Abstract
Rationale
Tobacco withdrawal is a key factor in smoking relapse, but important questions about the withdrawal phenomenon remain.
Objectives
This research was intended to provide information about two core components of withdrawal (negative affect and craving): 1) how various withdrawal symptom profile dimensions (e.g., mean level, volatility, extreme values) differ between negative affect and craving; and 2) how these dimensions relate to cessation outcome.
Methods
Adult smokers (N=1504) in a double-blind randomized placebo-controlled smoking cessation trial provided real-time withdrawal symptom data four times per day for 4 weeks (2 weeks pre-quit and 2 weeks post-quit) via palmtop computers. Cessation outcome was biochemically confirmed 8-week point-prevalence abstinence.
Results
Examination of craving and negative affect dimensions following a cessation attempt revealed that craving symptoms differed from negative affect symptoms, with higher means, greater variability, and a greater incidence of extreme peaks. Regression analyses revealed that abstinence was associated with lower mean levels of both craving and negative affect and fewer incidences of extreme craving peaks. In a multivariate model, the increase in mean craving and negative affect scores each uniquely predicted relapse.
Conclusions
Real-time reports revealed different patterns of abstinence-related negative affect and craving and that dimensions of both predict cessation outcome, suggesting that negative affect and craving dimensions each has motivational significance. This underscores the complexity of withdrawal as a determinant of relapse and the need to measure its distinct components and dimensions.
Keywords: Withdrawal, smoking cessation, craving, negative affect, tobacco, nicotine
Introduction
Withdrawal plays a central role in theories of drug dependence, is a major criterion used to determine whether an agent is addictive (U.S. Dept. of Health and Human Services 1988), causes significant distress for those attempting to reduce or stop their drug use, and appears to play an important role in relapse (Javitz et al. 2009; Piasecki et al. 2003a). Consequently, withdrawal is a vital research topic in the effort to understand and treat tobacco use and dependence.
Recent research has yielded important information about tobacco withdrawal and its motivational significance. For instance, withdrawal symptoms such as craving, negative affect, inability to concentrate, hunger, and insomnia all seem to index decreased tobacco intake amongst dependent smokers (Dawkins et al. 2009; Hughes 2007a; b; Leventhal et al. 2010). Factor analyses of withdrawal questionnaire items tend to yield two main factors: one influencing negative affect items and one influencing craving items; other withdrawal items such as hunger and insomnia typically load onto the main factors or other minor factors (Hughes 2007b; Piasecki et al. 2000; Welsch et al. 1999; cf. Javitz et al. 2009). These two types of symptoms (negative affect and craving) tend to be more strongly related to relapse or later smoking status than are other symptoms such as hunger (Piasecki et al. 2000; Zhou et al. 2009). Research has also shown that tobacco withdrawal reliably increases several distinct symptom profile dimensions—average intensity, trajectory or slope in the days following the quit day, and volatility or occasion-to-occasion variability (Piasecki et al. 1998; Piasecki et al. 2003a; Piasecki et al. 2000)—and that each of these dimensions may predict later smoking status (Piasecki et al. 2003b; Piasecki et al. 2000).
While much is now known about the nature of tobacco withdrawal, important questions remain. For instance, much of the extant research on withdrawal profile dimensions (e.g., scatter/volatility, slope) used omnibus withdrawal measures averaged across all types of withdrawal symptoms (e.g., average of anxiety, craving, insomnia ratings; Piasecki et al. 2003a; b; c; Piasecki et al. 2000), rather than more discrete withdrawal components (i.e., specific symptom) such as negative affect or craving. Because the latter components can be discriminated via factor analysis, and some data suggest that they share distinct relations with smoking relapse (e.g., Perkins et al. 2009; Piasecki et al. 2000; Shiffman et al. 2004a), it seems important to explore these specific components of the withdrawal syndrome (Shiffman et al. 2006b). Second, most research on profile dimensions used paper-pencil measures of withdrawal rather than real-time assessments (e.g., ecological momentary assessment or EMA), and it is possible paper-pencil measures could have produced a less accurate assessment of withdrawal symptoms (e.g., completing all assessments just before attending a study visit resulting in recall bias; Hughes 2007c; Shiffman et al. 2006b; Stone & Shiffman, 2002). Third, prior withdrawal research did not systematically assess the extent to which each of the withdrawal profile dimensions (e.g., slope) changed from pre- to post-quit. Assessment of pre- to post-quit change is one means of determining whether a measure reflects deprivation-induced withdrawal symptoms per se, versus reflecting pre-existing traits or response styles (Hughes 2007c).
