Abstract
Obesity is a serious and growing public health problem in the United States and the world. While weight loss is associated with significant benefits in obesity-related co-morbidities, successful long-term weight loss maintenance is extremely difficult. This limited success is primarily due to biologic mechanisms that clearly favor weight regain. The weight-reduced state is associated with not only reductions in energy expenditure and changes in substrate metabolism but also in increased energy intake. Measures of appetite (increased hunger, reduced satiety) clearly change with weight loss. These changes in appetite may be mediated by alterations of peripheral appetite-related signals, such as leptin and meal-related gut peptides, promoting energy intake. Furthermore, significant changes in the neuronal response to food-related cues in the weight- reduced state have also been shown, stressing the importance of the interactions between homeostatic and non-homeostatic regulation of energy intake. In summary, the weight-reduced state is clearly associated with a dysregulation of energy balance regulation, resulting in a milieu promoting weight regain, and thus being one of the major obstacles of “treating” obesity and reducing its comorbidities. This paper will review the adaptations in the central regulation of energy intake that occur after weight-loss or in the weight-reduce state in humans, including changes in peripheral appetite-related signals and neuroimaging studies examining the brain’s response to weight loss.
Keywords: reduced-obese, weight-reduced, fMRI, neuroimaging, appetite
1. Introduction
1.1. Case Presentation
LM is a 42 year old woman who presents to your office wanting help with her weight. She reports slow gradual weight gain over the past 10 years, especially after her two pregnancies. Most of her family is overweight or obese. She has tried just about every diet there is. They seem to work at first with the most success being a 25 pound weight loss, but with each diet she seems to regain the weight over time. She cannot understand why. “My metabolism must be low; I don’t eat at all,” she says. You perform a 24-hour recall which suggests very little energy intake. She denies eating out of hunger or emotion. She denies significant snacking or ingestion of “empty” liquid calories. She goes to the gym to “work out” several times per week. “What else can I do?” she asks in frustration.
LM’s situation is all too common. Calorie restriction typically does result in short-term weight loss in most individuals. Maintenance of this weight loss, however, is another story. So why does LM regain the weight? She claims that she does not eat very much. You know that for her to gain weight she must be in positive energy balance, so she must be eating more than she perceives. Is this her true perception, does she not sense energy intake correctly? Or is she purposely under-reporting? She claims that she does not eat out of hunger. Does she really not “feel” hunger? Is her interpretation of her physiologic signals at fault? Or is this all a behavioral or cognitive problem?
1.2. Obesity Prevalence
Obesity is a serious and growing public health problem. Despite efforts to promote healthy eating and physical activity behaviors and a tremendous push from the scientific community to better understand the pathophysiology of energy balance regulation, the prevalence of obesity and related metabolic disorders such as cardiovascular disease and type 2 diabetes continue to increase in the United States (US) and the world [1]. A majority of US adults are either overweight or obese, leaving only a minority with a “normal” body mass index (BMI). While genes undoubtedly play an important role in the development of obesity, this dramatic increase in the prevalence of obesity has occurred over a relatively short period of time in history. Genetic influences would not be expected to change over such a short period of time, suggesting that environmental factors are likely to be playing a significant role in the cause of this epidemic.
1.3. Weight Regain
Weight loss, specifically loss of body fat, is associated with benefits in all of the obesity-related comorbidities and mortality [2], but unfortunately while most weight loss interventions do result in short-term (3–6 months) success most individuals are not able to maintain this weight loss over the long-term [3–6]. In fact, it has been shown that less than 10% of individuals are able to maintain clinically meaningful weight loss for 5 years or longer [7]. This issue of weight regain after weight loss is one of the biggest obstacles that we face when treating overweight and obesity.
1.4. Potential Adaptations to the Weight-Reduced State
Significant advances over the last couple of decades have been made towards understanding the complex mechanisms responsible for the regulation of energy balance. The weight-reduced state is clearly associated with a dysregulation of or an adaptation in these mechanisms, resulting in a milieu promoting weight regain. Potential adaptations to the weight-reduced state include reduced energy expenditure, altered substrate metabolism, and/or increased energy intake (Table 1).
