Abstract
The study objective was to evaluate the effect of prescribing a low-carbohydrate diet (LCD) and a low-fat diet (LFD) on food cravings, food preferences, and appetite. Obese adults were randomly assigned to a LCD (n=134) or a LFD (n=136) for two years. Cravings for specific types of foods (sweets, high-fats, fast-food fats, carbohydrates/starches); preferences for high-sugar, high-carbohydrate, and low-carbohydrate/high-protein foods; and appetite were measured during the trial and evaluated during this secondary analysis of trial data. Differences between the LCD and LFD on change in outcome variables were examined with mixed linear models. Compared to the LFD, the LCD had significantly larger decreases in cravings for carbohydrates/starches and preferences for high-carbohydrate and high-sugar foods. The LCD group reported being less bothered by hunger compared to the LFD group. Compared to the LCD group, the LFD group had significantly larger decreases in cravings for high-fat foods and preference for low-carbohydrate/high-protein foods. Men had larger decreases in appetite ratings compared to women. Prescription of diets that promoted restriction of specific types of foods resulted in decreased cravings and preferences for the foods that were targeted for restriction. The results also indicate that the LCD group was less bothered by hunger compared to the LFD group and that men had larger reductions in appetite compared to women.
Keywords: low-carbohydrate diet, weight loss, diet, hunger, macronutrient composition
Introduction
Food craving can be defined as “an intense desire to consume a particular food (or type of food) that is difficult to resist” (1). Consumption of specific foods satisfy food cravings, while consumption of any number of foods can satisfy hunger (2). Food cravings are a common phenomenon, occurring in 58% to 97% of adults in samples from North America and New Zealand (3, 4). People who experience food cravings report 2 to 4 craving episodes per week (5, 6), which usually precipitate eating behavior (7) and consumption of the craved food or a similar food (4, 5). Food cravings can also precipitate binge eating episodes among females diagnosed with bulimia nervosa (8). Cravings are also associated with body mass among participants with type 2 diabetes (9), and cravings for high-fat foods are associated with obesity (1).
Food cravings and desire for restricted foods can contribute to the failure to comply with weight loss diets (10), and food cravings were the most frequently cited reason for not adhering to a diet during controlled feeding studies (11). Although food restriction was believed to contribute to food cravings (7), several studies have found that dieting or calorie restriction results in decreased food cravings, particularly among dieters participating in very-low-calorie (e.g., 200 kcal/day to 800 kcal/day) or monotonous diets (12–14). Some studies, however, found that food cravings either increase (2), or do not change during dieting (15). The use of different methods to measure food cravings likely contributes to the discrepant findings, and validated food craving instruments were only recently developed (1, 16–18).
Food cravings have also been viewed as a conditioned expression of hunger, where food cravings arise from pairing consumption of certain foods with hunger (19). Hunger is associated with food cravings among females (20), and it is hypothesized that the decrease in food cravings observed during dieting is mediated by decreased hunger (14). Dietary macronutrient composition could also affect hunger, and it has been reported that low-carbohydrate/high-protein diets have satiating properties (21–24) and promote greater short-term weight loss than low-fat diets (25–27). Nonetheless, the long-term effect of prescribing weight loss diets that vary in macronutrient composition on hunger, food cravings, and food preferences is unclear, as is the relation between changes in hunger and changes in food cravings.
Similar to food cravings, food preferences could contribute to the failure to comply with a weight loss diet. Exposure to food cues increases appetitive behavior (28), and children’s preference for foods high in fat is correlated with dietary fat intake (29), and similar findings have been found with adults (30). In addition, the food preferences of obese men include foods such as pizza and French fries and obese women prefer foods such as cake and doughnuts (31, 32). Hence, both genders prefer foods that have high energy content and that could impede weight loss efforts. The etiology of food preferences also might be similar to food cravings since conditioning is involved in the development of children’s food preferences (33). These findings suggest that changes in food cravings and food preferences might mirror each other during dieting.
The purpose of this study was to determine the long-term effects of prescribing a low-carbohydrate diet (LCD) and a low-calorie/low-fat diet (LFD) on changes in food cravings, food preferences, and appetite ratings during a two-year randomized controlled trial (RCT). Specifically, the aim was to examine the effects of prescription of the LCD and LFD on the outcome variables over the long-term rather than conducting a short-term inpatient study where macronutrient intake could be directly assessed. Hence, the conclusions from this study apply to obese participants who attempt to follow similar low-carbohydrate and low-fat diets and restrict certain macronutrients in their home environment. We hypothesized that: 1) promoting the restriction of specific food groups (macronutrients) during the LCD and LFD would result in larger decreases in cravings and preferences for those specific foods, 2) the LCD group would report less hunger compared with the LFD group, and 3) changes in hunger would be associated with changes in food cravings.
Methods
Ethics
Data for this study were collected as part of the multi-site randomized trial that was conducted at Temple University, Washington University-St. Louis (WU-St. Louis), and the University of Colorado Health Science Center (UCHSC) (34). The study protocol was approved by the Institutional Review Boards (IRB) of these three sites. In addition, the Pennington Biomedical Research Center’s IRB approved collection of data reported herein. All participants provided written informed consent.
