Abstract
SPARC (a secreted protein acidic and rich in cysteine) has a reputation for being potent anti-cancer and anti-obesity molecule. It is one of the first known matricellular protein that modulates interactions between cells and extracellular matrix (ECM) and is associated with the ‘balance’ of white adipose tissue (WAT) as well as lipogenesis and lipolysis during adipogenesis. Adipogenesis is an indication for the development of obesity and has been related to a wide variety of cancers including breast cancer, endometrial cancer, esophageal cancer, etc. Adipogenesis mainly involves ECM remodeling, changes in cell-ECM interactions, and cytoskeletal rearrangement. SPARC can also prevent hypertrophy of adipocytes and hyperplasia of adipocyte progenitors. In addition to SPARC’s inhibitory role in adipogenesis, it has also been known to be involved in cell cycle, cell proliferation, cell invasion, adhesion, migration, angiogenesis and apoptosis. Molecular cancer biology and clinical biochemistry have significantly enhanced our understanding of the mechanisms that motivate the anti-cancer and anti-obesity action of SPARC. Recent studies elucidating the signaling pathways that are activated by SPARC can help develop the beneficial aspects of SPARC for cancer therapy and obesity prevention. This review focuses on the anti-cancer role of SPARC as it pertains to obesity.
Keywords: SPARC, cancer, adipogenesis
Introduction
Recent contribution of research in the areas of physiology, biochemistry, molecular biology, endocrinology, nutrition, pathology and molecular genetics has resulted in new scientific advances that have enhanced the efficacy of cancer therapy. Despite multimodal treatment regimens including surgery, radiation, and chemotherapy, tumor recurrence is frequent, and most of these patients eventually die from progressive tumorigenesis.1 Many of these treatments are also toxic and can lead to long-term disabilities.2, 3 Consequently, finding novel ways to suppress tumor growth using low-toxicity therapies is a major goal of various cancer research laboratories. Many groups have demonstrated tumor growth inhibition by targeting potent intra- or extra-cellular molecules.4, 5 SPARC (Secreted protein, acidic and rich in cysteine), a well-known matricellular molecule is known to be involved in multiple processes in various cancers (Table. 1).6
Table 1.
Multifunctional SPARC in cancer
| Exogenous SPARC | Endogenous SPARC level | Down regulation of SPARC | DNA hypermethylation | Studies | References | |
|---|---|---|---|---|---|---|
| Ovarian cancer | Alters cell proliferation, apoptosis and tumor growth | Low level | Increases growth and reduces apoptosis | In vitro, in vivo | [108, 111] | |
| Prostate cancer | Low level | Enhances aggressive and metastatic behavior | Yes |
In vitro In vivo |
[49, 114] | |
| Neuroblastoma | Inhibits growth and angiogenesis Enhances autophagy and apoptosis |
Low level |
In vitro In vivo |
[87, 115] | ||
| Lung cancer | yes | [58] | ||||
| Breast cancer | Inhibits cell proliferation and metastasis Stimulates cell migration and invasion |
High level |
In vitro In vivo |
[83, 116–118] | ||
| Melanoma | Inhibits the growth of spheroids | High level | Inhibits invasion, adhesion and tumor growth |
In vitro In vivo |
[98, 119] | |
| Pancreatic | Inhibits cell migration and invasion | Low level | Increases growth and reduces apoptosis | yes |
In vitro In vivo |
[ 51, 92] |
| Leukemia cells AML cell lines |
Inhibits growth of cell lines, and induces cell cycle arrest | Low level | yes | [53] | ||
| Colorectal | Inhibits cell growth and enhances apoptosis | Low or High level | yes | [ 56, 57, 60, 112, 120] | ||
| Colon adenocarcinoma | Low | In vivo | [121] | |||
| Gliomas | Delay in tumor growth Reduces apoptosis and increases invasion |
High level |
In vivo in vitro |
[91, 122–123] | ||
| Medulloblastoma | Inhibits cell proliferation Induces autophagy, apoptosis |
In vivo in vitro |
[115] | |||
| Endothelial cells | Inhibits spreading, migration and proliferation. Induces apoptosis |
In vivo in vitro |
[115, 124–125] | |||
| Stromal cells | Modulates proliferation | High levels | yes |
In vivo in vitro |
[51, 85] |
SPARC is also known as osteonectin and basement-membrane protein and is secreted by endothelial cells.7–9 Initially discovered as a component of bone, SPARC is also expressed in epithelia showing high rates of turnover.10, 11 Immunocytochemical analysis of embryonic chicken cells in vitro and in vivo show the presence of SPARC in the nucleus. In addition, elution of soluble proteins and DNA from these cells show that SPARC may be a constituent of the nuclear matrix.12 These evidences suggest that SPARC mediates interactions between cells and components of the extracellular matrix. SPARC binds rapidly to precise components of the extracellular matrix (ECM) and modulates the interaction of cells.8 The ECM is a storehouse for a number of growth regulatory factors.13 Further, matrix proteins modulate cell proliferation and migration.14, 15 The stimulation of growth in vitro by ECM components has been attributed to morphological changes that result from interactions between cells and their supportive matrices.16 Nevertheless, many matrix-associated proteins also possess potential growth stimulatory activities that are autonomous of their adhesive properties. SPARC is expressed in many cell types and its expression increases during embryogenesis, adult bone tissues, wound healing, and tissue remodeling.