Table 1.
Potential Adaptations to the Weight-Reduced State.
Decreased Energy Expenditure
|
Changes in Substrate Metabolism
|
| Increased Energy Intake |
| Homeostatic Signals: changes in appetite-related hormones |
| Non-Homeostatic Signals: motivation, reward, attention, behavior |
This paper will review the adaptations in the central regulation of energy intake that occur after weight-loss or in the weight-reduce state in humans. After a brief review of energy intake regulation, the effects of weight loss on appetite will be discussed. This will be followed by a review of changes in peripheral appetite-related signals in the weight-reduced state. Finally, neuroimaging studies examining the brain’s response to weight loss will be discussed.
2. Regulation of Energy Intake
2.1. Homeostatic or Physiologic Regulation of Energy Intake
It is beyond the scope of this paper to review energy balance regulation in depth, and this topic has been previously reviewed [8–11]. The discovery of leptin has led to dramatic advances in the understanding of the homeostatic regulation of food intake. Adiposity signals, such as leptin and insulin, appear to trigger signals in the hypothalamus ultimately resulting in reduced energy intake and increased energy expenditure in a negative feedback manner. More recently gut peptides, such as ghrelin and PYY, have also been implicated in the regulation of energy intake again via hypothalamic signals. Furthermore, there appear to be interactions between the relatively acute gut signals with the more chronic adiposity signals.
2.2. “Non-Homeostatic” Regulation of Energy Intake
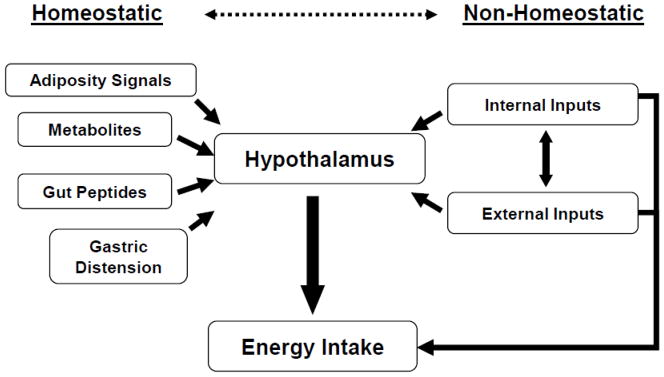
While a great deal has been learned about the homeostatic regulation of food intake and interactions with adiposity signals, it is clear, however, that the intake of food is a much more complex process that is not solely due to hypothalamic regulation. This is likely to be especially true in humans in which psychosocial factors play such a critical role and in which the process of eating is likely to be controlled by factors such as motivation, reward, and learned behaviors. Ultimately the decision to initiate food intake, how much to consume, and when to terminate a meal is affected by not only these homeostatic mechanisms but also by learned behaviors, cognitive factors, habits, social context, availability of food, and external sensory cues such as visual, smell, and taste inputs all impacting motivated behavior [12, 13]. Furthermore, the reinforcing and hedonic properties of food are also powerful modulators of feeding behaviors [11, 14]. This relates to the concept of “incentive” or “reinforcing” value of food. In summary, the regulation of energy intake is a complex process. Interactions between homeostatic and no-homeostatic signals are likely critical for the ultimate regulation of energy intake as depicted in Figure 1 [15].
Figure 1.
The integration of homeostatic and non-homeostatic signals in the regulation of energy intake [15].
