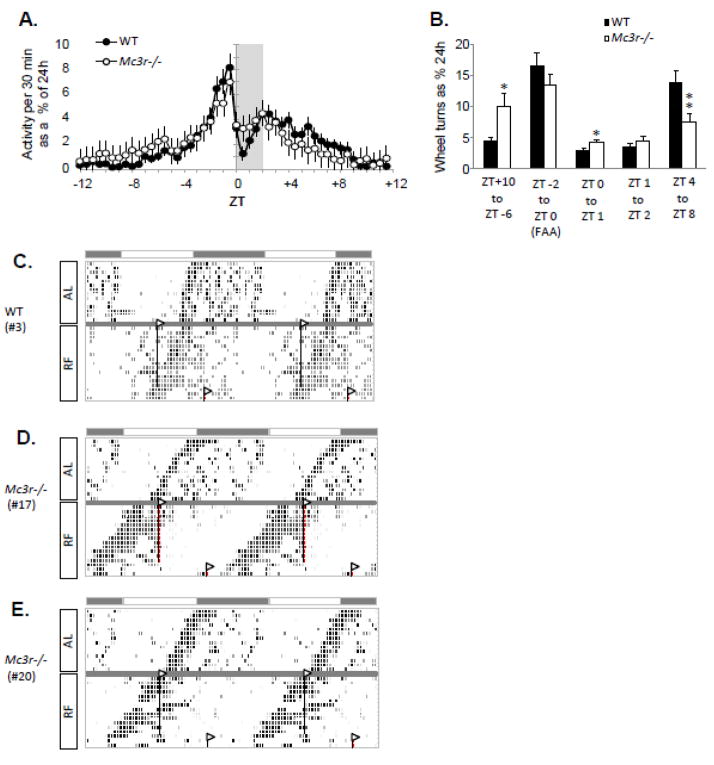
To begin to assess whether MC3R have a role in the expression of circadian rhythms, we completed a pilot study examining food entrainment using Mc3r−/− and control mice housed in constant dark (Fig. 4). This study involved older mice (around 9 months, n=18–20/group) which were allowed to free-run in constant dark for two weeks before being subjected to the restricted feeding protocol for a further two weeks.
Figure 4.
Preliminary results from an investigation of circadian rhythms in activity of WT and Mc3r−/− mice subject to RF in constant dark. (A) Averaged wheel running data for WT and Mc3r−/− mice as 30 min. bins. Each bin being the averaged of 3 d of activity after 2 wk of RF. ZT0 is the time of food presentation (1300 h). (B) Analysis of activity data separated into periods between and during meals. For this analysis, we separated this period into FAA (ZT-2 to ZT0 - the two hours prior to food presentation), the first and second hour after food presentation (most food is consumed in the first hour [81, 107]), and the period of post meal activity (ZT4 to ZT8). ZT+10 to ZT-6 is the 8 h period between meals when WT showed minimal activity. As predicted, WT subject to RF exhibited a period of increased activity of about 12 h (ZT-4 to ZT+8) centered around food presentation. As a group, Mc3r−/− mice appeared to exhibit FAA, with no significant difference from controls. However, activity between meals and in the first hour after food presentation was markedly different: they exhibited more activity the hour following food presentation (*, p<0.05), and were less active after the meal (**, p<0.01). (C-E) Analysis of individual mice indicated that the differences in activity shown in panel B were due to half of the Mc3r−/− mice not entraining to food presentation. Representative actograms showing activity of individual mice during the ad libitum free running period and subsequent RF are shown for a WT mouse (C), and for two Mc3r−/− mice which did not entrain to RF (D, E). Collectively, the analysis of wheel running data obtained in constant dark indicate that approximately half of the Mc3r−/− mice were not able to entrain their rhythm to nutrient intake after 2 wk of restricted feeding.
Analysis of activity of WT mice after 2 wk of restricted feeding during the subjective day indicated a peak in wheel running activity anticipating the presentation of food at 1300h, with inhibition of wheel running following food presentation (Fig. 4A). When the averaged activity of all the Mc3r−/− mice used in study was assessed, a subtle difference was observed in the distribution of activity around meal time (Fig. 4A, B). Significant differences in activity where observed during blocks of time well outside the timing of food presentation. When the activity of individual animals was inspected, it was evident that this difference was due to restricted feeding having no effect on the free-running activity of half of the Mc3r−/− mice (Fig. 4C-E). These results are preliminary, and future studies using younger animals and a more prolonged period of restricted feeding (up to 4–6 wk) are warranted.