Even though earlier studies linked SPARC overexpression with bone mineralization, current studies have shown that SPARC has pleiotropic effects on biological functions. Phenotypically, SPARC-null mice develop age-related abnormalities due to unusual differentiation of lenticular epithelial cells and partial fiber cell differentiation.17 SPARC over-expression has been observed in human colorectal cancers (CRCs) that are sensitive to chemotherapy, in contrast to therapy-refractory tumors.18 In vitro experiments have shown the influence of SPARC on several cellular processes (Table. 1). SPARC has been shown to inhibit cell adhesion to the extracellular matrix and modify cell shape.19 SPARC inhibits cell cycle progression. SPARC also binds to collagens and other extracellular matrix proteins and possibly plays a role in the organization of these components.20, 21 SPARC has been shown to bind several growth factors including platelet-derived growth factor and vascular endothelial growth factor and alter their biological activity.22, 23 SPARC indirectly influences the effects of basic fibroblast growth factor, and transforming growth factor-β.24, 25 SPARC functions as a chemotherapy sensitizer by stimulating tumor deterioration in response to radiation and chemotherapy in tumor xenograft models of chemotherapy-resistant tumors.26 Following topics will be considered in this review.
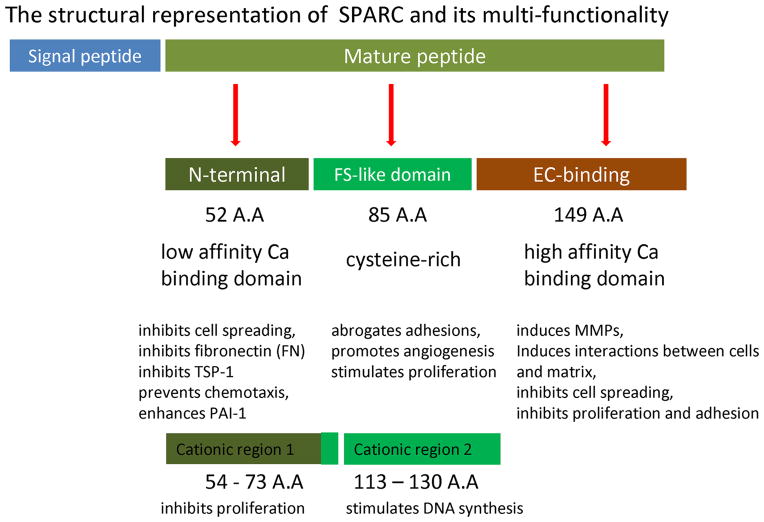
1. Structure of SPARC
SPARC is an extracellular matrix-associated glycoprotein and product of a single-copy gene mapped to mouse chromosome 11 and to the long arm of human chromosome 9. Sequence analysis of SPARC gene in bovine, mouse, and human has revealed a high degree of sequence similarity and an absence of canonical CAAT and TATA box sequences. Vertebrate SPARC cDNAs encode proteins of 298–303 amino acids that are post-translationally modified by N-linked glycosylation.27 The open reading frame (ORF) of SPARC gene is conceptually translated into a putative protein consisting of a typical hydrophobic signal peptide with 17 amino acids followed by mature protein (Fig.1).28 The signal peptide is removed during processing. The mature peptide of SPARC consists of three individual structural domains (Fig. 1) based on the predicted secondary structure and thought to mediate precise biological activities.29 1. An N-terminal highly acidic calcium-binding domain (low affinity) of 52 amino acids (Ala1-Glu52) that inhibits cell spreading, prevents chemotaxis, enhances plasminogen activator inhibitor-1(PAI-1) and decreases fibronectin (FN) and thrombospondin-1 (TSP-1).30 2. A cysteine-rich (contains 10 cysteine residues) FS-like domain is located between the N-terminus domain sequence and EC binding domain. This FS-like domain is 85 amino acids (Asn53-Pro137) long and consists of several internal disulfide bonds that stabilize two weakly interacting molecules. This domain abrogates focal adhesions, promotes angiogenesis and proliferation. 3. EC-binding domain (extracellular calcium-binding domain); high affinity, which is 149 amino acid residues (Cys138-Ile286) at the C-terminus, Val -Ile where the Valine residue serves as an amidation site, followed by a stop codon. This domain has two EF-hand motifs that bind to calcium with high affinity, and comprise almost entirely of α-helices. This region induces MMPs, interactions between cells and matrix, inhibits cell spreading, proliferation and adhesion.9, 31, 32 Follistatin-like domain of SPARC has been shown to control the proliferation of endothelial cells in vitro.33 Amino acids 54-73 (Cationic region 1) have been shown to inhibit the proliferation of sub-confluent endothelial cells, while amino acids 113-130 (cationic region 2) stimulates DNA synthesis (Fig.1). The role of phosphorylation in the function of SPARC is unclear. The posttranslational phosphorylation modification has been reported in bone osteonectin and in SPARC from PYS-2 cells, it was not found in SPARC produced by endothelial cells.9, 34, 35
Figure 1.