3. Adaptations in Intake Regulation to the Weight-Reduced State
3.1. Changes in “Appetite” with Weight Loss
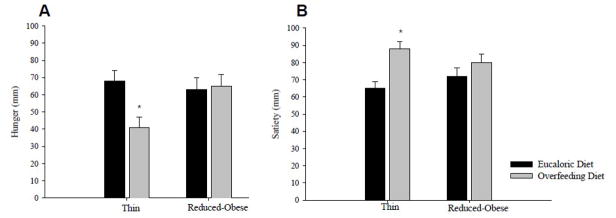
Most studies have shown that measures or ratings of appetite (hunger, prospective food consumption, desire to eat, etc) are enhanced and measures of satiety are reduced in the weight-reduced state [16–18]. These changes are more pronounced with greater perturbations in energy balance. We have observed that, while thin (obese-resistant) individuals quickly sense changes in energy balance (short-term overfeeding) with significant changes in subjective measures of hunger and satiety, reduced-obese individuals do not have the same response in hunger or satiety as seen in Figure 2, suggesting an inappropriate sensing of changes in energy balance [18]. In addition, reduced-obese individuals do not appear to properly compensate in subsequent energy intake [18]. The mechanisms for these responses in appetite to weight loss are not clear but are likely due to a complex interaction between physiologic or homeostatic signals and reward/motivation/behavior-related signals.
Figure 2.
Mean (±SEM) pre-meal hunger (A) and post-meal satiety (B) during eucaloric and overfeeding diet periods are shown [18]. Overfeeding resulted in significant reductions in mean pre-meal hunger and increases in mean post-meal satiety in thin as compared to reduced-obese individuals.
3.2. Changes in Appetite-Related Peripheral Signals
Adiposity related signals, such as leptin and insulin, are reduced with weight loss and the resultant reduced fat mass. This results in enhanced hunger and reduced satiety signaling in the brain as well as reduced energy expenditure [19, 20]. Of note, changes in leptin with weight loss have been shown to be predictive of weight regain and may therefore represent a key modulator of weight regain [21]. Gut-derived peptides such as ghrelin, PYY, and GLP-1 play important roles in the short-term meal-to-meal signaling of appetite and are also altered by weight loss, favoring and predicting increased food intake [21–25]. Gut peptide responses to weight loss, however, are variable likely due individual responsiveness to meals (macronutrients, fiber content, size), and thus may be a more or less important adaptive mechanism [25].
3.3. Changes in Neuronal Response
More recently it has become possible to examine the human brain’s response to food-related signals using neuroimaging techniques, allowing us to better understand the complex neural pathways and networks as they relate to food intake. Using Positron Emission Tomography (PET), DelParigi et al, examined regional cerebral blood flow (rCBF) in response to tasting and consuming a meal in normal weight, obese, and reduced-obese individuals [26]. In contrast to lean individuals, they found that both obese and reduced-obese individuals had similarly increased activation in response to taste in the insula, a brain region important in not only taste perception but also important in the control of complex aspects of eating behavior including anticipation and reward. In response to a meal (satiation) both obese and reduced-obese individuals had reductions in activity in the posterior hippocampus as compared to lean individuals. The authors concluded that the similarly “abnormal” neuronal responses to food in obese and reduced-obese individuals suggest that the predisposition to obesity may involve brain regions that are important in eating behavior. Using different methods and designs, Rosenbaum et al found significant changes in the neuronal response to visual food cues after weight loss [27]. Specifically, they examined the neuronal response to visual food cues using functional magnetic resonance imaging (fMRI) before and after 10% weight loss. They found significant differences in neuronal responses to food after weight loss in a network of brain regions involved in both the regulation of feeding behavior and high-level executive function. They also evaluated the effect of leptin “replacement” after weight loss and found that leptin modified the neural response to food cues in the weight stable reduced-obese subjects in a manner resembling the response in the obese state, in other words, reversing the effects of weight loss.
