Abstract
Context
Codeine has become a controversial choice for analgesia in children compared to other commonly available drugs.
Objectives
To evaluate whether an educational campaign shifted resident prescribing patterns away from codeine toward more appropriate analgesics.
Methods
Our intervention consisted of a pocket-sized reference card given to all trainees and key staff on an inpatient pediatric acute care unit; pediatrics residents also had the option to attend a one-hour lecture. The pocket-card recommended against codeine (including rationale), and gave prescription guidance for our institution’s preferred formulary alternative analgesics, which included tramadol and hydrocodone. We used inpatient prescribing data to track the prescribing of codeine and alternative medications over time.
Results
Following the interventions, there was a significant decrease in the percentage of patients receiving codeine (13.5% of patients received the drug in the year before, 5.4% in the year after, P<0.0001). Use of hydrocodone-containing analgesics increased overall during the same period (7.4% to 16%, P<0.0001) as did tramadol use (0.2% to 2.6%, P<0.0001). There were no changes in pain management satisfaction scores.
Conclusions
A simple, low-cost educational campaign consisting primarily of a pocket guide to analgesics markedly improved analgesic prescribing patterns, and that improvement extended to services not targeted by the didactic component of our educational campaign. Point-of-care decision support via a pocket-card may be sufficient for effecting change in medication prescribing patterns of trainees.
Keywords: Children, analgesics, codeine, trainees, point-of care reference
Introduction
Pain is an extremely common symptom in hospitalized children (1, 2). Historically, there have been significant gaps in pain management in children, with less effective pain control in children as compared to adults (3). Among trainees, there is significant variation in knowledge and prescribing practices for analgesics (4).
Codeine is a commonly used analgesic for moderate pain, although its use may be problematic. It is a weak opioid that must be metabolized into the active metabolite, morphine. As many as 10% of Caucasian patients (higher in North African populations) are slow metabolizers of codeine and are at risk for inadequate analgesia. Another 1–2% of the population are ultra-rapid metabolizers and are, therefore, at risk for morphine intoxication for themselves (5) as well as breastfed infants (6). Based on this information, many experts recommend caution when prescribing codeine as well as a need for attention to the relevant pharmacogenetics (7). It is not currently practical to test for these polymorphisms in all patients who might receive codeine. Therefore, our institutional experts recommend use of oral analgesics with more uniform pharmacokinetics. These include hydrocodone and tramadol, among others.
Pocket-cards improve trainee knowledge in palliative care (8), as well as in antibiotic prescribing (9). They also have recently been shown to improve compliance with evidence-based guidelines for asthma management (10). However, few studies have examined the role of pocket-cards in changing inpatient analgesic prescribing practices for children.
Our goal was to create an educational campaign to improve analgesic prescribing practices in our Children’s Hospital through the combination of lectures as well as pocket-sized prescribing reference cards. Our hypothesis was that after the educational campaign, there would be a decrease in codeine prescribing and in increase in the prescribing of recommended alternatives such as tramadol and hydrocodone.
Methods
Site and Subjects
Our study was carried out at the University of California San Francisco (UCSF) Children’s Hospital, a 175-bed tertiary care facility. Children on the inpatient unit are cared for primarily on a pediatric hospitalist service (pediatric hospitalists with pediatrics trainees), a medical subspecialty service (pediatric subspecialists with pediatrics trainees), a pediatric general surgery service (pediatric surgeons with general surgery trainees), or a pediatric surgical subspecialty service (pediatric surgical subspecialists with subspecialty surgery trainees). Surgical trainees rotate on pediatrics services for two to six months over the course of their multi-year training, depending on their program.
We studied non-intensive care unit patients aged 0–18 on the primary inpatient medical-surgical unit. Approval for retrospective review with waiver-of-consent was approved by the UCSF Committee on Human Research.
Intervention
Our intervention was an educational campaign comprising two main components: a one-hour lecture combined with a pocket-card containing specific analgesic prescribing information (Figure 1).
Fig. 1.
Analgesic reference card given to trainees.
Didactic Training on Pediatric Analgesic Prescribing
Our lecture provided an overview of pain management in children, including traditional pharmacologic analgesics as well as non-pharmacologic pain management strategies. Importantly, lectures provided a strong recommendation to avoid codeine-containing analgesics because of unpredictable pharmacokinetics. Hydrocodone-containing analgesics and tramadol were offered as enteral alternatives for patients with moderate pain.
