Abstract
Objective
To examine the association of statin use with clinical outcomes and circulating biomarkers in community-acquired pneumonia (CAP) and sepsis.
Design
Multicenter inception cohort study.
Setting
Emergency departments of 28 US hospitals.
Patients
1895 subjects hospitalized with CAP.
Interventions
None.
Measurements and Main Results
Our approach consisted of two different comparison cohorts, each reflecting methods used in prior publications in this area. We first compared subjects with prior statin use (prior use cohort), defined as a history of statin use in the week before admission, to those with no prior use. We then compared prior statin users whose statins were continued in-hospital (continued use cohort) to those with either no prior use or no inhospital use. We adjusted for patient characteristics, including demographics, comorbid conditions, and illness severity, and accounted for healthy user effect and indication bias using propensity analysis. We determined risk of severe sepsis and 90-day mortality. We measured markers inflammation (TNF, IL-6, IL-10), coagulation (antithrombin, Factor IX, plasminogen activator inhibitor, D-dimer, thrombin antithrombin complex), and lymphocyte cell surface protein expression during first week of hospitalization. There were no differences in severe sepsis risk between statin users and non-users for prior (30.8% vs. 30.7%, p=0.98) or continued statin use (30.2% vs. 30.8%, p=0.85) in univariate analyses, and after adjusting for patient characteristics and propensity for statin use. Ninety-day mortality was similar in prior statin users (9.2% vs. 12.0%, p=0.11) and lower in continued statin users (7.9% vs. 12.1%, p=0.02). After adjusting for patient characteristics and propensity for statin use, there was no mortality benefit for prior (OR=0.90 (0.63–1.29), p=0.57) or continued statin use (OR=0.73 (0.47–1.13), p=0.15). Only antithrombin activity over time was higher in statin subjects, yet the magnitude of difference was modest. There were no differences in other coagulation, inflammatory, or lymphocyte cell surface markers.
Conclusions
We found no evidence of a protective effect for statin use on clinical outcomes and only modest differences in circulating biomarkers in CAP, perhaps due to healthy user effects and indication bias.
Keywords: statins, community-acquired pneumonia, sepsis, coagulation, outcomes
INTRODUCTION
HMG-CoA reductase inhibitors (statins) are the most prescribed class of drugs in the world and significantly improve survival in patients with cardiovascular disease (1, 2). In addition to decreasing low density lipoproteins, statins have diverse pharmacological effects, including anti-inflammatory and anti-thrombotic properties (3). These effects have prompted speculation that statins may be useful in the treatment or prevention of severe sepsis (4), a syndrome defined as acute organ dysfunction secondary to infection and characterized by dysregulation of inflammation, coagulation, and other acute phase responses. Yet this speculation is also tempered by the very real possibility of increased serious side effects that might occur with more frequent use of statins in acutely ill subjects (5).
A variety of observational studies have examined the role of statins in the prevention or treatment of infection and sepsis, as recently reviewed (6–11). Most suggest a clinical benefit for statins, yet others show no benefit, and one shows possible harm. None of these has examined potential mechanisms of benefit. Furthermore, like all observation-based pharmacoepidemiologic studies, these studies are susceptible to a number of confounders and biases, analogous to studies of hormone replacement therapy (12). Indeed, any “benefit” of statins on outcomes of infection may be the result of these and other biases (7, 13).
Several randomized trials of statins in infection are either planned, underway, or recently completed (14–25). Unfortunately, these are small studies that are underpowered to address mortality or other clinically meaningful endpoints. Thus, there remains an unmet need to better understand what, if any, clinical benefit statins may have after appropriate consideration of confounders and biases. Of equal importance is the need to better understand the influence of statins on potential pathophysiologic mechanisms. We examined the association of statin use with clinical outcomes and measures of inflammation, coagulation, and lymphocyte cell surface protein expression in a large, multicenter inception cohort study, Genetic and Inflammatory Markers of Sepsis (GenIMS). GenIMS was specifically designed to explore risk factors, including statin use, for the development and progression of severe sepsis and death in patients hospitalized with community acquired pneumonia (CAP), the most common cause of severe sepsis. Our a priori hypotheses were that statin use would be associated with decreased rates of severe sepsis and death, reduced dysregulation of plasma markers of inflammation and coagulation, and changes in lymphocyte cell surface protein expression, but that some of these differences would be explained by patient characteristics, illness severity, indication bias, and healthy user effects.
MATERIALS AND METHODS
Subjects
GenIMS enrolled subjects in the Emergency Departments (ED) of 28 hospitals in Pennsylvania, Connecticut, Michigan, and Tennessee from December 2001 to November 2003. Details of the study design, eligibility criteria, and clinical definitions have been published elsewhere (26–30). Briefly, we enrolled subjects >18 years old, who had a clinical and radiological diagnosis of pneumonia per criteria of Fine et al. (31), and provided informed consent directly or by proxy. The study was approved by the Institutional Review Boards of all sites.
Clinical definitions and outcome variables
We ascertained comorbid conditions using the Charlson comorbidity index (32) and severity of illness using APACHE III (33) and the Pneumonia Severity Index (PSI) (34). We defined severe sepsis as pneumonia plus acute organ dysfunction following the 2001 International Consensus Criteria (35). We defined acute organ dysfunction as a new Sequential Organ Failure Assessment (SOFA) (36) score of ≥ 3 in any of six organ systems, based on the recent international Sepsis Occurrence in the Acutely ill Patient study (37). The initial empirical antibiotics received during the first 24 hours of hospitalization were considered adequate if compliant with the 2001 ATS Guidelines for the Management of Adults with Community-acquired Pneumonia (38), which were in place at the time of the study. We determined survival post-discharge by telephone and National Death Index search. We used 90-day mortality as our primary measure of survival, based on endpoint recommendations for sepsis trials from two recent international expert panels (39, 40).
