Abstract
A 2008 survey assessed the proportion of smokers in 8 geographic areas who reported trying snus. In test markets, 10% of smokers had tried snus in the past year. Among young adult men, the trial rate was 29%. Trial was more likely among Whites than among minorities, among respondents with lower education than among those with higher education, and among those without immediate plans to quit smoking than among those intending to quit in the next 30 days. The association between trial and low cessation motivation is an important target for research.
Tobacco control measures, such as increases in excise taxes on cigarettes, the spread of smoke-free ordinances, and growing antismoking norms, have resulted in a decline in cigarette smoking.1–4 In response, the tobacco industry has attempted to diversify its product offerings in the United States.5 In 2006, RJ Reynolds and Altria launched snus in several US test markets. This new tobacco product differs from conventional smokeless tobacco in that it (1) is lower in tobacco-specific nitrosamines, a major carcinogen; (2) does not require spitting; and (3) is packaged in small pouches that are placed under the upper lip and can be relatively unobtrusive when in use.
The introduction of snus in the United States has been controversial within the tobacco control community. Although snus is less harmful than cigarettes,6–9 some argue that promoting snus for harm reduction could lead to a delay in smoking cessation and the erroneous perception that the products are safe.8,10–15 Snus is currently being marketed to smokers as a way to enjoy tobacco in places where smoking is prohibited.16 To date, advertising messages do not include claims of reduced risk, but companies are seeking permission from the US Food and Drug Administration to make such health claims.17 It is difficult to predict how consumers will respond to these new spitless products, especially if they are advertised as less harmful than smoking.
The only published population-based study of awareness and trial of snus in US test markets took place in Indiana.18 Results indicated that about 20% of male smokers reported having tried 1 of the products, but trial was rare (1.4%) among female smokers. The Indiana study was limited by relatively small samples of smokers, and findings reflected only 1 test market area. The current study included a larger sample of smokers in 8 designated market areas. Three of these included test markets for several snus products. We investigated associations between snus trial, demographic characteristics, and smoking patterns.
METHODS
The data were from the first 2 waves of an ongoing longitudinal evaluation of EX®, a national mass media smoking cessation campaign. Survey items on snus trial were added to the second wave. The baseline sample included smokers from 8 designated market areas, 3 of which were test markets for snus: Portland, OR; Kansas City, MO; and Columbus, OH. Identical surveys also were administered in 5 designated market areas that had not been designated as snus test markets: Birmingham, AL; Fort Smith/Fayetteville, AR; Houston, TX; Phoenix/Prescott, AZ; and Pittsburgh, PA.
We conducted the baseline random-digit–dialed survey between February 5, 2008, and April 15, 2008, to identify smokers 18 to 49 years of age. Screening interviews identified 8489 eligible respondents. A total of 5616 (66%) completed the interview. The follow-up survey, conducted between August 23, 2008, and October 19, 2008, was completed by 4067 smokers (retention rate = 72%; overall response rate = 48% among known eligible households when The American Association for Public Opinion Research response rate method 3 was used).19
The outcome measure—confirmed trial of snus—was included only on the follow-up survey. The 3 criteria for confirmed trial were (1) affirming having heard of “New tobacco products … that come in teabaglike pouches that are put in the mouth under the lip (and) do not involve chewing, spitting or smoking”; (2) reporting having used such a product in the past 12 months; and (3) identifying the product as 1 of the following: Taboka, Camel Snus, Skoal Dry, Marlboro Snus, Triumph Snus, Grand Prix Snus, or Tourney Snus.
Predictors included the following variables all measured at baseline: residence in a test market (yes or no), gender, age, minority status (White, non-Hispanic vs other), education level (high school or less vs more than high school), nicotine dependence (smoked ≥ 20 per day and had first cigarette within 30 minutes of waking or not), quitting intentions (planned to quit within 30 days or not), and exposure to smoking bans at work and at home. We also assessed smoking cessation on the basis of smoking status reported at follow-up.
RESULTS
Unadjusted odds ratios showed that trying snus was significantly more likely among test market residents than among non–test market residents; among males than among females; among younger adults than among older adults; among White, non-Hispanic respondents than among minority respondents; and among those with no more than a high-school education than among those with higher levels of education (see Table 1). Confirmed trial was significantly associated with lack of intention to quit smoking within 30 days but was not associated with level of nicotine dependence or with exposure to smoking bans at work or at home. Smoking cessation between the baseline and the follow-up survey also was unrelated to snus trial.
TABLE 1.