Participants
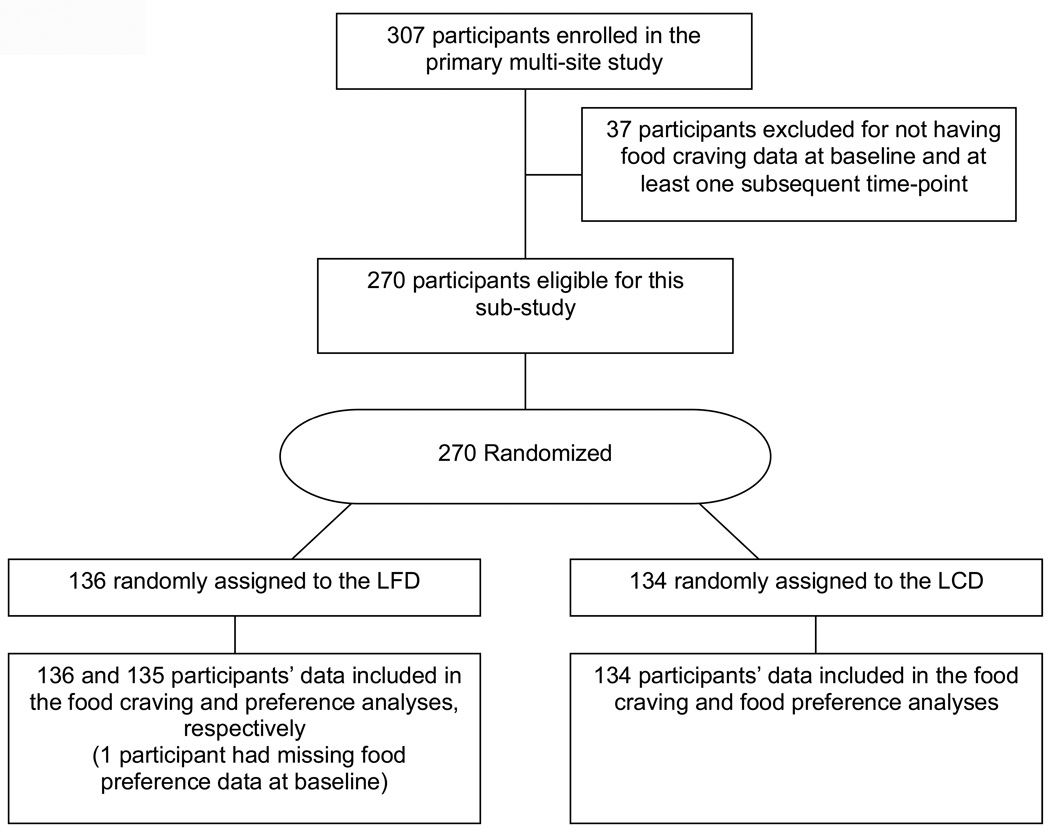
The main trial recruited 307 participants over a 2.5 year period, with 106 participants at Temple University, 108 at UCHSC and 93 at WU-St. Louis. Of these 307 participants, 270 participants’ data were included in the secondary analysis of trial data reported in this study. A conservative modified intent-to-treat approach was utilized (participants’ data were included in the analyses if they had baseline food craving data and data from at least one subsequent time-point). Hence, only 12% of participants’ were not included in this analysis. Adult participants (age 18 to 65 years) with BMI ≥ 30 kg/m2 and ≤ 40 kg/m2 from all racial and ethnic groups were recruited. A detailed description of the study sample is provided elsewhere (34). In brief, the exclusion criteria included: the presence or history of a chronic disease that is known to affect appetite, food intake, or metabolism (e.g., diabetes); use of prescription or over the counter medication known to affect appetite, food intake or energy expenditure (e.g., psychiatric medications, appetite suppressants); smoking; vegetarian diet; dyslipidemia medication; substance abuse; history of cardiovascular or protein wasting disease (e.g., Cushing’s syndrome); and for females, pregnancy, lactating or planning on becoming pregnant over the next two years. Hormone replacement therapy was allowed if the participant agreed to remain on the therapy throughout the study, though participants were excluded who used selective estrogen receptor modulators (tamoxifine).
Weight Loss Diets
Participants were randomly assigned to either a low-carbohydrate diet (LCD, n=134) or low-calorie/lowfat diet (LFD, n=136) in a one-to-one ratio. The CONSORT diagram depicting participant randomization is included in Figure 1.
Figure 1.
CONSORT Diagram.
Low-Carbohydrate Diet (LCD)
Participants assigned to the LCD were instructed to consume a low-carbohydrate diet, with unlimited consumption of fat and protein. During the first three months of the diet, participants were instructed to limit carbohydrate intake to 20 g/d in the form of low-glycemic index (GI) vegetables. Thereafter, participants were instructed to gradually increased carbohydrate intake (5g/d/wk) by consuming more vegetables, a limited amount of fruits, and eventually small quantities of whole grains and dairy products until a stable and desired weight was achieved. Participants were also encouraged to follow guidelines described in the Atkins’ New Diet Revolution book (35). Participants were instructed to focus on limiting carbohydrate intake and to eat foods rich in fat and protein until they were satisfied. The primary behavioral target was to limit carbohydrate intake.
Low-Fat Diet (LFD)
Participants in the LFD were instructed to limit calorie intake and decrease fat intake, though energy intake was the primary behavioral target. Women and men were initially prescribed 1200–1500 and 1500–1800 kcal/day diets, respectively. Participants were encouraged to consume approximately 30% kcal from fat, 15% from protein and 55% from carbohydrate.