Statin cohorts
Our approach consisted of two different comparison cohorts, each reflecting methods used in prior publications in this area. We first compared subjects with prior statin use (prior use cohort), defined as a history of statin use in the week before admission, to those with no prior use. We then compared prior statin users whose statins were continued in-hospital (continued use cohort) to those with either no prior use or no in-hospital use.
Laboratory procedures
Blood was drawn for biomarker assays at ED presentation and daily for the first week. Generally, day 1 blood samples were drawn at enrollment and subsequent samples were drawn at 8am. For logistic reasons, we did not obtain day 1 samples from subjects presenting after 11pm or on weekends and holidays. We assessed inflammation on days 1–7 in 1886 (99.5%) subjects by measuring plasma tumor necrosis factor (TNF), interleukin (IL)-6, and IL-10 levels. We assessed plasma coagulation markers (D-dimer, plasminogen activator inhibitor, antithrombin, Factor IX, and thrombin-antithrombin complex) on days 1–7 in the first 939 subjects (49.6%) enrolled using a commercial laboratory (Essoterix, Agoura Hills CA, USA). To determine whether statin users and non-users were equally likely to have bacterial infection, we measured plasma procalcitonin levels and compared the proportion of subjects across different statin groups stratified by procalcitonin levels (<0.1, 0.1–0.25, 0.25–0.5, >0.5 ng/ml). We assessed day 1 lymphocyte cell surface marker expression using an automated fluorescence-activated cell sorter (FACS) (FACSVantage SE, Becton Dickinson, Los Angeles, CA), which included CD3, CD4, CD5, CD 8, CD 14, CD19, CD64, CD120a, CD 120b, HLA-DR, TLR2, and TLR4. We analyzed these markers in a subset of 597 (31.5%) subjects enrolled in hospitals located within 60 miles of the University of Pittsburgh because samples for cell surface markers had to be analyzed within 48 hours. Details of assay methodology for these markers have been described previously (41, 42).
Statistical analyses
Analyses were performed using SAS 8.2 (SAS Institute, Cary, NC, USA) and SPSS 12.0 (SPSS Inc., Chicago, IL, USA) and assuming statistical significance at p<0.05. No corrections were made for multiple comparisons. We conducted univariate comparisons using Fisher's exact tests and t-test or nonparametric counterparts as appropriate. We tested for differences in inflammatory and coagulation markers by transforming values to their natural logs when skewed, using Tobit models (43) when ≥5% of values fell below detection thresholds, and constructing mixed models for repeated measures (44, 45). We tested for differences in day 1 lymphocyte cell surface protein expression using two-sample t-tests with a false discovery rate method to adjust for the multiple testing. We used multivariable models to compare severe sepsis rates and mortality between groups adjusting for potential confounders and accounting for clustering within specific hospitals by fitting the multivariable models using generalized estimating equations (GEE) (46). Four categories of potential confounders were eligible for inclusion in the multivariable models: demographics and comorbidities (age, race, gender, admitted from nursing home, comorbid illnesses, Charlson comorbidity); severity of illness (Pneumonia Severity Index (PSI) (47), Acute Physiology and Chronic Health Evaluation III (APACHE III) acute physiology score (APS) (33), and SOFA score); treatments received (pre-hospital antibiotic use, adequacy of initial hospital antibiotics (48), inpatient corticosteroid use); and healthy user indicators (insured, lives at home, functional status, former smoker, influenza and pneumovax vaccinations). Variables were entered into each model sequentially using forward stepwise regression, while examining several potential models at each step. Variables were retained in the model based on a significance level of p<0.05. To account for differential likelihood of receiving a statin, we constructed a propensity score for either prior or continued statin use (49). This score included age, diabetes and cardiac disease, aspirin and anticoagulant use, functional status, and living arrangement prior to hospitalization.
RESULTS
Baseline characteristics
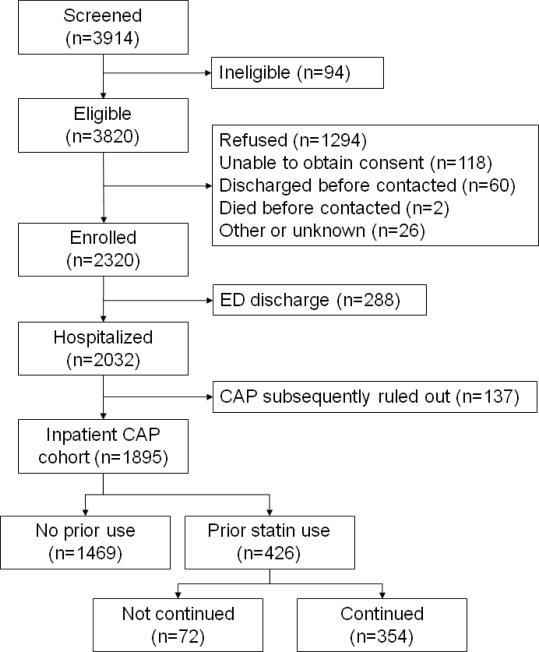
We enrolled 2320 subjects, excluding 288 (12%) discharged from the emergency department and 137 (6%) because their treating physicians subsequently excluded pneumonia as the cause of their illness (figure 1). Thus, the final inpatient analysis cohort was 1895. Four-hundred twenty-six (22.5%) were taking statins within the 7 days prior to admission (prior statin users), 354 (76.1%) of which had their statin continued in-hospital (continued statin users). Table 1 describes the baseline characteristics of the prior and continued statin use groups. As expected, both prior and continued statin users were older with greater comorbidity, including a more frequent history of cardiovascular disease and diabetes, as compared to statin non-users. Statin subjects were also less likely to be admitted from a nursing home and more likely to be white and to have higher Pneumonia Severity Index (PSI) scores on presentation, though this latter difference was due to a difference in age. There were no differences in severity of illness (APACHE III APS), degree of organ dysfunction (SOFA), treatments administered, or admission to an intensive care unit. Statin users had a greater likelihood of being a healthy user, with higher rates of insurance, living at home, good functional status, receipt of vaccinations, daily aspirin use, and having quit smoking. Among prior statin users, atorvastatin and simvastatin were used most commonly, accounting for 47.7% and 39.4% of subjects, while pravastatin (7.7%), lovastatin (3.5%), and fluvastatin (1.6%) were used less frequently.
