Abstract
Few studies have examined the potential effects of childbirth on the responses of the female vasculature – especially the resistance microvasculature of non-reproductive tissues. In the present study we have investigated the response of mesenteric microvascular resistance vessels to estrogen (E2), an important vasoactive hormone. Vessels were obtained from either nulliparous or postpartum female Sprague-Dawley rats, and isometric tension studies were performed. We found that E2 induced a concentration-dependent, endothelium-independent relaxation of microvessels precontracted with 10−5M phenylephrine; however, E2-induced relaxation was reduced by nearly half in vessels from postpartum animals compared to nulliparous controls. Inhibiting nitric oxide synthase activity with 10−4M L-NMMA or L-NPA (which exhibits selectivity for type 1 or nNOS) attenuated the relaxation effect of E2 on arteries from nulliparous animals. In contrast, L-NPA had little effect on arteries from postpartum animals, suggesting a reduced influence of nNOS after parturition. Moreover, expression of nNOS protein in microvessels was decreased 39% in the postpartum state compared to arteries from nulliparous animals. We propose that the impaired E2-induced relaxation response of microvessels from postpartum animals reflects a downregulation of NO production due to lower nNOS expressed in vascular smooth muscle cells. We measured a 73% decrease in serum E2 levels in the postpartum state compared to nulliparous animals. Because E2 has been shown to increase nNOS protein expression, we propose that lower E2 levels after parturition decrease expression of nNOS, leading to a reduced vasodilatory capacity of resistance microvessels.
Keywords: estrogen, parturition, postpartum, NOS, vascular smooth muscle cells
INTRODUCTION
Despite the fact that the need for gender-specific research on the cardiovascular system is now recognized, very little is known regarding the cardiovascular effects of one of the most important and biologically unique events of a woman’s life: parturition. Throughout pregnancy circulating concentrations of 17β-estradiol (E2), an important vasoactive hormone, increase [1]. After parturition, however, E2 levels decrease dramatically, and can remain low in postpartum women from 3 weeks to up to a full year [2-4]. Unfortunately, the postpartum period is poorly defined, and very little is known about how parturition influences cardiovascular regulation during the postpartum period.
Although multiple studies have examined the correlation between childbirth and decreased risk of ovarian cancer [5, 6], little research has been conducted on potential long-term effects of parturition on the cardiovascular system. A better understanding of how E2 affects cardiovascular function during the postpartum period is clearly needed because a great number of women resume E2-based oral contraceptive therapy soon after parturition. In other words, how do such artificially high levels of E2 impact vascular function at a time when physiological estrogen levels are naturally lower?
Several studies have characterized the effect of pregnancy on blood vessels. For example, pregnancy is associated with increased intimal thickness in carotid arteries [7]. In terms of arterial function, NO-mediated vasodilation of resistance vessels is enhanced in late pregnancy, however in this study the response was more likely associated with inflammation due to viral infection [8]. In addition, elevation of endogenous E2 during pregnancy increases NO-dependent modulation of mesenteric resistance artery tone [9]; however, the effects of E2 (endogenous or exogenous) on vascular function in the postpartum period were not studied. In fact, we can find no studies that have investigated effects of parturition on E2 signaling in non-reproductive or systemic resistance artery beds.
We have previously demonstrated that E2 induces vascular relaxation by stimulating a rapid, non-genomic transduction mechanism in both porcine and human coronary vascular smooth muscle (VSM) [10-12]. In addition to its well-known action on endothelial cells, we found that E2 also triggers phosphoinositol-3-kinase (PI3-kinase)-Akt-mediated stimulation of type 1, or neuronal, nitric oxide synthase (nNOS), which is expressed in VSM cells and regulates vascular tone via nitric oxide (NO) production [11, 13-17]. This ability of nNOS to influence vascular tone is especially important in situations where endothelial cells may be dysfunctional, such as in hypertension, diabetes, or age [18]. The purpose of the present study was to establish whether a similar E2 signaling pathway functions in mesenteric resistance arteries from nulliparous and parous female animals. Because the mechanism of E2 action in the systemic resistance vessels of parous – and especially postpartum – females is still largely unknown, we examined the effects of parturition on the response of these vessels to exogenous E2.
EXPERIMENTAL
Animals
Nulliparous and postpartum female Sprague Dawley (SD) rats (6-20 weeks old) were purchased from Harlan Laboratories or bred in-house under the Medical College of Georgia (Augusta, GA) Laboratory Animal Services program. Pilot studies were conducted on arteries from age-matched nulliparous and postpartum animals that had produced only one litter and were harvested between 1-3 days of giving birth. However, statistical analyses showed no differences between arteries from uni-parous vs. multi-parous animals or from time of harvest ranging from day of delivery up to 3 months postpartum when normal cycling had resumed. Thus these data are pooled. Animal use protocols were approved by the MCG Institutional Animal Care and Use Committee.
