Abstract
Aim
Endothelial membrane hyperpolarization mediated by KCa3.1 and KCa2.3 channels has been demonstrated to initiate endothelium-derived hyperpolarizing factor (EDHF)-type vasodilations. Moreover, pharmacological potentiation of KCa3.1/KCa2.3 channels has been suggested to improve EDHF-type vasodilations. Here we determined whether the KCa3.1/KCa2.3 activator SKA-31 and its derivative SKA-20 improve endothelial dysfunction in KCa3.1−/− and NOS3−/− mice.
Methods
Membrane potentials were measured using patch-clamp electrophysiology on carotid artery (CA) endothelial cells (CAEC) from wild-type (wt) and KCa3.1−/− mice. Endothelium-dependent vasodilations were determined by pressure myography in CA.
Results
SKA-31 (1 µmol L-1) activated KCa3.1 and KCa2.3 channels and induced membrane hyperpolarization in CAEC of wt (ΔMP –45mV). These responses were significantly reduced in CAEC of KCa3.1−/− (ΔMP –8mV). SKA-31 (200 nmol L-1, 500 nmol L-1) and SKA-20 (300 nmol L-1) significantly enhanced EDHF-vasodilations in wt. SKA-20 also improved vasodilations during NO-synthesis. In KCa3.1−/−, the defective EDHF-vasodilations were unchanged at 200 nmol L-1 SKA-31, but were significantly improved at 500 nmol L-1. EDHF-vasodilations were slightly enhanced at 300 nmol L-1 SKA-20, but vasodilations during NO-synthesis were unchanged. SKA-31 (500 nmol L-1) enhanced the impaired endothelium-dependent vasodilation in NOS3−/− mice 2-fold. Pharmacological inhibition of the soluble epoxide hydrolase by t-AUCB (1 µmol L-1) in contrast did not increase ACh-induced EDHF- or NO-mediated vasodilations in wt and KCa3.1−/−.
Conclusion
Normal and defective endothelium-dependent vasodilations in murine carotid arteries can be improved by pharmacological enhancement of KCa3.1/KCa2.3 functions. These findings further support the concept that pharmacological activation of endothelial KCa2.3/KCa3.1 could offer a novel endothelium-specific antihypertensive strategy.
Keywords: EDHF, KCa3.1, KCa2.3, soluble epoxide hydrolase, nitric oxide, endothelial dysfunction
Introduction
The endothelium regulates vascular tone by its ability to generate vasodilating autacoids and hyperpolarization events, which relaxe the underlying smooth muscle. The major endothelium-dependent vasodilator systems are the well-characterized NO-system (Furchgott and Zawadzki, 1980, Palmer et al., 1987) and the endothelium-derived hyperpolarizing factor (EDHF)-system (Feletou and Vanhoutte, 2006). While vasodilator actions by NO are well understood, the cellular mechanisms underlying the EDHF-phenomenon are still unclear and diffusible molecules such e.g. epoxyeicosatrienoic acids (EETs) as well as electrotonic gap-junctional-coupling mechanisms of endothelium and smooth muscle have been suggested to mediate smooth muscle hyperpolarization, subsequent closure of voltage-gated Ca2+-channels, and thus vasodilation (for extensive reviews see (Feletou and Vanhoutte, 2006, Grgic et al., 2009, Edwards et al., 2010)). Regardless of the still unresolved nature of the EDHF-phenomenon, there is compelling evidence that activation of endothelial Ca2+-activated K+ channels and the resulting hyperpolarization of the endothelium is a crucial step for initiation of EDHF-type vasodilations in a wide range of vascular beds from several species and in humans (Edwards et al., 1998, Grgic et al., 2009).
The significance of the NO as well as of the EDHF systems in endothelial function and thus blood pressure control has been demonstrated by the observations that genetic deficiency of the endothelial nitric oxide synthase or pharmacologic NO-synthase inhibition as well as deficiency of one or both of the endothelial KCa channels KCa3.1 and KCa2.3 (also known as IKCa and SKCa3) induce hypertension in mice (Taylor et al., 2003, Si et al., 2006, Brähler et al., 2009, Köhler and Ruth, 2010). Concerning the latter channels, the defects are stimulus dependent with strongly impaired ACh-induced EDHF-type vasodilations in KCa3.1−/− mice and impaired shear-stress-induced EDHF-type and NO-mediated vasodilations, as well as vasodilations to skeletal muscle contraction in mice deficient for KCa2.3 (Milkau et al., 2010). Moreover, in rodent models of cardiovascular disease and diabetes and more importantly in human cardiovascular pathologies, disturbances of these systems cause endothelial dysfunction, which has been suggested to contribute to pathophysiology (Ding et al., 2005, Liu et al., 2008, Feng et al., 2008, Vanhoutte et al., 2009, Brondum et al., 2009, Feletou et al., 2010). On the other hand, pharmacological stimulation of the KCa3.1/KCa2.3-EDHF-system raises the possibility to improve the diminished endothelial function or still intact endothelial function in hypertension or during vascular defects present in other cardiovascular disease states (for review see (Grgic et al., 2009)). That this is indeed possible was suggested by a recent study showing that the KCa3.1 channel activator NS309 (Strobaek et al., 2004) improved the diminished endothelial function in Zucker diabetic fatty (ZDF) rats (Brondum et al., 2009). Concerning hypertension, our own group recently showed that the novel KCa3.1/KCa2.3 activator naphtho[1,2-d]thiazol-2-ylamine (SKA-31) augmented EDHF-vasodilator responses and that i.p. injections of SKA-31 reduced blood pressure in both normotensive and in angiotensin-II-infused hypertensive mice (Sankaranarayanan et al., 2009). SKA-31 and its slightly more potent derivative anthra[2,1-d]thiazol-2-amine (SKA-20) are KCa3.1/KCa2.3 channel activating tool compounds (Fig. 1) that were developed out of the neuroprotectant riluzole (Domino et al., 1952). Similar to other “classic” KCa channel activators like EBIO (Devor et al., 1996) and NS309 (Strobaek et al., 2004), SKA-20 and SKA-31 increase the Ca2+ sensitivity of KCa channels resulting in an apparent leftward shift of their Ca2+ activation curve. Both compounds activate KCa3.1 with submicromolar EC50s and KCa2.3 with low micromolar EC50s and the less lipophilic and thus more “drug-like” SKA-31 was found to exhibit pharmacological properties that make it suitable for in vivo use. In contrast to NS309, which has an extremely short half-life in rodents, SKA-31 was found to have a plasma half-life of 12 hours in both mice and rats, allowing once daily administration in experiments probing the physiological role of KCa channels (Sankaranarayanan et al., 2009).