In response to limitations of the prior research, the current research will characterize the profile dimensions of tobacco withdrawal in approximately 1500 smokers from a placebo-controlled clinical trial of five different active pharmacotherapy conditions (Piper et al. 2009). As part of this trial, we collected real-time data on daily smoking status, withdrawal symptoms, and the occurrence of environmental events, pre- to post-quit, which allowed us to separately analyze the two major types of symptoms (i.e., negative affect and craving; cf. Shiffman et al. 2004b). Moreover, we expanded the range of profile dimensions tested by examining the mean level of post-quit symptoms, the percent of symptoms exceeding a threshold (i.e., the frequency of symptom peaks), symptom variability, and cue-induced symptom changes. These indicate the extent to which tobacco deprivation affects the mean level of symptoms, the tendency to experience extreme symptom values, the variability of symptoms, and symptomatic reaction to environmental events. Both prior data and theory suggest that any of these might play a role in smoking relapse (e.g., McCarthy et al. 2006; Piasecki et al., 2003b; Piper et al. 2008). Deprivation-induced cue reactivity will be examined because previous research suggests that deprivation modulates response to smoking cues (Gloria et al. 2009; McCarthy et al. 2006; McClernon et al. 2009; McDonough and Warren 2001) and because theory suggests that cue-induced responses may be important determinants of cessation outcomes (Siegel 1977; Wikler 1965). Pre- to post-quit change will be evaluated for each profile dimension to determine whether each dimension satisfies the withdrawal criterion that the dimension changes systematically as a function of tobacco abstinence (i.e., changes from pre- to post-quit). Finally, each dimension of each symptom will be tested on the basis of its ability to predict later smoking status and best-fitting prediction models of smoking status will be identified. We expect that the two major withdrawal symptoms (negative affect and craving) will show different patterns of profile dimensions, that their profile dimensions will be differentially affected by smoking reduction or deprivation, and that different profile dimensions of negative affect and craving will be predictive of later smoking status.
Methods
Recruitment and Inclusion/Exclusion Criteria
Participants were recruited via TV, radio and newspaper advertisements, community flyers, and earned media (e.g., radio and TV interviews, press releases) in the greater Madison and Milwaukee, WI areas. Primary inclusion criteria included: smoking at least 10 cigarettes per day for the past 6 months and being motivated to quit smoking. Exclusion criteria included: certain medications (including monoamine oxidase inhibitors, bupropion, lithium, anticonvulsants, and antipsychotics); any history of psychosis, bipolar disorder, or an eating disorder; consuming six or more alcoholic beverages daily 6 or 7 days a week; pregnancy or breast-feeding; and a serious health condition that would preclude use of study medications or adversely affect study participation. This study was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Procedure
Eligible participants provided written informed consent and then completed several baseline assessments, including a medical history screening, vital signs measurements, a carbon monoxide (CO) breath test and demographic, smoking history and tobacco dependence questionnaires. Participants were randomized to one of six treatment conditions: Bupropion SR (n=264); Nicotine lozenge (n=260); Nicotine patch (n=262); Nicotine patch + Nicotine lozenge (n=267); Bupropion SR + Nicotine lozenge (n=262) or Placebo (five placebo conditions that matched the five active conditions; n=189). All medications were provided for 8 weeks post-quit except the nicotine lozenge which was provided for 12 weeks post-quit (consistent with prescribing instructions). Randomization was conducted in a double-blind fashion using a randomization scheme with blocking on gender and race (White vs. non-White). All participants received six individual evidence-based counseling sessions (each lasting 10–20 minutes), designed to provide social support and training in problem-solving and coping skills. Bachelor-level case managers provided manualized counseling and were supervised by a licensed clinical psychologist.
Measures
Baseline Assessments
Participants completed questionnaires that assessed: gender, ethnicity, age, marital status, education level, employment, and smoking history features such as cigarettes smoked per day, age at smoking initiation, and number of prior quit attempts. Participants also completed the Fagerström Test of Nicotine Dependence (FTND; α=.61; Heatherton et al. 1991) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM; Piper et al. 2004) to assess tobacco dependence.
Ecological Momentary Assessment (EMA) Reports
Participants completed EMA reports four times a day (just after waking, prior to going to bed and at two other random times; all prompts were separated by at least an hour) for up to 2 weeks pre-quit and 2 weeks post-quit. Data analyzed in this research used reports from 10 days pre-quit to 10 days post-quit1. EMA reports asked participants to rate how they felt within the last 15 minutes in terms of: a) withdrawal symptoms (negative affect, craving, hunger, and difficulty concentrating) using items from the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al. 1999) but with a 10-point response scale to increase response variability as we have done in our previous research (McCarthy et al. 2006), b) an urge item from an adapted Questionnaire of Smoking Urges (Sweeney, Pillitteri & Kozlowski, 1996), c) self-efficacy, d) motivation, and e) cessation fatigue (i.e., “I'm tired of trying to quit smoking”). The EMA reports also assessed the number of alcoholic drinks consumed that day and number of cigarettes smoked, and the occurrence of stressors and temptation events since the last prompt. Participants were trained to interpret and respond appropriately to the EMA items.
Cessation Outcomes
The primary cessation outcome was CO-confirmed 7-day point-prevalence abstinence at 8 weeks post-quit. Alveolar CO was assessed using a Bedfont Smokerlyzer, and smokers with a CO < 10 ppm were considered abstinent. Participants who did not provide follow-up information were assumed to be smoking, using the intent-to-treat principle. We selected the 8-week outcome because it is sufficiently proximal so that it is likely to be influenced by early withdrawal symptoms but sufficiently distal to be a good predictor of long-term outcome (e.g., 6-month).
