Abstract
Background
Prostate cancer in men has a high mortality and morbidity due to metastatic disease. The pathobiology of prostate cancer metastasis is not well understood and cell lines and animal models that recapitulate the complex nature of the disease are needed. Therefore, the goal of the study was to establish and characterize a new prostate cancer line derived from a dog with spontaneous prostate cancer.
Methods
A new cell line (Leo) was derived from a dog with spontaneous prostate cancer. Immunohistochemistry and PCR were used to characterize the primary prostate cancer and xenografts in nude mice. Subcutaneous tumor growth and metastases in nude mice were evaluated by bioluminescent imaging, radiography and histopathology. In vitro chemosensitivity of Leo cells to therapeutic agents was measured.
Results
Leo cells expressed the secretory epithelial cytokeratins (CK) 8, 18 and ductal cell marker, CK7. The cell line grew in vitro (over 75 passages) and was tumorigenic in the subcutis of nude mice. Following intracardiac injection, Leo cells metastasized to the brain, spinal cord, bone, and adrenal gland. The incidence of metastases was greatest to the central nervous system (80%) with a lower incidence to bone (20%) and the adrenal glands (16%). In vitro chemosensitivity assays demonstrated that Leo cells were sensitive to velcade and an HDAC-42 inhibitor with IC50 concentrations of 1.9 nM and 0.95 μM respectively.
Conclusion
The new prostate cancer cell line (Leo) will be a valuable model to investigate the mechanisms of the brain and bone metastases.
Keywords: Prostate cancer, Brain metastasis, spinal cord metastasis, dog, canine, bone metastasis
Introduction
Prostate cancer is the most commonly diagnosed cancer in men accounting for 25% of all cancers in United States (1). Prostate cancer in advanced stages has a high incidence of bone metastases that causes severe skeletal complications resulting in morbidity and mortality. Dogs are the only nonhuman mammals that develop spontaneous prostate cancer, which shares many characteristics in clinical presentation and pathogenesis of the disease as in men (2). In addition, dogs also have extensive genomic homology with the humans, and recent sequencing of the dog genome and development of dog gene arrays make the dog an invaluable animal model to study the pathogenesis of prostate cancer in spontaneous or experimental settings (3). Previously, our laboratory established the ACE-1 canine prostate cancer cell line that metastasizes almost exclusively to bone and induced mixed osteoblastic and osteolytic lesions in nude mice similar to bone metastases in men with prostate cancer (4).
Although bone is the primary site of metastases in prostate cancer, metastases also occur in lymph nodes, brain, lung, adrenal gland and liver (5~7). Soft tissue metastases, alone or in combination with bone metastases, were detected in 33% of prostate cancer patients with metastases (8). Brain metastases occurs in a small percentage of patients (1%), but is often a terminal event. Median survival rate is 1 month in untreated patients with brain metastases, 3.5 months in patients who received radiotherapy and 9 months in patients who had stereotactic radiosurgery (9). Although brain metastases are uncommon in prostate cancer, the annual incidence of more than 150,000 cases of brain metastases from other cancers was reported in the US (10). It is important to develop new therapies for patients with brain metastases due to their serious consequences. Therefore, understanding the molecular pathogenesis of prostate cancer brain metastases will facilitate the development of novel treatment strategies.
Although numerous prostate cancer cell lines have been developed, few cell lines develop bone metastases and no cell lines have been reported to cause brain metastases (11). Despite the fact that the DU145 cell line was developed from a brain metastasis from a human prostate cancer patient, these cells have not demonstrated the ability to metastasize to the brain in animal models (12). Therefore, lack of prostate cancer cell lines that develop brain metastases have impeded studies of prostate cancer brain metastases.
In this investigation we report the establishment of a novel dog prostate cancer cell line and its ability to metastasize to numerous sites in the nude mouse including brain, spinal cord, bone and adrenal gland.
Materials and Methods
Establishment of a canine prostate carcinoma cell line (Leo)
Tissue from a spontaneously arising prostate carcinoma was collected immediately following euthanasia of a tumor-bearing, 5-year-old, castrated male mixed breed dog and washed three times in DMEM/F12 (Invitrogen Corp., Carlsbad, CA) containing 50 μg/ml primocin (Invivogen, San Diego, CA) and minced into approximately 1 mm3 pieces. The tissue pieces were digested in 500 units/ml of collagenase type 1 (Worthington Biochemical, Lakewood, NJ) in serum-free DMEM/F12 for 2 hours at 37°C on a rocker platform with gentle rocking. The digested tissue was washed three times with DMEM/F12 containing 50 μg/ml Primocin and 10% fetal bovine serum (FBS) (Invitrogen Corp.) and plated in a T-75 flask (Falcon; Becton-Dickinson, Franklin Lakes, NJ). Medium was changed every 3 days. Differential trypsinization was performed to eliminate the stromal cell contamination of epithelial cells once every two days by treating the cultures with 0.05% trypsin ethylenediamine-tetraacetic acid (EDTA) (Invitrogen Corp.). Detachment of the cells was monitored every two minutes to remove the stromal cells, which detached earlier than the epithelial cells. The first phase of detached cells (mostly stromal cells) was removed and the remaining epithelial cells were collected and replated.
Lentiviral yellow fluorescent protein-luciferase (YFP-Luc) vector production and transduction of Leo cell line
YFP-Luc lentiviral particles were produced by transient co-transfection of 293T cells with 10 μg packaging plasmid pCMVDR8.2, 2 μg envelope plasmid pMD.G and 10 μg transfer plasmid pHIVSIN-YFP-Luc using calcium phosphate (Sigma-Aldrich Co., St. Louis, MO) as described previously (13). The virus supernatant was collected at 24 hours, filtered through a 0.2 μm filter, and stored at −80° C until use. Leo cells were transduced with the retroviral YFP-Luc dual reporter gene and 8 μg/ml polybrene by spin inoculation at 2700 rpm for 1 hour at 32°c and transferred to a cell culture incubator overnight. Cells were maintained in DMEM/F12 medium containing 10% FBS and 50μg/ml Normocin (Invivogen).