2. Truncation of SPARC protein
Matrix metalloproteinases (MMP-2, -3, -7 and -13, plasmin and trypsin, have been shown to cleave SPARC in vitro, producing KGHK-containing fragment.7, 36 The presence of truncated form of SPARC protein has been recently reported in hepatocellular carcinoma (HCC) samples and esophageal carcinoma.37, 38 Immunoblotting studies show higher levels of truncated SPARC (24-27kDa) in tissues in comparison to intact SPARC (37-43 kDa).37 Interestingly, cell lysates do not show truncated SPARC indicating that the presence of truncated SPARC in tissues may possibly be associated with the existence of certain proteinases in the surrounding environment. Cleavage of SPARC by MMP3 produces peptides containing the KGHK sequence that can stimulate angiogenesis.36 Alpers et al.39 have suggested that AON-5031(anti-SPARC) recognizes the N-terminal part (amino acids 5–23) of the mature SPARC protein. So, the truncated protein should have the N-terminal half of intact SPARC, which contains the KGHK sequence located at amino acids 119–122 from the N-terminus (Genebank AN: BC072457). Truncated SPARC in the tumor sinusoidal area has been correlated to the tumor microvessel density (MVD), suggesting that truncated SPARC may play a significant pro-angiogenic role in HCC.37 Over-expression of SPARC leads to a decrease in MVD, at least in part, resulting in delayed tumor formation and reduction of tumor size. The decrease in MVD–CD34 observed in nude mice xenografts has been attributed to the high expression of anti-angiogenic intact-SPARC. In clinical samples, the positive relationship between SPARC protein expression and MVD has been probably due to the low expression of anti-angiogenic intact-SPARC and high expression of pro-angiogenic truncated-SPARC. Likewise, high expression of truncated-SPARC protein in tumor sinusoidal areas has been associated with increased tumor MVD while over-expression of full-length SPARC correlates with a decrease in tumor MVD, accompanied by a delay in tumor formation and a decrease in tumor size. Importance of exact sequence and the angiogenic properties of truncated- and intact-SPARC proteins observed in human liver cancer will be the subject of many future studies.
3. Receptors and modulators for SPARC
Even though receptors have been discovered for some of the matricellular molecules, it remains uncertain how SPARC cooperates with the cell surface to stimulate its effects. Recent research has suggested that stabilin-1 and integrins α5 and β3 may mediate some of the effects of SPARC.40, 41 Byzova and group 40 have shown that tumor cell migration attributed to SPARC is mediated by α5 β3 integrins and is controlled by an autocrine loop in which VEGF engages VEGFR-2. Kzhyshkowska and group,41 using a phage display, have proposed stabilin-1 as a cellular receptor for SPARC. Stabilin-1 interrelates with SPARC through the extracellular epidermal growth factor (EGF)-like domain containing the sequence FHGTAC. SiRNA knockdown of Stabilin-1 reduces SPARC expression, while elevated SPARC level is observed in Stabilin-1 over-expressing cells.42 Stabilin-1 mediates the internalization and delivery of SPARC to the endocytic pathway in stably transfected CHO cells. These observations show that stabilin-1 acts as a specific and efficient receptor in macrophages that mediates internalization of extracellular SPARC and its targeting to lysosomes. ShRNA of SOX-5 in nasopharyngeal carcinoma (NPC) cells causes a significant increase in SPARC. SOX-5 can bind directly to the SPARC promoter in a chromatin immunoprecipitation assay signifying that SOX-5 acts as a vital transcriptional repressor of SPARC.43 Exogenous TGF-β induces over-expression of both collagen and SPARC, while this response is significantly attenuated in fibroblasts, pre-transfected with SPARC small interfering RNA.44
4. DNA methylation
SPARC expression gets altered in both normal and tumor cells depending upon the tumor type and culture conditions. Higher levels of SPARC expression have been observed in some types of solid tumors, such as melanoma,45 glioblastomas,46 breast,47 and prostate48 while some others, such as endometrium, colorectal, and leukemia showed lower or undetectable levels of SPARC due to SPARC promoter hypermethylation (Table. 1).49–53 These studies imply that carcinogenic effect of SPARC is cell type specific and may depend on the tumor microenvironment. In general, alteration in DNA methylation involves DNA methyltransferases (1, 3a and 3b) leading to inactivation of many tumor suppressor genes during tumor growth.54, 55 DNA methylation is also involved in maintaining a normal balance between cell proliferation and apoptosis in some cancer cell lines.56, 57 Several studies have shown that changes in the methylation levels of SPARC promoter may lead to SPARC silencing leading to tumor growth. For example, low SPARC expression in lung cancer cell lines is due to DNA hypermethylation of the gene promoter. Consequently, treatment with 5-Aza-2’-deoxycytidine leads to higher expression of SPARC in lung cancer cells.58 Similar studies also demonstrated in colorectal cancer52 and pancreatic cancer cells.51 5-Aza-2’-deoxycytidine is a demethylating agent and inhibits DNA methyltransferase activity to upregulate SPARC expression. This mechanism leads to decreased cell viability and improved response of cancer cells to chemotherapy.52
5. SPARC in adipogenesis
Obesity is a disorder that results from excess white adipose tissue (WAT) and has been known as a risk factor for a wide variety of cancers including breast cancer, endometrial cancer, esophageal cancer, colon cancer, rectal cancer, pancreatic cancer, kidney cancer, ovarian cancer, cervical cancer and liver cancer.59, 60 Understanding the associations between obesity, overweight and different types of cancers, as well as the underlying biological mechanisms, remain a budding and presently very fascinating area of research. WAT is an endocrine organ that plays a central role in the regulation of energy metabolism mediating its biological effects by secreting a variety of peptide and protein hormones (adipokines; such as leptin, adiponectin, visfatin, retinol-binding protein-4, tumor necrosis factor and interleukin-6).60, 61 These adipokines target the central nervous system and peripheral tissues (WAT, liver and muscle) to modulate energy metabolism.62–64