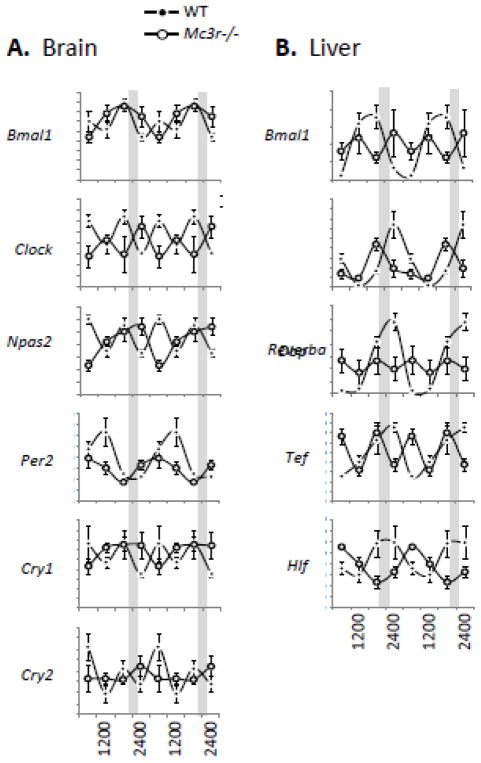
Overall, our analysis of wheel activity in Mc3r−/− mice housed in constant dark suggests that the master clock in the SCN is functional. MC3R are proposed to regulate inputs into a system which can over ride outputs from the SCN, and which is capable of operating an independent circadian rhythm. The location of this clock is not known. However, our assessment of clock activity by measuring the expression of clock genes in the brain (Fig. 5A) and liver (Fig. 5B) suggest the MC3R may regulate the synchronization of clocks throughout the body. On the other hand, it may be that the desynchronization of food intake from the circadian rhythm in Mc3r−/− mice housed in constant dark is producing a metabolic condition (e.g, glucose intolerance, dyslipidemia) which is interfering with the normal activity of clocks.
Figure 5.
Arrythmic circadian rhythms of clock gene expression in the brain and liver of Mc3r−/− mice housed in constant dark. Tissues were collected from WT and Mc3r−/− mice at 6h intervals (0600, 1200, 1800 and 2400 h) 4 days after a phase delay in food presentation, and the expression patterns of clock genes assessed in the brain (A) and liver (B) using published methods [81, 83]. The mice were not fed on the day of tissue collection.
6. FUTURE DIRECTIONS
In the time since the cloning of the melanocortin receptors and the establishment of the link between the melanocortin system and energy homeostasis, comparatively little attention has been given to the role of MC3Rs expressed in the brain. The results from the studies discussed here are the first to suggest that MC3R have a critical role in the regulation of homeostatic processes. The metabolic phenotype of Mc3r−/− mice during restricted feeding is particularly intriguing, indicating an inability to efficiently used glucose and the diversion of carbohydrates into lipogenic pathways. Signaling through MC3R during the transition between fasted and fed state thus appears to be important, if not essential, for the efficient utilization of glucose. Why this is not observed in the well-fed conditions is unclear. However, it raises the question about whether loss of MC3R function in the obese state could contribute to the Metabolic Syndrome. Indeed, we have observed that improvements in insulin sensitivity associated with the treatment of obese mice using potent melanocortin analogs is not dependent on functional MC4R [69]. That treatment of Mc4r−/− mice with potent melanocortin analogs improves glucose homeostasis raises the possibility that MC3Rs are a potential target for treating disorders of metabolism.
Whether and where MC3R in the brain regulate inputs into food entrainable oscillators governing the expression of circadian rhythms requires further investigation. However, the data shown in Fig. 4 and 5 are important for suggesting that MC3R regulate inputs into systems responsible for expression of a circadian rhythm in the absence of light. Future studies will identify specific neuronal pathways involved in the regulation of food entrainment and the regulation of glucose homeostasis by MC3R.