Subcutaneous and intracardiac left ventricular inoculation of Leo cells in nude mice
Subcutaneous (N=10) and intracardiac (N=25) injections of Leo YFP-Luc cells were performed in six-week-old male nu/nu mice (National Cancer Institute, Frederick, MD) under general anesthesia with a 3% isoflurane (Abbott Laboratories, North Chicago, IL)-oxygen mixture. All the animal experimental procedures were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee. Ten million Leo cells suspended in 250 μl of phosphate-buffered saline (PBS) (Invitrogen) were injected subcutaneously over the dorsal interscapular area of mice. Subcutaneous tumor growth in mice was measured weekly using Vernier calipers and bioluminescent imaging (BLI) and sacrificed after 6 weeks. Left ventricular cardiac injections were performed with 1×105 cells suspended in 100 μl of PBS using a 26 gauge needle. Subcutaneous and metastatic tumor growth and incidence were monitored using BLI (see below). Immediately after intracardiac injection of LeoYFP-Luc cells, a diffuse BLI signal originating from the tumor cells throughout the entire body was considered successful left ventricular injection. Mice were sacrificed after 6 weeks.
Bioluminescent imaging (BLI)
Mouse BLI was performed using an in vivo imaging system (IVIS 100, Caliper Life Sciences, Hopkinton, MA) as described previously (11). Briefly, 4.5 mg d-luciferin (Caliper Life Sciences) dissolved in 150 ul PBS was injected intraperitoneally into mice and imaging was carried out in an imaging chamber under general anesthesia with a 1.5% isoflurane-oxygen mixture until peak photon signal was attained. The photon signal intensity was quantified using LivingImage software version 2.50 (Caliper Life Sciences)
In vitro growth rate
Cells (5 × 105) from passage 70 were plated in 10 cm plates (Falcon) in quadruplicate in DMEM/F12 medium with 10% FBS and 50 μg/ml Normocin (Invivogen) and incubated at 37°C in 5% CO2. The cells were harvested using 0.25% trypsin (Fisher Scientific, Pittsburgh, PA) at 24, 48 and 72 hrs after plating and counted with an automated cell counter (Nexcelom Bioscience, Lawrence, MA) using trypan-blue dye exclusion to count live and dead cells. Doubling time was calculated using the formula: (t2-t1) × log(n2)/log(n2/n1), where n is the cell number at time points (t) (14).
Radiography of mice
High resolution radiographic images of mice were obtained using a Faxitron laboratory radiography system LX-60 (Faxitron X-ray Corp., Wheeling, IL) at 30KVp for 10 sec on day 28.
Histopathology and immunohistochemistry
Complete necropsies were performed on the mice and tissues were fixed in 10% neutral-buffered formalin at 4°C for 24 hr. Bones were decalcified in 10% EDTA (pH 7.4) for 2 weeks at 4° C and embedded in paraffin. The specimens were sectioned (5 μm) and were either stained with hematoxylin and eosin (H&E) or evaluated by immunohistochemistry using human antibodies for the presence of CK5/14, 8, 18, 7, vimentin, androgen receptor (AR) and prostate specific antigen (PSA) to characterize the prostate carcinoma cells (see Table 1 for a list of primary antibodies). Sections were deparaffinized in xylene (Hemo-De, Fisher Scientific, Bay Shore, NY) by two 3 min washes and rehydrated in 100%, 95% and 70% ethanol sequentially for 3 min and rinsed in water. Endogenous peroxidase activity was removed using 3% H2O2 (Dako Corp., Carpinteria, CA) for 5 min at room temperature (RT) and washed in PBS for 15 minutes. To block nonspecific binding of proteins, sections were incubated in Protein Block (Dako Corp.) for 15 minutes at RT and rinsed in PBS. Antigen retrieval was performed using target retrieval solution and heated for 30 min in an oven at 60°. Primary antibodies (see Table 1) were added to sections and incubated at RT for 30 minutes and sections were washed three times for 5 minutes in PBS/0.05% Tween. After washing, sections were incubated either with universal biotinylated goat anti-mouse or biotinylated goat anti-rabbit IgG secondary antibody (1:250 dilution in protein block reagent) for 30 min at RT and followed by three 5 minutes washes in PBS/0.05% Tween. Sections were incubated with avidin–biotin complex for 30 min according to the manufacturer's instructions (Vectastain ABC Kit, Vector Laboratories, Inc., Burlingame, CA). To visualize the peroxidase activity sections were incubated with 3,3'-diaminobenzidine (DAB) reagent (1:50 concentrate:reaction buffer) for 5 min at RT and rinsed in distilled water. The slides were counterstained with hematoxylin for 1 min, dried and coverslipped with xylene-based mounting media.
Table 1.
Anti-human antibodies used for immunohistochemistry of the primary prostate carcinoma
| Antibody | Species | Clone | Company | Dilution |
|---|---|---|---|---|
| MA903 (CK5/14) | mouse | 34βE12 | Enzo Life Sciences, Farmingdale, NY | 1:100 |
| CK18 | mouse | KS-B17.2 | Sigma Aldrich, St Louis, MO | 1:100 |
| CK8 | mouse | 4.1.18 | Chemicon, Temecula, CA | 1:30 |
| Vimentin | mouse | Clone V9 | Cell Marque, Hot Springs, AR | 1:50 |
| CK7 | mouse | OV-TL12/30 | Dako Corporation, Carpinteria,CA | 1:40 |
| Androgen Receptor (AR) | rabbit | N-20 | Santa Cruz Biotechnology, Inc, Santa Cruz, CA | 1:500 |
| PSA | rabbit | Polyclonal | Dako Corporation, Carpenteria, Ca | 1:150 |
RNA extraction and reverse transcription-polymerase chain reaction (RTPCR)
Total RNA was extracted from Leo cells, Ace-1 dog prostate cancer cells (4), dog transitional cell carcinoma (TCC) cells (15) and dog brain using Trizol reagent according to manufacturer's protocol (Invitrogen). Total RNA (2.5 μg) was reverse transcribed using the Superscript II First Strand cDNA synthesis kit (Invitrogen) and RT-PCR was performed for parathyroid hormone-related protein (PTHrP), matrix metalloproteinase (MMP-9), vascular endothelial growth factor (VEGF), beta2-microglubulin (B2M), receptor activator of NF-kappaB ligand (RANKL), osteoprotegerin (OPG), nerve growth factor (NGF), low-affinity nerve growth factor receptor (LNGFR), and neurotrophin tyrosine receptor kinase 1 (NTRK1) using canine-specific primers (Table 2).