Adipogenesis or adipocyte differentiation is a greatly controlled process that has been extensively studied for the last 35 years. Nutritional and hormonal signaling affects adipogenesis in a positive or negative manner, and factors involved in cell-matrix or cell-cell interactions are also crucial in regulating the preadipocyte differentiation process. Multiple signaling pathways regulate preadipocyte differentiation, including transforming growth factor-β (TGF-β), tumor necrosis factor-α, Wnt, insulin/ insulin-like growth factor-1, and other growth factors. The first characteristic feature of the adipogenesis process is the remarkable change in cell shape as the cells switch from fibroblastic to spherical shape. These morphological alterations are paralleled by changes in the level and kind of cytoskeletal components and the level of extracellular matrix (ECM) components.62 Adipogenesis is characterized by cell-associated ECM switches from a Fibronectin (FN)-enriched matrix into Laminin (LN)-rich basement membrane (BM); correspondingly, integrin expression changes from α5 (FN) to α6 (LN).65 SPARC is the first known matricellular protein associated to the ‘balance’ of WAT; during adipogenesis. SPARC increases the deposition of FN and the expression of its receptor, α5 integrin.65 SPARC also inhibits the deposition of LN during adipogenesis as well as expression of the α1 chain and its receptor, α6.65 Further, SPARC also down-regulates the secretion and deposition of LN in lens cells.66 LN and type IV collagen increase adipogenesis, in two-dimensional cell culture.67 In addition, SPARC interacts with types 1 and IV collagen for stability of basal lamina.29, 68 So, it is possible that SPARC inhibits adipogenesis in part by interference with the formation of basement membrane. SPARC is enriched in both mesenteric and subcutaneous WATs showing elevated levels of SPARC transcript.69 In humans, the plasma concentration of SPARC was correlated positively with body mass index.70 The excess of WAT is associated with increased TGF-β,71 which leads fibrosis and might enhance the expression of SPARC. But, other adipokines may override the effects of TGF-β, SPARC, and collagen.
SPARC-null mice show increased accumulation of WAT along with an increase in both adipocyte size and number as compared to wild type (WT) mice.72 SPARC-null mice might be highly susceptible to protease degradation and remodeling functions allowing adipocytes to develop a larger size, hence, contribute to hyperplastic WAT. The ECM remodeling associated with adipogenesis requires SPARC, to stabilize the tissue and maintain the balance of lipogenesis and lipolysis. SPARC, to some extent, can prevent hypertrophy of adipocytes and hyperplasia of adipocyte progenitors. Further SPARC-null mice contain higher levels of soluble type VI collagen in their skin,72 and it may influence the expression, folding, post-translational modification, and secretion of type VI collagen in WAT. Consequently, SPARC could control adipogenesis through type VI collagen. SPARC also interact with integrin α5β1 and this complex inhibits preadipocyte adhesion, focal adhesion kinase (FAK), integrin-linked kinase (ILK) activity and it leads to cell migration. SPARC also stimulates the phosphorylation and activation of FAK in glioma cells.73 Likewise SPARC can also inhibit the formation of adipocytes but enhances that of osteoblastocytes by its enrichment of the accumulation of β-catenin in both cytosol and the nucleus.66, 74 Both endogenous and exogenous SPARC enhance the accumulation of β-catenin. Wnt/Beta-catenin represses the expression of C/EBPα and PPARγ at early stages of adipocyte differentiation.75 Further, SPARC also inhibits the expression of C/EBPα and PPARγ at later stages of adipocyte differentiation. These evidence shows that SPARC suppresses the central transcriptional cascades of adipogenesis through the Wnt/Beta-catenin pathway. Further research is required to decipher the mechanisms underlying the inhibitory consequences of SPARC on adipogenesis in vivo.
6. Role of SPARC in cell cycle and proliferation
Generally, ‘vital functions’ of the cells are gauged by assaying for one of these parameters: cell viability, cell proliferation and cell growth. These ‘vital functions’ of the cells are mainly modulated by the signaling molecules or growth factors that convey information to cells during cellular differentiation or growth. Recent studies have shown that SPARC and TGF-β employ each other in a mutual relationship to modulate these cellular functions. SPARC and TGF-β acts as inhibitors of cell cycle progression and proliferation in several types of cells.31, 32, 76, 77 SPARC stimulates TGF-β expression in mesangial cells and, on the other hand, TGF-β stimulates SPARC expression in a number of cell types, including endothelial cells, fibroblasts, keratinocytes, and smooth muscle cells.20, 78-81 SPARC inhibits epithelial cell proliferation by commandeering the TGF-β signaling system, via coupling of C-terminal extracellular calcium (EC)-domain (i.e., SPARC-EC) to a TGF-β receptor/Smad2/3-dependent pathway.82 SPARC and TGF-β are both expressed in epithelial cells; however, the association between these signaling molecules in regulating epithelial cell behaviors remains to be addressed. SPARC negatively regulates breast cancer cell proliferation without stimulating metastasis.83 Elevated levels of SPARC increases cell de-adhesion from matrix in gliomas.84 SPARC also alters glioma growth by changing the tumor microenvironment and by repressing tumor vascularity through inhibition of VEGF expression.84 These results present a novel mechanism, whereby SPARC controls VEGF function by preventing the available growth factor. Haber et al.85 show that SPARC alters the proliferation of stromal but not melanoma cells. They also show that SPARC inhibits endothelial cell proliferation, spreading, and migration. In fact, low expression of integrins following exposure to SPARC considerably reduces cell proliferation and adhesion, in part by down-regulating the activation of Akt, FAK, MAPK 44/42 and Src.86 Treatment with exogenous SPARC significantly inhibits the growth of pancreatic cancer, colorectal cancers, neuroblastomas, and leukemia cells.18, 87
SPARC and its peptide 2.1 and 4.2 (from a disulfide-bonded domain of SPARC) inhibit DNA synthesis in BAEC, as determined by (3H) thymidine incorporation and delay the entry of cells into S phase.88 An inhibitory effect of SPARC on cell-cycle progression might facilitate the short-term withdrawal from the cell cycle that frequently happens after cellular responses to developmental or injury signals. Over-expression of SPARC protein and mRNA is frequently observed in non-proliferating, but actively secreting, Leydig, Sertoli cells and migrating cells 89, 90 showing that SPARC might direct a metabolic pathway in addition to cell cycle. SPARC is stimulated after cells have primarily proliferated and may function to withdraw cells temporarily from the cell cycle in preparation for migration.90 The recognition of SPARC as an anti-spreading factor for specific cells, together with its capability to direct noticeable changes in cell shape, suggests that the evident proliferation in the presence of SPARC may result from alteration in cell shape.90 Nevertheless, potential actions of SPARC on mitosis and cellular migration, need disconnection from ECM, have not been studied very well.