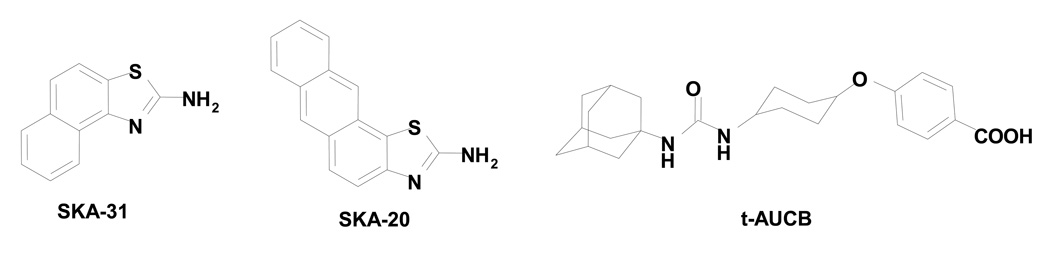
Figure 1. Chemical structures.
of the KCa3.1/KCa2.3 activators SKA-31 (naphtho[1,2-d]thiazol-2-ylamine) and SKA-20 (anthra[2,1-d]thiazol-2-amine), and the sEH inhibitor t-AUCB (trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid).
In the present study, we tested whether the KCa3.1/KCa2.3 activators SKA-31 and SKA-20 at concentrations activating either KCa3.1 or both, KCa3.1 and KCa2.3, improve endothelium-dependent vasodilations in wild-type mice and the defective vasodilations caused by genetic deficiency of KCa3.1 (defective EDHF-vasodilation) or of endothelial nitric oxide synthesis in NOS3−/− mice (loss of NO-mediated vasodilation). In addition, we tested whether an improvement of endothelium-dependent vasodilation can be achieved by inhibiting EETs degradation via pharmacological blockade of the soluble epoxide hydrolase (sEH) by trans-4-[4-(3-adamantan-1-ylureido)-cyclohexyloxy]-benzoic acid (t-AUCB). The results of our studies provide further evidence in support of the view that pharmacological activation of endothelial KCa3.1 and KCa2.3 channels may represent a potential endothelium-specific strategy for improving endothelial dysfunction in cardiovascular disease states.
Methods
Animals
KCa3.1−/− mice (Si et al., 2006) and corresponding wild-type mice (wt) were derived from our own breeding colonies at the local biomedical research laboratory. NOS3−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Patch-clamp electrophysiology
Carotid artery endothelial cells (CAEC) were isolated as described in detail elsewhere (Schmidt et al., 2010). In brief, freshly dissected carotid arteries (CA) were mounted on glass capillaries and filled with 0.25%/0.02% trypsin/EDTA solution. After incubation for 30 min, CAs were cut open longitudinally and the luminal surface was gently scrapped with a 10µl pipette tip. Detached single cells and cells clusters of 5 up to 20 cells were aspirated and were transferred into DMEM cell culture medium containing 10% fetal calf serum (all from Biochrom, Berlin, Germany). CAECs were allowed to settle down on cover slips for 2–3 h before experimentation. Currents and membrane potential were recorded with an Axon patch-clamp amplifier (Axon Instruments) using patch-pipettes with a resistance of 4–5 MΩ. The standard KCl-pipette solution for whole-cell patch-clamp experiments contained (in mmol L-1): 140 KCl, 1 Na2ATP, 1 MgCl2, 2 EGTA, 0.72 CaCl2 (0.1 µmol L-1 [Ca2+]free), and 5 HEPES, pH 7.2. The NaCl bath solution contained (mmol L-1): 137 NaCl, 4.5 Na2HPO4, 3 KCl, 1.5 KH2PO4, 0.4 MgCl2, 0.7 CaCl2, and 10 glucose (pH 7.4), adjusted with NaOH.