Figure 1.
Flow diagram of GenIMS CAP cohort.
Table 1.
Baseline characteristics of prior and continued statin use cohorts.
| Variable | No prior use N=1469 | Prior use N=426 | P-value | No continued use N=1541 | Continued use N=354 | P-value |
|---|---|---|---|---|---|---|
| Demographics and comorbidities | ||||||
| Age, yrs mean (SD) | 66.0 (17.92) | 71.6 (11.1) | <.0001 | 66.1 (17.7) | 72.2 (11.5) | <.0001 |
| Gender, % male | 51.3 | 54.2 | .29 | 51.3 | 54.8 | .24 |
| Race, % | ||||||
| White | 78.3 | 89.0 | <.0001 | 78.2 | 91.5 | <.0001 |
| Black | 17.8 | 8.9 | 17.9 | 6.5 | ||
| Other | 4.0 | 2.1 | 3.9 | 2.0 | ||
| Admitted from nursing home, % | 7.2 | 2.8 | .001 | 7.1 | 2.3 | .001 |
| Charlson, mean (SD) | 1.9 (2.3) | 2.2 (2.1) | .002 | 1.9 (2.2) | 2.2 (2.1) | .002 |
| Cardiovasc disease, % | 20.0 | 45.8 | <.0001 | 20.6 | 48.3 | <.0001 |
| Chronic resp disease, % | 37.9 | 37.8 | .96 | 37.5 | 39.6 | .48 |
| Diabetes, % | 17.2 | 31.0 | <.0001 | 17.8 | 31.1 | <.0001 |
| Chronic dialysis, % | 2.5 | 2.6 | .94 | 2.5 | 2.5 | .99 |
| Cirrhosis, % | 0.3 | 0.0 | .59 | 0.3 | 0.0 | .59 |
| Illness severity | ||||||
| PSI class, % | ||||||
| I or II | 24.7 | 12.9 | <.0001 | 24.5 | 11.6 | <.0001 |
| III | 21.7 | 17.6 | 21.4 | 18.1 | ||
| IV | 34.2 | 48.1 | 34.5 | 50.0 | ||
| V | 19.3 | 21.4 | 19.7 | 20.3 | ||
| Day 1 PSI, mean (SD) | 97.9 (39.9) | 106.2 (30.3) | <.0001 | 92.2 (39.6) | 106.5 (29.7) | <.0001 |
| Day1 PSI w/o age, mean (SD) | 36.8 (30.9) | 39.2 (27.4) | .13 | 37.0 (30.8) | 38.9 (27.1) | .24 |
| Day1 SOFA, mean (SD) | 2.4 (2.0) | 2.4 (1.8) | .98 | 2.4 (2.0) | 2.4 (1.8) | .77 |
| APACHE III APS, mean (SD) | 42.0 (14.4) | 42.1 (12.3) | .92 | 42.1 (14.4) | 42.0 (12.0) | .89 |
| Documented bacteremia, % | 7.2 | 8.0 | .56 | 7.3 | 7.6 | .82 |
| ICU admission, % | 15.3 | 18.3 | .14 | 15.6 | 17.5 | .39 |
| Mechanically ventilated, % | 7.2 | 6.3 | .56 | 7.3 | 5.4 | .18 |
| Treatments administered | ||||||
| Pre-hospital antibiotics, % | 18.0 | 16.2 | .39 | 17.7 | 17.2 | .85 |
| Adequate initial antibiotics†, % | 79.2 | 81.0 | .43 | 79.4 | 80.8 | .55 |
| Inpatient corticosteroid use, % | 39.0 | 36.2 | .28 | 38.9 | 35.9 | .28 |
| Healthy user indicators | ||||||
| Insured. % | 94.6 | 99.3 | <.0001 | 94.7 | 99.7 | <.0001 |
| Lives at home, % | 88.0 | 93.0 | .003 | 88.1 | 93.8 | .001 |
| Functional status, % | 61.3 | 67.8 | .014 | 61.3 | 69.2 | .005 |
| Influenza vaccination, % | 37.0 | 50.5 | <.0001 | 37.3 | 51.7 | <.0001 |
| Pneumovax, % | 34.4 | 50.5 | <.0001 | 35.0 | 51.4 | <.0001 |
| Former smoker, % | 40.3 | 49.3 | .0010 | 40.1 | 52.0 | <.0001 |
| Daily aspirin, % | 28.5 | 53.8 | <.0001 | 29.3 | 55.7 | <.0001 |
| Hospital characteristics | ||||||
| Teaching, % | 54.5 | 50.2 | .12 | 54.3 | 50.0 | .14 |
| Bed group, % | ||||||
| <100 | 1.9 | 1.9 | .39 | 1.8 | 2.3 | .53 |
| 100–249 | 43.3 | 39.0 | 43.1 | 39.0 | ||
| 250–499 | 39.2 | 41.1 | 39.3 | 41.2 | ||
| >=500 | 15.6 | 18.1 | 15.8 | 17.5 | ||
| State, % | ||||||
| CT | 29.1 | 26.8 | .02 | 28.8 | 27.4 | .02 |
| MI | 6.0 | 2.8 | 5.9 | 2.5 | ||
| PA | 59.9 | 66.2 | 60.2 | 66.4 | ||
| TN | 5.0 | 4.2 | 5.1 | 3.7 |
SD = standard deviation; w/o = without; PSI = Pneumonia Severity Index; SOFA = Sequential Organ Failure Assessment; APACHE III APS = Acute Physiology and Chronic Health Evaluation III acute physiology score, ICU = intensive care unit.