Force transduction studies
Approximately 2 mm rings were dissected from first- and second-order mesenteric arteries, ranging from 50-100 μM in lumen diameter, and were mounted on a Danish Myograph Technologies (DMT; Ann Arbor, MI) multi-wire myograph system (Model 610M Version 2.2) to measure vascular responses [19]. On average, 1-2 vessels were prepared from each animal. After determining optimal baseline tension by generating maximal response to phenylephrine (PE; Sigma Aldrich St. Louis, MO), arteries were adjusted to a baseline tension of 3.0 milliNewtons and incubated in oxygenated Krebs buffer of the following composition (mM): 122 NaCl, 4.7 KCl, 15.5 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 1.8 CaCl2, 11.5 glucose, pH 7.4 at 37.0°C. After 40 minutes equilibration, arteries were contracted to stable tension with 10−5 M PE (~ 5 minutes), and force measurement readings were recorded with Chart 5.0 (AD Instruments Colorado Springs, CO). We measured complete concentration-response relationships for PE and acetylcholine (ACh; Sigma Aldrich St. Louis, MO) (10−9 - 10−5M). Relaxation responses to 10−5M ACh verified the presence (relaxation ≥80%) or absence (relaxation ≥20%) of endothelium. The endothelial cell layer of some arteries was denuded by passing a hair through the lumen. Responses to E2 (10−9 to 10−6 M) were measured in arteries from both nulliparous and postpartum animals. Some arteries were incubated with the non-selective NOS inhibitor NG-Methyl-L-arginine (L-NMMA), or a more nNOS-selective inhibitor Nω-Propyl L-arginine (L-NPA; Sigma-Aldrich St. Louis, MO) at a standard concentration of 10−4M for 40 minutes before precontraction. After tension reached a stable plateau level, a complete concentration-response relationship was measured for E2.
Western blot
Frozen mesenteric arteries were pulverized and lysed with commercial lysis buffer (Fisher Scientific Pittsburgh, PA). Protein assays were performed using Bio-Rad (Hercules, CA) detergent compatible protein assay via colorimetric detection. Protein (100μg) was loaded into each lane for all Western blots, which were performed as described previously [11]. Antibodies against nNOS and β-actin (BD Transduction labs, San Jose, CA) were diluted 1:100 and 1:5000, respectively. The bound antibody was detected by enhanced chemiluminescence (SuperECL, Amersham Piscataway, NJ). ImageJ (NIH Bethesda, MD) was used for densitometry analysis.
ELISA
Serum 17β-estradiol concentration was assayed by EIA kit (Caymen Chemical, Ann Arbor, MI). Serum was collected from rats at euthanasia and frozen at −80°C until assayed.
Statistical Analysis
All data are expressed as mean ± standard error. Analysis between data points was made with a non-parametric 2-sided t-test with the Mann-Whitney post-test, and between groups with a 2-way ANOVA and Bonferroni post-test, as appropriate. Significance was set at p<0.05.
RESULTS
Exogenous E2 causes vasodilation via an NO-dependent mechanism
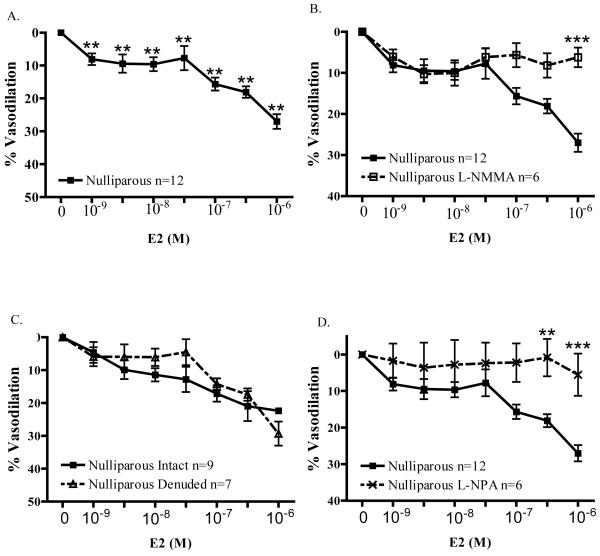
As illustrated in Figure 1A, E2 caused significant vasodilation (p<0.001) of precontracted mesenteric arteries from nulliparous (N) animals. In contrast to studies of larger, conduit vessels, significant E2-induced relaxation was observed at more physiological concentrations (e.g., 8.11 ± 1.77% relaxation at 1nM E2). Incubating arteries with 10−4M L-NMMA, a non-selective NOS inhibitor, effectively blocked E2-induced vasodilation. (Figure 1B) For example, the relaxation response to 1μM E2 (27.0 ± 2.2%; n=12) was inhibited 77% in the presence of L-NMMA (6.3 ± 2.4%; n=6; p<0.001). Interestingly, the relaxation response to E2 was endothelium-independent. As illustrated in Figure 1C, mechanical denudation had no effect on E2-induced relaxation of arteries from nulliparous females. Maximal relaxation to (1000nM) E2 was 22.4 ± 1.4% (n=6) in intact arteries, and was 31.7 ± 3.3% (n=6) in endothelium-denuded arteries.