The lecture was primarily targeted at pediatrics residents and was repeated, giving residents two opportunities to hear the lecture. Six months later, the lecture was given again as part of our Grand Rounds series with a slightly different target audience, including faculty and fellows. Attendance at lectures was not mandatory and attendance was not recorded. Based on rotation-assignments and timing of the lectures, we estimate that at least half of the pediatrics trainees attended one session. Less than one-half of the hospitalists attended a lecture, and less than one-quarter of subspecialty faculty or fellows attended. No faculty or trainees from the surgical departments attended.
Prescribing Pocket-Card
Our pocket-card was developed by two hospitalists, a pediatric anesthesiologist, a pediatric intensivist, and a pediatric clinical pharmacist. It included a stepwise approach to pain management modeled after the World Health Organization pain ladder (http://www.who.int/cancer/palliative/painladder/en/), as well as guidelines from national societies.
Key points on the card included information about the variable pharmacokinetics of codeine, a strong recommendation to avoid prescribing codeine, and guidance for how to prescribe non-opioids, opioids, and combination-opioid preparations (including considerations of contraindications, side effects, etc.). The pocket-card also included guidance regarding maximum acetaminophen dosing, including acetaminophen-containing combination analgesic preparations.
We distributed the pocket-card to pediatrics trainees via their intercampus mailboxes and to surgical trainees via clinical pharmacists at the beginning of their rotations on the pediatrics services. The card also was given to hospitalist attending physicians, clinical pharmacists, and key nursing staff. Clinical pharmacists were advised to use the pocket-cards to make recommendations when asked. Medication safety nurses checked written orders against the pocket-cards to verify safe dosing. We made no changes to the formulary or the availability of clinical pharmacists in making recommendations when asked. The study was designed post-hoc and, therefore, none of the care providers could have known that they were being studied.
Outcomes
Our primary outcome was analgesic prescribing practices. Using prescribing data, we determined whether patients had been prescribed any of our targeted analgesics (codeine-containing combination products, hydrocodone-containing combination products, and tramadol) as well as any of the following non-targeted analgesics: acetaminophen, ibuprofen, ketorolac, morphine, hydromorphone, and oxycodone-containing combination products.
Our secondary outcome was satisfaction with pain management, based on a parental satisfaction questionnaire.
Data Sources
Data on medication prescribing were collected from electronic inpatient prescribing databases. These databases include the names of medications prescribed, as well as the date the drug was ordered. We reviewed data during the 12-month period prior to the start of the intervention (7/1/07-6/30/08) and during the 12-month period beginning two months after the intervention (9/1/08-8/31/09).
Data describing parental satisfaction with pain control were collected through standard UCSF customer satisfaction surveys (administered by Press Ganey, a third party). These surveys are mailed to discharged patients, and they include a specific question assessing parental satisfaction with how well their child’s pain was controlled. Data were obtained in month-level aggregated groups. Our key parental satisfaction question asked parents to score: “How well your child’s pain was controlled,” on a Likert scale from 1–5. The data were translated to a 100-point scale (Very Poor = 0, Poor = 25, Fair = 50, Good = 75, Very Good = 100).
Data Analysis
We reviewed data for the entire unit, and also categorized patients based on admitting service to identify which residents were caring for the patients. For subgroup analysis, the pediatric hospitalist and pediatric medical subspecialty services were combined because all patients were cared for by pediatrics trainees. Surgical patients on the six high-volume subspecialties were grouped together (general surgery, plastic surgery, urological surgery, orthopedic surgery, neurosurgery, and otolaryngology head & neck surgery). The remaining services (see Appendix) were classified as “low-volume services.”
Appendix.
