Abstract
Purpose
Within schizophrenia cardiovascular disease (CVD) is highly prevalent secondary to atypical antipsychotic (AAP) use. Thorough assessments of diet, lifestyle, and endothelial functioning have not been done in this population. Omega 3 Fatty Acids (N-3 FAs) have garnered attention in relation to psychopathology as well as cardioprotection. This study examined the status of endothelial function within the schizophrenia population and determined pharmacogenetic, medication, dietary, and lifestyle factors associated with this functioning.
Methods
Schizophrenia subjects were screened for the metabolic syndrome along with physical activity, smoking, and variants related to folate pharmacogenetics in this cross-sectional analysis. Arteriole endothelial-dependent vasodilatation was measured using non-invasive peripheral arterial tonometry (RH-PAT, EndoPAT2000). A 24 hour dietary food recall was used to construct intake profiles using the Nutrition Data Systems for Research software (NDSR). We examined associations between AAP use and RH-PAT values, and the influence of N-3 FA dietary intake on this measure. Preliminary data are reported in 83 subjects with a mean age (± s.d.) of 45.89 (± 11.49), 64% were Caucasian (n = 53), 64% were male (n = 53), and 77% were receiving AAP treatment (n = 63).
Results
A significant positive relationship was found between RH-PAT values and N-3 FA intake (F=17.7(1,16), p=0.0007) in subjects not receiving AAPs. This relationship was lost in those treated with AAPs (F=0.25(1,43), p>0.6). Regression analysis confirmed the interaction effect of AAP treatment on the relationship between RH-PAT and N-3 FAs (p=0.0105). Endothelial dysfunction was also related to folate pharmacogenetic variants.
Conclusions
AAPs may counteract some vascular health benefits of a diet high in N-3 FAs. AAP use may necessitate a higher N-3 FA dose to regain these effects, but additional research is necessary to strengthen the preliminary findings. Pharmacogenetic variants related to folate and homocysteine metabolism may also increase endothelial dysfunction risk.
Keywords: Schizophrenia, Antipsychotics, Cardiovascular Disease, Omega 3 Fatty Acids, Endothelial Functioning
1. Introduction
The metabolic syndrome affects persons with schizophrenia significantly more than the general population, a fact related to the use of atypical antipsychotics (AAPs) (McEvoy et al. 2005). The metabolic syndrome comprises a cluster of risk factors including abdominal obesity; abnormalities in glucose metabolism, dyslipidemia, and elevated blood pressure, and was associated with an increased risk of cardiovascular disease (CVD) and diabetes (DM) (Davidson 2002). Our group, and others, have reported that occurrence of metabolic syndrome within schizophrenia is associated with pharmacogenetically regulated folate and homocysteine metabolism as well as folate exposure though dietary intake (Ellingrod et al. 2008;Garcia-Miss Mdel et al. 2010;van Winkel et al. 2010a;van Winkel et al. 2010b;Ellingrod et al. 2010). Presence of at least one variant allele for either of the key enzymes methylenetetrafolate reductase (MTHFR) 677C/T (rs1801133), 1298A/C (rs1801131) or catechol-o-methylatransferase (COMT) 158 Val/Met (rs4680) may increase the risk of metabolic syndrome within this population due to their roles in folate and homocysteine metabolism (Ellingrod et al. 2008;Tunbridge et al. 2008). While the literature related to metabolic syndrome risk within schizophrenia is rapidly expanding and lifestyle factors such as poor diet and a high incidence of cigarette smoking have also been identified as risk factors for increased CVD within this group (Vancampfort et al. 2010;DE Hert et al. 2009;Bobes et al. 2010), much remains unknown about the pathophysiology of CVD within schizophrenia. It is known that the vascular endothelium is adversely affected by the metabolic syndrome, (Benjamin et al. 2004;McVeigh et al. 1992;Brook et al. 2001;Lupattelli et al. 2000;Kuvin et al. 2003a;Tziomalos et al. 2010), increasing the cardiovascular disease (CVD) risk.
The endothelium is a vital organ that has garnered important attention due to its relationship to CVD) (Ross 1999;Haynes 2003;Rubinshtein et al. 2010). Physiologically the endothelium is comprised of a single layer of cells that line all blood vessels, and represents an important organ though its effects on regulating vasomotor tone, thrombosis, inflammation, platelet and leukocyte adhesion and vascular permeability (Lane et al. 2006). The presence of endothelial dysfunction increases the risk of CVD not only in individuals with established vascular disease, but also in those without (Kullo and Malik 2007). Thus measuring endothelial functioning may help to identity those at greatest risk for CVD. Unfortunately this measure has primarily been a research tool due to the invasive nature of this assessment as well as issues related to operator dependency (Bonetti et al. 2004). However, recently, measurement of fingertip pulse amplitude tonometry (RH-PAT) has introduced a non-invasive method for measuring small vessel (arteriole) endothelial-dependent vasodilatation and determining vascular health within the ambulatory clinic setting (Bonetti et al. 2004;Kuvin et al. 2003b;Kuvin et al. 2007). Pulse amplitude hyperemic response impairment has been associated with endothelial dysfunction and abnormal RH-PAT results have been correlated with the traditional invasive assessments of arteriole endothelial–dependent vasodilatation (Bonetti et al. 2004).
The aim of this investigation was to measure arteriole endothelial–dependent vasodilatation and the extent of endothelium dysfunction within the schizophrenia population using a non-invasive method, to determine the associated pharmacogenetic, medication, dietary, and lifestyle factors. We hypothesized that the prevalence of endothelial dysfunction would be similar to the prevalence of metabolic syndrome. Additionally we also proposed that presence of poorer arteriole endothelial–dependent vasodilatation and endothelial dysfunction would be related to AAP use, dietary and lifestyle measures, and the MTHFR and COMT genetic variants that regulate folate and homocysteine metabolism.