Adequate initial hospital antibiotic use within 24 hours of presentation according to American Thoracic Society guidelines [48].
Table 2 compares baseline characteristics of prior statins users stratified by whether their statin was continued in-hospital. Those whose statin was not continued (n=72) were younger, more than twice as likely to be mechanically ventilated and admitted from a nursing home, and less likely to have healthy user indicators, such as good functional status, receipt of vaccinations, daily aspirin use, and having quit smoking. Of these differences, only age, insurance and smoking status reached statistical significance, though use of mechanical ventilation (p=0.07) and daily aspirin use approached significance (p=0.08). Current and prior statin users were equally likely to have a bacterial infection, as evidenced by similar distribution of statin users and non-users across different strata of PCT levels (data not shown).
Table 2.
Baseline characteristics of prior statin users by whether their statin was continued.
| Variable | Prior not continued N=72 | Prior and continued N=354 | P-value |
|---|---|---|---|
| Demographics and comorbidities | |||
| Age, yrs mean (SD) | 68.9 (12.7) | 72.2 (10.7) | .02 |
| Gender, % male | 51.4 | 54.8 | .60 |
| Admitted from nursing home, % | 5.6 | 2.3 | .12 |
| Charlson, mean (SD) | 2.0 (2.0) | 2.2 (2.1) | .45 |
| Illness severity | |||
| Day 1 PSI, mean (SD) | 104.5 (33.6) | 106.5 (29.7) | .61 |
| Day 1 PSI w/o age, mean (SD) | 40.5 (28.8) | 38.9 (27.1) | .65 |
| Day 1 SOFA, mean (SD) | 2.5 (1.9) | 2.4 (1.8) | .55 |
| APACHE III APS, mean (SD) | 42.8 (13.8) | 42.0 (12.0) | .60 |
| Documented bacteremia, % | 9.7 | 7.6 | .55 |
| ICU admission, % | 22.2 | 17.5 | .35 |
| Mechanically ventilated, % | 11.1 | 5.4 | .07 |
| Treatments administered | |||
| Pre-hospital antibiotics, % | 11.1 | 17.2 | .20 |
| Adequate initial antibiotics†, % | 81.9 | 80.8 | .82 |
| Inpatient corticosteroid use, % | 37.5 | 35.9 | .79 |
| Healthy user indicators | |||
| Insured, % | 97.2 | 99.7 | .02 |
| Lives at home, % | 88.9 | 93.8 | .14 |
| Functional status, % | 61.1 | 69.2 | .18 |
| Influenza vaccination, % | 44.4 | 51.7 | .26 |
| Pneumovax, % | 45.8 | 51.4 | .39 |
| Former smoker, % | 36.1 | 52.0 | .01 |
| Daily aspirin, % | 44.4 | 55.6 | .08 |
SD = standard deviation; PSI = Pneumonia Severity Index; SOFA = Sequential Organ Failure Assessment; APACHE III APS = Acute Physiology and Chronic Health Evaluation III acute physiology score, ICU = intensive care unit.
Adequate initial hospital antibiotic use within 24 hours of presentation according to American Thoracic Society guidelines [48].
Clinical outcomes
There were 582 (30.7%) subjects who developed severe sepsis, most commonly (47.1%) on day one. In univariate analysis, there were no difference in severe sepsis rates between statin users and non-users, either for prior (30.8% vs. 30.7%, p=0.98) or continued use (30.2% vs. 30.8%, p=0.85). After adjusting for baseline characteristics neither prior nor continued statin use appeared to protect against the development of severe sepsis regardless of whether propensity for statin use was included (tables 3a [prior use] and 3b [continued use]). These findings persisted even when excluding severity of illness measures, which overlap with the diagnostic criteria for severe sepsis, from the models.
Table 3a–b.