In contrast, SPARC expression increases cell survival under stress commenced by serum removal through a decrease in apoptosis.91 Administration of SPARC quickly stimulates AKT phosphorylation, an effect that is blocked by a neutralizing SPARC antibody. AKT activation is crucial for the anti-apoptotic effects of SPARC as the reduced apoptosis and caspase activity linked with SPARC expression can be blocked with a specific AKT inhibitor or dominant-negative AKT.91 As tumor cells experience stressful microenvironments predominantly during the metastatic process, these results propose that SPARC functions, in part, to help tumor progression by facilitating tumor cells to survive under stressful environment. Greater MMP9 expression in the absence of SPARC has an additive effect in stimulating tumor development.92 The mechanisms of SPARC functions in cancer development remain multifaceted and depend on tumor cell type and the microenvironment.
7. Role of SPARC in cell invasion, adhesion and migration
Understanding the molecular mechanisms controlling invasion, adhesion and migration of tumor cells is essential for improvement of novel therapies to cancer. Invasion and metastasis are constituted of several steps, which are not well distinguished at the cellular and molecular levels. The early steps in invasion and metastasis by a tumor cell consist of the breakdown of cell-cell connections, disconnection of tumor cells, proteolysis of the ECM, and distribution throughout neovascularization. SPARC inhibits cellular adhesion. Several in vitro studies have shown that secretion of SPARC influences cell morphology by reducing the number of focal acquaintances and blocking the adhesion of cells to their substratum or to adjacent cells.93, 94 SPARC regulates cell-ECM communications that manipulate cell adhesion and migration. In vitro, pancreatic cancer cells over-expressing MMP9 show higher cell invasion and migration, which can be efficiently inhibited by the addition of SPARC.92 Over-expression of SPARC in gliomas induces brain tumor invasion and migration in vitro and in vivo, 95 whereas administration of SPARC siRNA into glioma cells results in down-regulation of SPARC expression, and considerable suppression in glioma cell migration in vitro96 and lower tumor cell survival and invasion.73 The level of SPARC correlates with total and phosphorylated HSP27. SPARC and HSP27 co-localize to invading cells in vivo. Inhibition of HSP27, mRNA reverse SPARC–stimulated changes in morphology, migration and invasion in vitro.95 These experiments indicate that HSP27-mediated actin polymerization, cell migration and contraction are novel downstream effectors of SPARC functions on cell morphology and migration. Variations in cell-cell and cell-matrix adhesion in tumors depend on the degree of cohesiveness and the mode of tumor growth. Abrogation of general cell adhesion function plays a vital role in the development of cancer. This change in tumor cell adhesion is essential because detachment of tumor cells is an initial step in the invasion of adjacent tissues and metastasis to distant sites. In vitro experiments have revealed that addition of exogenous SPARC to cultured cells inhibits cell distribution and stimulates cellular rounding. This effect leads to the detachment of cells.19 SPARC and TSP inhibit cell attachment and spreading and cause partial detachment and rounding of cells in vitro.9 Over-expression of SPARC in stably transfected F9 embryonal carcinoma cell lines results in rounding and aggregation.97 SPARC may change these cell-cell or cell-matrix connections by breaking cell-substrate bonds and stimulating the reorganization of actin cytoskeletal elements.19 SPARC plays a role in melanoma progression, because lower levels of SPARC limits the invasive and adhesive capabilities of melanoma cells in vitro, where as in vivo, down-regulation of SPARC in melanoma cells inhibits tumor development in nude mice, but also show an increase in polymorphonuclear leukocytes (PMNs) recruitment to this peri-tumoral region,98 because lower levels of SPARC stimulated greater migration of PMNs to the site of the tumor.99 High levels of SPARC in normal human melanocytes inhibits expression of E-cadherin and P-cadherin and stimulates a fibroblast-like morphology.100 However, this study does not explore whether melanocytes over-expressing SPARC produce tumors in vivo. Further notably, is it the secretion of SPARC by melanocytes or by adjacent myofibroblasts that is essential for tumorigenesis in this microenvironment?