Comprehensive Behavioral Treatment
Participants in both groups received a comprehensive treatment program to foster dietary adherence (36, 37). Participants attended weekly group sessions during weeks 1–20, biweekly sessions during weeks 22–40, and bimonthly sessions during weeks 48–104. Treatment included strategies to promote adherence, including stimulus control, self-monitoring, and relapse prevention. During group meetings, participants’ were coached on skills to help them adhere to the diet. The comprehensive behavioral treatment program was empirically based (36, 37) and is described in detail in an Appendix for the primary outcomes paper (34).
As noted earlier, the purpose of this study was to determine the long-term effects of prescribing a LCD and a LFD on changes in food cravings, food preferences, and appetite ratings during a two-year randomized controlled trial (RCT). Specifically, the aim was to examine the effects of prescription of the LCD and LFD on the endpoints of interests over the long-term, recognizing that macronutrient intake likely deviated from the prescriptions during the course of the two-year RCT even though the comprehensive behavioral treatment program was implemented to promote adherence. Hence, unlike a tightly controlled inpatient study where macronutrient intake is directly manipulated over the short-term, the conclusions from this study reflect the effect of obese participants being instructed to follow macronutrient restricted diets in their natural environment over two years. The strengths and limitations of this approach are discussed in the Discussion section.
Materials
Food-Craving Inventory (FCI)
The FCI is a reliable and valid measure of cravings for specific types of foods (1). Respondents rate the frequency of cravings over the past month for individual food items using a 5-point Likert scale (Never, scored as 1; Rarely, 2; Sometimes, 3; Often, 4; Always/almost every day, 5). The FCI consists of four scales (sweets, high-fats, carbohydrates/starches, and fast-food fats) (1). The sweets scale includes foods such as brownies and ice cream, and the high-fats scale includes foods such as bacon and fried fish. The carbohydrates/starches scale contains foods such as baked potatoes and pasta. The fast-food fats scale is composed of pizza, French fries, hamburgers and chips.
The FCI was used to measure food cravings at baseline (month 0) and months 3, 6, 12, 18, and 24. It was chosen because dieting has been found to decrease FCI scores (14) and FCI-identified cravings for specific foods are associated with consumption of corresponding types of food (38). Additionally, the FCI is a validated instrument to measure cravings for specific types of foods that are allowed or restricted on the LCD and LFD diets. Specifically, the LCD diet restricts or prohibits consumption of foods on the carbohydrates/starches, sweets, and fast-food fats scales. Alternatively, the LFD diet limits, but does not prohibit, consumption of foods on the high-fats, fast-food fats, and sweets scale.
Food Preference Questionnaire (FPQ)
The FPQ (30) assesses preferences for 72 foods in a 2 (High-Fat, Low-Fat) by 3 (High-Simple Sugar, High-Complex Carbohydrate, Low-Carbohydrate/High-Protein) matrix. Participants rate each food hedonically on a 9-point Likert scale by rating how much they like each food, with 1 = dislike extremely, 5 = neither like nor dislike, and 9 = like extremely. The FPQ has established reliability and validity (30).
The FPQ was used to measure preference for foods that participants were instructed to restrict on the LCD or LFD. Specifically, high-sugar foods that were high and low in fat (jelly, pudding) were restricted on the LCD diet. Similarly, high–carbohydrate foods that were high and low in fat (bagels, potato sticks) were restricted on the LCD diet. Low-carbohydrate/high-protein foods that were high- and low-fat (cheese, pot roast, ground turkey) were restricted on the LFD diet. Hence, the high-sugar, high-carbohydrate, and low-carbohydrate/high-protein factors of the FPQ were evaluated since these factors most accurately reflected the types of foods that participants were asked to restrict on each diet. The FPQ was administered at months 0, 3, 6, 12, 18, and 24.
Appetite Ratings
Visual analogue scales (VAS) were used to measure subjective ratings of appetite at months 0, 3, 6, 12, 18, and 24. The VAS items asked: “How hungry did you feel this week?”, “How much were you bothered or distracted by hunger this week?”, “How often did you want to eat in response to seeing or smelling food this week?”, and “How much did you think about food this week?”. The VAS consisted of a 100 mm line anchored from “not at all” to “extremely,” and participants placed a hash mark on the line that represented their level of appetite. The VAS were scored by measuring the distance from the left end of the line to the participant’s hash mark. VAS (39) and the “weekly” VAS used in this study (40) have been found to have satisfactory reliability and validity.
Design and procedures
A detailed description of this randomized trial is provided elsewhere (34). In brief, participants were instructed to follow their prescribed diet for two years (24 months) and they received extensive support to foster adherence to the diets, as detailed in the Comprehensive Behavioral Treatment section.
Data Analysis
Unless otherwise specified, alpha was set at .05. Mixed linear models were used to test if change from baseline on the outcome variables (food cravings, food preferences, and appetite ratings) differed significantly by group (LCD and LFD). Group and gender were fixed effects, and time was a repeated factor. The analyses controlled for site (Temple, WU-St. Louis, and UCHSC) and baseline values of the outcome variable were entered as covariates. The model included main effects for group, gender, and time, and tested if changes in the outcome variables were influenced by site. Time by group and time by gender interactions were also evaluated. Change scores at each time point (e.g., month 3 minus month 0) were examined and outlying values (values > 3 SDs from the mean) were eliminated from the analysis (the analyses were repeated without exclusion of outliers and the results did not meaningfully differ). Because the outcome variables were change scores, group main effects indicated that change over time differed by group and these were the primary comparisons of interest. As noted earlier, a conservative modified intent-to-treat approach was utilized, which relied on all participants’ data assuming that they completed a questionnaire at baseline and at least one other time point. PROC MIXED models were used that relied on likelihood-based methods for missing values when data were missing at random.1 These models accommodate correlations among repeated observations and thus allow inclusion of all available data.