2. Methods
2.1 Subjects
Subjects were included in this cross-sectional analysis if they meet the following inclusion criteria 1) Presence of a DSM-IV diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder, 2) Between the ages of 18–90 years of age, and 3) their physician determined that they should be treated with an antipsychotic which they had been receiving for at least 6 months. Subjects were excluded if they 1) were unwilling to participate, 2) lacked the ability to give informed consent, which was assessed using a short questionnaire asking subjects key questions about the study, 3) had medical records documenting the presence of type II diabetes before the onset of receiving an antipsychotic, or 4) currently had a active substance abuse diagnosis. The study protocol was approved by the University of Michigan Medical School Institutional Review Board (IRBMED) as well as the Washtenaw County Health Organization (WCHO).
2.2 Assessments
Subjects meeting study inclusion and exclusion criteria then underwent an informed consent process followed by a clinical interview that included the Structured Clinical Interview for DSM Diagnoses (SCID), a thorough assessment of current and past medication history, and an assessment of smoking status (including current and past use) that allowed us to calculate a smoking pack year history. Subjects were asked to fast for at least 8 hours before the study visit and each visit was scheduled to take place between 8 and noon. These visits were timed to be within 2 hours of the subject’s usually waking time based on appointment availability. Vital signs, as well as height, weight, and hip and waist circumference were also measured for each subject and Body Mass Index (BMI) as well as the Hip/Waist ratio was calculated from this data. Subjects were also asked to recall all foods eaten for the last 24 hours, which was repeated twice after the initial study visit for a total of three assessments. This information was then used to calculate average calorie intake as well as provide an average assessment of dietary folate and vitamin B12 intake using the Nutrition Data Systems for Research software (NDSR) developed by the Nutrition Coordinating Center (NCC) at the University of Minnesota for data entry and nutrient analysis (Schakel 2001). The output from this analysis provides values for more than 140 nutrients, nutrient ratios, and other compounds based on the information gathered from the three 24 hour food recounts. Each subject’s average physical activity level was calculated by using the Total Activity Measure 2 (TAM2) which measures total or moderate intensity physical activity by asking participants for the total time spent in activity at different activity levels per week (Orrell et al. 2007). A higher score indicates greater physical activity over the past week. Blood was drawn for the following fasting laboratory assessments: folate, vitamin B12, homocysteine, glucose, insulin, hemoglobin A1c, lipids (total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL)), and leptin. Subjects were given a diagnosis of metabolic syndrome if they presented any 3 of the following NCEP-ATP III criteria: 1) abdominal obesity characterized by waist circumference of >40 inches for men or >35 inches for women, 2) triglycerides ≥ 150 mg/dL, 3) HDL cholesterol <40 mg/dL for men and <50 mg/dL for women, 4) blood pressure ≥130/≥85 mmHg or treatment for hypertension, or 5) fasting glucose ≥100 mg/dL or treatment of diabetes (Davidson 2002),.
2.3 Endothelial Function Assessment
We accessed arteriole endothelial-dependent vasodilatation using the EndoPAT 2000 device (Itamar Medical Inc, Caesarea, Israel), which has been validated and described in previous studies as a non-invasive method using peripheral arterial tonometry (RH- PAT) signals (Tziomalos et al. 2010;Rubinshtein et al. 2010;Bonetti et al. 2004;Goor et al. 2004;Bonetti et al. 2003;Lavie et al. 2000;Halligan et al. 2004). Previous studies have demonstrated that this methodology provides a clinically useful metric of endothelial-dependent vasodilatation with a significant portion being nitric oxide (NO)-mediated in the arteriole system (Rubinshtein et al. 2010;Bonetti et al. 2004). Briefly, this assessment involves two specifically designed finger probes which are placed on the subject’s middle finger of each hand. These probes are then inflated through the use of pneumatic tubes connected to an inflating device controlled by a computer algorithm. Subjects are then instructed to remain motionless for 5 minute in order to obtain a baseline measurement. After this time the blood pressure cuff located on the subject’s non-dominate arm is inflated to 60 mmHg above baseline systolic or at least 200 mmHg of pressure for 5 minutes. Subjects remain motionless during this time while occlusion of arterial blood flow is confirmed by a reduction of the RH-PAT tracing to zero. After 5 minutes, the blood pressure cuff is rapidly deflated and the subject is instructed to stay motionless for another 5 minutes for the final assessment.
Arteriole endothelial–dependent vasodilatation is measured by the RH-PAT index which is automatically generated by the computer algorithm and calculated as the ratio of the PAT signal after blood pressure cuff release compared to the baseline evaluation. The final RH-PAT output is normalized for baseline signal and indexed to the contra lateral arm. Controversy still exists regarding the best way to use the RH-PAT index for identifying those with endothelial dysfunction. Previous reports have shown that an RH-PAT index of <1.35 was the best discriminator of presence versus absence of CVD and yielded 80% sensitivity and 85% specificity to identify endothelial dysfunction in subjects (Bonetti et al. 2004;Bonetti et al. 2003). Recently, though these same investigators have suggested a threshold of <1.67 based on their comparison to the gold standard method which is injection of acetylcholine during coronary catheterization (Bonetti et al. 2004). The newer threshold of <1.67 has also been adopted by the Itamar for clinical use, thus we chose to use <1.67 as our measure of endothelial dysfunction.