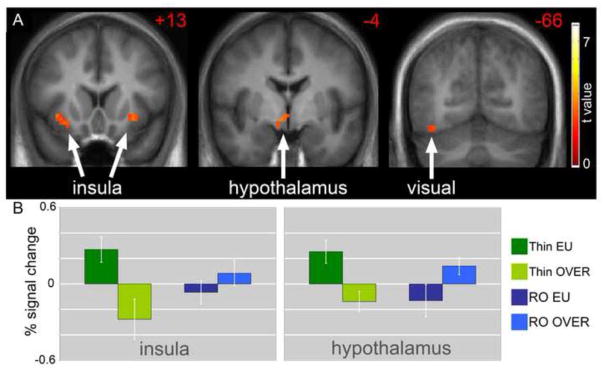
The energy balance state also appears to impact the neuronal response to food stimuli in reduced-obese individuals. We compared the neuronal response to visual food-cues by fMRI in reduced-obese as compared to thin individuals [28]. In the fasted state when in relative negative energy balance, thin individuals appear to be more sensitive to rewarding food cues than reduced-obese individuals with greater responses to images of foods of high hedonic value in the visual cortex and insula. Interestingly, however, two days of overfeeding reversed this effect (Figure 3). In positive energy balance the neuronal responses to food images were significantly attenuated in the thin and in fact there was also significantly reduced hypothalamic activity after overfeeding. Positive energy balance was not sensed in the reduced-obese individuals in the same manner as in thin individuals. There was persistent activation of brain regions important in the motivation and attention for food as if foods continue to appear interesting or rewarding despite overfeeding. In addition, there was also persistent hypothalamic activation with overfeeding in this group, further suggesting impaired interactions between visual cues and homeostatic regulation of energy balance. These findings suggest potential altered or abnormal sensing of energy balance in reduced-obese individuals potentially putting them at high risk for increased energy intake and subsequent weight regain.
Figure 3.
The difference in neuronal response with overfeeding as compared to eucaloric feeding in reduced-obese as compared to thin individuals in response to foods of high hedonic value is shown [28]. A. Greater deactivation of the insula, hypothalamus and visual cortex is noted in thin as compared to reduced-obese individuals (p < 0.01). B. Mean BOLD responses (± SEM) are shown for the insula and hypothalamus.
Finally, studies examining the neuronal response to food in successful weight losers can provide further insight into the central regulation of energy balance. Successful dieters from the National Weight Control Registry were found to have greater rCBF in the dorsal and dorsolateral prefrontal cortex in response to a meal as compared to both lean and obese non-dieters [29, 30]. Furthermore, dietary restraint was positively correlated with the response in these brain regions which are known to be important inhibitory behavior and cognitive control. It is not clear, though, how these individuals may be able to modulate these circuits. More recently these successful dieters were found to have increased frontal and visual cortical responses to food cues as measured by fMRI, further supporting greater inhibitory control in response to food cues than obese and lean controls [31]. While it is unclear whether these differences in neuronal responses were present before the weight loss they may still be an important mechanism for the success in long-term weight loss maintenance.
In summary, the reduced-obese state is associated with brain responses to food-related stimuli that would favor increased motivation and drive to eat regardless of potential homeostatic signaling. These differences in the central regulation of energy balance may favor increased energy intake and subsequent weight regain. Those who can successfully maintain the reduced-weight state for long periods of time appear to have increased activation of brain regions important in inhibitory behavior and cognitive control which may be one mechanism for their success.
4. Concluding Remarks
The majority of US adults and a significant number of children are overweight or obese. This epidemic of obesity is associated with serious comorbidities, increased mortality, reduced quality of life and a significant economic burden. While short-term weight loss is achievable in most and is associated with improvements in comorbidities, there is limited success with interventions to maintain the long-term weight loss that is necessary. This limited success is primarily due to biologic and behavioral mechanisms that clearly favor weight regain in the weight reduced state.
The regulation of energy balance is a complex process which includes the integration of peripheral, central and external signals. The brain is at the core of integrating homeostatic and non-homeostatic signals. Peripheral signals such as the adiposity-related hormone leptin, meal-derived gut peptides, nutrients, and gut distension are all key physiologic or homeostatic signals. Higher brain signals from cognitive, emotional, motivational and reward pathways are also central to the regulation of energy balance. Finally, external environmental signals such as food-related cues, social contexts, time cues, etc are also critical in the ultimate regulation of energy balance. In the development of obesity it appears that nonhomeostatic signals supersede or “over power” the biologic signals of energy balance. The obesigenic environment therefore wins the battle leading to excessive energy intake and reduced energy expenditure. After weight loss, as previously discussed, energy balance-related biologic signals are powerfully enhanced to promote increased energy intake and reduced energy expenditure setting up the individual for weight regain. This occurs in a setting, both environmental and psychosocial, that promote weight gain to begin with further increasing the risk of weight regain.