At each follow-up time point, correlation analysis was used to examine the association of change in food cravings, food preferences, and appetite ratings with change in body weight. Alpha was set at .01 for these analyses to control alpha inflation. A mediation analysis was planned to determine if change in food cravings was mediated by change in hunger.
Results
Participant Characteristics
The sample was predominantly white (76%) and female (68%) (Table 1). Mean ± SD baseline levels of food cravings were: sweets 2.6 ± 0.8, high-fats 1.9 ± 0.7, carbohydrates/starches 2.1 ± 0.7, and fast-food fats, 2.6 ± 0.8. These levels of food cravings are very similar to other samples (14) and also among the LCD and LFD groups. Baseline food preference scores were: high-sugar 5.9 ± 1.2, high-complex carbohydrate 5.5 ± 1.2, and low-carbohydrate/high-protein 5.8 ± 1.1, and were also very similar among the LCD and LFD groups. Site did not significantly influence change in any outcome variable.
Table 1.
Characteristics of the study sample.
| Total Sample (N = 270) |
LCD (n = 134) |
LFD (n = 136) |
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age | 45.2 | 9.8 | 45.8 | 9.3 | 44.6 | 10.2 |
| Height (cm) | 169.3 | 9.4 | 169.2 | 9.7 | 169.4 | 9.1 |
| Body Weight (kg) | 103.6 | 14.8 | 103.7 | 15.2 | 103.4 | 14.4 |
| Body Mass Index (kg/m2) | 36.0 | 3.3 | 36.1 | 3.3 | 35.9 | 3.3 |
| Food Craving Inventory | ||||||
| Sweets | 2.6 | 0.8 | 2.6 | 0.8 | 2.5 | 0.8 |
| High-Fats | 1.9 | 0.7 | 1.9 | 0.7 | 1.9 | 0.7 |
| Carbohydrates/starches | 2.1 | 0.7 | 2.1 | 0.7 | 2.2 | 0.7 |
| Fast-Food Fats | 2.6 | 0.8 | 2.6 | 0.9 | 2.6 | 0.8 |
| Food Preference Questionnaire | ||||||
| High-Sugar | 5.9 | 1.2 | 6.0 | 1.2 | 5.9 | 1.2 |
| High-Complex Carbohydrates | 5.5 | 1.2 | 5.5 | 1.2 | 5.5 | 1.2 |
| Low-carbohydrate/high-protein | 5.8 | 1.1 | 5.9 | 1.0 | 5.8 | 1.2 |
| n | % | n | % | n | % | |
| Gender | ||||||
| Male | 87 | 32% | 44 | 33% | 43 | 32% |
| Female | 183 | 68% | 90 | 67% | 93 | 68% |
| Race | ||||||
| White | 205 | 76% | 101 | 75% | 104 | 77% |
| African American | 58 | 21% | 29 | 22% | 29 | 21% |
| Asian | 2 | 1% | 0 | 0% | 2 | 1% |
| American Indian/Alaska Native | 4 | 2% | 3 | 2% | 1 | 1% |
| More than one race reported | 1 | <1% | 1 | % | 0 | 0% |
Food Craving (FCI) Change
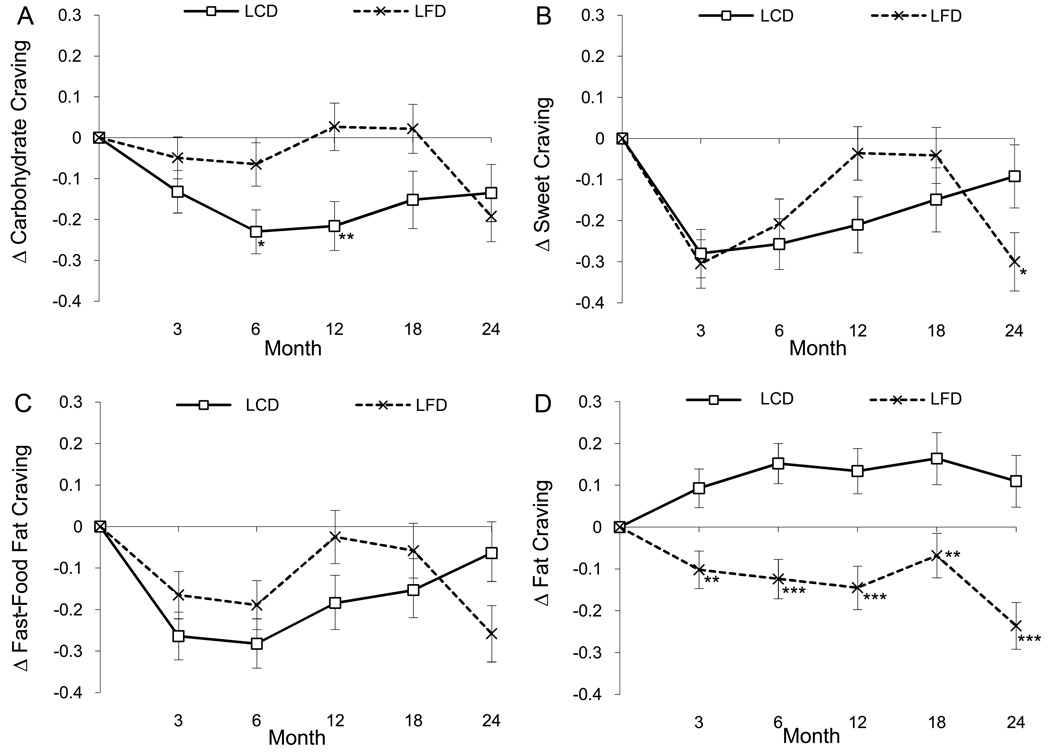
Compared to the LFD group, the LCD group had significantly larger decreases in carbohydrate/starches cravings (Figure 2A), F(1, 264) = 5.07, p = .025. Time by group interactions were found for sweet, F(4, 603) = 3.23, p = .012 (Figure 2B), and fast-food fat cravings, F(4, 615) = 2.45, p = .045 (Figure 2C), indicating that the LCD group had larger decreases in cravings for sweets and fast-food fats at many, but not all, time points. Compared to the LCD group, the LFD group had larger decreases in cravings for high-fat foods (Figure 2D), F(1, 276) = 34.43, p < .0001. Males had larger decreases in high-fat cravings (mean ± SEM; −0.05 ± 0.04) compared to females (0.04 ± 0.03), F(1, 282) = 4.10, p = .044.