Analytic Plan
The negative affect measure reflected the mean of the six negative affect WSWS items (Tense or anxious; Impatient; Bothered by negative moods such as anger, frustration, and irritability; Irritable or easily angered; Sad or depressed; and Hopeless or discouraged). The craving measure reflected the mean of the WSWS craving item (Bothered by a desire to smoke) and the craving item from the revised Questionnaire of Smoking Urges (Urge to smoke). First, the two withdrawal symptoms (negative affect and craving) were graphed to show the symptom profiles over time (pre- to post-quit) permitting an examination of the various withdrawal components (e.g., mean level, deviation which was indexed using standard deviation [SD] values, percentage of reports exceeding a threshold). We decided to use a threshold of 7 for craving and a threshold of 4 for negative affect, on the 1–10 scale, because scores of 7 and 4, respectively, constituted a deviation ≥ 1 standard deviation above the mean for craving and negative affect. We examined other thresholds, but 7 for craving and 4 for negative affect appeared to be most sensitive. Second, matched sample t-tests were used to analyze pre- to post-quit symptom changes for each symptom profile dimension to test whether the dimension changed as a function of the cessation attempt. This analysis was initially done using the full sample and then repeated excluding participants who smoked more than 5 cigarettes per day on average during the first 10 days post-quit (n = 47) to reduce potential bias due to regular smoking during the post-quit assessment period. The 5 cigarette cut-off represents half of the minimum smoking rate to qualify for study entry (10 cigarettes/day for the last 6 months), suggesting that smokers remaining in the analyses would still experience significant withdrawal symptoms. Previous research suggests that considerable smoking must occur before smokers suppress their withdrawal symptoms (Piasecki et al. 2003a). It should also be noted that only 34% of participants reported no smoking in the first 10 days post-quit and almost 80% reported smoking less than one cigarette per day, on average, in the first 10 days post-quit. This suggested that excluding those who smoked at all would create an unrepresentative sample, and also that most people smoked relatively little when they did smoke.
In our third set of analyses, we looked at which withdrawal profile dimensions of craving and negative affect were predictive of 8-week smoking cessation outcome using logistic regression (cf., Hosmer and Lemeshow 2000). Univariate models identified all significant predictors of outcome, and these were then used to assemble best-fitting models for craving and for negative affect separately, and then a joint model comprising both craving and negative affect predictors. To control for smoking and treatment's effects on withdrawal symptoms we then re-ran the same models and included the mean number of cigarettes smoked per day in the 10-days post-quit and treatment. The large positive skew of cigarettes per day required transforming the continuous variable into a categorical variable coded as 0 for no smoking, 1 for smoking less than one cigarette per day, on average, and 2 coded as smoking one or more cigarettes per day on average. Treatment was coded as 0 for placebo, 1 for monotherapy (nicotine patch, nicotine lozenge, bupropion) and 2 for combination pharmacotherapy (nicotine patch + nicotine lozenge, bupropion + nicotine lozenge). It should be noted that there were significant missing EMA data; of the possible 80 assessments (4 times per day for 20 days), the mean number of completed assessments was 50.9, with a median of 53. To explore this issue further, we created a variable for each subject representing the number of missing assessments, and studied this variable in relation to outcome and as a simultaneous predictor of outcome in the presence of our other predictors. Missing data (i.e., the number of possible assessments not completed) were significantly related to 8-week abstinence (Wald = 42.8, p < .001, OR = 1.03, 95% CI = 1.02, 1.03) such that smokers who relapsed had significantly more missing data. However, when we re-ran the models controlling for number of assessments completed, the results were all similar, suggesting that the non-missing at random data did not appear to significantly bias the results.
Results
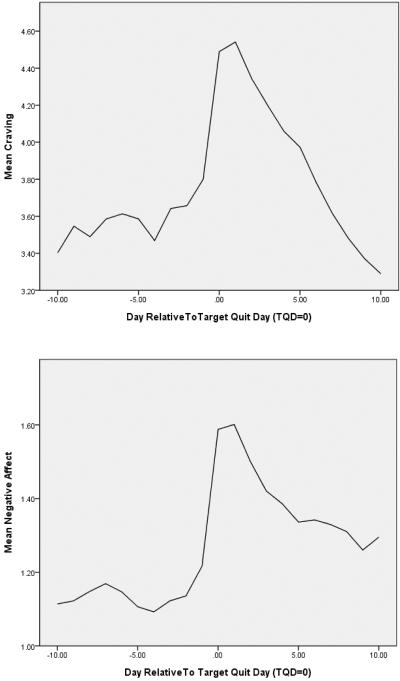
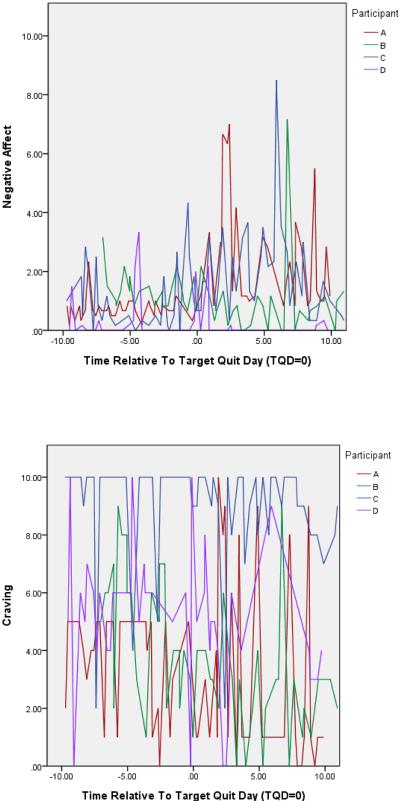
Negative affect and craving ratings show the characteristic rise-and-fall pattern consistent with withdrawal symptoms: the ratings increase precipitously on the quit day and then decline fairly steeply thereafter (see Figure 1; note the different metrics on the ordinates of the two figures). In the case of craving, symptom levels decrease, on average, below pre-quit levels by 10 days post-quit, whereas the decline is only partial for negative affect. However, examination of the negative affect and craving curves of 4 randomly selected smokers (see Figure 2) revealed that the symptom curves aggregated over the sample masked considerable heterogeneity and marked differences in patterns for the two symptoms. First, the negative affect scores were considerably lower than the craving scores; in fact, more than 75% of negative affect scores were 2 or less on a scale ranging from 1 to 10. While levels of negative affect were generally low, there were prominent, intermittent symptom spikes that appeared to be larger and more common in the post-quit period. Conversely, craving ratings were both higher and had more frequent large swings in symptom ratings. As can be seen in Figure 2, two subjects produced craving ratings that spanned the entire width of the scale (i.e., 10 points; the other two subjects used a range of 8 and 9 points for their ratings).