Final severe sepsis multivariable logistic regression models by prior statin use (top) and continued status use (bottom) with and without adjustment for propensity.
| Prior use | Prior use w/ propensity | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Prior statin use | 0.96 | (0.72, 1.29) | 0.81 | 0.98 | (0.71, 1.36) | 0.91 |
| Age | 1.01 | (1.00, 1.02) | 0.11 | 1.01 | (1.00, 1.02) | 0.04 |
| Gender | 1.21 | (1.00, 1.46) | 0.08 | 1.22 | (1.02, 1.45) | 0.06 |
| Functional status | 0.77 | (0.60, 0.99) | 0.08 | 0.78 | (0.61, 1.01) | 0.10 |
| Nursing home | 1.45 | (0.92, 2.28) | 0.11 | 1.43 | (0.94, 2.19) | 0.11 |
| Chronic renal disease | 0.28 | (0.11, 0.67) | 0.01 | 0.28 | (0.12, 0.66) | 0.01 |
| PSI w/o age | 1.02 | (1.01, 1.02) | 0.002 | 1.02 | (1.01, 1.02) | 0.001 |
| APACHE III APS | 1.04 | (1.03, 1.05) | 0.0002 | 1.04 | (1.03, 1.05) | 0.0002 |
| Continued use | Continued use w/ propensity | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Continued statin use | 0.96 | (0.70, 1.31) | 0.79 | 0.97 | (0.68, 1.40) | 0.89 |
| Age | 1.01 | (1.00, 1.02) | 0.11 | 1.01 | (1.00, 1.02) | 0.05 |
| Gender | 1.21 | (1.01, 1.46) | 0.08 | 1.22 | (1.02, 1.45) | 0.06 |
| Functional status | 0.77 | (0.60, 0.99) | 0.08 | 0.78 | (0.61, 1.01) | 0.10 |
| Nursing home | 1.45 | (0.92, 2.29) | 0.11 | 1.43 | (0.94, 2.19) | 0.11 |
| Chronic renal disease | 0.28 | (0.11, 0.67) | 0.01 | 0.28 | (0.12, 0.66) | 0.01 |
| PSI w/o age | 1.02 | (1.01, 10.2) | 0.002 | 1.02 | (1.01, 1.02) | 0.001 |
| APACHE III APS | 1.04 | (1.03, 1.05) | 0.0002 | 1.04 | (1.03, 1.05) | 0.0002 |
OR = odds ratio; 95% CI = 95% confidence interval; PSI = Pneumonia Severity Index; APACHE III APS = Acute Physiology and Chronic Health Evaluation III acute physiology score.
Unadjusted 90-day mortality was lower in statin users, though this difference was only significant for continued statin use (7.9% vs. 12.1%, p=0.02) as opposed to prior use (9.2% vs. 12.0%, p=0.11). Interestingly, prior statin users whose statin was not continued in-hospital had nearly twice the mortality of those whose statin was continued (15.3% vs. 7.9%, p=0.048), but similar rates of severe sepsis (33.3% vs. 30.2%, p=0.60). The final multivariable models of 90-day mortality with and without propensity adjustment are shown in tables 4a (prior use) and 4b (continued use). After adjustment for age, comorbidity, living at home, and illness severity, prior statin use showed no associated mortality benefit (adjusted OR [95% CI]: 0.74 [0.48–1.24], p=0.19), while continued statin use showed a marginally significant benefit (adjusted OR 0.60 [0.38–0.96], p=0.0496). After accounting for likelihood of statin use by including a propensity score in each model, there was no detectible benefit for either prior (adjusted OR 0.90 [0.63–1.29], p=0.57) or continued statin use (adjusted OR 0.73 [0.47–1.13], p=0.15). Among the subset that developed severe sepsis, there was no evidence of any mortality benefit for either prior or continued statin use (data not shown).
Table 4a–b.
Final 90d mortality multivariable logistic regression model by prior statin use (top) and continued status use (bottom) with and without adjustment for propensity.
| Prior use | Prior use w/ propensity | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Prior statin use | 0.74 | (0.48, 1.24) | 0.19 | 0.90 | (0.63, 1.29) | 0.57 |
| Age | 1.04 | (1.03, 1.06) | 0.001 | 1.05 | (1.04, 1.07) | 0.002 |
| Charlson | 1.09 | (1.03, 1.15) | 0.01 | 1.12 | (1.06, 1.18) | 0.01 |
| Lives at home | 0.60 | (0.44, 0.80) | 0.03 | 0.75 | (0.56, 1.01) | 0.10 |
| PSI w/o age | 1.02 | (1.01, 1.03) | 0.01 | 1.02 | (1.01, 1.03) | 0.01 |
| APACHE III APS | 1.03 | (1.01, 1.05) | 0.01 | 1.03 | (1.01, 1.05) | 0.01 |
| Continued use | Continued use w/ propensity | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Continued statin use | 0.60 | (0.38, 0.96) | 0.0496 | 0.73 | (0.47, 1.13) | 0.15 |
| Age | 1.04 | (1.03, 1.06) | 0.001 | 1.05 | (1.04, 1.07) | 0.002 |
| Charlson | 1.09 | (1.03, 1.15) | 0.01 | 1.12 | (1.06, 1.18) | 0.01 |
| Lives at home | 0.61 | (0.45, 0.82) | 0.04 | 0.75 | (0.56, 1.01) | 0.10 |
| PSI w/o age | 1.02 | (1.01, 1.03) | 0.01 | 1.02 | (1.01, 1.03) | 0.01 |
| APACHE III APS | 1.03 | (1.01, 1.05) | 0.01 | 1.03 | (1.01, 1.05) | 0.01 |
OR = odds ratio; 95% CI = 95% confidence interval; PSI = Pneumonia Severity Index; APACHE III APS = Acute Physiology and Chronic Health Evaluation III acute physiology score.