Figure 2.
Change (delta or Δ) in cravings are illustrated for carbohydrates (Panel A), sweets (Panel B), fast-food fats (Panel C), and high-fats (Panel D) by group (LCD and LFD). Asterisks denote the time-points at which significant differences were found between groups with post-hoc tests (*p < .05, **p < .01, p < .001).
Food Preference (FPQ) Change
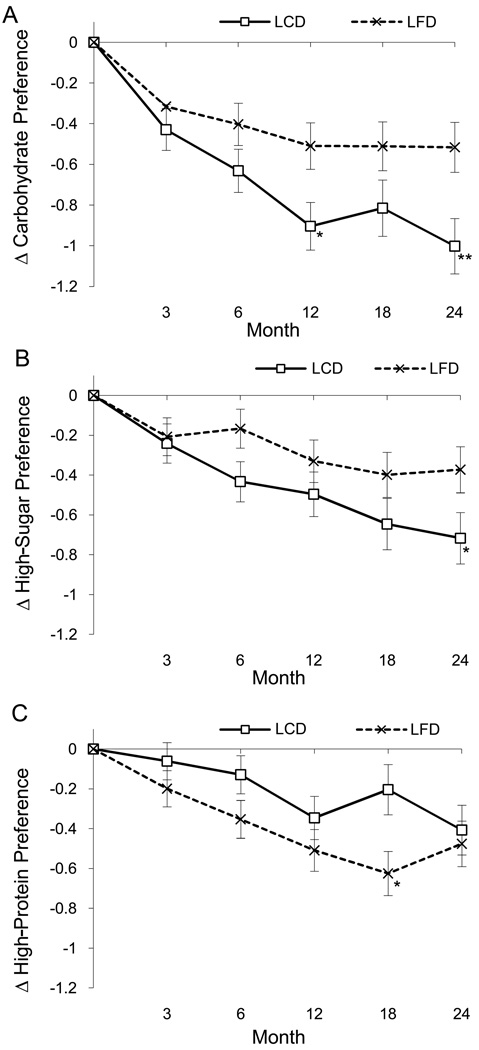
The LCD compared to the LFD group experienced significantly larger decreases in preference for high-carbohydrate, F(1, 268) = 8.55, p = .004 (Figure 3A), and high-sugar foods, F(1, 272) = 4.13, p = .043 (Figure 3B). A time by gender interaction also indicated that change in preference for high-sugar foods differed over time by gender, F(4, 609) = 2.52, p = .040, with women having larger decreases in sugar preference at month 6 and men at month 18. The LFD compared to the LCD group experienced larger decreases in preference for low-carbohydrate/high-protein foods (Figure 3C), F(1, 275) = 4.74, p = .03.
Figure 3.
Changes (delta or Δ) in preference for high-carbohydrate (Panel A), high-sugar (Panel B), and low-carbohydrate/high-protein foods (Panel C) by group (LCD and LFD). Asterisks denote the time-points at which significant differences were found between groups with post-hoc tests (*p < .05, **p < .01).
