Abstract
Background/Aims
Liver biopsies from patients with nonalcoholic fatty liver disease (NAFLD) sometimes exhibit non-zonal aggregates of hepatocytes with microvesicular steatosis, but its prevalence and significance are unclear. In this study, we have evaluated the frequency of microvesicular steatosis and assessed its association with histological markers of disease severity in a large sample of NAFLD liver biopsies.
Methods
Liver biopsies from a large cohort of adults who participated in two studies conducted by the NASH Clinical Research Network (NASH CRN) were included in this cross-sectional study. Liver histology was assessed centrally and various histological features scored in a systematic fashion. The relationship between microvesicular steatosis and various histological features that characterize NAFLD was tested by multiple logistic regression, after controlling for age, gender, race, body mass index, and diabetes.
Results
Among 1022 liver biopsies included, 102 (10%) had microvesicular steatosis. No demographic differences were noted between patients with or without microvesicular steatosis. The presence of microvesicular steatosis was associated with higher grades of steatosis (p<0.001), ballooning cell injury (p<0.001), presence of Mallory-Denk bodies (p<0.007), presence of megamitochondria (p<0.0001), higher NAS scores (p<0.0001), more advanced fibrosis (p<0.0001) and diagnosis of borderline or definite NASH (p<0.0001).
Conclusion
Microvesicular steatosis correlates with more advanced histology of NAFLD. Longitudinal studies are needed to address the role of microvesicular steatosis in mediating cellular injury and disease progression in NAFLD.
Keywords: Microvesicular steatosis, fatty liver, NASH, lipid droplets
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a well-defined clinico-pathological entity which encompasses a histologic spectrum ranging from a relatively benign steatosis or nonalcoholic fatty liver (NAFL) to a potentially progressive and aggressive form termed nonalcoholic steatohepatitis (NASH) [19, 21, 22]. NAFLD is often associated with characteristics of the metabolic syndrome and is considered to be the hepatic manifestation of the metabolic syndrome. Among obese patients, approximately 60% have steatosis, 20%–25% have NASH, and cirrhosis may be present in 2%–3% [2, 3, 11, 14]. Nonalcoholic steatohepatitis is characterized by the presence of inflammation and cell injury, i.e., hepatocyte ballooning, chronic inflammatory cell infiltrates and Mallory-Denk bodies, with or without fibrosis. Accumulating evidence from several cross-sectional studies suggests that oxidative stress plays a key role in the pathogenesis of NASH but it is not clear if this is the cause or consequence of liver injury. It was shown that there is strong correlation between the markers of oxidative stress, lipid peroxidation products and severity of NASH [1, 14, 25]. Although, there are several studies that identified various histological and non-histological variables to predict the presence of advanced histology in cross-sectional studies. To our knowledge, there are very few prospective studies that examined the significance of various histological features in the disease progression longitudinally.
Steatosis in NAFLD is usually seen as macrovesicular steatosis (large droplet steatosis) in which a single, large vacuole of fat fills up the hepatocyte and displaces the nucleus to the periphery. Often macrovesicular steatosis can be present with both large and small droplets that may be seen to coalesce. Macrovesicular steatosis alone is considered to have a good long-term prognosis with rare progression to fibrosis or cirrhosis. On the other hand, diffuse microvesicular steatosis denotes a separate clinical entity commonly characterized by encephalopathy and liver failure; the diseases share severe mitochondrial β-oxidation defects from genetic or acquired causes. Examples include acute fatty liver of pregnancy, Reyes syndrome, drugs or toxins [12, 13]. These diseases either resolve, or end in death if not managed with liver transplant; unlike NAFLD, these processes do not lead to chronic liver disease and cirrhosis. Histologically, microvesicular steatosis is characterized by distended hepatocytes with foamy appearing cytoplasm; small lipid vesicles (less than 1µm in diameter) may or may not be discernible. The nucleus is typically centrally located unlike in macrovesicular steatosis where the nucleus is displaced peripherally. Because of the diffuse cytoplasmic alteration, special staining such as oil red O may be required for its diagnosis. Microvesicular steatosis is also commonly present in the same hepatocytes that harbor visualizable mitochondria, known as “megamitochondria”.