At the end of the day, how does this relate back to our patient, LM? It is possible that her genetics or biology may simply favor nutrient storage and this is the primary cause of her weight regain. If this is the case then we must look for ways to alter her biologic signals possibly with pharmacotherapy for example. It may be, though, that she is insensitive to environmental cues especially after weight loss, and therefore would need help to change her food environment and/or look for strategies to help her to better ‘interpret’ external food-related signals. Finally, her ‘behaviors’ may be at inherently at fault. We would then need to look for better strategies to facilitate behavior change and to treat any underlying mental health and emotionally-related behaviors. Most likely her struggles with long term weight loss success are due to a combination of all of these issues.
Acknowledgments
I would like to acknowledge Dr Daniel Bessesen for his invaluable mentorship and Dr Jason Tregellas for his collaboration and expertise in neuroimaging. Support for this work was provided by the General Clinical Research Center M01 RR00051, the Clinical Nutrition Research Unit DK48520, and the National Center for Research Resources (NCRR) RR00192.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA. 303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119:688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- 4.Phelan S, Wing RR, Loria CM, Kim Y, Lewis CE. Prevalence and predictors of weight-loss maintenance in a biracial cohort: results from the coronary artery risk development in young adults study. Am J Prev Med. 39:546–554. doi: 10.1016/j.amepre.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 6.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 7.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. Int J Obes. 1989;13:123–136. [PubMed] [Google Scholar]
- 8.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14 (Suppl 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 10.Woods SC, Seeley RJ, Cota D. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annu Rev Nutr. 2008;28:295–311. doi: 10.1146/annurev.nutr.28.061807.155505. [DOI] [PubMed] [Google Scholar]
- 11.Lattemann DF. Endocrine links between food reward and caloric homeostasis. Appetite. 2008;51:452–455. doi: 10.1016/j.appet.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37:261–283. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 14.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 15.Cornier M-A. The effects of overfeeding and propensity to weight gain on the neuronal responses to visual food cues. Physiology & Behavior. 2009;97:525–530. doi: 10.1016/j.physbeh.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doucet E, St-Pierre S, Alméras N, Tremblay A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite. 2003;40:137–143. doi: 10.1016/s0195-6663(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert JA, Drapeau V, Astrup A, Tremblay A. Relationship between diet-induced changes in body fat and appetite sensations in women. Appetite. 2009;52:809–812. doi: 10.1016/j.appet.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite. 2004;43:253–259. doi: 10.1016/j.appet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801. doi: 10.1093/ajcn/68.4.794. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82:3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- 21.Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martinez JA, Casanueva FF. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 95:5037–5044. doi: 10.1210/jc.2009-2566. [DOI] [PubMed] [Google Scholar]
- 22.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 23.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of weight loss by a low-fat diet and a low-carbohydrate diet on peptide YY levels. Int J Obes. 34:1239–1242. doi: 10.1038/ijo.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran LJ, Noakes M, Clifton PM, Wittert GA, Le Roux CW, Ghatei MA, Bloom SR, Norman RJ. Postprandial ghrelin, cholecystokinin, peptide YY, and appetite before and after weight loss in overweight women with and without polycystic ovary syndrome. Am J Clin Nutr. 2007;86:1603–1610. doi: 10.1093/ajcn/86.5.1603. [DOI] [PubMed] [Google Scholar]
- 25.Beck EJ, Tapsell LC, Batterham MJ, Tosh SM, Huang XF. Oat beta-glucan supplementation does not enhance the effectiveness of an energy-restricted diet in overweight women. Br J Nutr. 2010;103:1212–1222. doi: 10.1017/S0007114509992856. [DOI] [PubMed] [Google Scholar]
- 26.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornier M-A, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The Effects of Overfeeding on the Neuronal Response to Visual Food Cues in Thin and Reduced-Obese Individuals. PLoS ONE. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DS, Pannacciulli N, Chen K, Salbe AD, Del Parigi A, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86:573–579. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31:440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 31.McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, Cohen R, Wing RR. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]