Appetite Rating Change
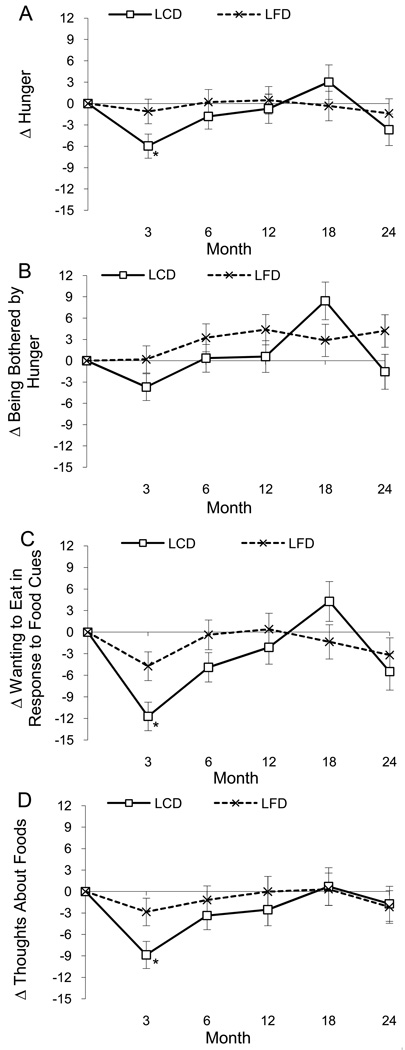
Significant time main effects were detected for three of the four appetite rating scales. Two scales decreased (wanting to eat in response to food cues and thoughts about foods) and one scale increased (being bother by hunger), but a significant time by group interaction, F(4,700) = 2.54, p = .039, qualified this increase. The interaction indicated that the LCD group was bothered less by hunger compared to the LFD group, except at one time point (month 18). No other time by group interactions were significant (p-values > .07). Decreased appetite ratings returned to baseline by month 12. Compared to women, men reported larger decreases in hunger, F(1,327) = 7.59, p = .006, being bothered by hunger, F(1, 325) = 5.39, p = .021, wanting to eat in response to seeing or smelling food, F(1, 320) = 17.74, p < .0001, and thoughts about food, F(1,313) = 5.90, p = .016.
Correlation Analyses
Similar to the findings of the main paper (34), weight loss in this sample was approximately 7.8%, 11.3%, 10.6%, 9.0% and 7.2% at 3, 6, 12, 18, and 24 months, respectively, and there were no differences in weight loss between the LCD and LFD groups at any time point. Greater weight loss was associated with larger reductions in cravings for sweets at month 3 (r = .22, p < .01) and high-fats at month 24 (r = .32, p < .01). Change in FPQ scale scores did not correlate significantly with weight loss at any time-point.
Mediation Analysis
A mediation analysis was planned to determine if change in hunger mediated the diet-induced decrease in food cravings. Nevertheless, the correlations between change in food cravings and change in hunger were small to modest (r-values = −.05 to .21, p-values = .01 to .96) and non-significant with alpha = .01, limiting the ability to perform the mediation analysis.
Discussion
The long-term effects of the prescribed macronutrient composition of weight loss diets on food cravings, food preferences, and hunger have important clinical implications in diet therapy for obesity. The results of the present study demonstrate that promoting the restriction of specific types of foods while dieting causes decreased cravings and preferences for the foods that are targeted for restriction. Promoting the restriction of carbohydrates resulted in decreased craving and preference for high-carbohydrate and sweet/high-sugar foods, whereas promoting the restriction of high-fat and high-protein foods decreased craving for high-fat foods and preference for high-protein foods. In addition, our data suggest that marked dietary monotony, such as liquid-only diets, is not necessary for a diet to result in food craving (or food preference) reduction. Neither the LFD or LCD were severely monotonous.
The belief that attempting to restrict food intake causes food cravings (7) can contribute to patients’ ambivalence toward dieting. The data reported herein suggests that these fears are unfounded, at least among obese adults who plan to follow either a low-fat or low-carbohydrate diet. The finding that food preferences and, to a lesser extent, food cravings remained suppressed during a two-year period indicates that the results are not spurious and that patients can expect these effects over the long-term. This information can be useful when counseling patients about diet/weight loss expectancies and when assessing patients’ readiness for dietary change.
It has been hypothesized that food cravings are a conditioned expression of hunger that result from pairing consumption of specific foods with hunger (19). This hypothesis is consistent with a biopsychosocial theory of food cravings, which suggests that food cravings can arise from pairing food intake with other conditions, such as emotional states or physical locations (41). If this hypothesis is true, there is less likelihood of pairing consumption of foods with stimuli that precipitate eating during a diet, which would limit the development of food cravings. Similarly, following the principles of conditioning, existing food cravings can be “extinguished”. This phenomenon could explain the decrease in food cravings observed during dieting. Because stimuli that were once associated with craving and eating foods are no longer paired with food intake, cravings will diminish. It is also appears that food preferences likely arise from similar conditioning phenomenon and are also susceptible to the same effects of extinction. These observations are not surprising, because food preferences vary among cultures and conditioning plays a central role in the development of children’s food preferences (33).
The VAS results provide important data on participants’ subjective experience during these two very different popular diets. Compared to the LFD group, the LCD group was bothered less by hunger during the majority of the two-year trial, though change on other appetite ratings did not differ significantly by group. These findings partially support our hypothesis and the assumption that low-carbohydrate/high-protein diets promote satiety (21–24) and are consistent with the conclusions of McClernon et al. (42) who found larger decreases in hunger during a low-carbohydrate, ketogenic diet compared to a low-fat diet.
This two year trial also demonstrated that decreases in appetite ratings during dieting (43) are transient and return to baseline by approximately one year, with some ratings exceeding baseline levels by month 18. It is possible that decreased adherence to the diets over time was associated with a rebound in appetite ratings and body weight did increase from its nadir during the second year of the trial. Our data also document robust gender effects, however, with men reporting larger decreases than women on all four appetite ratings that persist through year two. For men, three of the four appetite ratings remain below baseline for the duration of the two-year trial, while women’s ratings return or exceed baseline levels by month 6. These novel results warrant further investigation into the effects of dieting on men and women’s subjective ratings of appetite, and demonstrate the need to enroll males in randomized controlled weight loss trials, as this group is typically underrepresented.