Some liver biopsies from patients with NAFLD exhibit non-zonal aggregates of microvesicular steatosis. The significance of this finding, however, is not clear. Therefore, we conducted a study to examine the (a) prevalence of microvesicular steatosis in a large collection of well-characterized NAFLD liver biopsies and (b) relationship between microvesicular steatosis and various histological features that characterize NAFLD.
METHODS
This study was conducted on available liver biopsies from adult patients (age ≥ 18 years at time of biopsy) who were enrolled in the Nonalcoholic Fatty Liver Disease (NAFLD) Database study or the Pioglitazone vs. Vitamin E vs. Placebo for the Treatment of Non-diabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial conducted by the NASH Clinical Research Network (NASH CRN). The NAFLD Database is a prospective, observational study of patients with definite NAFLD, suspected NAFLD, definite cryptogenic cirrhosis, and suspected (clinical) cryptogenic cirrhosis. Patients with steatosis involving ≥ 5% hepatic parenchyma on liver biopsy with no significant alcohol consumption or other coexisting etiologies (e.g., autoimmune liver disease, hemochromatosis, primary biliary cirrhosis, etc.) were defined as having NAFLD. Significant alcohol consumption was defined as > 14 drinks/week in men or >7 drinks/week in women on average within the preceding 2 years. The details of alcohol consumption were obtained by physician interviews and by administration of the Alcohol Use Disorders Identification Test (AUDIT) and the Skinner Lifetime Drinking History questionnaires. PIVENS is a multi-center, randomized, placebo-controlled, double-masked clinical trial of treatment with pioglitazone, vitamin E, or placebo for non-diabetic patients with histologically-confirmed NASH. Only the liver biopsies taken prior to randomization and treatment were included in this study.
The Institutional Review Boards at each Clinical Center, including Indiana University School of Medicine, and the Data Coordinating Center reviewed and approved the protocols and each patient has signed an informed consent.
Clinical data
The demographic and clinical data were collected within 6 months of liver biopsy, including age at enrollment, gender, race, height, weight, history of diabetes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. Body mass index (BMI) was calculated as weight (Kg)/height (m2) and HOMA IR (Homeostasis Model Assessment for Insulin Resistance) was calculated in non-diabetic patients using simultaneously obtained fasting serum glucose and insulin using the formula: HOMA = (glucose [mmol/L]* insulin[µU/mL])/22.5).
Histopathological data
All liver biopsies were reviewed and centrally scored by the Pathology Committee according to the NASH CRN scoring system [16]. Hematoxylin and Eosin (H&E) staining (for all features except fibrosis) and Masson’s trichrome staining (fibrosis only) were used to perform the evaluations. The following histological features were considered for our study:
Steatosis
Macrovesicular steatosis
Graded from 0–3 based on the percentage of hepatocytes involved (0 = <5%; 1 = 5%–33%; 2 = 33%–66%; 3 = >66%). The assessment of macrovesicular steatosis was commonly done at 4× magnification (at most 10× magnification was used).
Microvesicular steatosis
Reported as being either present or absent. Initial assessment was done under lower magnification (4× to 10×) and confirmed under higher magnification, if necessary. Microvesicular steatosis was defined as the presence of non-zonal, contiguous patches of “foamy hepatocytes with centrally placed nuclei” on an H&E stained slide under light microscopy (Figures 1 and 2).