Figure 1.
A) E2 induced significant (***p<0.001) vasodilation compared to the pre-contracted baseline state in mesenteric resistance arteries from nulliparous rats from 1-1000nM E2 (n=12). B) 10−4M L-NMMA, a non-selective NOS inhibitor, effectively blocked E2-induced vasodilation of arteries from nulliparous rats (n=6; p<0.001). C) Removal of endothelium did not alter maximal (1000nM) E2-induced vasodilation (n=6). D) L-NPA 10−4M, a more selective nNOS inhibitor, attenuated vasodilation induced by 300nM E2 (n=6-12) or 1μM E2 (n=6-12; p<0.001).
Because the observed E2-induced relaxation was endothelium-independent, yet involved NO production, we next examined a putative role for nNOS expressed in microvascular smooth muscle. (Figure 1D) We found that L-NPA (10−4M), a more selective nNOS inhibitor [20], nearly abolished E2-induced relaxation. In the presence of L-NPA, 300nM E2-induced relaxation of arteries from nulliparous controls was attenuated by 95% (L-NPA: 0.9 ± 5.1%, n=6; control: 18.1 ± 1.8%, n=12; p<0.01). Similarly, L-NPA reduced relaxation due to 1000 nM E2 by 80% (5.5 ± 5.8%, n=6 vs. 27.0 ± 2.2%, n=12; p<0.001).
Characterization of vessels from postpartum animals
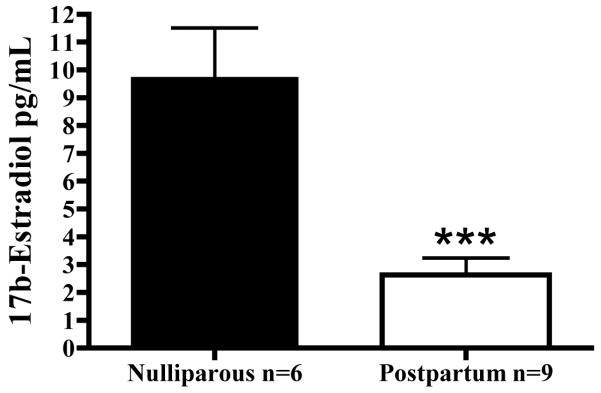
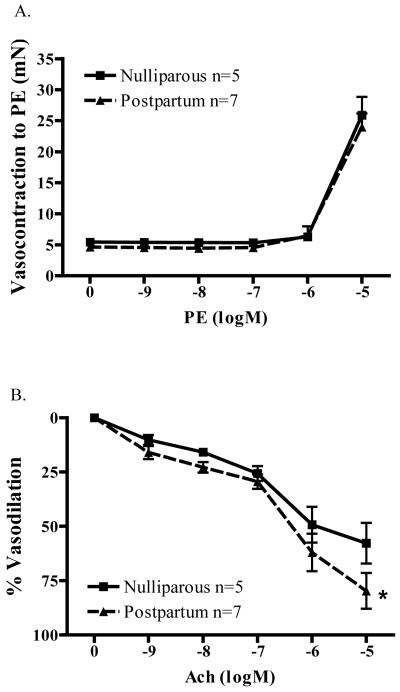
We next investigated whether parturition altered the response of microvessels to E2. Measured E2 levels are illustrated in Figure 2. We first measured endogenous serum E2 levels from postpartum (PP) animals, and found that plasma E2 was decreased during the postpartum period. Findings from EIA confirmed that E2 levels were decreased by more than 70% (73.01%; p=0.0002) in postpartum (2.63 ± 0.60 pg/mL; n=9) vs. nulliparous (9.76 ± 1.76 pg/mL; n=6) animals. This finding mirrored what is observed in postpartum women [2, 3]. In addition, we found that parturition did not alter the contractility of vessels. (Figure 3A) There was no difference in the concentration-response relationship for PE. Maximal force generation (10−5M PE) measured in nulliparous controls (N; 25.9 ± 2.9 mN; n=5) was similar to that measured in microvessels from postpartum animals (PP; 24.0 ± 2.2 mN; n=7).