Pressure myography
Experiments on freshly dissected CA were performed using a pressure myograph (P110, DMT) as described previously (Brähler et al., 2009). Perfusion and bath buffer (in mmol L-1): 145 NaCl, 1.2 NaH2PO4, 4.7 KCl, 1.2 MgSO4, 2 CaCl2, 5 glucose, 2 pyruvate, and 3 MOPS buffer (pH 7.4 at 37°C), and L-NNA (300 µmol L-1) and INDO (10 µmol L-1) to block NO- and prostaglandin-synthesis. SKA-31, SKA-20, or t-AUCB were dissolved in DMSO and appropriate amounts of 1 mmol L-1 stock solutions were added to the perfusion solution to give the desired final concentration. The DMSO concentration in the buffer did never exceeded 0.2%. CAs were pressurized to 80 mmHg and were perfused with a low flow rate of 30–150 µl min-1. After an equilibration period of 30 min, perfusion buffer was exchanged by a buffer containing either SKA-31, SKA-20, or t-AUCB. Thereafter, CAs were pre-constricted with increasing concentration of phenylephrine (PE, 1 nmol L-1-1 µmol L-1) in the bath solution. Thereafter, CAs were perfused with a flow rate of 600–1000 µl min-1 and with increasing concentrations of acetylcholine (ACh, 1 nmol L-1-10 µmol L-1). At the end of the experiment, maximal constriction was induced by exchanging the bath solution with a solution containing 60 mmol L-1 K+. Thereafter, maximal dilations were achieved by 10 µmol L-1 sodium nitroprusside (SNP) being added to the bath. Data are given as percentage of maximal dilation to SNP or percentage of maximal constriction to 60 mmol L-1 K+. Values of half maximal effective concentration (EC50) were calculated by fitting data points with the formula: y=Min+(Max-Min)/(1+10^((LOG(EC50)-x)*Hill slope)).
Statistics
Data are given as mean±SEM. For comparison of groups we employed One-way ANOVA followed by Newman-Keuls Test if more than two groups were compared. P<0.05 was considered significant.
Results
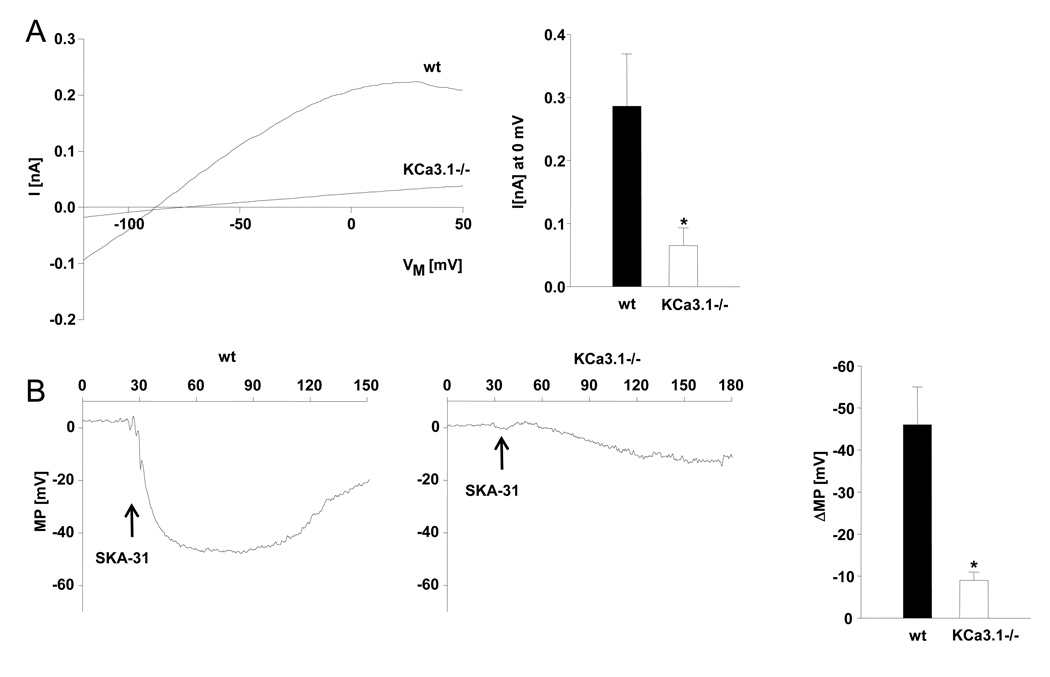
In whole-cell patch-clamp experiments, SKA-31 (1 µmol L-1) activated KCa3.1 and KCa2.3 currents in native CAEC clusters from wt mice (Fig. 2 A) as previously described in more detail (Sankaranarayanan et al., 2009). SKA-31 (1 µmol L-1) also potentiated KCa2.3 currents in CAEC clusters from KCa3.1−/− mice. However, current amplitudes were significantly smaller (Fig. 2 A). In current-clamp experiments, SKA-31 (1 µmol L-1) produced a shift towards negative membrane potentials (ΔMP –45 mV) in CAEC clusters from wt mice (Fig. 2 B). This shift was significantly smaller in CAEC clusters from KCa3.1−/− mice (ΔMP –8 mV) (Fig. 2 B, right panel).
Figure 2. SKA-31 activates endothelial KCa3.1 and KCa2.3 channels and induces membrane hyperpolarization of carotid artery endothelium.
A) On left: SKA-31 (1 µmol L-1) activated KCa currents in freshly isolated carotid artery endothelial cell clusters from wt and KCa3.1−/− mice. On right: summary of the current data (wt: n=3 clusters (6±1 cells), KCa3.1−/−: n=4 clusters (10±4 cells). B) On left: Membrane potential (MP) changes to SKA-31 (1 µmol L-1) in clusters from wt and KCa3.1−/−. CAEC clusters from both strain showed a similar depolarized resting MP (wt: 2±1 mV; KCa3.1−/−: 1±1 mV). On right: summary of the data of ΔMP (wt: n=4; KCa3.1−/−: n=4 cluster). Data are given as mean±SEM; *P<0.05, One-way ANOVA.