2.4 MTHFR and COMT genotyping
Genomic DNA was isolated from whole blood with the salt precipitation method (Lahiri and Nurnberger 1991). Genotyping was done with Pyrosequencing ™ Technology. Polymerase Chain Reaction (PCR) primers were designed using Oligo 6 (MBI, Cascade, CO, USA). Pyrosequencing primers were designed using Pyrosequencing single nucleotide polymorphism (SNP) Primer Design Version 1.01 software (http://www.pyrosequencing.com). Subjects were genotyped for the MTHFR 1298A/C (rs1801131) and 677C/T (rs1801133) variants as well as the COMT Val158Met (rs4680) variant. For these assays, forty-five PCR cycles were done for reactions in a 20μl volume with 1.5mM Mg2+. PCR products were visualized by electrophoresis on 1.5% agarose gels stained with ethidium bromide prior to Pyrosequencing. Briefly, Pyrosequencing is a primer based extension method of sequencing that utilizes a four-enzyme process performed in a single well. Nucleotides are incorporated to the open 3’ DNA strand in which pyrophosphate is released and used in a sulfurylase reaction emitting ATP. The ATP is then used by luciferace, which is converted to oxyluciferin. Light is discharged as a result of the reaction and collected by a CCD camera. The light is assembled into a readable format and represented as peaks, commonly called Pyrogram charts. Based on these programs, each DNA sample can be sequenced and then genotyped (Marsh et al. 2005).
3. Statistical Analysis
Subjects currently receiving olanzapine, clozapine, quetiapine, risperidone, or paliperidone were classified as receiving an atypical antipsychotic (AAP). Ziprasidone and aripiprazole were not included in this primary classification due to their lack of weight gain liability (Komossa et al. 2009b;Komossa et al. 2009a).. The criteria used for endothelial dysfunction was a RH-PAT value of <1.67 as previous discussed. Both the MTHFR and COMT variants have shown a relationship between CVD risk and/or hyperhomocysteinemia with presence of the 677T allele in subjects with the COMT 158 Val allele (Tunbridge et al. 2008;Klerk et al. 2002;Lewis et al. 2005). Therefore, for the MTHFR genotype subjects were grouped based on those caring at least one variant allele (677CT or CC and 1298 AC or CC) and those who were homozygous dominant (677TT or 1298CC). For the COMT genotype, Val allele carriers were included in one group and homozygous Met/Met subjects were included in another. This variant allele classification was done given to maximize power given the relatively small sample size of the group. Differences in mean values for the primarily outcomes and socio-demographic variables between genotype groups and AAP groups were determined by the use of one-way analysis of variance (ANOVA) for normally distributed variables (BMI, waist circumference, age, metabolic measures, smoking pack year history, RH-PAT, and dietary and lifestyle assessments). Chi squared analysis was used to compare dichotomous variables (e.g. gender, metabolic syndrome diagnosis, and endothelial dysfunction) by genotype and AAP groups. To examine the relationship between AAP use, dietary measures, MTHFR/COMT status, and endothelial dysfunction, a regression model was constructed using the RH-PAT index as the dependent variable and age, gender, dietary and lifestyle measures, AAP use, genotypes, and interactions as the independent variables. A p-value less than 0.05 was considered statistically significant due to the exploratory nature of this investigation.
4. Results
WE analyzed a total of 83 subjects with a schizophrenia spectrum diagnosis. The mean age (± s.d.) of subjects in years was 45.89 ± 11.49 (range = 22–70 years old). The majority of subjects (64%, n= 53) were Caucasian, with 27% (n= 22) reporting to be African American. Similar to the overall demographics of schizophrenia, 64% (n = 53) of our study group was male. The mean body mass index (BMI) of subjects was 33.2 ± 7.97 and 44% (n = 36) met metabolic syndrome criterion which is in line with the previous literature (McEvoy et al. 2005, Ellingrod et al. 2008). Fifty-nine percent of our group currently smoked cigarettes (n = 49) with a mean pack year history of 357.67 ± 225.41, however 76% (n = 62) reported a history of smoking.
We classified 77% (n= 63) of our group as users of an AAP at the time of the assessment was seen in 77% (n = 63) of our group. In looking at this closer the AAPs used most commonly were risperidone/pailperidone (n = 20), followed by clozapine (n = 18), quetiapine (n=14), and olanzapine (n = 11). Among those not receiving an AAP, 6 subjects use aripiprazole montherapy and 4 subjects used ziprasidone monotherapy. The rest of the non AAP users were receiving a typical antipsychotic agent such as haloperidol. Polypharmacy was also common within our group as 18 subjects were receiving more than one antipsychotic. Of these, 2 subjects were receiving two typical antipsychotics, and 2 were receiving two AAPs. The majority of polypharamacy could be attributed to the 14 individuals who were receiving an AAP with either ziprasione, aripiprazole, or a typical antipsychotic. For classification purposes, these subjects are included within the AAP group. In terms of other medications, 26% of subjects (n = 22) were also receiving a concurrent mood stabilizer (primarily valproate). In looking at the two groups (AAPs users and non-users) there was no difference in the percentage of patients concurrently receiving mood stabilizers (~ 25% for each). For antidepressant polypharamacy, overall 46% of subjects (n = 38) were also receiving an antidepressant, primarily a selective serotonin reuptake inhibitor (SSRI, n = 20). Forty-four percent of AAP users had concomitant therapy with an antidepressant compared to 58% of non-AAP users, which was not statistically different, but trended in that direction (χ2 = 19.06, p = 0.07). Table 1 outlines the demographic differences seen between AAP users and non-users. Although very few differences were found between the two groups, subejcts using AAPs, were younger (p = 0.01), and had trend for a higher physical activity (TAM2) score (p = 0.08) and lower polyunsaturated fatty acids to saturated fatty acids intake (PUFA:SFA) ratio (p = 0.06). Both MTHFR and COMT variants were in Hardy Weinberg equilibrium (p> 0.24 for all) with mean allele frequencies of 0.23 (MTHFR 677), 0.28 (MTHFR 1298) and 0.43 (COMT 158), which are similar to those reported in the dbSNP database for individuals of European descent. No demographic differences were found between any of the genotype groups based on the number of MTHFR and/or COMT allele present.
Table One.