Biomarkers
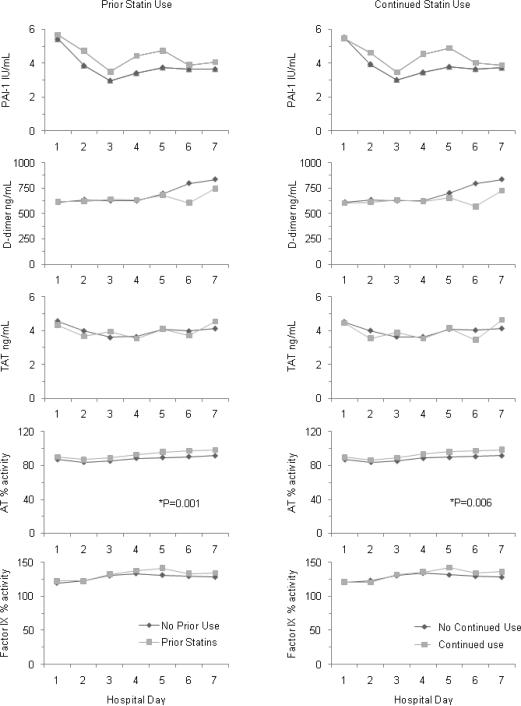
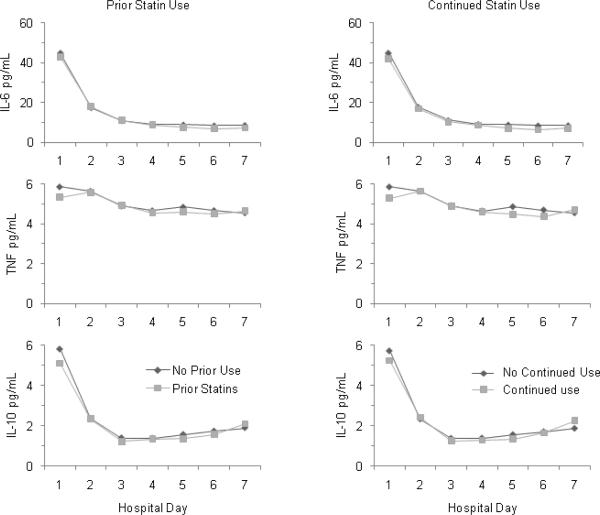
In the entire cohort, higher circulating IL-6 levels and lower antithrombin activity correlated with overall mortality. Both prior and continued statin subjects had higher antithrombin activity over hospital days one through seven (p=0.001 and p=0.006, respectively) compared to those without prior or continued statin use (figure 2). However, the magnitude of the antithrombin differences was modest, typically <5% absolute difference in antithrombin activity. For instance, on day 1, mean (SD) antithrombin activity was 90.1% (17.1%) vs. 87.0% (18.4%) in those with prior as opposed to no prior statin use, whereas >60% activity is considered normal. There were no differences between the two groups in the other coagulation markers (D-dimer, Factor IX, plasminogen activator inhibitor, thrombin antithrombin complexes); in the inflammatory markers (IL-6, IL-10, TNF) (figure 3); or in day 1 expression of lymphocyte cell surface proteins (CD3, CD4, CD5, CD 8, CD 14, CD19, CD64, CD120a, CD 120b, HLA-DR, TLR2, TLR4) (data not shown).
Figure 2.
Mean coagulation factor levels over hospital days 1 through 7 in 939 subjects hospitalized with CAP, stratified by prior (left) and continued (right) statin use. Statin users had higher antithrombin levels over time as compared to those without statin use. There were no significant differences for any of the other coagulation factors. Means are geometric means estimated from Tobit models when appropriate. Geometric means roughly approximate medians. AT = antithrombin; PAI-1 = plasminogen activator inhibitor-1; TAT = thrombinantithrombin complex. Normal values are : D-dimer ≤256 ng/ml, TAT ≤5.0 ng/ml, PAI activity ≤31 IU/ml, Factor IX activity ≥60%, and antithrombin activity ≥70%.
Figure 3.
Mean cytokine levels over hospital days 1 through 7 in 1886 subjects hospitalized with CAP, stratified by prior (left) and continued (right) statin use. There were no significant differences between groups for any of the measured cytokines. Means are geometric means estimated from Tobit models when appropriate. Geometric means roughly approximate medians. TNF = tumor necrosis factor; IL-6 = interleukin-6; IL-10 = interleukin-10.
DISCUSSION
In a large, prospective, multicenter cohort of patients hospitalized with CAP, we found no evidence of a protective effect for either prior or continued statin use on the development of severe sepsis. Prior statin use did not protect against 90-day mortality while continued use showed only a marginal benefit, which was no longer evident after accounting for likelihood of being a statin user. Furthermore, we found only modest differences in a single plasma marker of coagulation, antithrombin, with no observed differences for any of the other markers of coagulation, inflammation, or lymphocyte cell surface protein expression. Though we found no significant evidence of benefit for statins use and mortality, the adjusted odds ratios were all in favor of the drugs. This leaves open the possibility of a small yet clinically meaningful benefit for statins in CAP, one which is likely smaller than prior studies suggest, with implications for powering future randomized trials.