Figure 2.
Plasma E2 levels are decreased in postpartum animals compared to nulliparous animals. Each bar represents the mean E2 value ± SEM (n=6-9 animals; ***p<0.0002).
Figure 3.
A) Parturition does not change contractile responses of microvessels to phenylephrine (PE; n=5-7). B) Parturition increases the sensitivity of microvessels to acetylcholine (ACh)-induced vasodilation (n=5-7; *p<0.05).
In contrast to contractile responses, we found that ACh-induced relaxation of microvessels was enhanced in arteries from postpartum animals. (Figure 3B) When normalized to correct for basal arterial tone, mesenteric microvessels from postpartum animals had an enhanced relaxation response (79.8 ± 8.3%, n=7) as compared to nulliparous controls (57.8 ± 9.3%, n=5) to 10−5 M acetylcholine. (p<0.05)
E2-induced relaxation of vessels from postpartum animals
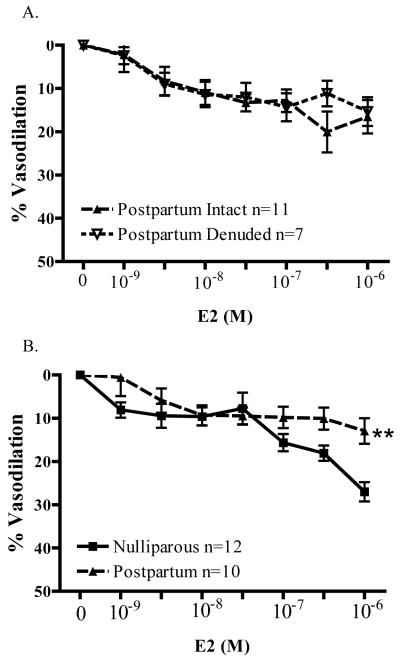
As observed in vessels from nulliparous animals, the relaxation response of arteries from postpartum (PP) animals to E2 was endothelium-independent. As illustrated in Figure 4A, there was no significant difference in E2-induced relaxation (even at 1000nM) between intact and endothelium-denuded mesenteric arteries (PP intact: 18.8 ± 3.8%, n=11 vs. PP denuded: 15.4 ± 3.3%; n=7). In the experiments illustrated in Figure 4B, however, we found that the relaxation response to E2 was impaired in arteries from PP animals compared to vessels from nulliparous animals. Although arteries from nulliparous animals exhibited significant relaxation to 1nM E2 (8.1 ± 1.8%, n=12), a 10-fold higher concentration of E2 (10nM) was required to elicit significant relaxation of arteries from PP animals (9.4 ± 2.3%, n=10). Further, maximal relaxation (1000nM E2) was reduced by nearly half in vessels from PP animals (PP: 13.9 ± 2.9% vs. N: 27.0 ± 2.2%; n=10). Statistical analysis of the entire concentration-response relationship to E2 revealed a significant depression of relaxation in microvessels from PP animals (p<0.01). Thus, parturition decreases the ability of arteries to relax in response to physiologically relevant concentrations of E2, and even more so at pharmacological concentrations.
Figure 4.
A) Endothelium-denudation does not alter E2-induced vasodilation in microvessels from postpartum female rats (n= 7-11). B) Mesenteric resistance arteries from postpartum rats (n=10) have significantly impaired vasodilation (**p<0.01) compared to the nulliparous group (n=10).
Estrogen-induced relaxation and NOS
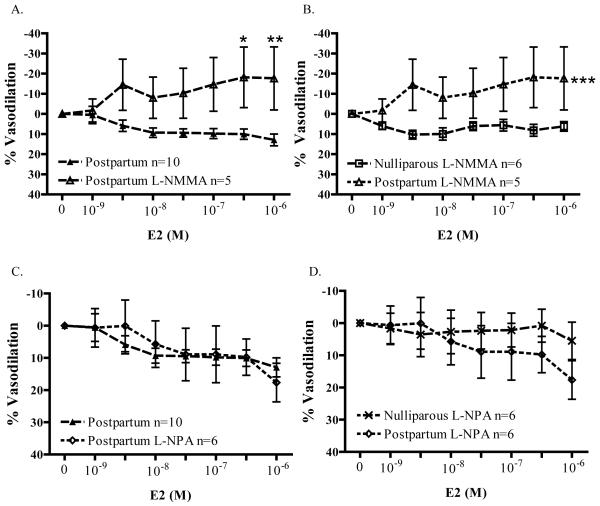
Microvessels from PP animals were pretreated with 10−4M L-NMMA to test whether E2-induced relaxation was NOS-dependent. Surprisingly, as illustrated in Figure 5A, 300nM E2 caused a further vasoconstriction of precontracted arteries from postpartum (PP) animals in the presence of L-NMMA (18.1 ± 15.1% contraction; n=5 vs. N, 10.1 ± 2.6% relaxation, n=10; p<0.05). In the presence of L-NMMA, 1000nM E2 continued to cause vasoconstriction: 17.5 ± 15.7% vasoconstriction vs. 13.0 ± 2.9% vasodilation (untreated PP controls; p<0.01). In contrast, as illustrated in Figure 5B, E2 did not contract vessels from nulliparous animals in the presence of L-NMMA (n=6).