Description of demographic differences between atypical antipsychotic (AAP) users and non-users
| Demographic variable | AAP users | AAP non-users | p-value |
|---|---|---|---|
| Age ± s.d (years) | 44.3 ± 11.5 | 51 ± 10.1 | 0.02 |
| Percent Caucasian/African American | 61.97/26.76 | 71.43/28.57 | 0.75 |
| Percent Male | 67 | 52 | 0.20 |
| Total Activity Score (TAM2) ± s.d (MET/minute) | 2926 ± 2592 | 1861 ± 1684 | 0.08 |
| Hip/Waist Ratio | 1.03 ± 0.09 | 1.04 ± 0.11 | 0.95 |
| BMI (kg/m2) | 33.5 ± 7.5 | 32.4 ± 9.6 | 0.58 |
| Systolic Blood Pressure ± s.d (mmHg) | 123.6 ± 17.5 | 122.5 ± 20.1 | 0.80 |
| Diastolic Blood Pressure ± s.d (mmHg) | 75.8 ± 12.9 | 74.3 ± 11.8 | 0.65 |
| Homocysteine ± s.d (umol/L) | 11.6 ± 4.3 | 12.9 ± 8.8 | 0.34 |
| RH-PAT ± s.d | 1.8 ± 0.5 | 1.7 ± 0.6 | 0.67 |
| Total Cholesterol ± s.d (mg/dl) | 168.6 ± 45.2 | 155.2 ± 37.8 | 0.23 |
| Triglycerides ± s.d (mg/dl) | 151.1 ± 103.7 | 114.8 ± 104.6 | 0.17 |
| High Density Lipoprotein ± s.d (mg/dl) | 46.3 ± 14.0 | 45.7 ± 11.6 | 0.85 |
| Low Density Lipoproteins ± s.d (mg/dl) | 105.0 ± 33.7 | 93.7 ± 32.1 | 0.18 |
| Glucose ± s.d (mg/dL) | 105.4 ± 48.4 | 106.8 ± 46.4 | 0.91 |
| Leptin ± s.d (ng/ml) | 22.2 ± 16.9 | 18.4 ± 21.1 | 0.47 |
| Percent meeting ATP-III Metabolic Syndrome Criteria | 45 | 47 | 0.84 |
| Number of ATP-III Metabolic Syndrome Criteria Met | 2.5 ± 1.5 | 2.1 ± 1.5 | 0.27 |
| Percent Current Smoker | 63 | 48 | 0.20 |
| Smoking Pack Year History | 221.8 ± 29.8 | 173.8 ± 54.0 | 0.44 |
| Mean Total N-3 FA intake ± s.d (grams/day) | 1.6 ± 0.9 | 1.5 ± 0.8 | 0.67 |
| Polyunsatured Fatty Acids (PUFA) ± s.d (grams/day) | 16.4 ± 8.7 | 16.0 ± 8.3 | 0.87 |
| Monounsatured Fatty Acids (MUFA) ± s.d (grams/day) | 29.6 ± 12.5 | 26.7 ± 8.9 | 0.35 |
| Total Saturated Fatty Acids (SFA) ± s.d (grams/day) | 28.9 ± 10.7 | 25.3 ± 8.9 | 0.66 |
| Mean PUFA:SFA Ratio ± s.d | 0.6 ± 0.3 | 0.8 ± 0.5 | 0.06 |
| n-3 PUFA ± s.d (grams/day) | 8.8 ± 25.8 | 1.5 ± 0.81 | 0.21 |
| n-6 PUFA ± s.d (grams/day) | 13.65 ± 8.1 | 14.38 ± 7.9 | 0.73 |
| Mean n-6:n-3 Ratio ± s.d | 8.7 ± 4.4 | 10.6 ± 5.5 | 0.14 |
| Mean Dietary Energy Consumed ± s.d (kcals/day) | 2035.7 ± 734.1 | 1907.0 ± 609.7 | 0.49 |
| Mean Dietary Fat Consumed ± s.d (grams/day) | 81.0 ± 31.7 | 74.4 ± 24.3 | 0.41 |
| Mean Dietary Carbohydrates Consumed ± s.d (grams/day) | 255.8 ± 99.5 | 233.8 ± 94.3 | 0.40 |
| Mean Dietary Fiber Consumed ± s.d (grams/day) | 17.3 ±9.1 | 15.1 ±8.4 | 0.35 |
The mean RH- PAT index was 1.76 ± 0.51 and 50% (n = 41) of subjects met criteria for endothelial dysfunction (RH-PAT index < 1.67) (Bonetti et al. 2004;Bonetti et al. 2003). Compared to the general population the overall RH-PAT index of our subjects is low. In looking at a group of health controls participating is a separate investigation, the mean RH-PAT index was 2.05 ±0.43. However these subjects were younger (mean age; 38 ± 12) consisted of more females (17 Female 8 Male) and overall of normal weight (mean BMI 25.7 ± 4.5), although they did come from the same geographical and study laboratory.