Table 2.
Dog-specific primers used for RT-PCR amplification
| Gene | Forward Primer (5'- 3') | Reverse Primer (5'- 3') |
|---|---|---|
| PTHrP | AGCTCGGCCGCCGGCTCAA | GGAAGAATCGTCGCCGTAAG |
| MMP9 | TGCAAAGTGAACATCTTCGACGCC | AGAAAGTCTTCTTGGTGAGCCCGT |
| VEGF | ATCGAGTACATCTTCAAGCCATCC | CTATGCTGCAGGAAACTCATCTCC |
| RANKL | AGGTTGGGCCAAGATCTCCAACAT | TCAGCTGAAGATACTCTGTGGCGA |
| OPG | AACTCATGACAATGTATGCTCTGG | ACGCTGAGCCAATTAGGAGT |
| NGF | GAGAAACGCTCACTGGGTTC | TGCTCCTGTGAGTCCTGTTG |
| LNGFR | CTGCAAAGCCTGCAATCTG | CCACCTCTTGAAGGCAATGT |
| NTRK1 | TAGGTGGCAGTTCCTTGTCC | GAACTTGCGGTAGAGGATGC |
| B2M | CTTGCTCCTCATCCTCCTC | TGACACGTAGCAGTTCAG |
PTHrP immunoradiometric assay
PTHrP concentrations were measured in the Leo cell conditioned medium at 0, 6 12, 24 hrs after the addition of serum-containing medium. Negative control medium was not conditioned by any cells. PTHrP concentration was quantified using a two-site immunoradiometric assay (DSL, Webster, TX) specific for PTHrP 1–80 using antibodies to the N-terminal region (amino acids 1 to 40) and mid-region (amino acids 57 to 80) according to manufacturer's instructions.
Analysis of Leo genomic imbalances by fluorescent in situ hybridization (FISH)
Cells were grown to ~80% confluence and 50 ng/ml of Karyomax (Invitrogen) were add to the medium and incubated at 37°C continued for 4 hrs. Chromosome preparations were generated following conventional procedures of hypotonic treatment and fixation in 3:1 methanol:glacial acetic acid. Chromosome preparations were counterstained with 80 ng/ml 4',6-diaminidino-2-phenylindole (DAPI) and mounted in antifade solution (Vectashield, Vector Laboratories). Images were acquired with a fluorescence microscope (Axioplan 2ie, Zeiss) equipped with a DAPI filter set and a cooled CCD camera (CoolSnapHQ, Photometrics, Tuscon, AZ) both driven by dedicated software (SmartCapture 3, Digital Scientific, Cambridge, U.K.). The digital image of each DAPI stained metaphase spread was processed using a high-pass spatial filter to reveal enhanced DAPI bands (16, 17). Chromosome counts were obtained from 50 metaphase preparations. To identify the position of the centromeres of the chromosomes, two BAC clones (330E21 and 326K03) from the CHORI-82 BAC library, which have been shown previously to hybridize to all autosomal centromeres of the canine genome (18), were labeled with SpectrumGreen-dUTP and hybridized to chromosome preparations of this cell line according to routine procedures (19).
Canine genome 2.0 arrays
RNA was extracted from Leo cells and purified with Absolutely RNA® Miniprep Kit (Stratagene, La Jolla, CA, USA). RNA was assayed for quality using Agilent RNA 6000 Nano Assay (Santa Clara, CA, USA). Only samples exhibiting minimal degradation as evidenced by RNA Integrity Numbers (RIN) greater than 8 were used for mircoarrays. Three samples at three separate passages were selected and array analysis with GeneChip Canine 2.0 Genome Arrays (Affymetrix, Santa Clara, CA, USA) was performed at OSU's Comprehensive Cancer Center Microarray Shared Resource. Background correction and normalization was performed to avoid technical bias, and gene expression level was summarized over the probeset using the RMA method. A Linear model was performed to detect differentially expressed genes (20). In order to improve the estimates of variability and statistical tests for differential expression, variance shrinkage method was employed for this study (21). The significance level was adjusted by controlling the mean number of false positives (22).
Data analysis
The half maximum inhibitory concentrations (IC50) of the drugs were calculated using Calcusyn software (Biosoft, Cambridge, UK).
Results
Histopathology of the prostate carcinoma
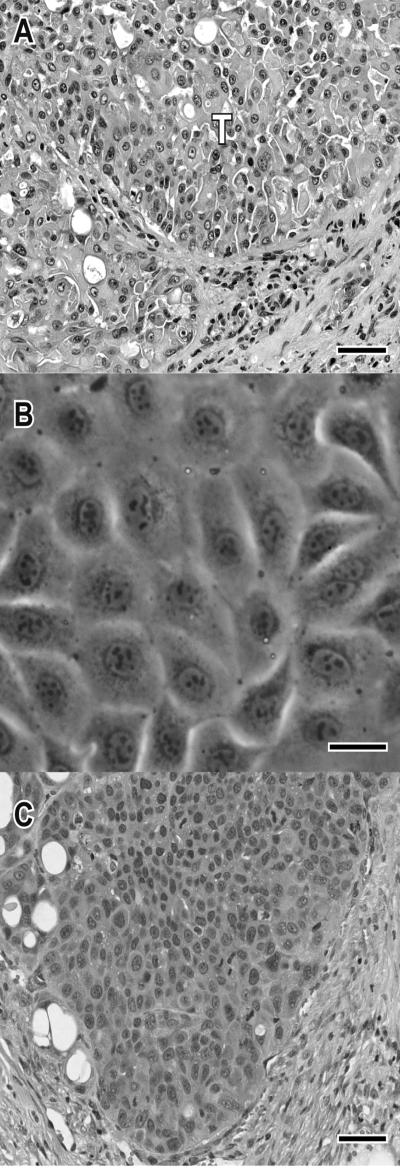
In the dog, the normal prostate gland was replaced by a highly invasive carcinoma consisting of multiple lobules of polygonal to columnar cells arranged in large sheets of cells and nests of cells separated by dense fibrous connective tissue (typical of the alveolar pattern of canine prostate cancer). Neoplastic cells had abundant cytoplasm with occasional cells containing a single, round, clear cytoplasmic vacuole which displaced the nucleus (signet ring formation). Binucleate cells were often apparent and up to 3 nucleoli were present in the nuclei (Fig 1A). Neoplastic tissue had regions of necrosis with secondary inflammation. The carcinoma invaded into the trigone region of urinary bladder and metastasized to the iliac lymph node, liver, lung and right proximal tibia. The central nervous system was not examined since the dog had no neurologic clinical signs.