Figure 5.
Nitric oxide synthase is a target of E2 action in the microvasculature. A) In the presence of 10−4M L-NMMA, 300nM E2 contracted arteries from postpartum animals (*p<0.05) compared to untreated controls (n=10). B) In the presence of 10−4M L-NMMA, E2-induced significant contraction of arteries from postpartum rats (n=5) compared to nulliparous rats (n=6, ***p<0.001). C) Inhibition of nNOS activity with 10−4M L-NPA did not alter E2-induced vasodilation of microvessels from postpartum animals (n=6). D) L-NPA (10−4M) eliminates significant differences in E2-induced relaxation of arteries from nulliparous (n=6) and postpartum rats (n=6).
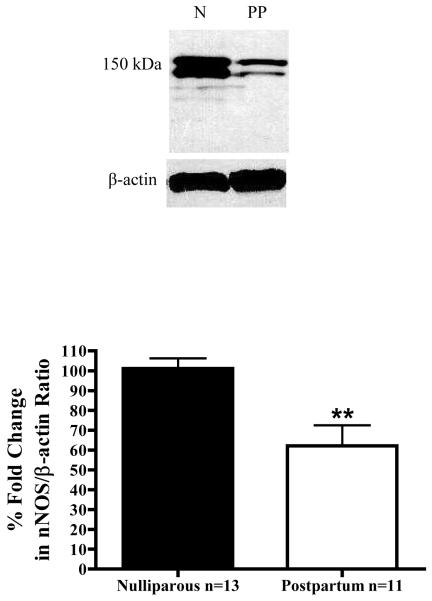
In contrast to findings with the non-selective NOS inhibitor L-NMMA, L-NPA did not alter responses to E2 in arteries from PP animals. (Figure 5C) Moreover, as illustrated in Figure 5D, a direct comparison of E2 concentration-response curves in L-NPA-treated arteries from the nulliparous and postpartum groups demonstrates that L-NPA eliminated any significant differences between the groups. In other words, NO produced from nNOS expressed in VSM appears to be a primary reason why arteries from nulliparous animals were more responsive to E2 than arteries from PP animals (where this mechanism appears to be attenuated). To test this hypothesis we then measured expression of nNOS protein in arteries from both groups, and found decreased expression of nNOS in the postpartum period. (Figure 6) Densitometry measurements of the nNOS/β-actin ratio, which included both nNOS bands, revealed that nNOS protein levels are decreased by 39% in arteries from postpartum animals (p=0.0077; n=4 immunoblots) compared to nulliparous controls. Thus, parturition decreases nNOS protein expression in mesenteric resistance arteries.
Figure 6.
Representative gel of nNOS and β-actin expression in microvessels from nulliparous (n=13) and postpartum (n=11) animals. Bar graph is the average densitometry readings (n=4 blots) ± SEM. (**p=0.0077)
DISCUSSION
Although the influence of gender on cardiovascular function is receiving increased attention, very few studies have examined the potential effects of childbirth on the responses of the female vasculature – especially the resistance microvasculature of non-reproductive tissues. The mesentery is the second-largest vascular bed, and is commonly employed as a model to study peripheral vascular resistance; however, far fewer studies have characterized the response of these microvessels from female animals. Therefore, there are at least two novel aspects of the present study: we provide important baseline data on responsiveness of the female resistance microvasculature, and further extend these findings to determine how these vessels respond during the postpartum (PP) period. To our knowledge, this study is the first to demonstrate that parturition alters how microvascular resistance arteries respond to estrogen, an important vasoactive hormone in women. Moreover, we propose a putative molecular mechanism (i.e., decreased expression of nNOS in VSM) that could account, at least in part, for the altered vascular responsiveness observed during the postpartum period.