Patient Categories
| Medical (n=2413) | High-Volume Pediatric Subspecialties (n=989) | Low-Volume Pediatric Subspecialties and Boarders (n=499) |
|---|---|---|
| General pediatrics | General surgery | Kidney transplant |
| Pulmonology | Plastic surgery | Liver transplant |
| Adolescent medicine | Urology | AGS |
| Gastroenterology | Orthopedic surgery | Cardiology |
| Endocrinology | Neurosurgery | CTS |
| Neurology (non-EEG) | Otolaryngology/Head & Neck surgery | Gynecology |
| Rheumatology | GYO | |
| Immunology | INN | |
| Nephrology | IVR | |
| MED | ||
| Neonatology | ||
| Neurology (EEG) | ||
| OBX | ||
| Ophthalmology | ||
| OSX | ||
| Vascular surgery | ||
| Well-baby | ||
We grouped the admission dates into three-month quarters for trending across the intervention time period. Chi-square analyses were performed to determine significant changes in prescribing and patient characteristics before and after the intervention. Analysis of variance was performed to determine if the mean number of analgesics prescribed changed after the intervention. All analyses were carried out in SAS version 9.2 (SAS Institute, Cary, NC).
Results
Study Population
During the 24-month period, there were 3901 admissions on our target inpatient unit (2413 medical, 989 high-volume subspecialties, and 499 low-volume specialties; categorization is described above) (Table 1).
Table 1.
Patient Demographics
| Pre (n=1897) n (%)a |
Post (n=2004) n (%)a |
|
|---|---|---|
| Age mean (SD) | 9.0 (5.7) | 8.8 (5.7) |
| Gender | ||
| Male | 974 (51.3) | 1044 (52.1) |
| Female | 923 (48.7) | 960 (47.9) |
| Race | ||
| White | 767 (40.4) | 788 (39.3) |
| Black | 133 (7.0) | 128 (6.4) |
| Asian | 204 (10.8) | 179 (8.9) |
| Other | 735 (38.8) | 855 (42.7) |
| Missing | 58 (3.1) | 54 (2.7) |
| Ethnicity | ||
| Hispanic | 651 (34.3) | 660 (32.9) |
| Payer type | ||
| Medicare | 44 (2.3) | 68 (3.4) |
| Medi-CAL/Medicaid | 989 (52.1) | 989 (49.4) |
| Private | 841 (44.3) | 922 (46.0) |
| Other | 23 (1.2) | 25 (1.3) |
| Admit source | ||
| ED | 475 (25.0) | 494 (24.7) |
| Outside hospital | 151 (8.0) | 143 (7.1) |
| Direct admit/other | 1271 (67.0) | 1367 (68.2) |
| Disposition | ||
| To home | 1880 (99.1) | 1979 (98.8) |
| Died | 3 (0.2) | 6 (0.3) |
| Other | 14 (0.7) | 19 (1.0) |
| Admit service | ||
| Medical | 1187 (62.6) | 1226 (61.2) |
| High-volume subspecialties | 480 (25.3) | 509 (25.4) |
| Low-volume subspecialties | 230 (12.1) | 269 (13.4) |
Note: There was no statistically significant difference between the patients before and after the intervention.
All P-values >0.05.
Trends in Targeted Analgesic Prescribing
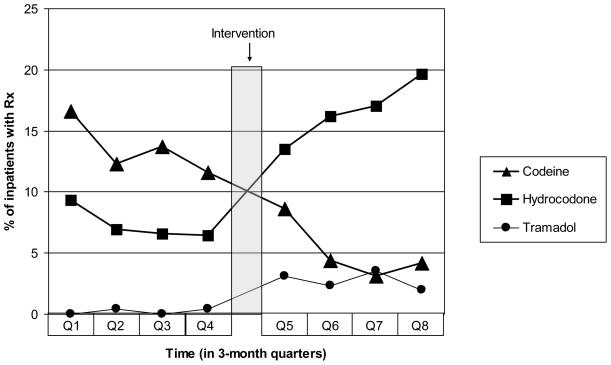
Prescriptions for codeine-containing compounds decreased markedly over the study period (Table 2). The overall decrease was from 13.5% to 5% (P<0.0001). This trend was present on both the medical and high-volume subspecialty services. During this same time period, prescriptions for hydrocodone-containing compounds and tramadol increased significantly in all groups. For further review, we divided the pre- and post-intervention periods into three-month quarters (Figure 2).
Table 2.