Figure 1.
Photomicrographs of primary prostate carcinoma, in vitro cell line (Leo) and subcutaneous xenograft. (A) Photomicrograph of hematoxylin and eosin (H&E)-stained primary prostate carcinoma. Note the solid carcinoma consisting of multiple lobules of polygonal to columnar cells (alveolar pattern) separated by fibrous connective tissue. Bar, 50 microns. (B) Phase contrast microscopy of Leo cells (passage 65). The polyhedral cells have a tightly packed cobblestone growth pattern. Bar, 50 microns. (C) Photomicrograph of H&E-stained subcutaneous Leo xenograft. Tumors were multilobular with large polygonal cells. Bar, 50 microns.
Development of Leo prostate cancer cell line
The prostate cancer cell line, Leo, was established from the primary prostate carcinoma tissue. The cell line has been passaged over 75 times in vitro. The cells grow in an anchorage-dependent manner in monolayer sheets with polyhedral cells exhibiting a tightly packed cobblestone growth pattern (Fig 1B).
Subcutaneous growth of Leo prostate carcinoma cells
Leo cells injected into the subcutaneous region of nude mice formed tumors that average 0.8 cm3 in 6 weeks (Fig 4C). The microscopic appearance of the Leo subcutaneous xenografts was similar to the primary carcinoma. (Fig 1C)
Figure 4.
In vitro and in vivo growth patterns of Leo cells. (A) In vitro growth curve of Leo cells. Data presented as mean + standard deviation of 4 replicates. (B) Graph represents the average intensity of the BLI signal measured at the indicated time points. (C) Graph represents the average tumor volume. Data presented as mean + standard deviation of 10 mice.
Immunohistochemistry of the primary prostate carcinoma
The primary prostate carcinoma was moderately positive for cytokeratin (CK) 8 (Fig 2A) and strongly expressed CK18 (Fig 2B), which are markers of prostatic secretory cells. The cancer cells were negative for basal cell cytokeratin markers CK5 and 14 (data not shown). Previously it was reported that prostate cancer can originate from ductal cells in dogs and express the cytokeratin marker, CK7 (23,24). The primary cancer cells expressed CK7 staining in the cytoplasm (Fig 2C). Androgen receptor, human prostate specific antigen (PSA), and vimentin were not expressed by the carcinoma cells (Data not shown). Dog prostate glands do not express human PSA, but have a related kallikrein, arginine esterase (25). The canine genomic array demonstrated that the Leo cells expressed arginine esterase at three different passages (passage 12, 13, and 14).
Figure 2.
Immunohistochemistry of the primary prostate carcinoma. (A) Cytokeratin 8 staining in the cytoplasm was moderately positive. (B) Cytokeratin 18 staining in the cytoplasm was strongly positive. (C) Cytokeratin 7 staining in cytoplasm was moderately positive.
RT-PCR of the Leo prostate cancer cell line
Leo cells expressed mRNA for MMP9 and VEGF (Fig 3), which have been reported to play an important role in the development of brain metastases (26,27). RANKL and OPG were expressed by the Leo cells (Fig 3) and might play a role in the bone metastases developed by Leo cells after intracardiac injection. Expression of PTHrP, NGF, LNGFR, and NTRK1 were not detectable. PTHrP concentrations in Leo cell conditioned media at different time points were similar to control medium without cells.
Figure 3.
mRNA expression of Leo cells. Expression of MMP9, VEGF, OPG, RANKL mRNA were detected using RT-PCR in canine prostate cancer cells lines; Ace-1 and Leo and a dog transitional cell carcinoma cell line (TCC). Expression of β2microglobulin was used as a control.
In vitro growth and in vivo tumorgenicity
Leo cells grew exponentially in vitro with a doubling time of approximately 38 hours under standard cell culture conditions (Fig 4A). Leo cells were transduced with YFP-Luc to monitor in vivo growth. The LeoYFP-Luc cells were injected into the subcutis of nude mice to test their tumorgenicity. Subcutaneous tumor growth was detected in all mice (n=10) and was progressive over 6 weeks (Fig 4B and C).
Metastases of Leo cells in nude mice
Left ventricular intracardiac injections of LeoYFP-Luc were performed in 25 male nude mice in two separate experiments. Metastatic ability of tumor cells was monitored weekly using BLI. At day 7, BLI signal was undetectable indicating that most injected Leo cells died. On day 14, metastatic foci were detected by BLI at different sites including the head, hind limbs, vertebrae and scapular region. BLI intensity of the metastatic foci increased at days 21 and 28. Metastases were detected in 23/25 mice. Mice were sacrificed on day 28 and ex vivo imaging of individual organs were performed immediately to localize the metastases. Metastases detected by BLI were confirmed by histopathological evaluation.
Histopathological analysis showed the presence of prostate carcinoma in the brain (19/25 mice), spinal cord (2/25 mice), adrenal gland (4/25), long bones (5/25) and scapula (1/25). Leo cells most commonly metastasized to brain (Fig 5A and B) when compared to other organs. Tumors in the brain ranged from 1mm to 5mm in diameter and multiple metastatic foci were observed in each brain section. Metastatic tumor in the brain and spinal cord (Fig 5C and D) was most frequently located within the white matter. The brain and spinal cord metastases were expansile with compression of surrounding neurophil consistent with tumor size. Central necrosis and hemorrhage were features of larger tumors within the brain. Adrenal metastases replaced the medulla and cortex of the adrenal gland (Fig 5E and F). In long bone metastases, such as tibia and femur, tumor cells caused osteolysis characterized by loss of cortical and trabecular bone in the metaphyseal region (Fig 6A, B and C). The single metastatic lesion to the scapula was osteoblastic and consisted of tumor cells surrounding the scapular bone and containing large radiating bands (50 um wide) of new woven bone proliferation lined by tall cuboidal osteoblasts (Fig 6D, E and F),
Figure 5.