Figure 1.
Mean craving and negative affect across all participants and all assessments from 10 days pre-quit to 10 days post-quit.
Figure 2.
Negative affect and craving curves for 4 randomly selected individual smokers
Table 1 illustrates how the withdrawal dimensions changed from pre- to post-quit for both symptoms. The matched-sample t-tests showed that tobacco deprivation (i.e., the quit attempt) resulted in an increase in all symptom dimensions. However, when we excluded the 47 participants who were smoking an average of five or more cigarettes per day during the first 10 days post-quit (to reduce the effects of post-quit suppression of symptoms by heavy smoking), craving SD no longer significantly increased from pre- to post-quit (t = −1.87, p = .06), although this could reflect reduced power to detect this effect.
Table 1.
Withdrawal phenomena that differ between pre-quit and post-quit using data aggregated across participants by pre-quit versus post-quit status
| Withdrawal measure | Pre-quit (SD) | Post-quit (SD) | Matched sample t-test (df) | p-value |
|---|---|---|---|---|
| Craving mean | 3.64 (2.36) | 4.14 (2.67) | −7.49 (1229) | <.001 |
| Craving SD | 1.93 (.98) | 1.99 (1.08) | −1.93 (1165) | .054 |
| Percent of craving reports greater than 7 | 14.61 (24.28) | 20.74 (28.72) | −7.96 (1229) | <.001 |
| Craving mean when faced with a temptation event | 4.69 (2.74) | 5.49 (2.84) | −6.66 (669) | <.001 |
| Craving mean when not faced with a temptation event | 3.41 (2.35) | 3.83 (2.68) | −6.07 (1186) | <.001 |
| Negative affect mean | 1.24 (1.23) | 1.53 (1.47) | −10.37 (1229) | <.001 |
| Negative affect SD | .91 (.62) | .95 (.68) | −2.15 (1165) | .03 |
| Percent of negative affect reports greater than 4 | .89 (5.31) | 1.62 (7.23) | −3.97 (1229) | <.001 |
| Negative affect mean when faced with a temptation event | 2.27 (1.84) | 2.48 (1.98) | −3.15 (669) | .002 |
| Negative affect mean when not faced with a temptation event | 1.08 (1.14) | 1.36 (1.39) | −10.36 (1186) | <.001 |
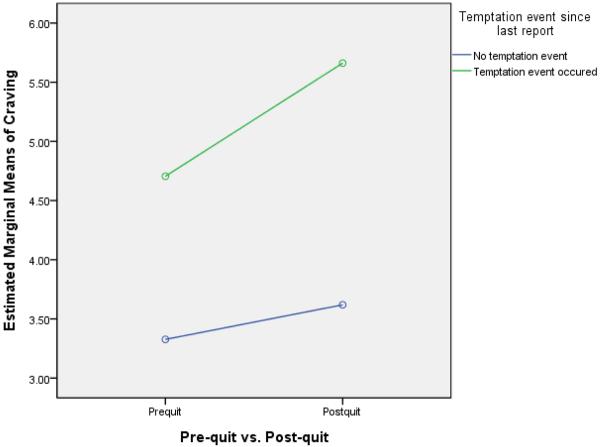
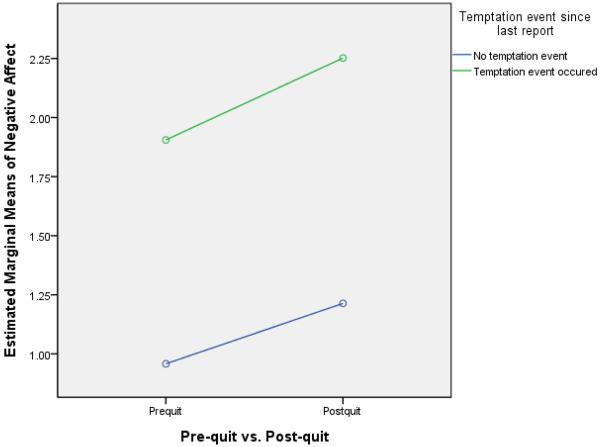
One of the withdrawal dimensions tested was the degree to which deprivation influenced reaction to a temptation event (i.e., “Since your last report, did anything happen that made you want to smoke?”). The pre- and post-quit rates of temptation event occurrence were, respectively, 18.9% and 19.5% of reports (χ2= 3.69, p = .055). Thus, deprivation was associated with a modest, nonsignificant increase in the occurrence of perceived temptation events. Matched-sample t-tests were then conducted to test the a priori notion that deprivation would affect all withdrawal symptom reports regardless of temptation context. These showed that there was a significant main effect of deprivation for both craving and negative affect in that both increased significantly from the pre- to the post-quit period with or without a temptation event (see Table 1). These results were consistent when the 47 participants smoking more than five cigarettes per day were excluded. When ratings were analyzed in a general linear model (comparing pre-quit to post-quit and a temptation event to no temptation event), results revealed a significant interaction between pre-quit/post-quit status and temptation status for craving (see Figure 3; F[1, 63340] = 105.17, p < .001) and for negative affect (see Figure 4; F[1, 63340] = 7.85, p = .005). This interaction reflects the fact that for both symptoms the highest ratings occurred when temptation events and deprivation were both present.