8. Role of SPARC in angiogenesis
Angiogenesis, the process of new blood vessel formation from pre-existing ones, plays a vital role in various pathological and physiological circumstances, including embryonic development, wound repair, tumor growth and inflammation.101 From a pathological point of view, angiogenesis is a major limiting step in tumor progression and is necessary for tumor development at metastatic sites. Angiogenesis consists of a sequence of phases that includes suspension of basement membrane, migration, and proliferation of endothelial cells, formation of vascular loop and formation of new basement membrane. These multiple steps are regulated by different factors such as growth factors, proteases, oxygen levels and extracellular matrix components, during which the endothelium gives rise to new vessels. Over the past two decades, matricellular proteins have expanded more attention in their role in regulating cellular functions and angiogenesis. SPARC has nominal effects on angiogenesis in vivo. Nevertheless, SPARC has some angiogenic properties.102 Microarray studies showed that SPARC and SPARC-like 1 are over-expressed in HCC and clustered with CD34 (a well-known angiogenic marker compared with non-tumorous liver cells, and both genes may play a role in HCC angiogenesis.103
SPARC modulates cell adhesion and proliferation and is thought to function in tissue remodeling and angiogenesis. SPARC binds to VEGF, thus inhibiting VFGF interaction with EC surface, ERK 1/2 activation and VEGF stimulated DNA synthesis.23 SPARC also binds to PDGF-AB and -BB, but not -AA, and inhibits the interaction of these growth factors with TK receptors.22 SPARC can also inhibit angiogenesis indirectly by regulating the expression of MMPs and TGF-β1 in vivo.25, 37, 92, 104, 105 SPARC, in combination with other known angiogenic factors, may act pleiotropically during angiogenesis. Angiostatin, a cleavage product of plasminogen, most probably acts through inhibition of angiogenesis. Over-expression of SPARC is correlated with a good prognosis in neuroblastoma, probably due to reduced angiogenesis.87 SPARC mediated inhibition of angiogenesis is indicated in SPARC-null mice that shows increased angiogenic activity in sponge cell invasion assays.106 The angiogenic activity of SPARC is complex, as different proteolytic fragments show contrasting effects. An EGF-like part of the follistatin-like domain in SPARC has been shown to be angiosuppressive.104 Whereas another fragment of this domain in SPARC consists of a KGHK amino acid sequence that is pro-angiogenic.20, 107 However, it is feasible that secreted modular calcium-binding protein (SMOC-2) apply its effects via the follistatin-like domain, which has been shown to have growth factor binding potential. In vivo, SPARC is also capable of suppression of VEGF-stimulated integrin activation and down-regulation of MMP-2 and -9.87, 92, 108 Recent research is paying attention on the angiostatin and angiogenic and antiangiogenic properties of SPARC. This research will offer a better understanding of how these key factors regulate angiogenesis.
9. Role of SPARC in apoptosis
Programmed cell death, apoptosis is vital for normal development and tissue homeostasis. Nevertheless uncontrolled apoptosis may occur after treatment with cytostatic chemicals. It is a patho-physiological process and is connected with the various human diseases.109 In general, there are two different pathways that initiate apoptosis: one is extrinsic pathway mediated by death receptors on the cell surface and the other is intrinsic pathway mediated by mitochondria.109, 110 In both extrinsic and intrinsic pathways, cysteine aspartyl-specific proteases (caspases) are stimulated that cleave cellular substrates, leading to the morphological and biochemical changes that feature apoptosis. Evidence that SPARC may promote apoptosis in cancer cells is presented by Yiu and colleagues, who show that exogenous treatment of various ovarian cancer cell lines with SPARC induces apoptosis.111 In support of this observation SPARC exposure increases cleaved caspase 3 in human ovarian carcinoma cells.108 SPARC induces apoptosis of endothelial cells in a dose-dependent manner, accomplishing maximal effect with 20μg/ml of SPARC.87 In colorectal cancer cell lines, over-expression of SPARC reduces cell viability and enhances apoptosis in cells exposed to various chemotherapeutic agents.18 This SPARC-mediated apoptosis occurs by activating the extrinsic pathway while enhancing the intrinsic pathway of apoptosis.112 The ability of SPARC to enhance apoptosis appears to involve its physical interaction with the N-terminus of caspase 8, which enhances the chemosensitivity of colorectal cancer cells through the apoptotic cascades.112 Apoptosis of colorectal cancer cells overexpressing SPARC can be further augmented following concomitant exposure to vitamin D and chemotherapy, by reducing phosphorylation of Akt and subsequent inactivation of the pro-survival pathway.26
Conclusion
Recent studies have putforth many promising molecules that are integral for metastatic progression of cancer cells including many proteases, angiogenic factors, and adhesive or deadhesive molecules. Depending on the type of SPARC is either associated with aggressive tumor phenotype (gliomas, melanoma, gastric cancers) or exhibits anti-tumor activity (ovarian, colorectal, neuroblastoma).113 The present review provides an overview of SPARC’s role as an anti-cancer molecule and its role in obesity.
Acknowledgments
This work was supported by grants National Cancer Institute at the National Institute of Health (grant numbers R01CA131294 and R21CA155686 to DS); CDMRP BCRP (BC030963 to DS); The Susan G. Komen for the Cure (BCTR0503526 to DS); and Mary K. Ash Foundation (grant to DS).