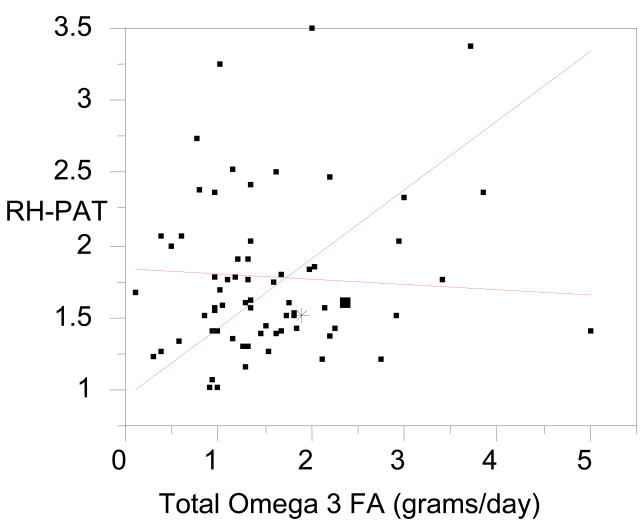
Overall, the RH-PAT index was not related to age (F = 0.18, df = 1, 79, p = 0.66), race (F = 0.23, df = 2, 78, p = 0.79), gender (F = 0.21, df = 1, 79, p = −.21), smoking status (F = 1.35, df = 1,79, p =0.24)) pack year history (F = 0.0001, df = 1, 77, p = 0.99), AAP use (F = 0.146, df = 1, 79, p = 0.70), or physical activity (TAM2) (F = 0.022, df = 1,80, p = 0.88). The RH-PAT index was negatively related to BMI, such that worse endothelial dysfunction was correlated with higher BMI (F = 5.08, df = 1, 79, p = 0.027), however it was not related to the hip/waist ratio (F = 0.19, df = 1, 79, p = 0.66) or a diagnosis of metabolic syndrome (F = 0.23, df = 1, 80, p = 0.64). There were no differences in the RH-PAT index based on AAP use or genotype status as stated previously. In looking at various dietary relationships in relation to arteriole endothelial–dependent vasodilatation, a significant positive relationship was found between the RH-PAT index and daily total omega-3 fatty acid (including both long and short chain fatty acids, N-3 FA) intake from the dietary recall data (F=17.7(1,16), p = 0.0007) as well as the PUFA:SFA ratio (F=9.72(1,16)p = 0.0066) in subjects not receiving AAPs. This relationship was not observed in subjects treated with AAPs (F=0.25(1,43), p > 0.6). Regression analysis confirmed the interaction effect of AAP on the relationship between RH-PAT and N-3 FA (F=4.09(3,59), p=0.0105) as well as PUFA:SFA ratio (F=2.76(3,59), p=0.05) (Figure 1). After controlling for age and TAM2 score (variables found to be different in AAP users–Table One), the AAP and N-3 FA interaction remained significant (t = −2.99, p = 0.0041) as did the PUFA:SFA ratio (t = −2.16, p = 0.035). These results were also seen for the interaction between N-3 FA and AAP use when subjects receiving aripiprazole and ziprasidone were included in the AAP group after controlling for age and TAM2 (F = 2.61(5,57), p = 0.034).
Figure 1. Interaction between N-3 FA intake and AAP use and RH-PAT index.
This figure shows a graphical represtnation of the interaction between n-3 FA intake and AAP use on Arteriole Endothelial Dependent Vasodilatation in schizophrenia subjects. The blue line represents a positive relationship between n-3 FA intake and the RH-PAT index in non-AAP users, while the red line shows the lack of a relationship between n-3 FA intake and the RH-PAT index in AAP users. A higher RH-PAT index score is an indicator of betters Arteriole Endothelial Dependent Vasodilatation.
For the pharmacogenetic analysis, there was a trend for the RH-PAT index to be related to the COMT Val allele (F = 3(1,78), p = 0.08) which continued after controlling for N-3 FA intake (F = 3.057(2, 60), p = 0.05). Thus in individuals with the COMT Val allele, the RH-PAT index was lower (mean 1.67) compared to those with the Met/Met genotype (mean = 2.00). Sixty percent of the COMT Val allele carriers (n = 37) meet endothelial dysfunction criteria compared to none in the Met/Met group (χ2 = 3.87, p = 0.0.04). We did not find a relationship between the MTHFR variants and arteriole endothelial–dependent vasodilatation.
5. Discussion
Overall this investigation found that 50% of schizophrenia subjects meet criteria for endothelial dysfunction (RH-PAT < 1.67) (Bonetti et al. 2004), which is to our knowledge the first time this has been reported. Given that at least 40% of schizophrenia subjects meet metabolic syndrome criteria, and that this population is at high risk for AAP associated metabolic complications, we hypothesized that at least 40% of our group would have an RH-PAT < 1.67, similar to the incidence of metabolic syndrome (McEvoy et al. 2005). We observed no evidence for a relationship between endothelial dysfunction and age, race, gender, smoking status, or AAP use (p = 0.15 for all), but was significantly related to the COMT Val allele, with 60% of those with this allelic variant meeting these criteria.
Previously investigations within the general population have reported that a naturally logarithmically transposed RH-PAT value (L-RH-PAT) of <0.4 carries a greater risk of CV adverse events such as cardiac death, myocardial infarction, revascularization or cardiac hospitalization (Rubinshtein et al. 2010). In looking at our group 35% had an L-RH-PAT index of <0.4 which is similar to the 40% prevalance of metabolic syndrome. As part of this project we had hypothesized that in subjects who pharmacogentically showed altered folate metabolism due to presence of MTHFR and COMT variants, poorer arteriole endothelial–dependent vasodilatation would be seen. Overall, we did not show a combined effect of the MTHFR and COMT variants on the RH-PAT index or endothelial dysfunction. We did see a relationship between endothelial dysfunction and the COMT Val allele. Additionally, within the COMT Val allele carriers, 43% had an L-PAT value of <0.4, compared to only 11% of those with the Met/met genotype. This is the first time this relationship has been reported within the schizophrenia literature and may be a factor behind the exaggerated risk for CVD.
Interestingly, the RH-PAT index, the L-RH-PAT measure, as well as presence of endothelial dysfunction, was not associated with cigarette smoking. This contradicts the literature within the general population, as cigarette smoking has been identified as a strong risk factor for endothelial dysfunction (Grassi et al. 2010;Puranik and Celermajer 2003). Nevertheless, the schizophrenia population has been hypothesized as having different risks elated to smoking, and possibly experiencing differences in nicotine metabolism, given the high percentage of consumers that smoke, and the reported equal or lower incidence of lung cancer compared to the general population (Tran et al. 2009;Catts et al. 2008). Although 59% of our study group currently smoked, the vast majority (76%) had a history of cigarette smoking. A better understanding of the relationship between cigarette smoking and arteriole endothelial–dependent vasodilatation with the schizophrenia population is needed and will be followed up in future studies.