Patients Prescribed a Targeted Analgesica
| Analgesic | Group | Pre- intervention n (%) |
Post- intervention n (%) |
P-value |
|---|---|---|---|---|
| Codeine (including combination products) | All patients | 256 (13.5) | 120 (5.1) | <0.0001 |
| Medical service | 49 (4.3) | 8 (0.7) | <0.0001 | |
| High-volume subspecialties | 199 (41.5) | 93 (18.3) | <0.0001 | |
| Hydrocodone (including combination products) | All patients | 138 (7.3) | 332 (16.6) | <0.0001 |
| Medical service | 37 (3.1) | 91 (7.4) | <0.0001 | |
| High-volume subspecialties | 81 (16.9 | 218 (42.8) | <0.0001 | |
| Tramadol | All patients | 4 (0.2) | 55 (2.7) | <0.0001 |
| Medical service | 3 (0.3) | 46 (3.8) | <0.0001 | |
| High-volume subspecialties | 0 | 7 (1.4) | 0.0156 |
Percent of patients in subgroups who were prescribed targeted analgesics, showing that after the intervention there was a decrease in prescribing of codeine-containing analgesics and an increase in hydrocodone-containing analgesics and tramadol.
Fig. 2.
Prescribing trends of targeted analgesics by quarter, for one year before and one year after the intervention, represented as percent of patients who received each analgesic. Triangles represent codeine prescribing, for which prescribing decreased significantly over the study period. Squares represent hydrocodone-containing analgesics and circles represent tramadol, both of which had significantly increased prescribing.
Trends in Non-Targeted Analgesics
Acetaminophen was the most commonly prescribed analgesic overall. After the intervention, it was still the most commonly prescribed but was used slightly less. Subgroup analysis showed that this trend was primarily driven by prescribing for the medical patients. In the high-volume subspecialties, there was an increase in acetaminophen prescribing.
Parenteral morphine was prescribed slightly more often after the intervention, but there was no significant change in enteral morphine or parenteral hydromorphone prescribing. Combined service data for all analgesics is shown in Table 3.
Table 3.
Patients Prescribed Any Analgesic or Naloxone
| Medication | Pre-intervention (n=1897) n (%) |
Post-intervention (n=2004) n (%) |
P-value |
|---|---|---|---|
| Hydrocodone (including combination products) | 138 (7.3) | 332 (16.6) | <0.0001 |
| Acetaminophen | 1003 (52.9) | 973 (48.6) | 0.0070 |
| Codeine (including combination products) | 256 (13.5) | 102 (5.1) | <0.0001 |
| Ibuprofen | 194 (10.2) | 204 (10.2) | 0.9613 |
| Ketorolac | 69 (3.6) | 78 (3.9) | 0.6761 |
| Oxycodone (including combination products) | 24 (1.3) | 29 (1.5) | 0.6237 |
| Tramadol | 4 (0.2) | 55 (2.7) | <0.0001 |
| Morphine/parenteral | 460 (24.3) | 541 (27.0) | 0.0496 |
| Morphine/enteral | 20 (1.1) | 14 (0.7) | 0.2322 |
| Dilaudid/parenteral | 170 (9.0) | 172 (8.6) | 0.6760 |
| Dilaudid/enteral | 14 (0.7) | 4 (0.2) | 0.0131 |
| Any analgesic | 1355 (71.4) | 1303 (65.0) | <0.0001 |
| Naloxone | 23 (1.2) | 9 (0.5) | 0.0082 |
Trends in Overall Analgesic Prescribing
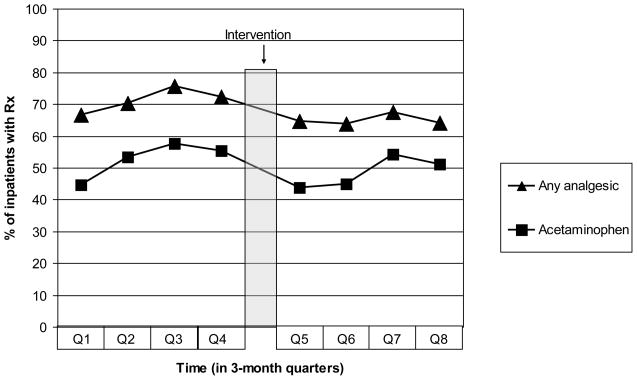
Overall, there was a statistically significant decrease in analgesic doses prescribed during the study period. The percent of patients receiving any analgesic decreased from 71% in the pre-intervention period to 65% after the intervention (P<0.0001). This was mirrored by a decrease in acetaminophen from 53% to 49% (P=0.0070) (Figure 3).
Fig. 3.