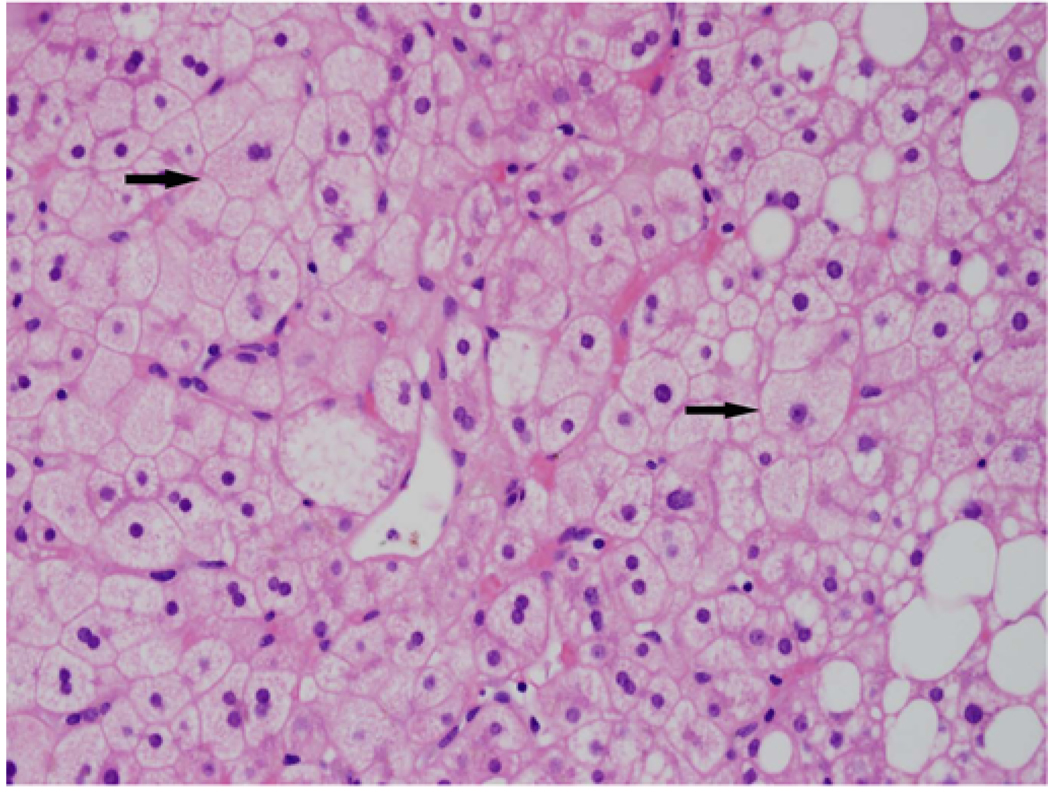
Figure 1.
H and E stained sections shows microvesicular steatosis and scattered mixed small and large droplet macrovesicular along the right; note the uniform involvement of hepatocytes. The hepatocytes have an almost "foamy" appearance
Figure 2.
Trichrome stained slide showing a patch of microvesicular steatosis in zone 3. Megamitochondria can be observed (arrows).
Lobular inflammation
Lobular inflammation was graded from 0–3 based on inflammatory foci per 20× magnification (0 = none; 1 = 1–2/20×; 2 = up to 4/20×; 3 = >4/20×).
Ballooning
Hepatocyte ballooning was limited to three categories (0 = none, 1 = few or 2 = many) based on the reproducible cut-off points as described previously [16].
NAFLD activity score (NAS score)
NAS score was calculated from the sum of scores for steatosis (0–3), lobular inflammation (0–3) and hepatocyte ballooning (0–2).
Fibrosis
was graded based on the modified Brunt classification; 0 = no fibrosis, 1 = perisinusoidal or periportal fibrosis, 2 = both perisinusoidal and periportal fibrosis, 3 = bridging fibrosis, 4 = cirrhosis [5].
Additional Features
Other histological features such as megamitochondria and presence of Mallory-Denk bodies were graded on a two-point scale as either 0 (none/rare) or 1 (many).
Diagnosis of NASH
Diagnosis of steatohepatitis was based on pattern recognition and was categorized into three groups: not NASH, borderline NASH or definite NASH.
Statistical methods
Baseline patient characteristics were compared across the two groups (presence and absence of microvesicular steatosis) using chi-square tests for categorical variables (Fisher’s Exact Test was used for race, due to small expected numbers), and the Wilcoxon rank-sum test for continuous measures. Nominal, two-sided P-values were used and were considered to be statistically significant if P<0.05. The relationship between the presence of microvesicular steatosis and different histological features including grades of macrovesicular steatosis, lobular inflammation, hepatocyte ballooning, fibrosis, Mallory bodies, megamitochondria, NAS score, steatohepatitis diagnosis, and selected biochemical values such as AST and ALT was explored using multiple logistic regression analyses. Using the presence vs. absence of microvesicular steatosis as the outcome measure, separate logistic regression models were fit for each of the histological features listed above, controlling for age at enrollment (years), gender, race (Caucasian vs. other), BMI (kg/m2), and presence of diabetes. All analyses were performed using SAS statistical software (version 9.1, SAS Institute, Cary, NC) and Stata (Release 10.0, Stata Corporation, College Station, Texas).