In addition to reporting the prevalance of endothelial dysfunction within a schizophrenia study group, this investigation is also the first to examine the relationship between arteriole endothelial–dependent vasodilatation and dietary and lifestyle assessments within the schizophrenia population. Our research group has been focusing on the role of folate and homocysteine metabolism, as this is pharmacogenetically regulated and has been associated with CVD, however this is controversial within the general population (Ellingrod et al. 2008;Klerk et al. 2002;Lewis et al. 2005). Thus, as part of this currently project, we felt it important to measure overall dietary folate exposure and in doing so were able to measure other dietary nutrients and components using the 24 hour food recall. Very little is known about the dietary habits of schizophrenia patients, particularly in relation to folic acid intake, however recent work has shown some promising results in relation to folate exposure (Goff et al. 2004). Regardless understanding the relationship between dietary measure and CVD in this group is completely unexamined. Most ordinarily, patients with schizophrenia have poorer dietary habits compared to the general population. Henderson and colleagues were the first to observe the dietary intake of schizophrenia patients and found that compared to the National Health and Nutrition Examination Survey (NHANES) data, the schizophrenia group consumed significantly less (p<0.001) calories, carbohydrate, protein, mono- and polyunsaturated fat, folate, sodium, and significantly more caffeine than the NHANES group (Henderson et al. 2006). In looking at our dietary assessments, we found that fatty acid intake, specifically, total N-3 FA intake was related to the arteriole endothelial–dependent vasodilatation within our study population. This is the first time to our knowledge that this relationship has been reported within schizophrenia. While the idea of altered fatty acid metabolism within schizophrenia is not new, our group is looking at a very different phenotype compared to what is currently in the literature. Previous work done by other investigators reported on the “Niacin Induced Skin Flush” in schizophrenia (Messamore 2003). Briefly lack of a flush from niacin administered topically to schizophrenia subjects may to be due to a suggestive depletion of arachadonic acid and other essential fatty acids (Messamore 2003). Unfortunately, we did not measure the niacin skin flush within our study. Additionally it has been reported that in first-episode patients, levels of red blood cell essential polysaturated fatty acid levels were reduced and that risperidone or olanzapine administration for 6 months levels normalized (Evans et al. 2003). N-3 FAs are of interest within schizophrenia and have been proposed as a possible treatment intervention and/or preventative measure (Kuehn 2010;Amminger et al. 2010). The polyunsaturated fatty acids (PUFA), which include N-3 FAs have been identified as “cardioprotective” due to their beneficial effect on reducing inflammation, their role in maintaining membrane fluidity, and regulation of blood pressure and blood clotting (Shukla et al. 2010;Manerba et al. 2010;Wall et al. 2010). We found a significant relationship between N-3 FA and PUFA intake and greater arteriole endothelial–dependent vasodilatation in those not receiving AAPs, while this relationship did not exist in those receiving AAP. These relationships maintained significance after controlling for differences in age and physical activity seen between the two groups. These data may suggest that the proposed fatty acid “cardio-protective” effects may not be seen in those receiving AAPs or that a higher N-3 FA dosage is needed within this group. We hypothesized that AAP use may be related to increases in BMI, blood pressure, or metabolic laboratory values (cholesterol, triglycerides, or glucose), but no differences were noted between these two groups. As seen in Table 1 the AAP group was younger and reported more physical activity compared to non AAP users. Most interestingly, the mean intake of N-3 FA was higher in the AAP group compared to those not receiving AAPs, however this was not statistically significant.
Within the cardiovascular literature, the cardioprotective effects of the N-3 FAs is still controversial. Although older literature has suggested a cardiprotective effect of N-3 FA supplementation (Marik and Varon 2009), two new large randomized double blind placebo controlled N-3 FA supplementation trails have recently called these results into question. Both the “omega study” and the “alpha and omega study” found that in well treated individuals’ at high risk for CVD, N-3 FA supplementation did not confer any long term benefit (Kromhout et al. 2010). It is important to note that these trials took place within the general population and consisted of subjects who had already experienced at least one major cardiovascular event (i.e. myocardial infarction). Arguably, this population is very different than our subjects but also at high risk for CVD. The long term benefits from using N-3 FA supplements may be different than benefits seen in subjects who obtain these fatty acids from a composite healthier diet, presumably due to an overall healthier lifestyle. While the majority of our subjects reported engaging in regular physical activity, over 40% meet the NCEP-ATP-III metabolic syndrome criteria (Davidson 2002) indicating a fair amount of CVD. Additionally as stated earlier, previous reports suggest diets of schizophrenia subjects are often not as “healthy” as those within the general population (Henderson et al. 2006). Our data provide new insight into the role of N-3 FAs within schizophrenia but unfortunately create many new questions that must be answered. While the basic pharmacology of the AAPs is known as it relates to serotonin and dopamine neurotransmission, the secondary pharmacology has yet to be determined. Within the schizophrenia literature it has been suggested that pharmacologically AAPs may alter lipid metabolism or cause perturbations within the inflammatory cascade, both of which may be mechanisms for this altered relationship between N-3 FA and arteriole endothelial–dependent vasodilatation within this group (Lauressergues et al. 2010;Saetre et al. 2007). Unfortunately, given the assessments included in our current research, we are unable to further elucidate any mechanisms behind the AAP’s effect, so for now, we will be unable to answer this question.