List of Abbreviations
- CRCs
Colorectal cancers
- 3-D
3-dimensional
- EGF
Epidermal growth factor
- EC
Extracellular calcium
- ECM
Extracellular matrix
- FN
Fibronectin
- FAK
Focal adhesion kinase
- HSP
Heat shock protein
- HCC
Hepatocellular carcinoma
- ILK
Integrin-linked kinase
- LN
Laminin
- MMP
Matrix metalloproteinases
- MVD
Microvessel density
- NPC
Nasopharyngeal carcinoma
- NMR
Nuclear magnetic resonance
- ORF
Open reading frame
- PAI-1
Plasminogen activator inhibitor-1
- PDGF
Platelet-derived growth factor
- PMNs
Polymorphonuclear leukocytes
- SMOC-2
Secreted modular calcium-binding protein
- SPARC
Secreted protein acidic and rich in cysteine
- TSP-1
Thrombospondin-1
- TGF-β
Transforming growth factor
- VEGF
Vascular endothelial growth factor
- WAT
White adipose tissue
- WT
Wild type
Footnotes
Author’s Contributions: GPCN and DS have been involved in drafting and revising the manuscript critically. All authors read and approved the final manuscript.
Competing Interests: The author(s) declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garzia L, Andolfo I, Cusanelli E, Marino N, Petrosino G, De Martino D, et al. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS One. 2009;4:e4998. doi: 10.1371/journal.pone.0004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kombogiorgas D, Sgouros S, Walsh A, Hockley A, Stevens M, Grundy R, et al. Outcome of children with posterior fossa medulloblastoma: a single institution experience over the decade 1994–2003. Child's Nerv Sys. 2007;23:399–405. doi: 10.1007/s00381-006-0258-5. [DOI] [PubMed] [Google Scholar]
- 3.Yang S, Wang K, Cho B, Kim Y, Lim S, Park S, et al. Radiation-induced cerebellar glioblastoma at the site of a treated medulloblastoma. J Neurosurgery: Pediatrics. 2005;102:417–422. doi: 10.3171/ped.2005.102.4.0417. [DOI] [PubMed] [Google Scholar]
- 4.Gogineni V, Kargiotis O, Klopfenstein J, Gujrati M, Dinh D, Rao J. RNAi-mediated Downregulation of Radiation-induced MMP-9 Leads to Apoptosis via Activation of ERK and Akt in IOMM-Lee cells. Inte J Oncol. 2009;34:209–218. [PMC free article] [PubMed] [Google Scholar]
- 5.Guessous F, Li Y, Abounader R. Signaling pathways in medulloblastoma. J Cellular Physiol. 2008;217:577–583. doi: 10.1002/jcp.21542. [DOI] [PubMed] [Google Scholar]
- 6.Podhajcer O, Benedetti L, Girotti M, Prada F, Salvatierra E, Llera A. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer and Metastasis Reviews. 2008;27:691–705. doi: 10.1007/s10555-008-9146-7. [DOI] [PubMed] [Google Scholar]
- 7.Sage E, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- 8.Sage E, Tupper J, Bramson R. Endothelial cell injury in vitro is associated with increased secretion of an Mr 43,000 glycoprotein ligand. J Cellular Physiol. 1986;127:373–387. doi: 10.1002/jcp.1041270305. [DOI] [PubMed] [Google Scholar]
- 9.Sage H, Johnson C, Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984;259:3993–4007. [PubMed] [Google Scholar]
- 10.Termine J, Kleinman H, Whitson S, Conn K, McGarvey M, Martin G. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 11.Porter P, Sage E, Lane T, Funk S, Gown A. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43:791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- 12.Gooden M, Vernon R, Bassuk J, Sage E. Cell cycle-dependent nuclear location of the matricellular protein SPARC: association with the nuclear matrix. J Cellular Biochem. 1999;74:152–167. [PubMed] [Google Scholar]
- 13.Flaumenhaft R, Rifkin D. The extracellular regulation of growth factor action. Mol Biol Cell. 1992;3:1057–1065. doi: 10.1091/mbc.3.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingber D, Folkman J. How does extracellular matrix control capillary morphogenesis. Cell. 1989;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- 15.Ingber D, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingber D. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Nat Acad Sci USA. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassuk JA, Birkebak TED, Rothmier JD, Clark JM, Bradshaw AMY, Muchowski PJ, et al. Disruption of theSparcLocus in Mice Alters the Differentiation of Lenticular Epithelial Cells and Leads to Cataract Formation. Exp Eye Res. 1999;68:321–331. doi: 10.1006/exer.1998.0608. [DOI] [PubMed] [Google Scholar]
- 18.Tai I, Dai M, Owen D, Chen L. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest. 2005;115:1492–1502. doi: 10.1172/JCI23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy-Ullrich J, Lane T, Pallero M, Sage E. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca2+-binding EF-hand. J Cellular Biochem. 2004;57:341–350. doi: 10.1002/jcb.240570218. [DOI] [PubMed] [Google Scholar]
- 20.Ford R, Wang G, Jannati P, Adler D, Racanelli P, Higgins P, Staiano-Coico L. Modulation of SPARC Expression during Butyrate-Induced Terminal Differentiation of Cultured Human Keratinocytes: Regulation via a TGF-(β)-Dependent Pathway. Exp Cell Res. 1993;206:261–275. doi: 10.1006/excr.1993.1146. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki T, Göhring W, Mann K, Maurer P, Hohenester E, Knäuper V, et al. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem. 1997;272:9237–9243. doi: 10.1074/jbc.272.14.9237. [DOI] [PubMed] [Google Scholar]