Why have we failed to demonstrate a benefit for statins where the majority of previous studies have shown benefit? The answer, we believe, lies in limitations of prior studies and our handling of potential sources of bias and confounding. We used detailed, prospectively collected information about baseline medical conditions, severity of illness, and treatments received, such as adequacy of initial antibiotic therapy. Such granularity is uncommon in previous statin studies, especially those based on administrative data. The healthy user effect occurs when adherence to a treatment is a surrogate marker for engaging in a broad spectrum of health-promoting behaviors that are themselves linked to the outcome of interest. Indeed, statin users in our study were universally more likely to have healthy user indicators, such as being insured, living at home, being of good functional status, receiving of vaccinations, taking a daily aspirin, and quitting smoking, findings which are supported by other work in this area (50–53) and likely to positively influence mortality in CAP (54).
Indication bias can be seen when the choice to use or continue a drug is driven by factors associated with outcome. Prior studies suggest that worse outcomes in subjects whose statins were discontinued supports protective effect of statin. However, for a medication that is taken chronically, the decision to continue the drug in-hospital primarily comes down to how sick the patient is and whether they are able to take medications by mouth. In our study, subjects whose statins were discontinued were more than twice as likely as continued users to be mechanically ventilated. Medications taken for chronic disease are often withheld in mechanically ventilated patients who tend to have higher mortality. The inclusion of propensity for continued statin use in the mortality models universally moved the adjusted odds ratio closer to unity and the p-value toward greater degrees of insignificance.
Our study has many strengths. First, we used a prospective cohort design, specifically designed to explore risk factors, including statin use, for the development and progression of severe sepsis and death in CAP [45]. By focusing on CAP, our study was less vulnerable to spurious differences in case-mix, though limiting the study to CAP also limits generalizeability of our findings to other infections. Second, we recruited subjects at multiple centers to study a large number of subjects and determine that our findings were consistent across centers, suggesting our findings are robust and likely generalizeable to CAP patients elsewhere. Third, unlike previous studies, we were able to assess circulating biochemical indices of the inflammatory and coagulation pathways, and lymphocyte cell surface protein expression to understand potential mechanisms. We focused on these pathways because modulation of inflammation and coagulation has been most closely tied to improved outcome in sepsis [46,47]. Our results suggest that statins modified the coagulation response to infection, albeit only slightly, but had no discernable effect on circulating inflammatory cytokines or immune cell surface protein expression. Further studies, presumably under the auspices of a randomized interventional trial of statin therapy, will be necessary to extend and confirm these preliminary observations.
The key limitations of this study stem from its observational cohort design, which is hypothesis generating and cannot prove cause and effect. Even so, findings from this study enhance our understanding of prior work in this area and provide robust estimates of statins' effects on both mechanisms and outcomes that may inform the design of future randomized trials. Our findings are potentially biased by failure to account for unmeasured differences in patient characteristics, yet we are unaware of any likely unmeasured confounders that would significantly alter our findings. Another limitation was that we could only explore the association of statins in general, and were underpowered to determine differences with particular statin agents or doses. The number of subjects in whom statins were discontinued after hospitalization was small and therefore, we cannot draw inferences about effect of discontinuation of statin therapy. Finally, we measured coagulation and cell surface markers in a subset. We were underpowered to detect small differences, though the clinical relevance of such findings would be unclear.
CONCLUSIONS
In conclusion, we found little to no evidence of a protective effect for statin use on meaningful clinical outcomes in CAP and a suggestion that healthy user effects and indication bias may be important elements to consider in any observational study of statin use in the setting of infection. Furthermore, the near complete absence of differences in coagulation, inflammation, and cell surface protein expression call into question the use of these measures as surrogate outcomes for any randomized controlled trial of statins in infection.
ACKNOWLEDGMENTS
We are indebted to all the investigators, coordinators, and clinical and laboratory staff of all participating hospitals; and the research assistants and secretaries associated with the study for their efforts. GenIMS was funded by NIGMS R01 GM61992 with additional support from GlaxoSmithKline for enrollment, clinical data collection, and coagulation assays and from Diagnostic Products Corporation and BRAHMS Diagnostica for the cytokine assays. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Source of support: GenIMS was funded by NIGMS R01GM61992 with additional support from GlaxoSmithKline for enrollment, clinical data collection, and coagulation assays and from Diagnostic Products Corporation and BRAHMS Diagnostica for the cytokine assays.
This study was funded by NIH.
Footnotes
The authors have not disclosed any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 3.Undas A, Brummel KE, Musial J, et al. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103:2248–2253. doi: 10.1161/01.cir.103.18.2248. [DOI] [PubMed] [Google Scholar]
- 4.Almog Y. Statins, inflammation, and sepsis: hypothesis. Chest. 2003;124:740–743. doi: 10.1378/chest.124.2.740. [DOI] [PubMed] [Google Scholar]
- 5.Kruger PS, Freir NM, Venkatesh B, et al. A preliminary study of atorvastatin plasma concentrations in critically ill patients with sepsis. Intensive Care Med. 2009;35:717–721. doi: 10.1007/s00134-008-1358-3. [DOI] [PubMed] [Google Scholar]
- 6.Kopterides P, Falagas ME. Statins for sepsis: a critical and updated review. Clin Microbiol Infect. 2009;15:325–334. doi: 10.1111/j.1469-0691.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 7.Saint Martin L, Tandθ D, Goetghebeur D, et al. Statin use does not affect the outcome of acute infection: a prospective cohort study. La Presse Mθdicale. 2010;39:e52–e57. doi: 10.1016/j.lpm.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Janda S, Young A, Fitzgerald JM, et al. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care. 2010;25:656–22. doi: 10.1016/j.jcrc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Bjorkhem-Bergman L, Bergman P, Andersson J, et al. Statin treatment and mortality in bacterial infections--a systematic review and meta-analysis. PLoS One. 2010;%19(5):e10702. doi: 10.1371/journal.pone.0010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra V, Flanders SA. Does statin use improve pneumonia outcomes? Chest. 2009;136:1381–1388. doi: 10.1378/chest.09-0941. [DOI] [PubMed] [Google Scholar]
- 11.Viasus D, Garcia-Vidal C, Gudiol F, et al. Statins for community-acquired pneumonia: current state of the science. Eur J Clin Microbiol Infect Dis. 2010;29:143–152. doi: 10.1007/s10096-009-0835-0. [DOI] [PubMed] [Google Scholar]
- 12.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Majumdar SR, McAlister FA, Eurich DT, et al. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [3-10-2008];Statins in Sepsis Study (STATInS) 2008 http://wwwanzctrorgau/ACTRN12607000028404aspx.