Trend in prescribing any analgesic compared to trend in prescribing acetaminophen, for one year before and one year after the intervention, represented as percent of patients who received each analgesic. Triangles represent the percent of patients prescribed any analgesic, and squares represent acetaminophen. Both lines followed similar trends over time.
After the intervention there was a slightly larger group of patients who were not prescribed any analgesic. However, there was no decrease in the average number of analgesics per patient (P=0.8925). Detailed data are shown in Table 4.
Table 4.
Average Number of Analgesics Per Patient
| Patients receiving: | Pre n(%) | Post n(%) |
|---|---|---|
| 0 analgesics | 542 (28.6) | 701 (35.0) |
| 1 analgesic | 716 (37.7) | 576 (28.7) |
| 2 different analgesics | 408 (21.5) | 395 (19.7) |
| 3 different analgesics | 147 (7.8) | 227 (11.3) |
| 4 different analgesics | 51 (2.7) | 76 (3.8) |
| 5 different analgesics | 25 (1.3) | 21 (1.1) |
| 6 different analgesics | 6 (0.3) | 8 (0.4) |
| 7 different analgesics | 2 (0.1) | 0 |
| Mean # of analgesics per patient | 1.24 (1.15) | 1.25 (1.24) |
Note: There was no statistically significant change.
Trends in Naloxone Prescribing
During the study period, naloxone prescribing decreased from 1.2% to 0.5% of patients (P=0.0082).
Patient Satisfaction
Based on aggregated monthly data, the average parental satisfaction score for child’s pain management was 83.8 pre-intervention and 84.4 post-intervention (P=0.6958).
Discussion
A simple campaign consisting primarily of a widely distributed reference card appeared to produce a marked improvement in analgesic prescribing patterns across all major prescribing practitioner groups on our inpatient unit. Specifically, we observed a decrease in the use of codeine-containing analgesics in favor of analgesics such as tramadol or hydrocodone. Moreover, our data suggest continued effects, implying that changes in practice may have continued over time.
Interestingly, improvements in analgesic prescribing were seen for patients who were cared for by trainees who did not have exposure to all components of the intervention (surgery residents did not participate in the lecture curriculum). The highly significant changes in prescribing practices in this group suggests that providing point-of-care decision support via a pocket-card may be sufficient for effecting change. Lectures may not provide additional benefit.
Some of our intervention’s effectiveness may have been enhanced by our clinical pharmacists who also used the reference card. In our institution, clinical pharmacists round with the medical teams and co-follow all surgical patients. In addition, pharmacists review charts and may make unsolicited recommendations. The availability and role of clinical pharmacists was the same before and after the intervention.
Clinical pharmacists interacting directly and indirectly with prescribing physicians can contribute to improved prescribing practices in other settings (11). Most recommendations for surgical patients are reactive, based on order-review; therefore the decrease in codeine prescribing is unlikely to be due to the intervention on a patient-by-patient basis. At least one clinical pharmacist was involved in developing the card, and the cards were disseminated within that group. Therefore, we do believe that the card had a powerful impact that extended beyond the physician community to include the clinical pharmacists.
The trend toward an overall decrease in analgesic prescriptions is notable. While we have no direct evidence, it is possible that the decrease in analgesic usage overall was in part driven by increased attention to risk of acetaminophen overdosing in patients who also were prescribed an acetaminophen-containing combination product (the decrease in total analgesic usage was mirrored by the decrease in acetaminophen usage). At minimum, it is clear that increased awareness of pain recommendations did not translate into increased prescribing of analgesics. We would have liked to document that there was no detrimental change in average pain scores during hospitalization or post-operative periods with these interventions. We are reassured that our parental satisfaction scores did not worsen during our study period.
The decrease in naloxone prescriptions is likely multifactorial in origin. Direct chart review revealed that most naloxone that was prescribed was not actually administered. Naloxone is commonly prescribed prophylactically for patients who receive patient-controlled analgesia (PCA). This study was not designed to evaluate changes in PCA usage. However, we believe that the decrease in naloxone usage reflects a higher level of comfort and safety with analgesics prescribed after the intervention.
The study has several important limitations. The intervention was originally designed as a bundled educational and practice-improvement program. As such, there was no true control group and we were limited in the data available to us for our retrospective study. We cannot document the degree to which target trainees got the full intervention because attendance was not taken at the lecture. Because of the natural turnover in our Graduate Medical Education program, front-line prescribers during the pre-intervention year were not the same as in the intervention period and post-intervention year. Some residents (particularly non-pediatrics residents) might not have received the reference card or might not have carried it. However, prescribing by trainees who did not carry the card may have been impacted by pharmacists and nurses who had access to the card and made recommendations.