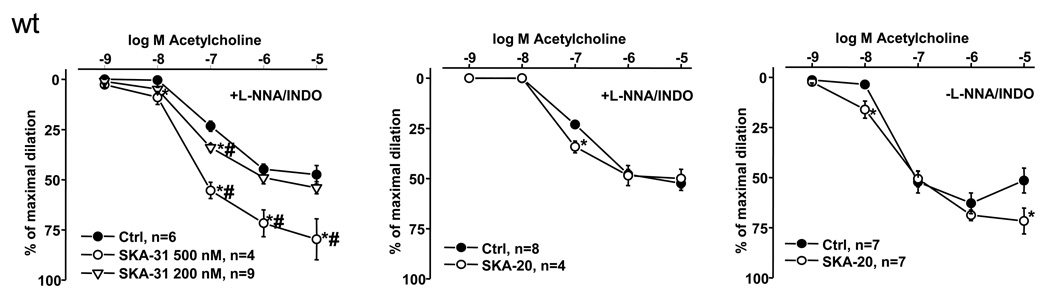
To test the efficacy of SKA-31 and SKA-20 to augment EDHF-type vasodilations, we performed pressure myography on phenylephrine-pre-constricted CAs of mice in the presence of the NO-synthase inhibitor L-NNA and the cyclooxygenase blocker indomethacin (to selectively study EDHF-responses) and in the continuous presence or absence of either SKA-31 (200 and 500 nmol L-1) or SKA-20 (300 nmol L-1). These studies revealed that EDHF-vasodilator responses at a physiological ACh concentration of 100 nmol L-1 were enhanced 1.5-fold and 2-fold by 200 nmol L-1 and 500 nmol L-1 SKA-31, respectively) and 1.5-fold by 300 nmol L-1 SKA-20 (Fig. 3). Moreover, at 10 nmol L-1 ACh, a concentration at which EDHF-vasodilation is virtually undetectable, the presence of SKA-31 resulted in small but appreciable vasodilation responses shifting the dose response curve for ACh slightly to the left (Ctrl: EC50 100 ± 8 nmol L-1 vs. 500 nmol L-1 SKA-31: EC50 51 ± 16 nmol L-1). In contrast, at higher, supra-physiological ACh concentrations there were no obvious potentiating effects by 200 nmol L-1 SKA-31 or by 300 nmol L-1 SKA-20, while the higher concentration of SKA-31 (500 nmol L-1) was able to enhance vasodilation 1.5-fold at these concentrations. Also during intact NO and prostaglandins synthesis (in the absence of L-NNA and indomethacin) 300 nmol L-1 SKA-20 significantly augmented the vasodilation in response to 10 nmol L-1 ACh and still increased vasodilation at ACh concentrations as high as 10 µmol L-1 (Fig. 3, right panel). However, a shift in the dose response curve was not evident (Ctrl: EC50 36 ± 23 nmol L-1 vs. 300 nmol L-1 SKA-20: EC50 39 ± 1 nmol L-1). At this high concentration, vasodilation was smaller compared to a lower 1 µmol L-1 concentration because the action of endothelium-derived contracting factors (EDCFs, in particular PGH2 PGF2α (Vanhoutte and Tang, 2008, Wong et al., 2009)) counteracted the NO- and EDHF-mediated vasodilation.
Figure 3. Potentiating effects of SKA-31 and SKA-20 on endothelium-dependent vasodilation of murine carotid arteries.
ACh-induced EDHF-mediated vasodilation in wt mice in the absence (Ctrl) or in the continuous presence of SKA-31 (200 and 500 nmol L-1, on left) or of SKA-20 (300 nmol L-1, in middle). On right: Potentiation of ACh-induced vasodilation by SKA-20 (300 nmol L-1) during intact NO- and prostaglandin-synthesis (without L-NNA and INDO). n refers to the number of animals studied. Data are given as mean±SEM; *P<0.05 vs. Ctrl; #P<0.05 500 nmol L-1 vs. 200 nmol L-1 SKA-31; One-way ANOVA followed by Newman-Keuls Test for comparison of more than 2 data sets (in left panel).