RESULTS
Demographic and clinical data
Microvesicular steatosis was present by light microscopy in 102 of 1022 liver biopsies (10%) reviewed. When groups with and without microvesicular steatosis were compared, there were no significant differences in demographic and clinical features, including age, sex, race/ethnicity, presence of diabetes, BMI, or serum aminotransferase levels (Table 1). There was statistically significantly relationship between the length of liver biopsy and the presence of microvesicular steatosis. The median (25th, 75th percentile) length of liver biopsies with microvesicular steatosis was significantly higher than length of biopsies without microvesicular steatosis (20 (16–25) mm vs, 16 (11–24) mm, p<0.0001).
Table 1.
Baseline patient characteristics and selected clinical data of patients (n=1022)
| Microvesicular Steatosis | |||
|---|---|---|---|
| Present (n=102) | Absent (n=920) | p-value® | |
| Age at enrollment (yrs) | 50.2 ±12.5 | 49.5 ± 11.9 | 0.4 |
| Females (%) | 66 | 63 | 0.6 |
| Caucasians (%) | 88 | 84 | 0.5 |
| BMI (kg/m2)€ | 36.1 ± 7.6 | 34.4 ±6.4 | 0.1 |
| Diabetes (%) | 24.5 | 29.5 | 0.3 |
| HOMA–IR† | 4.4 (3.4–5.9) | 4.0 (2.6–6.3) | 0.3 |
| AST (U/L)* | 50 (36–71) | 45 (32–65) | 0.07 |
| ALT (U/L)* | 63 (46–102) | 65 (43–97) | 0.6 |
Values with age, gender, race, and BMI are expressed as mean with standard deviation.
HOMA-IR: Homeostatic model assessment for Insulin resistance, only non-diabetic patients with insulin and glucose measurements within 6 months of liver biopsy were included (n=505).
IQR- median interquartile ranges of AST and ALT values obtained within 6 months of liver biopsy (n=650).
Only patients with height and weight measurements within 6 months of the liver biopsy were included (n=636).
P-values derived from chi-square tests (gender, diabetes), Fisher’s Exact Test (race), and Wilcoxon rank sum test for continuous variables.
Microvesicular steatosis and histological features of NASH
a. Macrovesicular steatosis, lobular inflammation, hepatocyte ballooning and fibrosis
Microvesicular steatosis was present across all grades of macrovesicular steatosis, but was clearly associated with more severe macrovesicular steatosis (Table 2). In contrast, there was no significant association between microvesicular steatosis and presence or degree of lobular inflammation. However, there was an association between the presence of microvesicular steatosis and hepatocyte ballooning, as microvesicular steatosis was seen at a significantly higher frequency in liver biopsies with few ballooned hepatocytes (OR: 3.0, 95% CI-1.5–5.8), and with even greater frequency in liver biopsies with many ballooned hepatocytes (OR: 3.6, 95% CI 2.0–6.8). Finally, the presence of microvesicular steatosis was significantly associated with more advanced fibrosis (Grade 3 and 4 combined) (OR: 2.3, 95%, CI: 1.4–3.6).
Table 2.