Our findings reveal that E2 induces relaxation of microvessels from females via an endothelium-independent mechanism that appears to involve production of NO, as NOS inhibitors were quite effective at inhibiting E2-induced vasodilation. Because attenuating the influence of eNOS (via endothelium denudation) did not alter the response of microvessels to E2, we propose that nNOS expressed in VSM is an important target of E2 action in the microvasculature. These findings are supported by a previous report of nNOS expression in VSM of rat mesenteric arteries [21], and our previous findings that E2 stimulates nNOS activity in coronary artery smooth muscle from either porcine or human arteries [11, 22, 23]. Furthermore, we observed that inhibiting the binding of L-arginine to NOS with L-NMMA could convert the expected vasodilatory response of E2 into a vasoconstrictor effect. We observed a similar phenomenon in porcine coronary arteries, and attributed this unusual response to E2-stimulated superoxide production due to uncoupled NOS activity [22]. The present findings have now documented E2-induced vasoconstriction in the microvasculature as well.
The present study indicates that expression of nNOS protein was decreased in the microvasculature during the postpartum state, and this finding is consistent with others demonstrating that uterine arteries from late-term pregnant rats exhibit decreased nNOS expression [24]. Similarly, no expression of nNOS was detected in uterine, omental, or renal arteries from pregnant sheep [25]. Though, Alexander et al. [26] found increased nNOS expression in whole kidney homogenates from postpartum rats, implying that nNOS expression may heterogeneous with respect to organ and tissue. Nonetheless, our findings provide evidence for nNOS expression in microvascular VSM, and that pregnancy decreases expression of this protein. Our findings now extend this attenuation of vascular nNOS expression into the postpartum period as well. We also measured greatly decreased serum E2 levels during the postpartum period, which is commonly observed in humans [2-4].
It was possible that the observed decrease in postpartum E2 levels contributed to lower nNOS protein expression in mesenteric microvessels. This hypothesis is supported by previous studies indicating that expression of nNOS is greater in hearts from female rabbits compared to males [27], and other studies demonstrating that E2 stimulates nNOS expression in neutrophils from either men or women [28]. Furthermore, nNOS expression and activity is reduced in neurons from ovariectomized rats, while exogenous E2 supplementation restores nNOS expression [29]. Thus, there is consistent evidence from a variety of cell types that E2 promotes nNOS expression and activity. As E2 levels fall during the postpartum period, we predict there is a corresponding loss of nNOS protein in the microvasculature. Hence, there should also be a corresponding decrease in the ability of nNOS to influence microvascular reactivity. Because our data indicate that the vasodilatory effect of E2 on microvessels is mediated primarily by nNOS-derived NO, this mechanism could account for the observed hyporesponsiveness of microvessels from postpartum animals to E2. Moreover, we found that L-NPA, an inhibitor with some selectivity for nNOS, essentially abolished E2-induced relaxation of vessels from normal animals, but had very little effect on E2-induced vasodilation of vessels from postpartum animals. These functional studies are consistent with our biochemical observations, and strongly support our hypothesis of decreased nNOS influence in the postpartum period. Lastly, it should be noted that arteries from reproductive tissues exhibit a greater sensitivity to E2 than vessels from non-reproductive tissues, such as mesentery [30]. Therefore, responsiveness of arteries from reproductive tissues may be altered to an even greater extent in the postpartum period than we observed in vessels from non-reproductive tissue (i.e., mesentery).
We found E2-induced relaxation to be endothelium-independent in arteries from both nulliparous (N) and postpartum rats, thus implicating VSM cells as an E2 target in the microcirculation. These findings mirror our previous findings of endothelium-independent effects of E2 on porcine coronary arteries [11]. While vascular endothelial cells (EC) play a major role in regulating vessel tone, the impact of this role lessens when these cells are damaged or become dysfunctional, as occurs in diabetes, atherosclerosis, hypertension, smoking, and age [31, 32]. In addition, EC dysfunction may be both a cause and a consequence of cardiovascular diseases in pregnancy (e.g., preeclampsia) [33]. Thus, understanding this EC-independent signaling mechanism may provide an important alternative method to regulate vascular tone in face of EC dysfunction. In addition, because E2-induced relaxation of mesenteric resistance arteries was impaired up to 3 months after parturition, understanding the regulatory signaling pathways in VSM may prove to be useful in assessing the cardiovascular risk of healthy, postpartum women receiving exogenous E2 – as commonly occurs in oral contraceptives, which many women being receiving during the postpartum period.