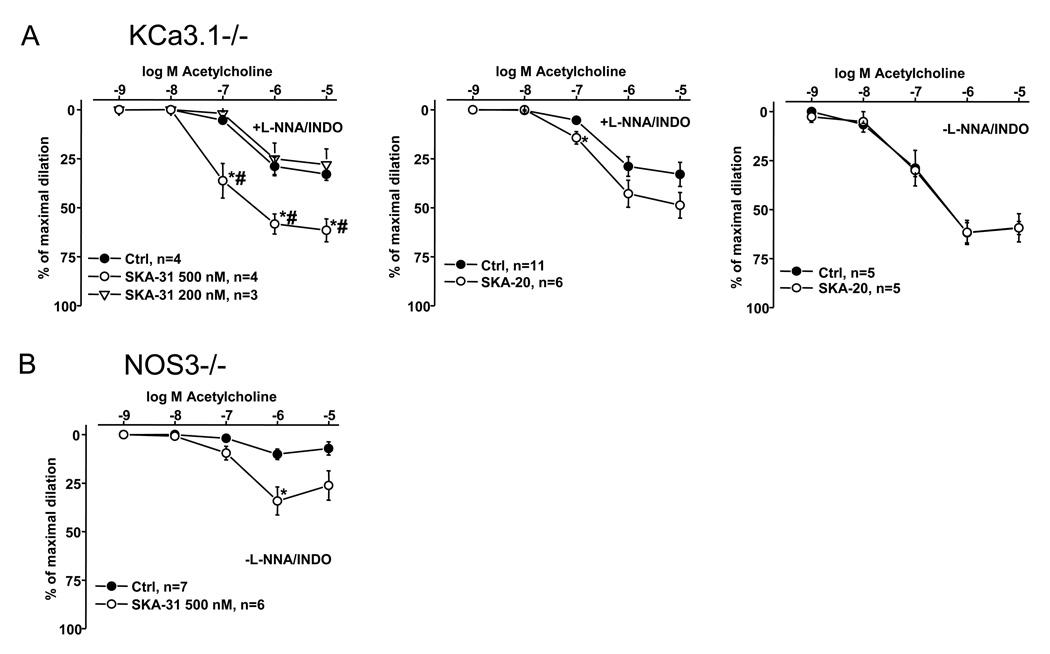
In contrast to wt, SKA-31 (200 nmol L-1), which predominantly affects KCa3.1, did not improved the severely defective EDHF-type vasodilation in KCa3.1−/− mice (Ctrl: EC50 264 ± 4 nmol L-1 vs. 200 nmol L-1 SKA-31: EC50 353 ± 1 nmol L-1) (Fig. 4A, on left). However, a higher concentration of SKA-31 (500 nmol L-1) clearly improved the defective EDHF-vasodilation starting at 100 nmol L-1 ACh albeit not at 10 nmol L-1 ACh as seen in wt (Fig. 4A, left panel). Nonetheless, the dose-response was shifted to the left (500 nmol L-1 SKA-31: EC50 80 ± 12 nmol L-1). Similarly, SKA-20 at 300 nmol L-1 slightly improved the response at 100 nmol L-1 ACh (Ctrl: EC50 272 ± 4 nmol L-1 vs. 300 nmol L-1 SKA-20: EC50 238 ± 14 nmol L-1) (Fig. 4A, panel in middle), without reaching the wild-type level (Ctrl: EC50 118 ± 16 nmol L-1; 300 nmol L-1 SKA-20: EC50 73 ± 20 nmol L-1), but failed to improve the larger vasodilation during intact NO/prostaglandin-synthesis (Ctrl: EC50 112 ± 30 nmol L-1 vs. 300 nmol L-1 SKA-20: EC50 100 ± 20 nmol L-1) (Fig. 4A, right panel).
Figure 4. Potentiating effects of SKA-31 and SKA-20 on endothelium-dependent vasodilation of murine carotid arteries in KCa3.1−/− mice and NOS3−/− mice.
A) ACh-induced EDHF-mediated vasodilation in the absence (Ctrl) or in the continuous presence of SKA-31 (200 and 500 nmol L-1, on left) or of SKA-20 (300 nmol L-1, in middle) in KCa3.1−/− mice. On right: Potentiation of ACh-induced vasodilation by SKA-20 (300 nmol L-1) during intact NO- and prostaglandin-synthesis. B) ACh-induced vasodilation in the absence (Ctrl) or in the continuous presence of SKA-31 (500 nmol L-1) in NOS3−/− mice. Note that experiments were conducted during intact prostaglandin-synthesis (without INDO). Data are given as mean±SEM; *P<0.05 vs. Ctrl; #P<0.05 500 nmol L-1 vs. 200 nmol L-1 SKA-31; One-way ANOVA followed by Newman-Keuls Test for comparison of more than 2 data sets (in left panel).
NOS3−/− mice showed a severely impaired ACh-induced endothelium-dependent vasodilation of CA during intact prostaglandin synthesis (Fig 4B). Intriguingly, 500 nmol L-1 SKA-31 was able to significantly enhance (by 2-fold) the response.
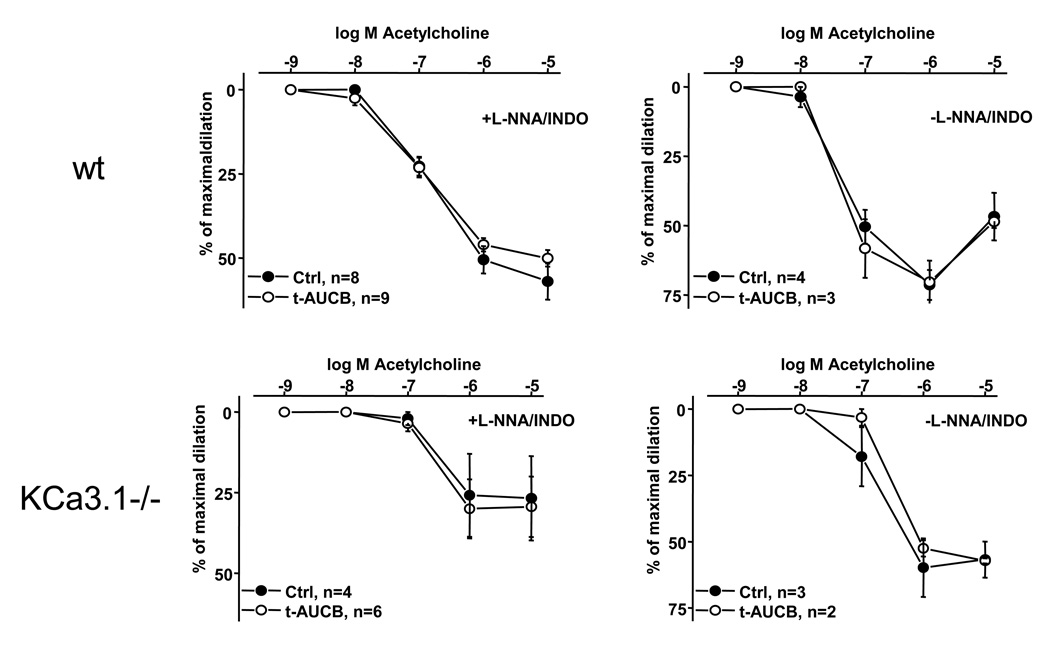
Epoxyeicosatrienoic acids (EETs) generated by CyP450 enzymes have been shown to serve as a diffusible EDHF in a variety of vascular beds (Archer et al., 2003, Imig and Hammock, 2009). We therefore tested whether pharmacological inhibition of the EETs degrading sEH by t-AUCB (Fig. 1)(Hwang et al., 2007) is similarly capable of improving EDHF-type or combined NO- and EDHF-vasodilator responses in wt and the reduced responses in KCa3.1−/−. However, selective inhibition of sEH by 1 µmol L-1 t-AUCB had no effect on ACh-induced EDHF- or NO-mediated vasodilations in CAs from wt and KCa3.1−/− mice under our experimental conditions (Fig. 5).