Relationship between microvesicular steatosis and histological features of NASH from multiple logistic regression analysis
|
Total N |
% with Microvesicular Steatosis |
Odds Ratio (95% CI) |
p-value |
|
|---|---|---|---|---|
| Steatosis | ||||
| <5% (reference) | 65 | 3.1% | ||
| 5–33% | 401 | 7.0% | 2.2 (0.5–9.5) | |
| 34–66% | 318 | 10.4% | 3.6 (0.8–15.4) | <0.001 |
| >66% | 238 | 16.4% | 6.4 (1.5–27.4) | |
| Lobular inflammation | ||||
| <2 under 20X mag (reference)¶ | 538 | 9.1% | ||
| 2–4 under 20Xmag | 372 | 11.3% | 1.2 (0.8–1.9) | 0.68 |
| >4 under 20Xmag | 112 | 9.8% | 1.2 (0.6–2.5) | |
| Ballooning | ||||
| None (reference) | 340 | 4.4% | ||
| Few | 260 | 11.9% | 3.0 (1.5–5.8) | <0.001 |
| Many | 422 | 13.3% | 3.6 (2.0–6.8) | |
| Fibrosis stage | ||||
| 0: None (reference) | 249 | 4.0% | ||
| 1: Zone 3 or portal/periportal | 274 | 7.3% | 1.6 (0.7–3.6) | |
| 2: Zone 3 and periportal | 180 | 14.4% | 4.3 (2.0–9.3) | <0.0001 |
| 3: Bridging | 204 | 13.7% | 4.4 (2.0–9.6) | |
| 4: Cirrhosis | 107 | 15.9% | 5.7 (2.4–13.6) | |
| Megamitochondria | ||||
| Rare/Absent (reference) | 860 | 6.7% | 5.0 (3.1–7.9) | <0.0001 |
| Many | 162 | 27.2% | ||
| Mallory-Denk bodies | ||||
| Rare/absent (reference) | 723 | 8.6% | ||
| Present | 299 | 13.4% | 1.9 (1.2–2.9) | <0.007 |
| NAFLD Activity Score (NAS, range 0–8) | 4.4±1.7 | 1.4(1.2–1.6) | <0.0001 | |
| Presence of NASH | ||||
| No (reference) | 230 | 1.7% | ||
| Borderline/Suspicious | 206 | 8.7% | 6.9 (2.0–23.9) | <0.0001 |
| Definite | 585 | 13.7% | 12.0 (3.7–38.8) | |
Lobular inflammation grades 0 and 1 combined into <2 under 20X mag as the reference category.
b. Megamitochondria and Mallory-Denk bodies
The odds of finding microvesicular steatosis were 1.9 times greater in liver biopsies with Mallory-Denk bodies (95% CI: 1.2–2.9) compared with biopsy samples without. Similarly, there was a strong relationship between microvesicular steatosis and the presence of megamitochondria (OR: 5.0, 95% CI: 3.1–7.9) (Table 2).
c. NAS and diagnosis of NASH
There was a clear association of the presence of microvesicular steatosis and increasing NAS scores. For every one-point increase in NAS score, there was a 1.4-fold increase in the likelihood of observing microvesicular steatosis (95% CI: 1.2–1.6, p<0.0001). There was also a strong relationship between microvesicular steatosis and a diagnosis of NASH. Thus, in comparison to “not NASH”, the odds ratio for borderline NASH was 6.7(95% CI: 2.0–3.7, p<0.0001) and 12.0 for definite NASH (95% CI: 3.7 – 38.8, p<0.0001) (Table 2).
DISCUSSION
To our knowledge, this is first study to evaluate the presence and significance of microvesicular steatosis in relation to common and well-established histological features of NASH. It describes the findings of a large, multicenter study of liver biopsies from patients with well-characterized NAFLD. Our study makes several important observations that add incremental knowledge to our understanding of NAFLD and NASH.
The Pathology Committee of the NASH CRN has taken significant caution in defining different types of steatosis; microvesicular steatosis, in particular, is a finding that describes clusters of foamy hepatocytes distributed in an azonal pattern. These foamy appearing “microvesicular patches” are different from the small and/or the medium sized droplets that are seen with macrovesicular steatosis of NAFLD.
It has been hypothesized that large fat droplets are formed by the fusion of small droplets initially found on the surface of endoplasmic reticulum [4, 20]. The strong relationship we noted between micro-and macrovesicular steatosis is indirectly supportive of this hypothesis, but due to its cross-sectional study design, our study is not able to discern the longitudinal relationship between micro-and macrovesicular steatosis.