Figure 3.
Interaction effects of the occurrence of temptation events in the pre-quit vs. post-quit period on craving.
Figure 4.
Interaction effects of the occurrence of temptation events in the pre-quit vs. post-quit period on negative affect.
Withdrawal components (defined as post-quit values minus pre-quit values) were then used to predict 8-week CO-confirmed point-prevalence abstinence. We did not include craving standard deviation because it did not significantly differ from pre- to post-quit after controlling for smoking in the post-quit period. In univariate regression analyses (see Table 2), controlling for treatment and post-quit smoking, we found that most profile dimensions of negative affect and craving predicted cessation outcome, with the exception of: a) frequency of intense negative affect (percentage of scores of greater than 4); b) variability in negative affect; and c) mean level of negative affect during a temptation event. The results were similar without the covariates and when we did not use the intent-to-treat principle but allowed participants who were missing outcome data to be coded as missing. We also found that the results were similar when the models controlled for the number of EMA reports completed (i.e., missing data).
Table 2.
Withdrawal parameters that predict 8-week cessation outcome in univariate logistic regression analyses, controlling for post-quit smoking and treatment
| Withdrawal measure | Wald | p-value | OR | OR 95% CI |
|---|---|---|---|---|
| Change in craving mean (post-pre) | 19.58 | <.001 | .89 | .84, .93 |
| Change in percent of craving reports greater than 7 (post-pre) | 9.41 | .002 | .99 | .988, .997 |
| Change in craving mean when faced with a temptation event (post-pre) | 6.19 | .01 | .93 | .88, .985 |
| Change in craving mean when not faced with a temptation event (post-pre) | 14.38 | <.001 | .90 | .85, .95 |
| Change in negative affect mean (post-pre) | 8.73 | .003 | .82 | .72, .94 |
| Change in negative affect SD (post-pre) | 2.83 | .09 | .83 | .67, 1.03 |
| Change in percent of negative affect reports greater than 4 (post-pre) | 1.81 | .18 | .99 | .97, 1.01 |
| Change in negative affect mean when faced with a temptation event (post-pre) | .92 | .34 | .95 | .86, 1.05 |
| Change in negative affect mean when not faced with a temptation event (post-pre) | 4.77 | .03 | .85 | .74, .98 |
Notes. Post-quit smoking is a categorical variable due to the skewed distribution, where 0 = no smoking, 1 = smoking less than 1 cigarette per day on average, and 2 = smoking more than 1 cigarette per day on average. Treatment is a categorical variable with 0 = placebo, 1 = monotherapy (i.e., patch, lozenge and bupropion) and 2 = combination therapy (i.e., patch + lozenge, bupropion + lozenge). Results were similar when the models controlled for the number of EMA reports completed (i.e., missing data) and when not controlling for mean cigarettes per day and treatment with the exception that change in negative affect SD became significant (Wald = 5.62, p = .02, OR = .79, 95% CI = .66, .96). Change in craving standard deviation was not included as an outcome predictor because it did not differ significantly between pre- and post-quit, suggesting that it may not be a withdrawal symptom.
The various dimensions of craving and negative affect were meaningfully correlated (r's ranged from .20 to .80 for the craving variables and .26 to .55 for the negative affect variables), although distinct, suggesting potential value in studying the dimensions as individual predictors. Therefore, it was important to determine whether the various dimensions of each symptom provided orthogonal prediction of outcome. Optimal prediction models were then built separately first using only craving variables and then using only negative affect variables (Hosmer and Lemeshow 2000). As shown in Table 3, increase in mean levels of craving from pre- to post-quit was the only significant predictor of cessation even when other craving dimensions (e.g., proportion of intense cravings reported) were added to the model. For the negative affect models (see Table 3), we found that a greater increase in mean negative affect post-quit, relative to pre-quit, was related to lower cessation success. However, contrary to expectation, an increase in mean levels of negative affect in a temptation context actually predicted a greater likelihood of cessation success. Finally, when we combined the craving and negative affect models, we found that cessation failure was predicted by increased mean levels of craving and negative affect from pre- to post-quit and by reduced affective reaction to temptation events. In other words, the increase in chronic levels of post-quit craving and negative affect, relative to the prequit levels, heightened the risk of cessation failure. But, to the extent that individuals reported stronger affective reactions to episodic temptation events post-quit versus pre-quit, they were more likely to be successful in the quit attempt2. All of the best fitting models were similar when we did not use any covariates.