Levels of Snus Trial in Demographic and Smoking Subgroups Among 4067 Smokers in 8 Designated Market Areas: United States, August 23–October 19, 2008
| Tried Snus, No. (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Snus test market | |||
| Yes | 1704 (10.4) | 4.69* (2.87, 7.66) | 4.87* (2.89, 8.21) |
| No (Ref) | 2363 (2.4) | 1.00 | 1.00 |
| Gender | |||
| Male | 1840 (8.0) | 4.21* (2.36, 7.50) | 4.00* (2.22, 7.24) |
| Female (Ref) | 2227 (2.0) | 1.00 | 1.00 |
| Age group, y | |||
| 18–24 | 616 (12.3) | 9.21* (5.33, 15.93) | 9.46* (5.21, 7.19) |
| 25–35 | 1094 (5.6) | 3.89* (2.20, 6.90) | 3.77* (2.12, 6.69) |
| 36–49 (Ref) | 2357 (1.5) | 1.00 | 1.00 |
| Race/Ethnicity | |||
| White, non-Hispanic | 3013 (6.5) | 2.69* (1.43, 5.05) | 2.02* (1.07, 3.83) |
| Hispanic minority (Ref) | 1054 (2.5) | 1.00 | 1.00 |
| Education level | |||
| ≤ High school | 2414 (6.5) | 1.96* (1.27, 3.03) | 1.62 (0.99, 2.66) |
| > High school (Ref) | 1653 (3.4) | 1.00 | 1.00 |
| Heavy smoking | |||
| Yes | 1521 (6.1) | 1.25 (0.81, 1.94) | 1.32 (0.74, 2.34) |
| No (Ref) | 2546 (5.0) | 1.00 | 1.00 |
| Plans to quit | |||
| Not in next 30 d | 3397 (5.9) | 2.38* (1.25, 4.54) | 2.37* (1.12, 5.00) |
| Next 30 d (Ref) | 670 (2.6) | 1.00 | 1.00 |
| Smoking ban at work | |||
| Yes | 1612 (6.1) | 1.61 (0.95, 2.73) | 1.31 (0.75, 2.29) |
| No | 864 (6.6) | 1.74 (0.98, 3.09) | 1.21 (0.66, 2.24) |
| Doesn't work or works at home (Ref) | 1591 (3.9) | 1.00 | 1.00 |
| Smoking ban at home | |||
| Yes | 1708 (5.8) | 1.20 (0.79, 1.84) | 1.19 (0.67, 2.10) |
| No (Ref) | 2359 (4.9) | 1.00 | 1.00 |
| Smoking status at follow-up | |||
| Quit | 217 (5.9) | 1.12 (0.53, 2.35) | 1.92 (0.80, 4.61) |
| Still smoking (Ref) | 3850 (5.3) | 1.00 | |
Note. CI = confidence interval; OR = odds ratio.
*Significant at the .05 level of confidence. Numbers are unweighted; percentages are weighted.
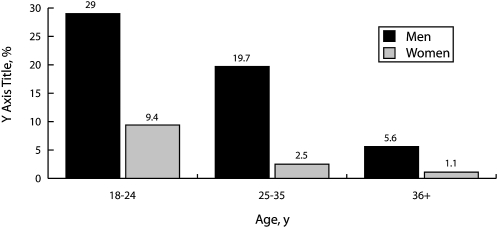
A multivariate logistic regression examined the independent effect of each of the predictors while controlling for the other predictors and confirmed that snus trial was almost 5 times more likely to occur in test markets than in non–test markets, 4 times more likely among males than among females, and 9 times more likely among young adult smokers aged 18 to 24 years than among smokers older than 36 years. White smokers were twice as likely as minorities to try snus, and those with at most a high-school education were about 1.6 times more likely to try snus than were those with higher education. Finally, those with no immediate plans to quit were more than twice as likely to try snus as were those reporting an intention to quit in the next 30 days. Figure 1 shows that in the test market areas, almost one third of the youngest adult male smokers and about one tenth of the youngest adult female smokers had tried the new snus products. The products, as currently marketed, apparently have not been of much interest to smokers older than 36 years.
FIGURE 1.
Trial of snus products by gender among 1074 smokers residing in test markets: Portland, OR; Kansas City, MO; and Columbus, OH, 2008.
DISCUSSION
Although the current findings support earlier work reporting that these oral tobacco products are more likely to be tried by men than by women, our study was the first to identify the clear age gradient showing a high magnitude of interest among those aged 18 to 24 years, particularly males (29% of whom tried snus), and progressively lower levels of trial in the older age groups.
The fact that smokers who had no immediate plans to quit were more likely to try snus prompts the following question: Does having low motivation to quit smoking lead one to try snus, or does trying snus lower one's motivation to quit? We cannot determine the answer because we assessed snus trial only at follow-up, and trial might have occurred before the baseline measurement of quit intentions. If low motivation preceded snus trial (i.e., if snus is attractive to individuals who do not want to give up smoking but want to use it where smoking is banned), then using snus instead of going outside to smoke could potentially reduce health risks. However, if trial of snus leads to the lowering of motivation to quit smoking, then health risks would increase if the cessation rate were actually reduced by the product.