In vivo BLI and histopathologic evaluation of nude mice 28 days following intracardiac injection of LeoYFP-Luc cells (A) was performed in ventral (a) and lateral (b, c) recumbency and demonstrated metastasis to the cranium. (B) H&E-stained coronal section of brain revealed a large metastatic carcinoma (T, right side) with multiple small metastases in the brain parenchyma. BLI evaluation of spinal cord metastasis (C). Histopathologic evaluation (D) showed a metastatic carcinoma (T) in the white matter of spinal cord (H&E). BLI evaluation revealed a metastasis to the lumbar region (E) which was microscopically confirmed as an adrenal metastasis (F). Tumor cells are indicated by (T), adrenal cortex by (CT) and adrenal medulla by (M). H&E
Figure 6.
Evaluation of bone metastasis on day 28 following intracardiac injection of LeoYFP-Luc in nude mice. (A) BLI showed metastasis to the left hind limb. (B) Radiographic evaluation showed osteolysis (arrow) in the metaphyseal region of the humerus. (C) Histopathologic evaluation showed that tumor (T) replaced bone marrow in the metaphysis and diaphysis of humerus. The metastasis caused extensive bone osteolysis (arrow) characterized by loss of cortical (CT) and trabecular bone. Metastasis to the scapula was detected by BLI (D). Radiographs demonstrated a radiodense lesion (E) which was histologically composed of carcinoma interspersed with radiating trabeculae of woven bone (F).
In vitro chemosensitivity
The in vitro chemosensitivity of the Leo cells to different doses of calcitriol, piroxicam, (S)-HDAC-42 and velcade was measured using the MTT assay at 72 hrs. All the drugs inhibited cell growth in a dose-dependent manner. The half maximum inhibitory concentrations (IC50) were calculated using Calcusyn software and were 6.5 μM for calcitriol (Fig 7A), 1.9 nM for velcade (Fig 7B), 0.95 μM for (S)-HDAC-42 (Fig 7C) and 518 μM for piroxicam (Fig 7D). The in vitro IC50 concentrations of calcitriol and piroxicam were higher than what is accepted as physiologically achievable in patients. Leo cells were most sensitive to (S)-HDAC-42 and velcade compared to calcitriol and piroxicam.
Figure 7.
Dose-response of Leo cell viability to calcitriol (A), velcade (B), (S)-HDAC-42 (C) and piroxicam (D) treatment. An MTT assay was performed with 6 replicates for each dose for 72 hours. IC50 values for calcitriol, piroxicam, (S)-HDAC-42 and velcade were 6.8 μM, 518 μM, 0.95 μM, and 1.9 nM respectively. Bars represent mean + standard deviation of 6 replicates.
Genomic imbalances in Leo cells
All of the 50 cells evaluated for chromosome number were hyperdiploid, containing between 120–138 chromosomes, with a mean of 131. Seventy five percent of the cells had a count between 130–135. Typical metaphase spreads of Leo cells (Fig 8Ai and Aii) showed that most of the chromosomes were short armed and 6–8 chromosomes were bi-armed. Centromeres were not detected in three chromosomes using BAC clones that were previously shown to hybridize to all canine autosomes, suggesting that these three aberrant chromosomes may contain centromeres from the sex chromosomes (18).
Figure 8.

Ai, Aii) Typical metaphase chromosome preparations derived from Leo cells, exhibiting chromosome numbers in excess of 130 per cell. In addition to the high number of chromosome number, there are several bi-armed chromosomes indicative of structural as well as numerical changes. Bi) Hybridization of two BAC clones that map to all autosomes of the canine genome revealed that centromeres were detected in all but nine chromosomes (arrows). Bii) Overexposure of the same metaphase spreads was unable to detect even a weak signal at the centromeres of three (red, yellow and white arrowheads) of the nine chromosomes.
DISCUSSION
Understanding the pathogenesis of prostate cancer and development of novel therapeutic agents can be improved with additional unique cell lines and in vivo animal models that mimic the condition in men (7). Therefore, development of new cell lines and animal models that closely recapitulate the clinical disease in patients are crucial to understand the molecular heterogeneity and pathogenesis of prostate cancer. Dogs not only develop spontaneous prostate cancer but also share similarities to the clinical presentation of prostate cancer in men (2,11,28). In the present study, we described the establishment of a novel dog prostate cancer cell line (Leo) and characterization of its tumorgenicity and metastatic ability to various organs in nude mice.
The Leo cell line was developed from a 5-year-old castrated male mixed breed dog. The Leo cells formed tumors in the subcutis of nude mice demonstrating its tumorgenicity in vivo. Following intracardiac inoculation, the Leo cells metastasized to multiple sites including the brain, spinal cord, bones, and adrenal gland. The Leo prostate cancer cells exhibited a remarkable tropism for developing metastases in the brain based on the 80% incidence of brain metastases. It has been reported that MMP9 is upregulated in most brain metastases (26). MMP9 belongs to matrix metalloprotease family of enzymes that degrades the extracellular matrix leading to invasion of tumor cells (29). High expression of MMP9 by Leo cells suggests that it might play a significant role in the development of brain metastases. Increased VEGF expression by cancer cells helps in angiogenesis and transendothelial migration by regulating the permeability of brain microvascular endothelial cells (30). The Leo cells express VEGF, which may be important in the development of brain metastases seen in this model. Although VEGF and MMP9 may facilitate brain metastasis, other factors must also be involved because the Ace-1 and TCC cell lines also produce VEGF and MMP9 and do not metastasize to the brain.
The brain is a rich source of NGF, which is important in the development and maintenance of nervous tissue (31). NGF regulates its action by binding and activating to two types of neurotrophin receptors, a low affinity nerve growth factor receptor (LNGFR or P75) and neurotrophin receptor tyrosine kinase (NTRK) (32). However, expression of NGF, LNGFR and NTRK1 were not detectable in Leo cells. Leo cells had severe chromosomal imbalances such as hyperdiploidy and bi-armed chromosomes. Further evaluation of the chromosome imbalances will be important to understand the molecular pathogenesis of metastasis of the Leo cells.