Figure 5. The sEH-inhibitor t-AUCB did not influence vasodilation of murine carotid arteries.
Upper panel on left: ACh-induced EDHF-mediated vasodilation in wt mice in the continuous presence or absence (Ctrl) of t-AUCB (1 µmol L-1). On right: ACh-induced vasodilation during intact NO- and prostaglandin-synthesis. Lower panel on left: ACh-induced EDHF-mediated vasodilation in KCa3.1−/− in the continuous presence or absence (Ctrl) of t-AUCB (1 µmol L-1). On right: ACh-induced vasodilation during intact NO- and prostaglandin-synthesis. Note that ACh-induced EDHF-mediated vasodilations and vasodilations in the absence of L-NNA and INDO are severely impaired in KCa3.1−/− if compared wild-type responses. n refers to the number of animals studied. Data are given as mean±SEM.
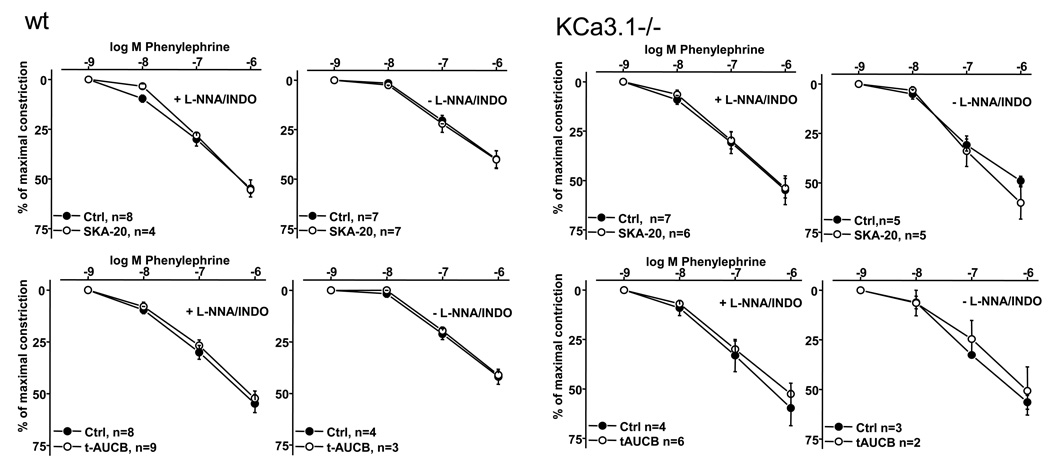
Of note, SKA-20, SKA-31, and t-AUCB had no sizeable impact on phenylephrine-induced constrictions (Fig. 6) or on endothelium-independent vasodilation to the NO-donor sodium nitroprusside (data not shown) which indicated that SKA-20 and SKA-31 at the concentration used here acted at the endothelial level and did not interfere with endothelium-independent smooth muscle cell functions.
Figure 6. SKA-20 and t-AUCB did not influence phenylephrine-induced constriction.
of carotid arteries from wt (left panels) and KCa3.1 (right panels) in either the presence or absence of L-NNA (300 µmol L-1) and INDO (10 µmol L-1). Data are given as mean±SEM.
Discussion
The aim of the present study was to evaluate whether pharmacological potentiation of KCa3.1 and KCa2.3 function is capable of improving normal and defective endothelial function in KCa3.1−/− (impaired EDHF-vasodilation) and NOS3−/− mice (loss of endothelial NO-formation).
Our study shows that SKA-31 activated endothelial KCa3.1 and KCa2.3 channels and elicits membrane hyperpolarization in wt and KCa3.1−/− mice. However, these responses were substantially reduced in KCa3.1−/−. This demonstrates that the SKA-31-induced membrane hyperpolarization was mediated mainly by an effect on KCa3.1 and only to a minor extent by KCa2.3 channel activation.
Our pressure myography experiments demonstrate that SKA-31 (200 nmol L-1) and SKA-20 (300 nmol L-1) potentiated ACh-induced EDHF-type responses in wt animals. This potentiation was stronger at a higher concentration of SKA-31 (500 nmol L-1) which fully activates KCa3.1 and –although to a much lesser degree- is also effective on KCa2.3 (Sankaranarayanan et al., 2009). This potentiation of ACh-induced endothelium-dependent vasodilation was not restricted to pure EDHF-vasodilations since the SKA-31 derivative SKA-20 also improved the ACh-induced vasodilation under conditions of intact NO and prostaglandins synthesis. Moreover, SKA-20-mediated potentiation of EDHF- and possibly NO-mediated responses seem to limit the actions of EDCFs because the counteracting actions of EDCFs on vasodilations at a high ACh concentration were reduced by SKA-20.