Acknowledgments
The authors thank Dr. Jennifer Rood, Dr. Robert Koza, Dr. Jose Galgani, Brian Goh, Emily Meyer, Armand Centanni, Ramani Singh Rasile and Xiying Wu for technical assistance over the course of the studies described here. We thank Dr Roger Cone and Oregon Health & Science University for providing the Mc3r−/− mice used in these experiments. Funding for this work was provided by the National Institutes of Health (DK073189 to A.A.B.), the Pennington Biomedical Research Foundation and The Scripps Research Institute. A.A.B. also acknowledges the support of the Pennington Biomedical Research Foundation and Clinical Nutrition Research Unit Center grant, “Nutritional Programming: Environmental and Molecular Interactions” (1P30 DK072476), sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 2.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam CK, Chari M, Lam TK. CNS regulation of glucose homeostasis. Physiology (Bethesda) 2009;24:159–70. doi: 10.1152/physiol.00003.2009. [DOI] [PubMed] [Google Scholar]
- 4.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–51. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reaven GM. Why Syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005;1:9–14. doi: 10.1016/j.cmet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Accili D. Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes. 2004;53:1633–42. doi: 10.2337/diabetes.53.7.1633. [DOI] [PubMed] [Google Scholar]
- 7.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 8.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 9.Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89:2526–39. doi: 10.1210/jc.2004-0276. [DOI] [PubMed] [Google Scholar]
- 10.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–61. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 11.Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: Findings from animal models. Best Pract Res Clin Endocrinol Metab. 2010;24:785–800. doi: 10.1016/j.beem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsey KM, Bass J. Obeying the clock yields benefits for metabolism. Proc Natl Acad Sci U S A. 2009;106:4069–70. doi: 10.1073/pnas.0901304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 15.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephan FK. The "other" circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–92. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 17.Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 18.Smith GP, Stephen C. Woods: A precocious scientist. Physiol Behav. doi: 10.1016/j.physbeh.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 20.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 21.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–49. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 22.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 23.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, et al. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148:3987–97. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 24.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixit VD, Mielenz M, Taub DD, Parvizi N. Leptin induces growth hormone secretion from peripheral blood mononuclear cells via a protein kinase C- and nitric oxide-dependent mechanism. Endocrinology. 2003;144:5595–603. doi: 10.1210/en.2003-0600. [DOI] [PubMed] [Google Scholar]
- 26.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414–24. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–21. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes. 2001;50:425–35. doi: 10.2337/diabetes.50.2.425. [DOI] [PubMed] [Google Scholar]
- 29.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–92. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 30.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, et al. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 31.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 11:286–97. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–47. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–9. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 2011;59:1657–66. doi: 10.2337/db09-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–7. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obici S. Molecular Targets for Obesity Therapy in the Brain. Endocrinology. 2009 doi: 10.1210/en.2009-0409. [DOI] [PubMed] [Google Scholar]
- 38.Coppari R, Ramadori G, Elmquist JK. The role of transcriptional regulators in central control of appetite and body weight. Nat Clin Pract Endocrinol Metab. 2009;5:160–6. doi: 10.1038/ncpendmet1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin AC, Zheng H, Berthoud HR. An expanded view of energy homeostasis: Neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 60:17–23. doi: 10.2337/db10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 214:242–53. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 42.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–85. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez-Juarez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004;279:49704–15. doi: 10.1074/jbc.M408665200. [DOI] [PubMed] [Google Scholar]
- 44.Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–9. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–88. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature. 2009;462:307–14. doi: 10.1038/nature08532. [DOI] [PubMed] [Google Scholar]
- 48.Farooqi IS, O'Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–77. doi: 10.1038/ncpendmet0966. [DOI] [PubMed] [Google Scholar]