Analgesic choice is dependent on many factors, including input from attending physicians. There is an emerging literature on “physician champions,” (12) and we did not track which attending physicians were involved in the choice of analgesic. Individual physician champions may have had a small impact before our formal intervention began. Our data were collected from the inpatient pharmacy prescribing database because the goal was to track what trainees are actually prescribing. Although we cannot verify whether or not patients actually received the ordered medications, we were able to document that the prescribing practices changed.
We have shown that trainee prescribing practices can be improved by distributing reference cards. Prescribing recommendations as well as advisory notes for specific analgesics are ideally addressed with Computerized Provider Order Entry (CPOE) systems. However, many hospitals still have not successfully implemented CPOE programs. In these settings, quick-reference point-of-care decision support tools such pocket-sized reference cards have significant advantages. Cards are inexpensive, can be customized to reflect institutional formularies, can provide advisories specific to various patient populations, and can provide additional resources to providers.
Acknowledgments
Dr. Auerbach was supported by NHLBI grant K24HL098372 during the course of this study.
The authors thank Larkin R. Callaghan, MA, who provided significant support toward the creation of the reference card, and Sarah Scarpace Lucas, PharmD, who provided significant clinical pharmacology guidance.
Footnotes
Disclosures
The authors have no conflicts of interest to declare in relationship to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston CC, Abbott FV, Gray-Donald K, Jeans ME. A survey of pain in hospitalized patients aged 4–14 years. Clin J Pain. 1992;8(2):154–163. doi: 10.1097/00002508-199206000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Cummings EA, Reid GJ, Finley GA, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain. 1996;68(1):25–31. doi: 10.1016/S0304-3959(96)03163-6. [DOI] [PubMed] [Google Scholar]
- 3.Palermo TM, Drotar DD, Lambert S. Psychosocial predictors of children’s postoperative pain. Clin Nurs Res. 1998;7(3):275–291. doi: 10.1177/105477389800700305. [DOI] [PubMed] [Google Scholar]
- 4.Saroyan JM, Schechter WS, Tresgallo ME, et al. Assessing resident knowledge of acute pain management in hospitalized children: a pilot study. J Pain Symptom Manage. 2008;36(6):628–638. doi: 10.1016/j.jpainsymman.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Tremlett M, Anderson BJ, Wolf A. Pro-con debate: is codeine a drug that still has a useful role in pediatric practice? Paediatr Anaesth. 2010;20(2):183–194. doi: 10.1111/j.1460-9592.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Public health advisory: Use of codeine by some breastfeeding mothers may lead to life-threatening side effects in nursing babies. Silver Spring, MD: FDA; 2007. [Google Scholar]
- 7.Madadi P, Koren G. Pharmacogenetic insights into codeine analgesia: implications to pediatric codeine use. Pharmacogenomics. 2008;9(9):1267–1284. doi: 10.2217/14622416.9.9.1267. [DOI] [PubMed] [Google Scholar]
- 8.Mikhael J, Baker L, Downar J. Using a pocket card to improve end-of-life care on internal medicine clinical teaching units: a cluster-randomized controlled trial. J Gen Intern Med. 2008;23(8):1222–1227. doi: 10.1007/s11606-008-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deuster S, Roten I, Muehlebach S. Implementation of treatment guidelines to support judicious use of antibiotic therapy. J Clin Pharm Ther. 2010;35(1):71–78. doi: 10.1111/j.1365-2710.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- 10.To T, Wang C, Dell SD, et al. Can an evidence-based guideline reminder card improve asthma management in the emergency department? Respir Med. 2010;104(9):1263–1270. doi: 10.1016/j.rmed.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartzberg E, Rubinovich S, Hassin D, et al. Developing and implementing a model for changing physicians’ prescribing habits-- the role of clinical pharmacy in leading the change. J Clin Pharm Ther. 2006;31(2):179–185. doi: 10.1111/j.1365-2710.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 12.Reinertsen JLGA, Rupp W, Whittington JW. IHI Innovation Series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2007. Engaging physicians in a shared quality agenda. [Google Scholar]