The potentiating effects of SKA-31 can be accounted for in large part by potentiation of KCa3.1 because the lower concentration of SKA-31, which predominantly affects KCa3.1, did not improve the severely defective EDHF-vasodilation in KCa3.1−/− mice. Nonetheless, a higher concentration of SKA-31 and to a smaller extent also SKA-20 improved the defective EDHF-vasodilation. These potentiating effects in KCa3.1−/− mice can be explained by the different sensitivities of KCa3.1 and KCa2.3 to SKA-20 and SKA-31. Both compounds are roughly 10-fold more potent on KCa3.1. SKA-31 activates KCa3.1 with an EC50 of 260 nmol L-1 and KCa2.3 with an EC50 of 2.9 µmol L-1. SKA-20 is slightly more potent and has EC50s of 115 nmol L-1 for KCa3.1 and of 1.2 µmol L-1 for KCa2.3 (Sankaranarayanan et al., 2009). Thus 300 nmol L-1 SKA-20 and of 500 nmol L-1 SKA-31 already have a significant potentiating effect on the residual KCa2.3 channel, which explains the improvement of EDHF-type vasodilations that both compounds exhibit in KCa3.1−/− mouse CAs. Our results thus also demonstrate that the endothelial dysfunction at the EDHF-level, which is caused by loss of KCa3.1, can be partially compensated for by potentiation of KCa2.3.
Genetic deficiency of endothelial nitric oxide synthase in mice (NOS3−/−) resulted in a severely impaired ACh-induced endothelium-dependent vasodilation of CA during intact prostaglandin synthesis. The residual weak response is most likely mediated by the KCa3.1/KCa2.3 EDHF-system, which appears to at least partially override the antagonistic actions of the EDCF-system in murine CAs. Intriguingly, SKA-31 at concentrations that activate both KCa3.1 and KCa2.3 considerably improved EDHF-vasodilations demonstrating that pharmacological enhancement of the KCa3.1/KCa2.3 EDHF-system is capable of at least partially correcting the endothelial dysfunction caused by loss of endothelial NO formation and possibly even to limit the actions of EDCFs in this type of endothelial dysfunction.
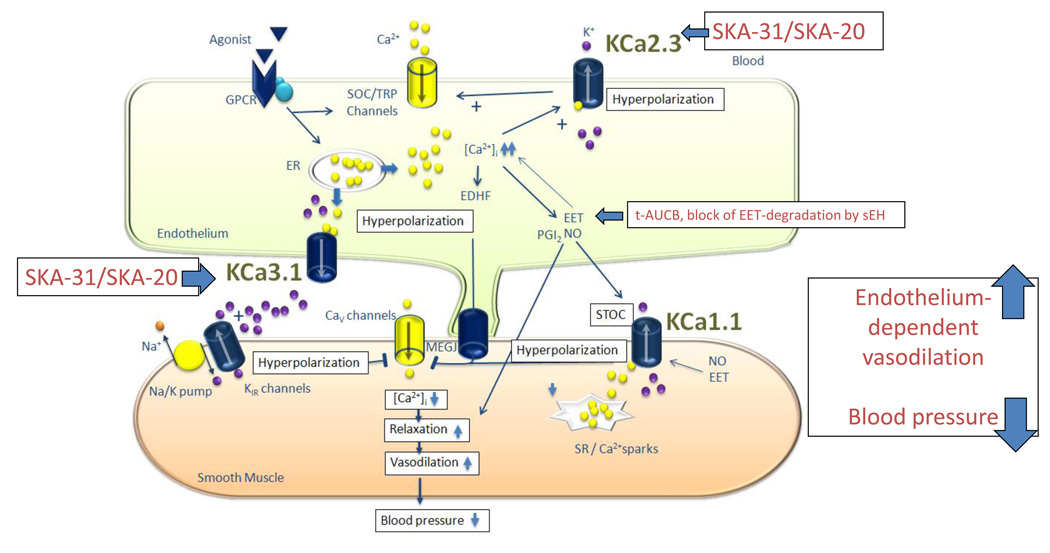
Besides the KCa3.1/KCa2.3 EDHF-system, epoxyeicosatrienoic acids (EETs) generated by CyP450 enzymes have been proposed to act as diffusible EDHFs targeting smooth muscle KCa1.1 channels (BK channels) (Fig. 7). Moreover, this EDHF-system has been suggested to be particularly important in situations of endothelial dysfunction (Archer et al., 2003, Imig and Hammock, 2009). However, in contrast to the activators of KCa3.1 and KCa2.3, selective pharmacological inhibition of the EET-degrading enzyme sEH failed to improve normal as well defective EDHF-vasodilations in carotid arteries of wt and KCa3.1−/− mice, respectively. Inhibition of sEH also did not alter the vasodilator responses during intact NO and prostaglandin synthesis in either genotype. This indicates that endogenous EETs production and metabolism might be low in the wt CA and that the EETs-EDHF-system does not compensate for EDHF-signaling defects caused by the loss of KCa3.1. Albeit sEH inhibition had thus no improving effects on endothelial function in these murine conduit arteries, EETs were shown to cause endothelium dependent hyperpolarization and relaxation on the vascular smooth muscles in a variety of arteries from experimental animals and humans; however, this is not a universal finding in all arteries (Larsen et al., 2006, Fleming et al., 2007, Campbell and Fleming, 2010). Other recent findings suggested that the EETs are vasodilatory in mesenteric arteries, largely through their ability to activate endothelial NO synthase (eNOS) and NO release, and they do not cause EDHF responses in mice (Hercule et al., 2009). Furthermore, the main site of the blood pressure reducing actions of EETs and sEH inhibitors is thought to be the microvasculature of the kidney (Chiamvimonvat et al., 2007, Imig and Hammock, 2009), which might explain why we did not observe any effects of the sEH inhibitor on our CA preparation.