Table 3.
Best-fitting multivariate models using craving only, negative affect only, and then the combination of craving and negative affect variables to predict 8-week abstinence, controlling for smoking in the first 10 days post-quit and treatment
| Withdrawal measure | Wald | p-value | OR | OR 95% CI |
|---|---|---|---|---|
| Craving only model | ||||
| Change in craving mean (post-pre) | 19.58 | <.001 | .89 | .84, .93 |
| Negative affect only model | ||||
| Change in negative affect mean (post-pre) | 21.07 | <.001 | .56 | .43, .71 |
| Change in negative affect mean when faced with a temptation event (post-pre) | 5.55 | .02 | 1.19 | 1.03, 1.37 |
| Combined craving and negative affect model | ||||
| Change in craving mean (post-pre) | 6.15 | .01 | .90 | .83, .98 |
| Change in negative affect mean (post-pre) | 12.92 | <.001 | .62 | .48, .80 |
| Change in negative affect mean when faced with a temptation event (post-pre) | 5.43 | .02 | 1.18 | 1.03, 1.37 |
Models were the same controlling for the number of EMA reports completed.
Discussion
In our analyses of different profile dimensions of negative affect and craving, we found a post-quit increase in: the mean level of negative affect and craving, the variability of negative affect and craving, the number of extreme ratings of negative affect and craving, and negative affect and craving responses in the face of temptation events. These data may be useful for counseling smokers about what changes to expect when they try to quit smoking—craving and affective distress increase rapidly, tend to remain chronically high for at least several days, are highly variable, and tend to increase more markedly during exposure to temptation events (see Figure 1 and Table 1). While these data suggest that smokers' craving and negative affect symptoms tend to respond similarly during a quit attempt, there appear to be striking differences as well. Relative to their reports of negative affect, smokers' reports of craving are much more intense on average and much more frequently show large swings in intensity (Figure 1). For example, smokers reported extreme craving levels (> 7) on 20% of their post-quit reports but extreme negative affect (>4) on only 1.6% of those reports. Further, craving scores returned to the 10-day pre-quit level, or below, by 10-days post-quit, consistent with previous research (Shiffman et al. 2006b), whereas negative affect did not, suggesting that, on average, the latter is more refractory.
Because these symptom dimensions were all adjusted for pre-quit levels, these data afford the opportunity to determine whether deprivation associated changes in these withdrawal dimensions index a heightened likelihood of relapse. The results of univariate tests show that many of the withdrawal dimensions significantly predict 8-week smoking status (Table 2). The only dimensions that were not significant predictors were the number of extreme values in negative affect, the variability in negative affect, and temptation-related increases in negative affect.
In the best-fitting models predicting 8-week smoking status, change in mean level of craving from pre- to post-quit was the only significant predictor amongst craving dimensions, while change in mean level of negative affect and temptation-related increases in negative affect from pre- to post-quit were the only significant predictors amongst affect dimensions (Table 3). Tests of models built with both symptoms revealed that all three of those dimensions—change in mean level of craving, change in mean level of negative affect, and temptation-related increases in negative affect from pre- to post-quit—accounted for independent prediction of 8-week outcome.
The results suggest that those who are least likely to quit and stay quit are those who experience a greater increase in overall craving and negative affect after quitting. It is important to recognize that the craving and negative affect means do not reflect chronic, steady high levels of symptoms per se, but rather integrate symptom intensity information across multiple dimensions (e.g., peaks and troughs, trajectories). Therefore, the relation between relapse and the change in mean symptom levels could reflect the influences of multiple, more discrete symptom patterns such as chronic high levels and symptom peaks, and this inclusiveness may account for the mean symptom level's superior predictive validity. In sum, the univariate tests of the individual dimensions reveal that many dimensions are, in fact, related to later abstinence status, and the strong performance of the symptom means in the prediction models does not mean that other symptom dimensions are motivationally inert. The best-fitting models may be suggesting which dimensions integrate risk information most efficiently, rather than informing us about discrete causal paths.
It is interesting that both craving and negative affect contributed independent or orthogonal predictive validity with regard to cessation outcome. Recent research has tended to implicate craving rather than negative affect as a more consistent or stronger risk factor for cessation failure (McCarthy et al. 2006; Shiffman and Kirchner 2009). Negative affect may have been related to outcome in the present research versus in earlier studies because of the sample size and resulting statistical power, poor reliability in estimating linear trend characteristics of negative affect in previous studies, the use of more sensitive and valid EMA data versus paper-pencil measures, or the fact that the symptom measures in the present research reflected deprivation-related increases (i.e., the influence of prequit levels were statistically controlled in this study). With regards to the last point, negative affect may be more likely to index smoking motivation when it arises from tobacco deprivation than when it arises from other sorts of events (see Perkins et al. 2010). In any event, the current results suggest that while craving and negative affect tend to be intercorrelated (Baker et al. 2004), each possesses independent predictive validity with respect to cessation outcome which adds to the evidence that withdrawal symptoms are important determinants of cessation success, and that heterogeneity exists within such symptoms with regards to profile characteristics and motivational impact (Hughes 2007b).