There appears to be contrasting association between micro- and macrovesicular steatosis and histological markers of NASH such as hepatocyte ballooning, Mallory bodies and fibrosis. In this study, the presence of microvesicular steatosis was significantly associated with histological hallmarks of cellular injury and cytoskeletal damage such as hepatocyte ballooning and Mallory-Denk bodies. This is in contrast to macrovesicular steatosis which does not consistently correlate with features of cell injury and steatohepatitis. For example, in an earlier study from our group, we failed to observe significant relationship between higher grades of macrovesicular steatosis and prominent degrees of ballooning or Mallory-Denk bodies [10]. Interestingly, we found a significant association between the presence of microvesicular steatosis and advanced fibrosis. This finding also differs with the widely recognized finding that macrovesicular steatosis may disappear as the disease progresses into advanced fibrosis and cirrhosis [21, 24]. It is unclear if this finding suggests different etiology for the small droplets or regression in the size of lipid droplets with increasing fibrosis or implies that microvesicular steatosis, rather than macrovesicular steatosis, is an independent predictor of advanced forms of NASH.
Hepatocellular ballooning is an important histological parameter in the diagnosis of NASH and a few longitudinal studies have confirmed it to be a major distinguishing feature indicating a greater risk of disease progression [6, 21, 26]. In a recent study published by Caldwell et al, the ballooned hepatocytes were examined by H & E stain, fat-specific oil red stain, anti-keratin-18 immunohistochemistry stained sections and they are found to have multiple small lipid droplets, megamitochondria, dilated endoplasmic reticulum, mallory-denk bodies and cytoskeletal damage suggested by keratin-18 deficiency [7]. These small intrahepatocelluar lipid droplets have limiting phospholipid membranes that are shown to be the site of initiation of oxidative stress, a process implicated in the pathogenesis of NASH. In a study, Ikura et al demonstrated that oxidized phosphotidylcholine (oxidatively damaged phospholipid) was seen at the surface of the small lipid droplet within the ballooned hepatocytes suggesting that the source of oxidative injury is the small lipid droplet [15]. This unifies the concept of oxidative stress mediated injury to hepatocyte and the pathogenic role of small lipid droplet. The strong association seen between microvesicular steatosis, hepatocellular ballooning and mallory denk bodies in our study leads to a plausible explanation that the presence of microvesicular steatosis on a H & E stain may represent a severe form of NASH. It is also possible that oxidative injury may be the difference between microvesicular steatosis and ballooning degeneration.
Diffuse microvesicular steatosis denotes a separate clinical entity with a grave prognosis and is generally attributed to severe genetic or acquired defects in mitochondrial β-oxidation [12, 13]. Megamitochondria are regarded as the most striking change in the mitochondrial morphology observed in at least 5–15% of hepatocytes distributed in azonal fashion in NASH patients [8, 9, 18]. Collective data from previous studies indicate that mitochondrial morphological features reflect a true functional impairment in the mitochondria [17, 23] while a few studies suggest that their presence merely represent an adaptive process to oxidative stress. As we observed significant association between megamitochondria and microvesicular steatosis, it is tempting to speculate that presence of both these histological features may indicate significant mitochondrial dysfunction.
There are some limitations to our study. Given that this is a cross-sectional study, it is not possible to assess the impact of microvesicular steatosis on disease progression and outcomes. This possibility is best answered with longitudinal study of NAFLD patients with well characterized hepatic histology. The other limitation is that our histological evaluation was done by H&E stain under light microscopy at lower magnifications and thus the true prevalence of microvesicular steatosis may be underreported in this study. Fat specific oil red O stains are better predictors of the prevalence of lipid droplets. Drugs and toxins such as alcohol have been frequently implicated in microvesicular steatosis. Although significant alcohol consumption has been systematically excluded in all the patients, a detailed medication history is not obtained. However, drug induced microvesicular steatosis may be less likely a confounder in 10% of microvesicular steatosis group as it tends to be clinically acute and histologically diffuse often leading to death.
The strengths of our study are the central review process which involves at least 5 liver pathologists and the fact that the foci of microvesicular steatosis were carefully discerned on routine histochemical staining, as is done by all histology laboratories. Another strength is that this is a large series of very carefully characterized patients from multiple centers.
In summary, microvesicular steatosis is seen up to 10% of liver biopsies in patients with NAFLD and its presence signifies advanced histological features such as ballooning, inflammation, steatohepatitis, and fibrosis. Longitudinal studies are needed to further characterize the role of microvesicular steatosis in the progression of liver disease in patients with NAFLD. If other groups can reproduce our findings, then microvesicular steatosis should be systemically looked for and reported by pathologists when they are examining the liver biopsies from patients with NAFLD.