Dog prostate cancers can have mixed histologic features of adenocarcinoma and ductal differentiation (2). It has been suggested that prostate cancer in dogs may originate from prostate ductal cells that are positive for CK7 (23,24). Expression of CK7 in addition to secretory epithelial cytokeratins, 8 and 18, by the Leo cells suggests that they have differentiated towards both secretory and ductal cells. Ductal cells with CK 7 expression in dogs may also have urothelial differentiation (24).
The androgen receptor in dogs is expressed in prostate secretory and basal epithelial cells, ductal cells, stromal cells and the prostatic urethra (23). However, in neutered dogs the AR is not expressed. Lack of expression of AR in the Leo cell line, developed from a prostate carcinoma in a neutered dog, is consistent with previous reports and typical of an androgen-insensitive canine prostate carcinoma (23). The Leo cells were negative for vimentin, which is generally expressed by stromal cells although it has been reported in some prostate cancer cells suggesting epithelial to mesenchymal transition (4). Prostate specific antigen, a biomarker of prostate cancer in human, is expressed in glandular epithelium and liquefies the semen by degrading semenogelin (33,34). PSA belongs to the family of kallikrein genes and it is encoded by kallikrein 3. In human 15 kallikreins were identified whereas in dogs only 14 kallikreins are present. The human kallikrein 3 gene encoding PSA in humans is absent in dogs (35). However, dog kallikrein 2, encoding arginine esterase (AE), shares 58% amino acid homology with human PSA and both genes are regulated by androgens (36,37). Polyclonal and monoclonal PSA antibodies cross-react with human kallikreins 1 and 2 since the degree of amino acid homology is 80% (38). A few reports have demonstrated staining for PSA (using antibodies to human PSA) in normal dog prostate and canine prostate cancers (39). Presumably this may be due to cross-reactivity to canine arginine esterase. However, since dogs lack a homolog to human PSA, PSA assay and immunohistochemistry generally are not useful in dogs (39). The Leo cells did express arginine esterase.
The bone metastases that developed in nude mice with the Leo cells were predominantly osteolytic similar to that observed in the dog with the tibial metastasis. Dog prostate cancer metastases to bones mostly affect the axial skeleton and proximal long bones and can be osteoblastic, osteolytic or mixed (2). We have also developed a nude mouse model of dog prostate cancer using the Ace-1 cells that develop a 100% incidence of mixed osteoblastic and osteolytic bone metastases (11). The Leo cells have a unique metastatic pattern in vivo with a relatively low incidence of metastases to bone (20%). The RANKL and OPG axis has been shown to play an important role in regulating osteolytic bone metastases (40). RANKL expressed by prostate cancer cells binds to RANK receptor expressed on the surface of osteoclast precursors and induces their differentiation and maturation into active osteoclasts. OPG expressed by cancer cells acts as decoy receptor for RANKL and prevents it from binding to RANK resulting in inhibition of osteoclastogenesis (41). Leo cells, similar to the Ace-1 prostate cancer cell line, express high levels of both RANKL and OPG. The RANKL may be responsible for the induction of osteoclastic bone resorption, and the amount of OPG is not adequate to inhibit the osteoclastic bone resorption in the metastases. The Leo cells likely do not make significant osteoblastic factors that are a characteristic of the Ace-1 cells.
Velcade (PS341), a proteosome inhibitor, was shown to inhibit prostate cancer growth and osteolytic bone metastases in preclinical models (42). Leo cells were very sensitive to velcade with an IC50 of 1.9 nm. Serum concentrations of velcade in human patients of 40 to 90 ng/L were shown to have antitumor activity without serious adverse effects (43,44). Therefore, it will be important to test the efficacy of velcade on tumor growth and metastases in the Leo model of prostate cancer. A novel HDAC-42 inhibitor developed at Ohio State University was very effective in inhibiting the growth of Leo cells in vitro with an IC50 of 0.95 μm at after 72 hours of treatment. This novel phenylbutyrate-derived HDAC inhibitor was shown to possess potent anti-tumor activity in prostate cancer by inducing apoptosis (45). Despite the fact that calcitriol and piroxicam had inhibited the Leo cell growth, the IC50 concentrations were too great to achieve relevant serum levels in clinical patients.
One of the most important findings of the Leo xenografts was the consistent and frequent development of brain metastases in nude mice. To our knowledge, this is the first prostate cancer cell line that consistently develops brain metastases in vivo. This provides a unique translational model to investigate the pathogenesis of brain metastases and develop potent therapeutic strategies to treat or prevent brain metastases. Though the incidence of bone metastases was not high, it may be feasible to develop a subline of the Leo cells with a high rate of bone metastases, which would be a valuable model to study the skeletal complications of prostate cancer. Therefore, the new Leo prostate cancer cell line provides a valuable tool to help understand the molecular mechanisms responsible for the pathogenesis of prostate cancer and metastases to the brain and bone.
Table 3.
Incidence of LeoYFP-Luc metastases by anatomic location at 28 days following intracardiac injection
| Site of Metastasis | Incidence |
|---|---|
| Total mice with metastases | 23/25 |
| Brain | 19/25 |
| Spinal cord | 2/25 |
| Bone | 5/25 |
| Adrenal gland | 4/25 |
Acknowledgements
This work was supported by the National Cancer Institute (2P01 CA93900) and The Ohio State University College of Veterinary Medicine (520026).