6. Limitations
Despite our best research efforts this investigation has some limitations. Most notably this is a cross-sectional study that only had one assessment for the arteriole endothelial–dependent vasodilatation. Additionally lack of a specific control may limit our results and the use of the 24 hour food recount for quantifying an average daily intake is not optimal, despite our use of this assessment in triplicate in an effort to obtain a good approximation of each subject’s usual diet. Lack of blood levels of many of the dietary measurements hinder our ability to relate the body’s handling of these nutrients in relation to AAP associated metabolic complications. Additionally, our measurement of physical activity was based on subject self-report which is prone to both systematic and random errors and only a small minority of our subjects were being treated with any type of cardiovascular medication (i.e. statin medication). Thus, this brings up the question as to if N-3 FAs would be more beneficial in our subjects if primary cardiovascular treatment was first undertaken. Given recent research within the cardiovascular literature, uncertainty now exists as to the benefits of N-3 FA supplementation in “well medicated” individuals (Marik and Varon 2009;Kromhout et al. 2010). Lastly, the sample size included in this post-hoc analysis is rather small compared to others within the general population that have examined arteriole endothelial–dependent vasodilatation and given the number of variables available to test in regards to the RH-PAT variable, we did not correct for multiple statistical testing. Despite these limitations, this investigation is the first to report on arteriole endothelial–dependent vasodilatation with the schizophrenia population and will add substantially to the literature as we continue our quest to understand the metabolic complications from AAP use.
7. Summary
In summary, as part of this investigation we found 50% of individuals with schizophrenia met criteria for arteriole endothelial–dependent vasodilatation when measured using the non-invasive EndoPAT2000. This percentage is similar to the 40% incidence of metabolic syndrome seen in this population. This investigation is the first, to our knowledge, to report on the prevalance of endothelial dysfunction (RH-PAT < 1.67) within the schizophrenia population. Additionally we found an interaction between total dietary N-3 FA intake and AAP use, where the positive relationship found between arteriole endothelial–dependent vasodilatation and N-3 FA intake was negated in subjects receiving AAPs. This loss of association was not due to an increase in other components related to metabolic syndrome development which may mean that the AAPs themselves have a direct effect in removing the “cardio protective” effects of the N-3 FAs. Gaining a better mechanistic understanding of this relationship is unfortunately beyond the scope of this current project, but the results of this study will provide the framework for continued work in this area.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amminger GP, Schfer M, Papageorgiou K, Klier C, Cotton S, Harrigan S, Mackinnon A, McGorry P, Berger G. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109(5):613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- Bobes J, Arango C, Garcia-Garcia M, Rejas J. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: an analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res. 2010;119(1–3):101–109. doi: 10.1016/j.schres.2010.02.1030. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88(11):1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- Catts VS, Catts SV, O’Toole BI, Frost AD. Cancer incidence in patients with schizophrenia and their first-degree relatives - a meta-analysis. Acta Psychiatr Scand. 2008;117(5):323–336. doi: 10.1111/j.1600-0447.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- Davidson MH. A symposium: National Cholesterol Education Program Adult Treatment Panel III: Impact and implementation of the new guidelines. Introduction Am J Cardiol. 2002;89(5A):1C–2C. [PubMed] [Google Scholar]
- DE Hert M, Schreurs V, Vancampfort D, VAN Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15–22. doi: 10.1002/j.2051-5545.2009.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingrod VL, Grove TB, Bly MJ, Taylor SF. Methylenetetrahydrofolate Reductase (MTHFR) 677C/T and Catechol-O-Methyltransferase (COMT) Val158Met Variants and Endothelial Functioning in Schizophrenia and Bipolar Subjects Treated with Antipsychotics. Journal of Pharmacy Practice. 2010;23(2):171. [Google Scholar]
- Ellingrod VL, Miller DD, Taylor SF, Moline J, Holman T, Kerr J. Metabolic syndrome and insulin resistance in schizophrenia patients receiving antipsychotics genotyped for the methylenetetrahydrofolate reductase (MTHFR) 677C/T and 1298A/C variants. Schizophr Res. 2008;98(1–3):47–54. doi: 10.1016/j.schres.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69(6):393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Garcia-Miss Mdel R, Perez-Mutul J, Lopez-Canul B, Solis-Rodriguez F, Puga-Machado L, Oxte-Cabrera A, Gurubel-Maldonado J, Arankowsky-Sandoval G. Folate, homocysteine, interleukin-6, and tumor necrosis factor alfa levels, but not the methylenetetrahydrofolate reductase C677T polymorphism, are risk factors for schizophrenia. J Psychiatr Res. 2010;44(7):441–446. doi: 10.1016/j.jpsychires.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Goff DC, Bottiglieri T, Arning E, Shih V, Freudenreich O, Evins AE, Henderson DC, Baer L, Coyle J. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry. 2004;161(9):1705–1708. doi: 10.1176/appi.ajp.161.9.1705. [DOI] [PubMed] [Google Scholar]
- Goor DA, Sheffy J, Schnall RP, Arditti A, Caspi A, Bragdon EE, Sheps DS. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol. 2004;27(3):137–141. doi: 10.1002/clc.4960270307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi D, Desideri G, Ferri L, Aggio A, Tiberti S, Ferri C. Oxidative Stress and Endothelial Dysfunction: Say No to Cigarette Smoking! Curr. Pharm Des. 2010 doi: 10.2174/138161210792062867. [DOI] [PubMed] [Google Scholar]
- Halligan SC, Murtagh B, Lennon RJ, Pumper GM, Mathew V, Higano ST, Lerman A. Effect of long-term hormone replacement therapy on coronary endothelial function in postmenopausal women. Mayo Clin Proc. 2004;79(12):1514–1520. doi: 10.4065/79.12.1514. [DOI] [PubMed] [Google Scholar]