We observed increased relaxation to ACh in mesenteric arteries from postpartum animals. Zhang et al. [9] also found that pregnancy enhanced the relaxation response to ACh, and suggested that this effect was due to increased NO bioavailability because of E2-stimulated eNOS activity or E2-mediated scavenging of reactive oxygen species (ROS). However, we observed no difference between EC-intact vs. denuded microvessels in response to E2, suggesting that eNOS-dependent NO production is not increased in the postpartum period. While postpartum animals did demonstrate increased relaxation to ACh, they also had lower blood E2 concentrations than nulliparous controls. These findings tend to discount the hypothesis that E2 scavenging of ROS would increase NO-mediated relaxation. Nonetheless, our findings suggest that the increased relaxation to ACh reflects altered E2-independent signaling that continues through pregnancy and into the postpartum period. Increased eNOS activity and expression have been reported in human uterine arteries during pregnancy [34], and we speculate that enhanced NO production from eNOS might be compensatory to loss of nNOS in the postpartum period. While we did not assess eNOS protein expression directly, we did measure E2-induced vasodilation in EC-intact and –denuded arteries and found no functional difference. Therefore, it seems unlikely that eNOS expression influences altered responses to E2 in postpartum rats.
In summary, the present study has investigated the reactivity of mesenteric resistance arteries from nulliparous and postpartum animals, and found that endothelium-independent, E2-induced relaxation is impaired in arteries from postpartum animals. This impairment appears to be dependent upon decreased expression of nNOS protein in microvascular smooth muscle, and correlates with reduced serum E2 levels measured during the postpartum period. This reduced vasodilatory response of the microvasculature may be an underlying factor that could contribute to the development of hypertension in the later stages of pregnancy and into the postpartum period. We propose that restoration of normal nNOS function might be a potential therapeutic target of future studies seeking to normalize function of the resistance microvasculature during the postpartum period.
ACKNOWLEDGEMENTS
This study was supported by NIH HL073890, the Department of Pharmacology & Toxicology, Medical College of Georgia / Georgia Health Sciences University, and the College of Graduate Studies, Georgia Health Sciences University. Thanks to Cathy Royal for editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no potential conflict of interest, financial or otherwise.
REFERENCES
- [1].Risberg A, Olsson K, Lyrenas S, Sjoquist M. Plasma vasopressin, oxytocin, estradiol, and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet Gynecol Scand. 2009;88:639–46. doi: 10.1080/00016340902919002. [DOI] [PubMed] [Google Scholar]
- [2].Bernstein L, Pike MC, Ross RK, Judd HL, Brown JB, Henderson BE. Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst. 1985;74:741–5. [PubMed] [Google Scholar]
- [3].Bridges RS, Byrnes EM. Reproductive experience reduces circulating 17beta-estradiol and prolactin levels during proestrus and alters estrogen sensitivity in female rats. Endocrinology. 2006;147:2575–82. doi: 10.1210/en.2005-0917. [DOI] [PubMed] [Google Scholar]
- [4].Figuero E, Carrillo-de-Albornoz A, Herrera D, Bascones-Martinez A. Gingival changes during pregnancy: I. Influence of hormonal variations on clinical and immunological parameters. J Clin Periodontol. 2010;37:220–9. doi: 10.1111/j.1600-051X.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- [5].Sueblinvong T, Carney ME. Current understanding of risk factors for ovarian cancer. Curr Treat Options Oncol. 2009;10:67–81. doi: 10.1007/s11864-009-0108-2. [DOI] [PubMed] [Google Scholar]
- [6].Cetin I, Cozzi V, Antonazzo P. Infertility as a cancer risk factor - a review. Placenta. 2008;29(Suppl B):169–77. doi: 10.1016/j.placenta.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [7].Skilton MR, Bonnet F, Begg LM, Juonala M, Kahonen M, Lehtimaki T, Viikari JS, Raitakari OT. Childbearing, child-rearing, cardiovascular risk factors, and progression of carotid intima-media thickness: the Cardiovascular Risk in Young Finns study. Stroke. 2010;41:1332–7. doi: 10.1161/STROKEAHA.110.579219. [DOI] [PubMed] [Google Scholar]
- [8].Gombos RB, Hemmings DG. Differential effects on nitric oxide-mediated vasodilation in mesenteric and uterine arteries from cytomegalovirus-infected mice. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.01113.2009. [DOI] [PubMed] [Google Scholar]
- [9].Zhang Y, Stewart KG, Davidge ST. Endogenous estrogen mediates vascular reactivity and distensibility in pregnant rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2001;280:H956–61. doi: 10.1152/ajpheart.2001.280.3.H956. [DOI] [PubMed] [Google Scholar]