- 15. [3-10-2008];The influence of Statins on Blood Vessel Function in Severe Sepsis. 2008 http://wwwanzctrorgau/ACTRN12607000393459aspx.
- 16. [3-10-2008];Statins for the Early Treatment of Sepsis (SETS) 2008 http://clinicaltrialsgov/show/NCT00528580.
- 17. [3-10-2008];Simvastatin and severe Sepsis: a randomised controlled Trial (SimSepT) 2008 http://wwwcontrolled-trialscom/ISRCTN92093279/
- 18. [3-10-2008];Hydroxymethylglutaryl-CoA reductase inhibition in Acute lung injury to Reduce Pulmonary oedema and inflammation (HARP) 2008 http://wwwcontrolled-trialscom/ISRCTN70127774/
- 19. [3-10-2008];Randomised double-blind placebo-controlled trial of 40 mg/day of Atorvastatin on reduction in severity of SEPSIS in ward patients (ASEPSIS) 2008 http://wwwcontrolled-trialscom/ISRCTN64637517/
- 20. [3-8-2008];Effect of Rosuvastatin in Abdominal Sepsis. 2008 http://clinicaltrialsgov/ct2/show/NCT00357123.
- 21. [3-8-2008];Statin for Immunomudulation in Sepsis. 2008 http://clinicaltrialsgov/ct2/show/NCT00452608.
- 22. [3-10-2008];Simvastatin in Patients With Septic Shock. 2008 http://clinicaltrialsgov/ct2/show/NCT00450840.
- 23. [5-20-2009];Statin Therapy in the Treatment of Sepsis. 2009 http://clinicaltrialsgov/ct2/show/NCT00676897.
- 24. [5-20-2009];Pravastatin and Ventilator Associated Pneumonia (EPRAVAP) 2009 http://clinicaltrialsgov/ct2/show/NCT00702130.
- 25.Kruger PS, Freir NM, Venkatesh B, et al. A preliminary study of atorvastatin plasma concentrations in critically ill patients with sepsis. Intensive Care Med. 2009;35:717–721. doi: 10.1007/s00134-008-1358-3. [DOI] [PubMed] [Google Scholar]
- 26.Huang DT, Weissfeld LA, Kellum JA, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52:48–58. doi: 10.1016/j.annemergmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reade MC, Weissfeld L, Angus DC, et al. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm Med. 2010;10:15. doi: 10.1186/1471-2466-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayr FB, Yende S, D'Angelo G, et al. Do hospitals provide lower quality of care to black patients for pneumonia? Crit Care Med. 2010;38:759–765. doi: 10.1097/CCM.0b013e3181c8fd58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milbrandt EB, Reade MC, Lee M, et al. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol Med. 2009;15:438–445. doi: 10.2119/molmed.2009.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–36. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 34.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 35.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 36.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 37.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 38.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J, Guyatt G, Bernard GR, et al. New strategies for clinical trials in patients with sepsis and septic shock. Crit Care Med. 2001;29:880–886. doi: 10.1097/00003246-200104000-00039. [DOI] [PubMed] [Google Scholar]
- 40.Angus DC, Carlet J, the 2002 Brussels Roundtable Participants Surviving intensive care: A report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29:368–377. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- 41.Yende S, van der PT, Lee M, et al. The influence of pre-existing diabetes mellitus on the host immune response and outcome of pneumonia: analysis of two multicentre cohort studies. Thorax. 2010;65:870–877. doi: 10.1136/thx.2010.136317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang DT, Weissfeld LA, Kellum JA, et al. Risk Prediction With Procalcitonin and Clinical Rules in Community-Acquired Pneumonia. Annals of Emergency Medicine. 2008;52:48–58. doi: 10.1016/j.annemergmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epstein MP, Lin X, Boehnke M. A tobit variance-component method for linkage analysis of censored trait data. Am J Hum Genet. 2003;72:611–620. doi: 10.1086/367924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown H, Prescott R. Applied mixed models in medicine: statistics in practice. John Wiley & Sons; New York: 2000. pp. 1–428. [Google Scholar]
- 45.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 46.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 47.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 48.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 49.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–695. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 50.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 51.Majumdar SR, McAlister FA, Eurich DT, et al. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119:2051–2057. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dublin S, Jackson ML, Nelson JC, et al. Statin use and risk of community acquired pneumonia in older people: population based case-control study. BMJ. 2009;338:b2137. doi: 10.1136/bmj.b2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majumdar SR, McAlister FA, Eurich DT, et al. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]