In addition to influencing craving and negative affect, tobacco deprivation can enhance cue reactivity and cue reactivity in turn may affect smoking motivation (Acri and Grunberg 1992; Gloria et al. 2009; McCarthy et al. 2006; Shiffman et al. 2006a; Shiffman et al. 1996; Spiga et al. 1994; cf Perkins 2009a). However, the current research found that increased negative affect reactivity in response to smoking cues/temptation events during deprivation was predictive of smoking cessation success, but only after controlling for overall level of negative affect. (In the univariate test, without statistical control for mean level of negative affect, the magnitude of temptation-associated negative affect was nonsignificantly related to smoking cessation failure). It is unclear why greater negative affect in response to temptation was related to better outcomes in multivariate models. It seems unlikely that this could be due to conditioned withdrawal symptoms that manifest as negative affect (e.g., Siegel 1977). Why would greater conditioned withdrawal symptoms aid a person in quitting? Instead, it could be that once withdrawal symptoms per se are statistically controlled, affective reaction then represents some other process, such as distress or irritation at being confronted with smoking cues. Thus, such reactions might reflect rejection of such cues and frustration at being exposed to them. It is also possible that such reactions index cue awareness, and this may have spurred coping response execution, which has been associated with successful resolution of temptation events (O'Connell et al. 2008, although cf. Shiffman et al. 1996). In any event, the current findings are in general agreement with recent findings by McRobbie and colleagues (McRobbie et al. 2008) that general affective status seems more predictive of cessation outcome than is cue-elicited reactivity (cf. Perkins 2009b).
There are limitations to be considered when interpreting these results. First, analyzing withdrawal symptoms in the context of intermittent smoking (i.e., withdrawal symptoms among smokers who may have smoked 1–2 cigarettes during the first few days of their quit attempt) is complex. It is important to note that we did not have daily biochemical verification of smoking status during this study. However, when we did control for self-reported smoking during the post-quit period we found similar results. Another limitation involves whether participants accurately reported on the occurrence of targeted events; e.g., whether they experienced a temptation event (i.e., something that made them want to smoke). For instance, we cannot know with certainty the causal direction between reporting a temptation event and higher craving; elevated craving might have caused people to report temptation events rather than vice versa. However, the number of temptation events reported increased only slightly during the cessation attempt, while craving reports increased significantly. This provides some modest support for the notion that temptation events were not slaves to participants' affective or motivational state. In addition, participants were given pre-assessment training to use the item to report events or triggers. Our use of the ratings 7 and 4 for the thresholds of “peak” craving and negative affect reports, respectively, is another possible limitation of this research. These thresholds may not capture optimal cut-scores for a symptom “peak” and therefore, may underestimate peak effects. However, these scores represent reports that are at least 1 standard deviation from the mean and appeared to be the most sensitive indices of relapse risk. Finally, smokers in this study were highly motivated to quit and willing to engage in a long-term study thereby perhaps limiting the generalizability of these finding.
Conclusions
Using real-time reports of symptoms, we found that tobacco deprivation affected participants' negative affect and craving reports quite differently. For instance, relative to negative affect, craving reports were higher on average, more variable, and more frequently extreme in nature. This finding suggests that—in addition to differentiation based on covariance patterns in static measures (Cappelleri et al. 2005; Etter and Hughes 2006; Welsch et al. 1999)—the two types of withdrawal measures are distinguished by their dynamic patterns across the post-quit period. The results also show that many of the dynamic dimensions of both craving and affect were significantly increased by reductions in tobacco use, consistent with the notion that craving and affect do indeed reflect withdrawal processes. This finding is significant in that doubts about craving's sensitivity to tobacco deprivation caused it to be excluded from the DSMIV list of withdrawal symptoms (American Psychiatric Association 1994). The results also show that numerous craving and affective profile dimensions are predictive of later smoking, suggesting their motivational significance. When combined into one model, craving and negative affect each accounted for unique prediction of later smoking. The results underscore the complexity of withdrawal as a determinant of relapse following a quit attempt, as well as the need to measure withdrawal symptoms in a more molecular manner, distinguishing both symptom (e.g., craving, negative affect) and dynamic component (e.g., mean level, variability). Finally, these results suggest that smokers attempting to quit might benefit from education regarding typical patterns of both negative affect and craving and benefit from training in ways to cope with different dimensions of the withdrawal phenomenon.
Acknowledgements
Funding: This research was conducted at the University of Wisconsin, Madison and was supported by grant #P50 DA019706 from NIH/NIDA and by grant #M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources, NIH. Dr. Piper was supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), National Institutes of Health (NIH). Dr. Cook was supported by K08DA021311. Dr. Baker was supported via NCI 1K05CA139871. Dr. Loh was partially supported by U. S. Army Research Office grant W911NF-09-1-0205. Medication was provided to patients at no cost under a research agreement with GlaxoSmithKline (GSK); no part of this manuscript was written or edited by anyone employed by GSK.
Footnotes
Data from the first and last two days of recording were unrepresentative of the other recording days based on both completion rate and mean values.
While our previous research using HLM analyses has generally returned nonsignificant results in regard to negative affect once accounting for craving, the estimates for negative affect generally show poorer reliability due to the variability in symptom endorsement, especially the intermittent symptom spikes, as seen in the current analysis.