Figure 7. Schematic illustration of the action of the KCa3.1/KCa2.3 activator SKA-31 and SKA-20 on endothelial function and vasodilations.
Cav, voltage-dependent Ca2+ channel; CYP, cytochrome P450 epoxygenase; EETs, epoxyeicosatrienoic acids; ER, endoplasmic reticulum; GPCR, G-protein coupled receptor, KCa1.1, large-conductance Ca2+-activated K+ channel; KCa3.1, intermediate-conductance Ca2+-activated K+ channel; Kir, inwardly-rectifying K+ channel; KCa2.3, small-conductance Ca2+-activated K+ channel subtype 3; MEGJ, myoendothelial gap-junction; sEH, soluble expoxide hydrolase, SOC, store operated channels, SR, sarcoplasmic reticulum; STOC, spontaneous transient outward currents; t-AUCB, trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid = sEH inhibitor; TRP, transient receptor potential channels.
From a more clinical perspectives, many cardiovascular pathologies have been associated with endothelial dysfunction (Vanhoutte et al., 2009), a complication contributing to impaired vasodilator responses and long-term pathologic arterial remodeling and end-organ damage. The present results further support the concept that pharmacologic activation of endothelial KCa3.1 and KCa2.3 has the potential to improve endothelial function and vasodilation in cardiovascular disease states and may serve as a novel endothelium-specific anti-hypertensive strategy (Köhler et al., 2010). At present these compounds should still be considered tool compounds and further chemical optimization, achievement of tissue specificity and improved bioavailability together with a proof of efficacy in large mammals is required to demonstrate their cardiovascular protective actions. In contrast, the t-AUCB related sEH inhibitor AR9281 (Imig and Hammock, 2009) has already been evaluated in clinical trials for the treatment of mild hypertension and type-2 diabetes; however the AR9281 treatment failed to be effective herein. A possible reason could be a low in-vivo potency of the AR9281 compound at the dosage used (AR9281 is 10-times less potent than t-AUCB). Nonetheless, animal studies suggested that pharmacologic sEH blockade or genetic deficiency of the enzyme exert cardiovascular protective effects on brain, heart and kidneys and sEH blockers may be useful for treating pulmonary hypertension and in the prevention of atherosclerosis (Imig and Hammock, 2009, Simpkins et al., 2009, Wang et al., 2010). In conclusion, a pharmacologic activation of endothelial KCa channels as well as pharmacologic enhancement of the availability of endothelial relaxing factors such as EETs, at least under some circumstances, could be therapeutic options to treat cardiovascular disease (Fig. 7).
Acknowledgements
These studies have been supported by Novo Nordisk Fonden and the Deutsche Forschungsgemeinschaft (KO1899-10/11 to R.K.) and by the National Institutes of Health National Institute of Neurological Disorders and Stroke (Grant R21-NS052165 to H.W) and by the National Institute of Environmental Health Sciences (R01 ES002710 to C.M. B.D.H.). BDH is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- Brondum E, Kold-Petersen H, Simonsen U, Aalkjaer C. NS309 restores EDHF-type relaxation in mesenteric small arteries from type 2 diabetic ZDF rats. Br J Pharmacol. 2009;159:154–165. doi: 10.1111/j.1476-5381.2009.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brähler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Köhler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl- secretion by benzimidazolones. I. Direct activation of a Ca(2+)-dependent K+ channel. Am J Physiol. 1996;271:L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- Ding H, Hashem M, Wiehler WB, Lau W, Martin J, Reid J, Triggle C. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. Br J Pharmacol. 2005;146:1110–1118. doi: 10.1038/sj.bjp.0706417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Unna KR, Kerwin J. Pharmacological properties of benzazoles. I. Relationship between structure and paralyzing action. J Pharmacol Exp Ther. 1952;105:486–497. [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Feletou M, Köhler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010;12:267–275. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Feng J, Liu Y, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, Nishimura KK, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest. Circulation. 2008;118:S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Köhler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses - relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves A, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler R, Kaistha BP, Wulff H. Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets. 2010;14:143–155. doi: 10.1517/14728220903540257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflugers Arch. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sellke EW, Feng J, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human skeletal muscle microvascular endothelial dysfunction related to cardiopulmonary bypass. Surgery. 2008;144:239–244. doi: 10.1016/j.surg.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkau M, Kohler R, de Wit C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J. 2010;24:3572–3579. doi: 10.1096/fj.10-158956. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Köhler R, Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Dubrovska G, Nielsen G, Fesus G, Uhrenholt TR, Hansen PB, Gudermann T, Dietrich A, Gollasch M, De Wit C, Kohler R. Amplification of EDHF-type vasodilatations in TRPC1-deficient mice. Br J Pharmacol. 2010;161:1722–1733. doi: 10.1111/j.1476-5381.2010.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Köhler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–2095. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, Olesen SP, Christophersen P, Skaaning-Jensen B. Activation of human IK and SK Ca2+ - activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! J Physiol. 2008;586:5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Ulu A, Zhang LN, Hammock B. Soluble epoxide hydrolase in atherosclerosis. Curr Atheroscler Rep. 2010;12:174–183. doi: 10.1007/s11883-010-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, Chen ZY, Vanhoutte PM, Gollasch M, Huang Y. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]