The correlation analyses found that weight loss was not associated with change in food preferences, but was associated with reductions in cravings for sweets at month 3 and high-fats at month 24. Although additional correlations would be considered significant if a less conservative alpha level was adopted, the results indicate that the relation between weight loss and reductions in food cravings is not robust, which is consistent with the results from other studies (14).
The small to modest correlations between change in food cravings and change in hunger limited our ability to perform a mediation analysis to determine if change in cravings were mediated by change in hunger. Nevertheless, the pattern of correlations observed in this study suggests that change in hunger is not a likely mediator of change in food cravings during dieting.
This study had many strengths. First, validated instruments were used to measure food cravings, food preferences, and subjective ratings of appetite. Second, the Food Craving Inventory was used in a previous study that found dieting to reduce food cravings (14), and the present study contributes to the literature by examining the effect two different types of diets on food cravings. Finally, weight loss was very similar between the LCD and LFD groups (34); therefore, differential weight loss between the groups did not confound the results. The study was also associated with limitations that warrant consideration when interpreting the results. First, knowledge of participants’ macronutrient intake during the two-year study would be very beneficial, yet dietary intake data are not available. Therefore, it is not known how closely participants adhered to the specific macronutrient composition of the diets, though participants were provided with a comprehensive behavioral treatment program to foster adherence. Hence, the results of this study can be generalized to people who attempt to follow similar weight loss diets in their natural environment. Inpatient feeding studies are needed to determine the precise effect of macronutrient restriction on food cravings in controlled conditions, and future research is warranted to determine the relation between macronutrient intake in free-living conditions and food cravings. Second, the macronutrient composition of the LCD changed over time, though these changes reflect popular low-carbohydrate diets that gradually increase carbohydrate intake after a period of more severe carbohydrate (e.g., (35)).
In summary, promoting the restriction of certain types of foods while dieting results in decreased cravings and preferences for the foods that are targeted for restriction. These results provide empirical data to address dieters’ concerns that food restriction/dieting will increase cravings. Further, the results demonstrate that food or calorie restriction is an effective strategy to reduce food cravings. Lastly, the low-carbohydrate group was bothered less by hunger during the majority of the two-year trial, and men experienced larger reductions in appetite compared to women.
Figure 4.
Change (delta or Δ) in ratings of hunger (Panel A), being bothered by hunger (Panel B), wanting to eat in response to seeing or smelling food (Panel C), and thinking about food (Panel D) by group. The time by group interaction for being bothered by hunger (Panel B) was significant (p < .039). Asterisks denote the time-points at which significant differences were found between groups with post-hoc tests (*p < .05).
Acknowledgements
This work was supported by the National Institutes of Health grants AT1103, K23 DK068052, UL1 RR024992, UL1RR024134 and UL1 RR000051 and Clinical Nutrition Research Unit grants P30 DK072476-059001 and DK56341. The funding source had no role in study design, conduct or reporting. The authors would like to extend their profound gratitude to the study volunteers for participating in this study.
Footnotes
Clinical Trials.gov Identifier: NCT00143936
Potential Conflicts of Interest. The authors report no conflicts of interest.
A secondary analysis that only included participants who provided data at months 0 and 24 (i.e., completers) was also conducted. The proportion of participants who completed questionnaires at months 0 and 24 (completers) did not differ by sex or diet assignment, and completers did not differ from non-completers in height, body weight, or BMI. Further, the results differed little between the completers analysis and the modified intent-to-treat approach; therefore, only results from the latter analysis are reported.
References
- 1.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–114. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 2.Pelchat ML, Schaefer S. Dietary monotony and food cravings in young and elderly adults. Physiol Behav. 2000;68:353–359. doi: 10.1016/s0031-9384(99)00190-0. [DOI] [PubMed] [Google Scholar]
- 3.Gendall KA, Joyce PR, Sullivan PF. Impact of definition on prevalence of food cravings in a random sample of young women. Appetite. 1997;28:63–72. doi: 10.1006/appe.1996.0060. [DOI] [PubMed] [Google Scholar]
- 4.Weingarten HP, Elston D. Food cravings in a college population. Appetite. 1991;17:167–175. doi: 10.1016/0195-6663(91)90019-o. [DOI] [PubMed] [Google Scholar]
- 5.Hill AJ, Heaton-Brown L. The experience of food craving: a prospective investigation in healthy women. J Psychosom Res. 1994;38:801–814. doi: 10.1016/0022-3999(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 6.Hill AJ, Weaver CF, Blundell JE. Food craving, dietary restraint and mood. Appetite. 1991;17:187–197. doi: 10.1016/0195-6663(91)90021-j. [DOI] [PubMed] [Google Scholar]
- 7.Weingarten HP, Elston D. The phenomenology of food cravings. Appetite. 1990;15:231–246. doi: 10.1016/0195-6663(90)90023-2. [DOI] [PubMed] [Google Scholar]