We thank Tim Vojt for help with preparation of the figures, Wessel Dirksen for help with laboratory experiments and Alan Fletcher for help with the histology.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics, 2008. CA: A Cancer Journal for Clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Waters DJ, Sakr WA, Hayden DW, Lang CM, McKinney LA, Murphy GP, Radinsky R, Ramoner R, Richardson RC, Tindall DJ. Workgroup 4: Spontaneous Prostate Carcinoma in Dogs and Nonhuman Primates. The Prostate. 1998;36:64–7. doi: 10.1002/(sici)1097-0045(19980615)36:1<64::aid-pros12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, Breen M, Kitchell B, McNeil E, Modiano JF. The Dog as a Cancer Model. Nature Biotechnology. 2006;24:1065–6. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- 4.LeRoy BE, Thudi NK, Nadella MVP, Toribio RE, Tannehill-Gregg SH, van Bokhoven A, Davis D, Corn S, Rosol TJ. New Bone Formation and Osteolysis by a Metastatic, Highly Invasive Canine Prostate Carcinoma Xenograft. The Prostate. 2006;66:1213–22. doi: 10.1002/pros.20408. [DOI] [PubMed] [Google Scholar]
- 5.Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic Patterns of Prostate Cancer: An Autopsy Study of 1,589 Patients. Human Pathology. 2000;31:578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 6.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. New England Journal of Medicine. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 7.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, MacVicar GR, Varambally S, Harwood J, Bismar TA. Androgen-Independent Prostate Cancer is a Heterogeneous Group of Diseases Lessons from a Rapid Autopsy Program. Cancer Research. 2004;64:9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Jones E, Gulley J, Arlen P, Chen C, Figg W, Dahut W. Routine Interval Computed Tomography in Detecting New Soft Tissue Disease in Patients with Androgen-Independent Prostate Cancer (AIPC) and Only Bone Metastasis. Journal of Clinical Oncology. 2006;24:4621. doi: 10.1111/j.1464-410X.2006.06654.x. [DOI] [PubMed] [Google Scholar]
- 9.Tremont-Lukats IW, Bobustuc G, Lagos GK, Lolas K, Kyritsis AP, Puduvalli VK. Brain Metastasis from Prostate Carcinoma. Cancer. 2003;98:363–8. doi: 10.1002/cncr.11522. [DOI] [PubMed] [Google Scholar]
- 10.Marosi C. Chemotherapy in Patients with Brain Metastases. Magazine of European Medical Oncology. 2008;1:11–3. [Google Scholar]
- 11.Thudi NK, Martin CK, Nadella MVP, Fernandez SA, Werbeck JL, Pinzone JJ, Rosol TJ. Zoledronic Acid Decreased Osteolysis but Not Bone Metastasis in a Nude Mouse Model of Canine Prostate Cancer with Mixed Bone Lesions. The Prostate. 2008;68:1116–25. doi: 10.1002/pros.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a Human Prostate Carcinoma Cell Line (DU 145) International Journal of Cancer. 1978;21:274–81. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 13.Nadella MV, Kisseberth WC, Nadella KS, Thudi NK, Thamm DH, McNiel EA, Yilmaz A, Boris-Lawrie K, Rosol TJ. NOD/SCID Mouse Model of Canine T-Cell Lymphoma with Humoral Hypercalcaemia of Malignancy: Cytokine Gene Expression Profiling and in Vivo Bioluminescent Imaging. Veterinary and Comparative Oncology. 2008;6:39–54. doi: 10.1111/j.1476-5829.2007.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright K, El Masri W, Osman A, Roberts S, Trivedi J, Ashton B, Johnson W. The Cell Culture Expansion of Bone Marrow Stromal Cells from Humans with Spinal Cord Injury: Implications for Future Cell Transplantation Therapy. Spinal Cord. 2008;46:811–7. doi: 10.1038/sc.2008.77. [DOI] [PubMed] [Google Scholar]
- 15.Kaewsakhorn T, Kisseberth WC, Capen CC, Hayes KA, Calverley MJ, Inpanbutr N. Effects of Calcitriol, Seocalcitol, and Medium-Chain Triglyceride on a Canine Transitional Cell Carcinoma Cell Line. Anticancer Research. 2005;25:2689–96. [PubMed] [Google Scholar]
- 16.Breen M, Bullerdiek J, Langford CF. The DAPI Banded Karyotype of the Domestic Dog (Canis Familiaris) Generated using Chromosome-Specific Paint Probes. Chromosome Research. 1999;7:401–6. doi: 10.1023/a:1009224232134. [DOI] [PubMed] [Google Scholar]
- 17.Breen M, Jouquand S, Renier C, Mellersh CS, Hitte C, Holmes NG, Chéron A, Suter N, Vignaux F, Bristow AE. Chromosome-Specific Single-Locus FISH Probes Allow Anchorage of an 1800-Marker Integrated Radiation-hybrid/linkage Map of the Domestic Dog Genome to all Chromosomes. Genome Research. 2001;11:1784. doi: 10.1101/gr.189401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas R, Duke S, Karlsson E, Evans A, Ellis P, Lindblad-Toh K, Langford C, Breen M. A Genome Assembly-Integrated Dog 1 Mb BAC Microarray: A Cytogenetic Resource for Canine Cancer Studies and Comparative Genomic Analysis. Cytogenetic and Genome Research. 2008;122:110–21. doi: 10.1159/000163088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breen M, Hitte C, Lorentzen TD, Thomas R, Cadieu E, Sabacan L, Scott A, Evanno G, Parker HG, Kirkness EF, Hudson R, Guyon R, Mahairas GG, Gelfenbeyn B, Fraser CM, Andre C, Galibert F, Ostrander EA. An Integrated 4249 Marker FISH/RH Map of the Canine Genome. BMC Genomics. 2004;5:65. doi: 10.1186/1471-2164-5-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, Normalization, and Summaries of High Density Oligonucleotide Array Probe Level Data. Biostatistics. 2003;4:249. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 21.Smyth GK. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Statistical Applications in Genetics and Molecular Biology. 2004;3:1027. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 22.Gordon A, Glazko G, Qiu X, Yakovlev A. Control of the Mean Number of False Discoveries, Bonferroni and Stability of Multiple Testing. Annals of Applied Statistics. 2007;1:179–90. [Google Scholar]