ACKNOWLEDGEMENTS
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD). Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, M01RR00188, UL1RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359). Following are the all the members of the NASH CRN who were instrumental in conducting the study at each of the participating institutions: Clinical Centers: Baylor College of Medicine, Houston, TX: Stephanie Abrams, MD; Diana Arceo, MD, MS; Denise Espinosa; Leanel Angeli Fairly, RN; Case Western Reserve University Clinical Centers: MetroHealth Medical Center, Cleveland, OH: Arthur J. McCullough, MD; Patricia Brandt; Diane Bringman, RN (2004–2008); Srinivasan Dasarathy, MD; Jaividhya Dasarathy, MD; Carol Hawkins, RN; Yao-Chang Liu, MD (2004–2009); Nicholette Rogers, PhD, PA-C (2004–2008); Margaret Stager, MD (2004–2009); Cleveland Clinic Foundation, Cleveland, OH: Arthur J. McCullough, MD; Srinivasan Dasarathy, MD; Mangesh Pagadala, MD; Ruth Sargent, LPN; Lisa Yerian, MD; Claudia Zein, MD; California Pacific Medical Center: Raphael Merriman, MD; Anthony Nguyen; Children’s National Medical Center, Washington DC: Parvathi Mohan, MD; Kavita Nair; Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Stephanie DeVore; Rohit Kohli, MD; Kathleen Lake; Stavra Xanthakos, MD; Duke University Medical Center, Durham, NC: Manal F. Abdelmalek, MD; Stephanie Buie; Anna Mae Diehl, MD; Marcia Gottfried, MD (2004–2008); Cynthia Guy, MD; Meryt Hanna; Paul Killenberg, MD (2004–2008); Samantha Kwan, MS (2006–2009); Yi-Ping Pan; Dawn Piercy, FNP; Melissa Smith; Indiana University School of Medicine, Indianapolis, IN: Elizabeth Byam, RN; Naga Chalasani, MD; Oscar W. Cummings, MD; Ann Klipsch, RN; Jean P. Molleston, MD; Linda Ragozzino, RN; Girish Subbarao, MD; Raj Vuppalanchi, MD; Johns Hopkins Hospital, Baltimore, MD: Kimberly Pfeifer, RN; Ann Scheimann, MD; Michael Torbenson, MD; Mount Sinai Kravis Children’s Hospital: Nanda Kerkar, MD; Sreevidya Narayanappa; Frederick Suchy, MD; Northwestern University Feinberg School of Medicine/Children’s Memorial Hospital: Mark H. Fishbein, MD; Katie Jacques; Ann Quinn, RD; Cindy Riazi, RN; Peter F. Whitington, MD; Seattle Children’s Hospital & Research Institute, WA: Melissa Coffey; Sarah Galdzicka, Karen Murray, MD; Melissa Young; Saint Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Jose Derdoy, MD; Joyce Hoffmann; Debra King, RN; Andrea Morris; Joan Siegner, RN; Susan Stewart, RN; Brent A. Neuschwander-Tetri, MD; Judy Thompson, RN; University of California San Diego, San Diego, CA: Cynthia Behling, MD, PhD; Janis Durelle; Tarek Hassanein, MD (2004–2009); Joel E. Lavine, MD, PhD; Rohit Loomba, MD; Anya Morgan; Steven Rose, MD (2007–2009); Heather Patton, MD; Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Tanya Stein, MD (2005–2009); University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Kiran Bambha, MD (2006–2010); Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Danuta Filipowski, MD; Bo Gu (2009–2010); Raphael Merriman, MD (2002–2007); Mark Pabst; Monique Rosenthal (2005–2010); Philip Rosenthal, MD; Tessa Steel (2006–2008); University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD; Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN, BSN; Melissa J. Contos, MD; Michael Fuchs, MD; Amy Jones; Velimir AC Luketic, MD; Puneet Puri, MD; Bimalijit Sandhu, MD (2007–2009); Arun J. Sanyal, MD; Carol Sargeant, RN, BSN, MPH; Kimberly Noble; Melanie White, RN, BSN (2006–2009);Virginia Mason Medical Center, Seattle, WA: Kris V. Kowdley, MD; Jody Mooney, MS; James Nelson, PhD; Sarah Ackermann; Cheryl Saunders, MPH; Vy Trinh; Chia Wang, MD; Washington University, St. Louis, MO: Elizabeth M. Brunt, MD; Resource Centers: National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD; National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, MD; National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH (Project Scientist); Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Frederick L. Brancati, MD, MHS (2003–2009); Jeanne M. Clark, MD, MPH; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005); Milana Isaacson, BS; Wana Kim, BS; Alison Lydecker, MPH (2006–2008), Pamela Mann, MPH (2008–2009); Laura Miriel; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- PIVENS
Pioglitazone vs. Vitamin E vs. Placebo for the treatment of Non-diabetic patients with NASH
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST:
Guarantor of the article: Naga Chalasani, MD.