- 49.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 50.Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab. 2009;20:203–15. doi: 10.1016/j.tem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 52.Tung YC, Piper SJ, Yeung D, O'Rahilly S, Coll AP. A comparative study of the central effects of specific proopiomelancortin (POMC)-derived melanocortin peptides on food intake and body weight in pomc null mice. Endocrinology. 2006;147:5940–7. doi: 10.1210/en.2006-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lechan RM, Fekete C. Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis. Peptides. 2006;27:310–25. doi: 10.1016/j.peptides.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 54.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–8. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 55.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 56.Nijenhuis WA, Oosterom J, Adan RA. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15:164–71. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- 57.Buch TR, Heling D, Damm E, Gudermann T, Breit A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. J Biol Chem. 2009;284:26411–20. doi: 10.1074/jbc.M109.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 30:2472–9. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–40. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 60.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–36. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 62.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, et al. Agrp Neurons Mediate Sirt1's Action on the Melanocortin System and Energy Balance: Roles for Sirt1 in Neuronal Firing and Synaptic Plasticity. J Neurosci. 2010;30:11815–25. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 65.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–35. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 66.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–54. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–54. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 68.Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339–44. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar KG, Sutton GM, Dong JZ, Roubert P, Plas P, Halem HA, et al. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides. 2009;30:1892–900. doi: 10.1016/j.peptides.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW, et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J. 2005;19:1482–91. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 71.Fekete C, Marks DL, Sarkar S, Emerson CH, Rand WM, Cone RD, et al. Effect of Agouti-related protein in regulation of the hypothalamic-pituitary-thyroid axis in the melanocortin 4 receptor knockout mouse. Endocrinology. 2004;145:4816–21. doi: 10.1210/en.2004-0476. [DOI] [PubMed] [Google Scholar]
- 72.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 74.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90:8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 76.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 77.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–22. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 78.Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–90. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–96. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellacott KL, Murphy JG, Marks DL, Cone RD. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148:6186–94. doi: 10.1210/en.2007-0699. [DOI] [PubMed] [Google Scholar]
- 81.Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, et al. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–55. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Begriche K, Sutton GM, Fang J, Butler AA. The role of melanocortin neuronal pathways in circadian biology: a new homeostatic output involving melanocortin-3 receptors? Obes Rev. 2009;10 (Suppl 2):14–24. doi: 10.1111/j.1467-789X.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sutton GM, Begriche K, Kumar KG, Gimble JM, Perez-Tilve D, Nogueiras R, et al. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010;24:862–72. doi: 10.1096/fj.09-142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi S, Wong LS, Yamat C, Dallman MF. Hypothalamic ventromedial nuclei amplify circadian rhythms: do they contain a food-entrained endogenous oscillator? J Neurosci. 1998;18:3843–52. doi: 10.1523/JNEUROSCI.18-10-03843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 86.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103:12150–5. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, et al. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci. 2009;29:1447–60. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- 88.Davidson AJ. Lesion studies targeting food-anticipatory activity. Eur J Neurosci. 2009;30:1658–64. doi: 10.1111/j.1460-9568.2009.06961.x. [DOI] [PubMed] [Google Scholar]
- 89.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–42. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 91.Burris TP. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol Endocrinol. 2008;22:1509–20. doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin L, Wu N, Lazar MA. Nuclear receptor Rev-erbalpha: a heme receptor that coordinates circadian rhythm and metabolism. Nucl Recept Signal. 8:e001. doi: 10.1621/nrs.08001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2010;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–13. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, Challet E. Lack of food anticipation in Per2 mutant mice. Curr Biol. 2006;16:2016–22. doi: 10.1016/j.cub.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 96.Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, et al. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- 97.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi JS, Shimomura K, Kumar V. Searching for genes underlying behavior: lessons from circadian rhythms. Science. 2008;322:909–12. doi: 10.1126/science.1158822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–96. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 101.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A. 2009;106:13582–7. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, et al. Reduced Anticipatory Locomotor Responses to Scheduled Meals in Ghrelin Receptor Deficient Mice. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci. 2009;30:1718–29. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- 106.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 330:379–85. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trevaskis JL, Gawronska-Kozak B, Sutton GM, McNeil M, Stephens JM, Smith SR, et al. Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity (Silver Spring) 2007;15:2664–72. doi: 10.1038/oby.2007.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Getting SJ. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther. 2006;111:1–15. doi: 10.1016/j.pharmthera.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 110.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, et al. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–50. [PubMed] [Google Scholar]
- 111.Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci U S A. 2010;107:7024–9. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]