As the US Food and Drug Administration begins to implement regulation of tobacco products, it is essential that surveillance mechanisms of these new smokeless tobacco products be established. Because snus has the potential both to reduce exposure to tobacco toxins and to delay cessation, it is critical to better understand how the products will be used if we are to reduce the toll of the tobacco epidemic.
Acknowledgments
This study was funded by the American Legacy Foundation. L. Biener's effort was funded by the National Cancer Institute (grant 3 R01 CA086257-07S3).
We are grateful for the insight and intervention of Donna Vallone, who recognized the opportunity to add questions about snus to an ongoing survey, and for the assistance of Amy L. Nyman, who carried out most of the data analysis.
Human Participant Protection
This study was approved by the human subjects review committees of Westat, the data collection contractor, and Copernicus Group IRB, the external institutional review board used by the American Legacy Foundation.
References
- 1.Biener L, Hamilton WL, Siegel M, Sullivan EM. Individual, social-normative, and policy predictors of smoking cessation: a multilevel longitudinal analysis. Am J Public Health. 2010;100:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton WL, Biener L, Brennan RT. Do local tobacco regulations influence perceived smoking norms? Evidence from adult and youth surveys in Massachusetts. Health Educ Res. 2008;23:709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roeseler A, Burns D. The quarter that changed the world. Tob Control. 2010;19(suppl 1):i3–i15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis JA, Abramsohn EM, Park HY. Policy-driven tobacco control. Tob Control. 2010;19(suppl 1):i16–i20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpert HR, Koh H, Connolly GN. Free nicotine content and strategic marketing of moist snuff tobacco products in the United States: 2000–2006. Tob Control. 2008;17:332–338 [DOI] [PubMed] [Google Scholar]
- 6.Levy DT, Mumford EA, Cummings KM, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: estimates of a panel of experts. Cancer Epidemiol Biomarkers Prev. 2004;13:2035–2042 [PubMed] [Google Scholar]
- 7.Foulds J, Ramstrom L, Burke M, Fagerstrom K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob Control. 2003;12:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savitz DA, Meyer RE, Tanzer JM, Mirvish SS, Lewin F. Public health implications of smokeless tobacco use as a harm reduction strategy. Am J Public Health. 2006;96:1934–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J, Ye W, Zendehdel K, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet. 2007;369(9578):2015–2020 [DOI] [PubMed] [Google Scholar]
- 10.Hatsukami DK, Henningfield JE, Kotlyar M. Harm reduction approaches to reducing tobacco-related mortality. Annu Rev Public Health. 2004;25:377–395 [DOI] [PubMed] [Google Scholar]
- 11.Zhu SH, Wang JB, Hartman A, et al. Quitting cigarettes completely or switching to smokeless tobacco: do US data replicate the Swedish results? Tob Control. 2009;18:82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomar SL. Epidemiologic perspectives on smokeless tobacco marketing and population harm. Am J Prev Med. 2007;33(6 suppl):S387–S397 [DOI] [PubMed] [Google Scholar]
- 13.Roosaar A, Johansson ALV, Sandborgh-Englund G, Axell T, Nyren O. Cancer and mortality among users and nonusers of snus. Int J Cancer. 2008;123:168–173 [DOI] [PubMed] [Google Scholar]
- 14.Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products: new tobacco-delivery systems. Am J Prev Med. 2007;33(6 suppl):S368–S378 [DOI] [PubMed] [Google Scholar]
- 15.Zeller M, Hatsukami D. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob Control. 2009;18:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpenter CM, Connolly G, Ayo-Yusuf O, Wayne GF. Developing smokeless tobacco products for smokers: an examination of tobacco industry documents. Tob Control. 2009;18:54–59 [DOI] [PubMed] [Google Scholar]
- 17.Kesmodel D. Philip Morris pushes smokeless: firm wants FDA to position tobacco product as safer alternative to cigarettes. Wall Street Journal. January 6, 2010. Available at: http://online.wsj.com/article/SB10001424052748704160504574640433243035824.html?KEYWORDS=Philip+Morris+pushes+smokeless. Accessed June 30, 2010
- 18.Biener L, Bogen K. Receptivity to Taboka and Camel Snus in a U.S. test market. Nicotine Tob Res. 2009;11:1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The American Association for Public Opinion Research Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 6th ed 2009. Available at: http://www.aapor.org/Content/NavigationMenu/ResourcesforResearchers/StandardDefinitions/StandardDefinitions2009new.pdf. Accessed May 10, 2010