Disclosures: Megan E. Piper, Tanya R. Schlam, Jessica W. Cook, Megan A. Sheffer, Stevens S. Smith, Wei-Yin Loh, Daniel M. Bolt, Su-Young Kim, Jesse T. Kaye and Kathryn R. Hefner have no potential conflicts of interest to disclose.
References
- Acri JB, Grunberg NE. A psychophysical task to quantify smoking cessation-induced irritability: the reactive irritability scale (RIS) Addict Behav. 1992;17:587–601. doi: 10.1016/0306-4603(92)90068-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostice and statistical manual of mental disorders. 4th edn. American Psychiatric Association, American Psychiatric Association; 1994. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Curr Med Res Opin. 2005;21:749–60. doi: 10.1185/030079905X43712. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104:850–8. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Hughes JR. A comparison of the psychometric properties of three cigarette withdrawal scales. Addiction. 2006;101:362–72. doi: 10.1111/j.1360-0443.2005.01289.x. [DOI] [PubMed] [Google Scholar]
- Gloria R, Angelos L, Schaefer HS, Davis JM, Majeskie M, Richmond BS, Curtin JJ, Davidson RJ, Baker TB. An fMRI investigation of the impact of withdrawal on regional brain activity during nicotine anticipation. Psychophysiology. 2009;46:681–693. doi: 10.1111/j.1469-8986.2009.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. Second Edition, 2nd edn. John Wiley & Sons, Inc., John Wiley & Sons, Inc.; 2000. [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007a;9:329–39. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007b;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Measurement of the effects of abstinence from tobacco: a qualitative review. Psychol Addict Behav. 2007c;21:127–37. doi: 10.1037/0893-164X.21.2.127. [DOI] [PubMed] [Google Scholar]
- Javitz HS, Brigham J, Lessov-Schlaggar CN, Krasnow RE, Swan GE. Association of tobacco dependence and quit attempt duration with Rasch-modeled withdrawal sensitivity using retrospective measures. Addiction. 2009;104:1027–35. doi: 10.1111/j.1360-0443.2009.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35:1120–30. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. J Abnorm Psychol. 2006;115:454–66. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough BE, Warren CA. Effects of 12-h tobacco deprivation on event-related potentials elicited by visual smoking cues. Psychopharmacology (Berl) 2001;154:282–91. doi: 10.1007/s002130000647. [DOI] [PubMed] [Google Scholar]
- McRobbie H, Hajek P, Locker J. Does the reaction of abstaining smokers to the smell of other people's cigarettes predict relapse? Addiction. 2008;103:1883–7. doi: 10.1111/j.1360-0443.2008.02340.x. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Schwartz JE, Shiffman S. Do resisted temptations during smoking cessation deplete or augment self-control resources? Psychol Addict Behav. 2008;22:486–95. doi: 10.1037/0893-164X.22.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Acute responses to nicotine and smoking: implications for prevention and treatment of smoking in lower SES women. Drug Alcohol Depend. 2009a;104(Suppl 1):S79–86. doi: 10.1016/j.drugalcdep.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009b;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Briski J, Fonte C, Scott J, Lerman C. Severity of tobacco abstinence symptoms varies by time of day. Nicotine Tob Res. 2009;11:84–91. doi: 10.1093/ntr/ntn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biol Psychiatry. 2010;67:707–14. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: Two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–51. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003a;112:3–13. [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003b;112:14–27. [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Exp Clin Psychopharmacol. 2003c;11:276–85. doi: 10.1037/1064-1297.11.4.276. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J Consult Clin Psychol. 2004;72:139–54. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–62. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology (Berl) 2006a;184:637–44. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Temptations to smoke after quitting: A comparison of lapsers and maintainers. Health Psychology. 1996;15:455–61. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR. Cigarette-by-cigarette satisfaction during ad libitum smoking. J Abnorm Psychol. 2009;118:348–59. doi: 10.1037/a0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, Hickcox M, Waters A, Balabanis M. Natural history of nicotine withdrawal. Addiction. 2006b;101:1822–32. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of unrestricted smoking patterns. J Abnorm Psychol. 2004a;113:166–71. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West R, Gilbert D. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res. 2004b;6:599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Siegel S. Learning and psychopharmacology. Appleton-Century-Crofts, Appleton-Century-Crofts; 1977. [Google Scholar]
- Spiga R, Bennett RH, Schmitz J, Cherek DR. Effects of nicotine on cooperative responding among abstinent male smokers. Behav Pharmacol. 1994;5:337–343. doi: 10.1097/00008877-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Capturing momentary self-report data: a proposal for reporting guidelines. Ann Behav Med. 2002;24:236–43. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Pillitteri JL, Kozlowski LT. Measuring drug urges by questionnaire: Do not balance scales. Addict Behav. 1996;21:199–204. doi: 10.1016/0306-4603(95)00044-5. [DOI] [PubMed] [Google Scholar]
- U.S. Dept. of Health and Human Services . The health consequences of smoking: Nicotine addiction. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 1988. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7:354–61. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wikler A. Conditioning factors in opiate addictions and relapse. In: Kassebaum DMWGG, editor. Narcotics. McGraw-Hill; New York: 1965. pp. 85–100. [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34:365–73. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]