- 8.Waters A, Hill A, Waller G. Bulimics' responses to food cravings: is binge-eating a product of hunger or emotional state? Behav Res Ther. 2001;39:877–886. doi: 10.1016/s0005-7967(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 9.Delahanty LM, Meigs JB, Hayden D, Williamson DA, Nathan DM. Psychological and behavioral correlates of baseline BMI in the diabetes prevention program (DPP) Diabetes Care. 2002;25:1992–1998. doi: 10.2337/diacare.25.11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swinburn B, Egger G. The runaway weight gain train: too many accelerators, not enough brakes. Bmj. 2004;329:736–739. doi: 10.1136/bmj.329.7468.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall DM, Most MM. Dietary adherence in well-controlled feeding studies. J Am Diet Assoc. 2005;105:1285–1288. doi: 10.1016/j.jada.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Harvey J, Wing RR, Mullen M. Effects on food cravings of a very low calorie diet or a balanced, low calorie diet. Appetite. 1993;21:105–115. doi: 10.1016/0195-6663(93)90003-3. [DOI] [PubMed] [Google Scholar]
- 13.Lappalainen R, Sjoden PO, Hursti T, Vesa V. Hunger/craving responses and reactivity to food stimuli during fasting and dieting. Int J Obes. 1990;14:679–688. [PubMed] [Google Scholar]
- 14.Martin CK, O'Neil PM, Pawlow L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity (Silver Spring) 2006;14:115–121. doi: 10.1038/oby.2006.14. [DOI] [PubMed] [Google Scholar]
- 15.Gilhooly CH, Das SK, Golden JK, et al. Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes (Lond) 2007;31:1849–1858. doi: 10.1038/sj.ijo.0803672. [DOI] [PubMed] [Google Scholar]
- 16.Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA. The development and validation of the State and Trait Food-Cravings Questionnaires. Behavior Therapy. 2000;31:151–173. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- 17.Toll BA, Katulak NA, Williams-Piehota P, O'Malley S. Validation of a scale for the assessment of food cravings among smokers. Appetite. 2008;50:25–32. doi: 10.1016/j.appet.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin CK, McClernon FJ, Chellino A, Correa J, editors. Food cravings: A central construct in food intake behavior, weight loss, and the neurobiology of appetitive behavior. New York: Springer; in press. [Google Scholar]
- 19.Gibson EL, Desmond E. Chocolate craving and hunger state: implications for the acquisition and expression of appetite and food choice. Appetite. 1999;32:219–240. doi: 10.1006/appe.1998.0207. [DOI] [PubMed] [Google Scholar]
- 20.Steel D, Kemps E, Tiggemann M. Effects of hunger and visuo-spatial interference on imagery-induced food cravings. Appetite. 2006;46:36–40. doi: 10.1016/j.appet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–899. doi: 10.1016/S0140-6736(04)16986-9. [DOI] [PubMed] [Google Scholar]
- 22.Kushner RF, Doerfler B. Low-carbohydrate, high-protein diets revisited. Curr Opin Gastroenterol. 2008;24:198–203. doi: 10.1097/MOG.0b013e3282f43a87. [DOI] [PubMed] [Google Scholar]
- 23.Nickols-Richardson SM, Coleman MD, Volpe JJ, Hosig KW. Perceived hunger is lower and weight loss is greater in overweight premenopausal women consuming a low-carbohydrate/high-protein vs high-carbohydrate/low-fat diet. J Am Diet Assoc. 2005;105:1433–1437. doi: 10.1016/j.jada.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87:1558S–1561S. doi: 10.1093/ajcn/87.5.1558S. [DOI] [PubMed] [Google Scholar]
- 25.Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 26.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 27.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 28.Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 29.Fisher JO, Birch LL. Fat preferences and fat consumption of 3- to 5-year-old children are related to parental adiposity. J Am Diet Assoc. 1995;95:759–764. doi: 10.1016/S0002-8223(95)00212-X. [DOI] [PubMed] [Google Scholar]
- 30.Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav. 1998;63:919–928. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 31.Meiselman HL, Waterman D. Food preferences of enlisted personnel in the Armed Forces. J Am Diet Assoc. 1978;73:621–629. [PubMed] [Google Scholar]
- 32.Wyant KW, Meiselman HL. Sex and race differences in food preferences of military personnel. J Am Diet Assoc. 1984;84:169–175. [PubMed] [Google Scholar]
- 33.Birch LL. Development of food acceptance patterns in the first years of life. Proc Nutr Soc. 1998;57:617–624. doi: 10.1079/pns19980090. [DOI] [PubMed] [Google Scholar]
- 34.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins RC. Dr. Atkins' new diet revolution. New York: Avon Books, Inc.; 1998. [Google Scholar]
- 36.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;82:230S–235S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- 37.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 38.Martin CK, O'Neil PM, Tollefson G, Greenway FL, White MA. The association between food cravings and consumption of specific foods in a laboratory taste test. Appetite. 2008;51:324–326. doi: 10.1016/j.appet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 40.Womble LG, Wadden TA, Chandler JM, Martin AR. Agreement between weekly vs. daily assessment of appetite. Appetite. 2003;40:131–135. doi: 10.1016/s0195-6663(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 41.Rogers PJ, Smit HJ. Food craving and food "addiction": a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav. 2000;66:3–14. doi: 10.1016/s0091-3057(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 42.McClernon FJ, Yancy WS, Jr, Eberstein JA, Atkins RC, Westman EC. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity (Silver Spring) 2007;15:182–187. doi: 10.1038/oby.2007.516. [DOI] [PubMed] [Google Scholar]
- 43.Wadden TA, Stunkard AJ, Day SC, Gould RA, Rubin CJ. Less food, less hunger: reports of appetite and symptoms in a controlled study of a protein-sparing modified fast. Int J Obes. 1987;11:239–249. [PubMed] [Google Scholar]