- Haynes WG. Triglyceride-rich lipoproteins and vascular function. Arterioscler Thromb Vasc Biol. 2003;23(2):153–155. doi: 10.1161/01.atv.0000053181.59330.79. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Borba CP, Daley TB, Boxill R, Nguyen DD, Culhane MA, Louie P, Cather C, Eden Evins A, Freudenreich O, Taber SM, Goff DC. Dietary intake profile of patients with schizophrenia. Ann Clin Psychiatry. 2006;18(2):99–105. doi: 10.1080/10401230600614538. [DOI] [PubMed] [Google Scholar]
- Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG MTHFR Studies Collaboration Group. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Hunger H, Schwarz S, Bhoopathi PS, Kissling W, Leucht S. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009a;4(4):CD006627. doi: 10.1002/14651858.CD006627.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Schmid F, Hunger H, Schwarz S, El-Sayeh HG, Kissling W, Leucht S. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009b;4(4):CD006569. doi: 10.1002/14651858.CD006569.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout D, Giltay E, Geleijnse J. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Early interventions for schizophrenia aim to improve treatment outcomes. JAMA. 2010;304(2):139–40. 145. doi: 10.1001/jama.2010.874. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J Am Coll Cardiol. 2007;49(13):1413–1426. doi: 10.1016/j.jacc.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12(1):13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Patel AR, Sidhu M, Rand WM, Sliney KA, Pandian NG, Karas RH. Relation between high-density lipoprotein cholesterol and peripheral vasomotor function. Am J Cardiol. 2003a;92(3):275–279. doi: 10.1016/s0002-9149(03)00623-4. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003b;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19(19):5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H, Smith J, Davies JS. Noninvasive assessment of preclinical atherosclerosis. Vascular Health and Risk Management. 2006;2(1):19–30. doi: 10.2147/vhrm.2006.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauressergues E, Staels B, Valeille K, Majd Z, Hum D, Duriez P, Cussac D. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedebergs Arch Pharmacol. 2010;381(5):427–439. doi: 10.1007/s00210-010-0499-4. [DOI] [PubMed] [Google Scholar]
- Lavie P, Shlitner A, Sheffy J, Schnall RP. Peripheral arterial tonometry: a novel and sensitive non-invasive monitor of brief arousals during sleep. Isr Med Assoc J. 2000;2(3):246–247. [PubMed] [Google Scholar]
- Lewis SJ, Ebrahim S, Davey Smith G. Meta-analysis of MTHFR 677C->T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ. 2005;331(7524):1053. doi: 10.1136/bmj.38611.658947.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupattelli G, Lombardini R, Schillaci G, Ciuffetti G, Marchesi S, Siepi D, Mannarino E. Flow-mediated vasoactivity and circulating adhesion molecules in hypertriglyceridemia: association with small, dense LDL cholesterol particles. Am Heart J. 2000;140(3):521–526. doi: 10.1067/mhj.2000.108508. [DOI] [PubMed] [Google Scholar]
- Manerba A, Vizzardi E, Metra M, Cas L. n-3 PUFAs and cardiovascular disease prevention. Future Cardiology. 2010;6(3):343–350. doi: 10.2217/fca.10.19. [DOI] [PubMed] [Google Scholar]
- Marik P, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32(7):365–372. doi: 10.1002/clc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S, King CR, Garsa AA, McLeod HL. Pyrosequencing of clinically relevant polymorphisms. Methods Mol Biol. 2005;311:97–114. doi: 10.1385/1-59259-957-5:097. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35(8):771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- Messamore E. Relationship between the niacin skin flush response and essential fatty acids in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2003;69(6):413–419. doi: 10.1016/j.plefa.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Orrell A, Doherty P, Miles J, Lewin R. Development and validation of a very brief questionnaire measure of physical activity in adults with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2007;14(5):615–623. doi: 10.1097/HJR.0b013e3280ecfd56. [DOI] [PubMed] [Google Scholar]
- Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis. 2003;45(6):443–458. doi: 10.1053/pcad.2003.YPCAD13. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46–46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schakel SF. Maintaining a Nutrient Database in a Changing Marketplace: Keeping Pace with Changing Food Products A Research Perspective. Journal of Food Composition and Analysis. 2001;14(3):315–322. [Google Scholar]
- Shukla S, Gupta S, Ojha S, Sharma S. Cardiovascular friendly natural products: a promising approach in the management of CVD. Natural product research. 2010;24(9):873–898. doi: 10.1080/14786410903417378. [DOI] [PubMed] [Google Scholar]
- Tran E, Rouillon F, Loze JY, Casadebaig F, Philippe A, Vitry F, Limosin F. Cancer mortality in patients with schizophrenia: an 11-year prospective cohort study. Cancer. 2009;115(15):3555–3562. doi: 10.1002/cncr.24383. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Warden DR, Johnston C, Refsum H, Smith AD. Polymorphisms in the catechol-O-methyltransferase (COMT) gene influence plasma total homocysteine levels. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):996–999. doi: 10.1002/ajmg.b.30700. [DOI] [PubMed] [Google Scholar]
- Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutrition, metabolism, and cardiovascular diseases. 2010;20(2):140–146. doi: 10.1016/j.numecd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Moons T, Peerbooms O, Rutten B, Peuskens J, Claes S, van Os J, De Hert M. MTHFR genotype and differential evolution of metabolic parameters after initiation of a second generation antipsychotic: an observational study. Int Clin Psychopharmacol. 2010a doi: 10.1097/YIC.0b013e32833bc60d. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Rutten BP, Peerbooms O, Peuskens J, van Os J, De Hert M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res. 2010b doi: 10.1016/j.schres.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Knapen J, Probst M, van Winkel R, Deckx S, Maurissen K, Peuskens J, De Hert M. Considering a frame of reference for physical activity research related to the cardiometabolic risk profile in schizophrenia. Psychiatry Res. 2010;177(3):271–279. doi: 10.1016/j.psychres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Wall R, Ross RP, Fitzgerald G, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]