- [10].Han G, Yu X, Lu L, Li S, Ma H, Zhu S, Cui X, White RE. Estrogen receptor alpha mediates acute potassium channel stimulation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther. 2006;316:1025–30. doi: 10.1124/jpet.105.093542. [DOI] [PubMed] [Google Scholar]
- [11].Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, White RE. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol. 2007;293:H314–21. doi: 10.1152/ajpheart.01342.2006. [DOI] [PubMed] [Google Scholar]
- [12].White RE, Han G, Maunz M, Dimitropoulou C, El-Mowafy AM, Barlow RS, Catravas JD, Snead C, Carrier GO, Zhu S, Yu X. Endothelium-independent effect of estrogen on Ca2+-activated K+ channels in human coronary artery smooth muscle cells. Cardiovasc Res. 2002;53:650–61. doi: 10.1016/s0008-6363(01)00428-x. [DOI] [PubMed] [Google Scholar]
- [13].Svedas E, Nisell H, Vanwijk MJ, Nikas Y, Kublickiene KR. Endothelial dysfunction in uterine circulation in preeclampsia: can estrogens improve it? Am J Obstet Gynecol. 2002;187:1608–16. doi: 10.1067/mob.2002.127378. [DOI] [PubMed] [Google Scholar]
- [14].Lekontseva OCS, Davidge ST. Neuronal Nitric Oxide Synthase Mediates Estrogen-Induced Relaxation in Rat Mesenteric Resistance Arteries. Canadian Cardiovascular Congress. 2010 [Google Scholar]
- [15].White RE. Estrogen and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- [16].Kavdia M, Popel AS. Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. J Appl Physiol. 2004;97:293–301. doi: 10.1152/japplphysiol.00049.2004. [DOI] [PubMed] [Google Scholar]
- [17].Rosenfeld CR, Chen C, Roy T, Liu X. Estrogen selectively up-regulates eNOS and nNOS in reproductive arteries by transcriptional mechanisms. J Soc Gynecol Investig. 2003;10:205–15. doi: 10.1016/s1071-5576(03)00049-2. [DOI] [PubMed] [Google Scholar]
- [18].Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [19].Angus JA, Wright CE. Techniques to study the pharmacodynamics of isolated large and small blood vessels. J Pharmacol Toxicol Methods. 2000;44:395–407. doi: 10.1016/s1056-8719(00)00121-0. [DOI] [PubMed] [Google Scholar]
- [20].Fedorov R, Hartmann E, Ghosh DK, Schlichting I. Structural basis for the specificity of the nitric-oxide synthase inhibitors W1400 and Nomega-propyl-L-Arg for the inducible and neuronal isoforms. J Biol Chem. 2003;278:45818–25. doi: 10.1074/jbc.M306030200. [DOI] [PubMed] [Google Scholar]
- [21].Kwon SY, Groszmann RJ, Iwakiri Y. Increased neuronal nitric oxide synthase interaction with soluble guanylate cyclase contributes to the splanchnic arterial vasodilation in portal hypertensive rats. Hepatol Res. 2007;37:58–67. doi: 10.1111/j.1872-034X.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- [22].White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1468–75. doi: 10.1152/ajpheart.01173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Han G, Ma H, Chintala R, Fulton DJ, Barman SA, White RE. Essential role of the 90-kilodalton heat shock protein in mediating nongenomic estrogen signaling in coronary artery smooth muscle. J Pharmacol Exp Ther. 2009;329:850–5. doi: 10.1124/jpet.108.149112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 - estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293:H3713–9. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- [25].Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol. 1997;272:H1730–40. doi: 10.1152/ajpheart.1997.272.4.H1730. [DOI] [PubMed] [Google Scholar]
- [26].Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension. 1999;33:435–9. doi: 10.1161/01.hyp.33.1.435. [DOI] [PubMed] [Google Scholar]
- [27].Chen J, Petranka J, Yamamura K, London RE, Steenbergen C, Murphy E. Gender differences in sarcoplasmic reticulum calcium loading after isoproterenol. Am J Physiol Heart Circ Physiol. 2003;285:H2657–62. doi: 10.1152/ajpheart.00557.2003. [DOI] [PubMed] [Google Scholar]
- [28].Molero L, Garcia-Duran M, Diaz-Recasens J, Rico L, Casado S, Lopez-Farre A. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men: regulation by estrogen. Cardiovasc Res. 2002;56:43–51. doi: 10.1016/s0008-6363(02)00505-9. [DOI] [PubMed] [Google Scholar]
- [29].Grohe C, Kann S, Fink L, Djoufack PC, Paehr M, van Eickels M, Vetter H, Meyer R, Fink KB. 17Beta-estradiol regulates nNOS and eNOS activity in the hippocampus. Neuroreport. 2004;15:89–93. doi: 10.1097/00001756-200401190-00018. [DOI] [PubMed] [Google Scholar]
- [30].Burger NZ, Kuzina OY, Osol G, Gokina NI. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+ Am J Physiol Endocrinol Metab. 2009;296:E503–12. doi: 10.1152/ajpendo.90517.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch. 2010;459:977–94. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- [32].Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch. 2010;459:807–16. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- [34].Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–63. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]