Specific author contributions: All the members of the NASH CRN were involved in the study design and data acquisition; Data analysis and statistical support were provided by Laura A Wilson; Drs Tandra, Chalasani and Vuppalanchi were involved in the data collection, data analysis, data interpretation and manuscript preparation. Drs Yeh, Cummings, Unalp-Arida were involved in the preparation of the manuscript. Dr. Elizabeth Brunt participated in the manuscript preparation and critical revision of the article; All of the authors drafted, edited and approved the final manuscript draft.
Potential competing interests: The authors declare that they do not have anything to disclose regarding funding from industries or conflicts of interest with respect to this manuscript.
REFERENCES
- 1.Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, et al. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54(7):987–993. doi: 10.1136/gut.2004.057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen T, Christoffersen P, Gluud C. The liver in consecutive patients with morbid obesity: a clinical, morphological, and biochemical study. Int J Obes. 1984;8(2):107–115. [PubMed] [Google Scholar]
- 3.Andersen T, Gluud C. Liver morphology in morbid obesity: a literature study. Int J Obes. 1984;8(2):97–106. [PubMed] [Google Scholar]
- 4.Bostrom P, Rutberg M, Ericsson J, Holmdahl P, Andersson L, Frohman MA, et al. Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler Thromb Vasc Biol. 2005;25(9):1945–1951. doi: 10.1161/01.ATV.0000179676.41064.d4. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM, Neuschwander-Tetri BA, Oliver D, Wehmeier KR, Bacon BR. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35(9):1070–1082. doi: 10.1016/j.humpath.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, et al. Hepatocellular ballooning in NASH. J Hepatol. 2010;53(4):719–723. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell SH, Chang CY, Nakamoto RK, Krugner-Higby L. Mitochondria in nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8(3):595–617. doi: 10.1016/j.cld.2004.04.009. x. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96(2):519–525. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48(5):829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 12.Fromenty B, Berson A, Pessayre D. Microvesicular steatosis and steatohepatitis: role of mitochondrial dysfunction and lipid peroxidation. J Hepatol. 1997;26(Suppl 1):13–22. doi: 10.1016/s0168-8278(97)82328-8. [DOI] [PubMed] [Google Scholar]
- 13.Fromenty B, Pessayre D. Impaired mitochondrial function in microvesicular steatosis. Effects of drugs, ethanol, hormones and cytokines. J Hepatol. 1997;26(Suppl 2):43–53. doi: 10.1016/s0168-8278(97)80496-5. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Monzon C, Martin-Perez E, Iacono OL, Fernandez-Bermejo M, Majano PL, Apolinario A, et al. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol. 2000;33(5):716–724. doi: 10.1016/s0168-8278(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 15.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43(3):506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Krahenbuhl S. Alterations in mitochondrial function and morphology in chronic liver disease: pathogenesis and potential for therapeutic intervention. Pharmacol Ther. 1993;60(1):1–38. doi: 10.1016/0163-7258(93)90020-e. [DOI] [PubMed] [Google Scholar]
- 18.Le TH, Caldwell SH, Redick JA, Sheppard BL, Davis CA, Arseneau KO, et al. The zonal distribution of megamitochondria with crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2004;39(5):1423–1429. doi: 10.1002/hep.20202. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 20.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7(5):373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 21.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 22.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38(4):999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 24.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 25.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37(1):56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 26.Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128(5):837–847. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]