- 23.Leav I, Schelling KH, Adams JY, Merk FB, Alroy J. Role of Canine Basal Cells in Prostatic Post Natal Development, Induction of Hyperplasia, Sex Hormone-Stimulated Growth; and the Ductal Origin of Carcinoma. The Prostate. 2001;47:149–63. doi: 10.1002/pros.1058. [DOI] [PubMed] [Google Scholar]
- 24.LeRoy B, Nadella M, Toribio R, Leav I, Rosol T. Canine Prostate Carcinomas Express Markers of Urothelial and Prostatic Differentiation. Veterinary Pathology. 2004;41:131–40. doi: 10.1354/vp.41-2-131. [DOI] [PubMed] [Google Scholar]
- 25.Chapdelaine P, Gauthier E, Ho-Kim MA, Bissonnette L, Tremblay RR, Dube JY. Characterization and Expression of the Prostatic Arginine Esterase Gene, a Canine Glandular Kallikrein. DNA and Cell Biology. 1991;10:49–59. doi: 10.1089/dna.1991.10.49. [DOI] [PubMed] [Google Scholar]
- 26.Arnold SM, Young AB, Munn RK, Patchell RA, Nanayakkara N, Markesbery WR. Expression of p53, Bcl-2, E-Cadherin, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinases-1 in Paired Primary Tumors and Brain Metastasis. Clinical Cancer Research. 1999;5:4028–33. [PubMed] [Google Scholar]
- 27.Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Davis DW, McConkey DJ, Fidler IJ. Expression of Vascular Endothelial Growth Factor is Necessary but Not Sufficient for Production and Growth of Brain Metastasis 1. Cancer Research. 2000;60:4959–67. [PubMed] [Google Scholar]
- 28.Teske E, Naan E, Van Dijk E, Van Garderen E, Schalken J. Canine Prostate Carcinoma: Epidemiological Evidence of an Increased Risk in Castrated Dogs. Molecular and Cellular Endocrinology. 2002;197:251–5. doi: 10.1016/s0303-7207(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 29.Nathoo N, Chahlavi A, Barnet GH, Toms SA. Pathobiology of Brain Metastases. British Medical Journal. 2005;58:237–42. doi: 10.1136/jcp.2003.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TH, Avraham HK, Jiang S, Avraham S. Vascular Endothelial Growth Factor Modulates the Transendothelial Migration of MDA-MB-231 Breast Cancer Cells through Regulation of Brain Microvascular Endothelial Cell Permeability. Journal of Biological Chemistry. 2003;278:5277–84. doi: 10.1074/jbc.M210063200. [DOI] [PubMed] [Google Scholar]
- 31.Misko T. Nerve Growth Factor in Neuronal Development and Maintenance. Journal of Experimental Biology. 1987;132:177–90. doi: 10.1242/jeb.132.1.177. [DOI] [PubMed] [Google Scholar]
- 32.Bothwell M. Functional Interactions of Neurotrophins and Neurotrophin Receptors. Annual Review of Neuroscience. 1995;18:223–53. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez J, Thompson IM. Prostate-Specific Antigen: A Review of the Validation of the most Commonly used Cancer Biomarker. Cancer. 2004;101:894–904. doi: 10.1002/cncr.20480. [DOI] [PubMed] [Google Scholar]
- 34.Robert M, Gibbs BF, Jacobson E, Gagnon C. Characterization of Prostate-Specific Antigen Proteolytic Activity on its Major Physiological Substrate, the Sperm Motility Inhibitor Precursor/Semenogelin I†. Biochemistry. 1997;36:3811–9. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- 35.Elliott MB, Irwin DM, Diamandis EP. In Silico Identification and Bayesian Phylogenetic Analysis of Multiple New Mammalian Kallikrein Gene Families. Genomics. 2006;88:591–9. doi: 10.1016/j.ygeno.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Dube J. Search for Androgen Response Elements in the Proximal Promoter of the Canine Prostate Arginine Esterase Gene. Journal of Andrology. 1995;16:304–11. [PubMed] [Google Scholar]
- 37.Clements J. The Glandular Kallikrein Family of Enzymes: Tissue-Specific Expression and Hormonal Regulation. Endocrine Reviews. 1989;10:393–419. doi: 10.1210/edrv-10-4-393. [DOI] [PubMed] [Google Scholar]
- 38.Yousef GM, Diamandis EP. An Overview of the Kallikrein Gene Families in Humans and Other Species: Emerging Candidate Tumour Markers. Clinical Biochemistry. 2003;36:443–52. doi: 10.1016/s0009-9120(03)00055-9. [DOI] [PubMed] [Google Scholar]
- 39.Lai CL, van den Ham R, van Leenders G, van der Lugt J, Mol JA, Teske E. Histopathological and Immunohistochemical Characterization of Canine Prostate Cancer. The Prostate. 2008;68:477–88. doi: 10.1002/pros.20720. [DOI] [PubMed] [Google Scholar]
- 40.Wittrant Y, Theoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D, Redini F. RANKL/RANK/OPG: New Therapeutic Targets in Bone Tumours and Associated Osteolysis. Biochimica Biophysica Acta-Reviews on Cancer. 2004;1704:49–57. doi: 10.1016/j.bbcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Dai J, Yao Z, Lu Y, Dougall W, Keller ET. Soluble Receptor Activator of Nuclear Factor κB Fc Diminishes Prostate Cancer Progression in Bone. Cancer Research. 2003;63:7883–90. [PubMed] [Google Scholar]
- 42.Whang P, Gamradt S, Gates J, Lieberman J. Effects of the Proteasome Inhibitor Bortezomib on Osteolytic Human Prostate Cancer Cell Metastases. Prostate Cancer and Prostatic Diseases. 2005;8:327–34. doi: 10.1038/sj.pcan.4500823. [DOI] [PubMed] [Google Scholar]
- 43.Moreau P, Coiteux V, Hulin C, Leleu X, van de Velde H, Acharya M, Harousseau JL. Prospective Comparison of Subcutaneous Versus Intravenous Administration of Bortezomib in Patients with Multiple Myeloma. Haematologica. 2008;93:1908–11. doi: 10.3324/haematol.13285. [DOI] [PubMed] [Google Scholar]
- 44.Attar EC, DeAngelo DJ, Supko JG, D'Amato F, Zahrieh D, Sirulnik A, Wadleigh M, Ballen KK, McAfee S, Miller KB. Phase I and Pharmacokinetic Study of Bortezomib in Combination with Idarubicin and Cytarabine in Patients with Acute Myelogenous Leukemia. Clinical Cancer Research. 2008;14:1446–54. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
- 45.Kulp SK, Chen CS, Wang DS, Chen CY, Chen CS. Antitumor Effects of a Novel Phenylbutyrate-Based Histone Deacetylase Inhibitor,(S)-HDAC-42, in Prostate Cancer. Clinical Cancer Research. 2006;12:5199–206. doi: 10.1158/1078-0432.CCR-06-0429. [DOI] [PubMed] [Google Scholar]