- 22.Raines E, Lane T, Iruela-Arispe M, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Nat Acad Sci USA. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupprion C, Motamed K, Sage E. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- 24.Hasselaar P, Sage E. SPARC antagonizes the effect of basic fibroblast growth factor on the igration of bovine aortic endothelial cells. J Cellular Biochem. 2004;49:272–283. doi: 10.1002/jcb.240490310. [DOI] [PubMed] [Google Scholar]
- 25.Francki A, Bradshaw A, Bassuk J. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J Biol Chem. 1999;274:32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- 26.Taghizadeh F, Tang M, Tai I. Synergism between vitamin D and secreted protein acidic and rich in cysteine–induced apoptosis and growth inhibition results in increased susceptibility of therapy-resistant colorectal cancer cells to chemotherapy. Mol Cancer Ther. 2007;6:309–317. doi: 10.1158/1535-7163.MCT-06-0517. [DOI] [PubMed] [Google Scholar]
- 27.Motamed K. SPARC (osteonectin/BM-40) Int J Biochem Cell Biol. 1999;31:1363–1366. doi: 10.1016/s1357-2725(99)00090-4. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen H, Brunak S, Von Heijne G. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng Design and Sele. 1999;12:3–9. doi: 10.1093/protein/12.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Maurer P, Hohenadl C, Hohenester E, Göhring W, Timpl R, Engel J. The C-terminal portion of BM-40 (SPARC/osteonectin) is an autonomously folding and crystallizable domain that binds calcium and collagen IV. J Mol Biol. 1995;253:347–357. doi: 10.1006/jmbi.1995.0557. [DOI] [PubMed] [Google Scholar]
- 30.Xie R, Long G. Role of N-linked glycosylation in human osteonectin. J Biol Chem. 1995;270:23212–23217. doi: 10.1074/jbc.270.39.23212. [DOI] [PubMed] [Google Scholar]
- 31.Bradshaw A, Sage E. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Q, Sage E. SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem. 1999;47:1495–1506. doi: 10.1177/002215549904701201. [DOI] [PubMed] [Google Scholar]
- 33.Lane T, Iruela-Arispe M, Johnson R, Sage E. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell biol. 1994;125:929–943. doi: 10.1083/jcb.125.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchiyama A, Suzuki M, Lefteriou B, Glimcher M. Isolation and chemical characterization of the phosphoproteins of chicken bone matrix: heterogeneity in molecular weight and composition. Biochemistry. 1986;25:7572–7583. doi: 10.1021/bi00371a047. [DOI] [PubMed] [Google Scholar]
- 35.Engel J, Taylor W, Paulsson M, Sage H, Hogan B. Calcium binding domains and calcium-induced conformational transition of SPARC/BM-40/osteonectin, an extracellular glycoprotein expressed in mineralized and nonmineralized tissues. Biochemistry. 1987;26:6958–6965. doi: 10.1021/bi00396a015. [DOI] [PubMed] [Google Scholar]
- 36.Sage E, Reed M, Funk S, Truong T, Steadele M, Puolakkainen P, et al. Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J Biol Chem. 2003;278:37849–37857. doi: 10.1074/jbc.M302946200. [DOI] [PubMed] [Google Scholar]
- 37.Lau C, Poon R, Cheung S, Yu W, Fan S. SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma. J Pathol. 2006;210:459–468. doi: 10.1002/path.2068. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita K, Upadhay S, Mimori K, Inoue H, Mori M. Clinical significance of secreted protein acidic and rich in cystein in esophageal carcinoma and its relation to carcinoma progression. Cancer. 2003;97:2412–2419. doi: 10.1002/cncr.11368. [DOI] [PubMed] [Google Scholar]
- 39.Alpers C, Hudkins K, Segerer S, Sage E, Pichler R, Couser W, et al. Localization of SPARC in developing, mature, and chronically injured human allograft kidneys. Kidney Int. 2002;62:2073–2086. doi: 10.1046/j.1523-1755.2002.00680.x. [DOI] [PubMed] [Google Scholar]
- 40.Byzova T. Integrins in bone recognition and metastasis. J Musculosk Neuro Inter. 2004;4:374. [PubMed] [Google Scholar]
- 41.Kzhyshkowska J, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Gratchev A, et al. Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J Immunol. 2006;176:5825–5832. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- 42.Kzhyshkowska J, Gratchev A, Schmuttermaier C, Brundiers H, Krusell L, Mamidi S, et al. Alternatively activated macrophages regulate extracellular levels of the hormone placental lactogen via receptor-mediated uptake and transcytosis. J Immunol. 2008;180:3028–3037. doi: 10.4049/jimmunol.180.5.3028. [DOI] [PubMed] [Google Scholar]
- 43.Huang D, Lin Y, Jan P, Hwang Y, Liang S, Peng Y, et al. Transcription factor SOX-5 enhances nasopharyngeal carcinoma progression by down-regulating SPARC gene expression. J Pathol. 2008;214:445–455. doi: 10.1002/path.2299. [DOI] [PubMed] [Google Scholar]
- 44.Zhou H, Xia X, Xu Z. An RNA polymerase II construct synthesizes short-hairpin RNA with a quantitative indicator and mediates highly efficient RNAi. Nucleic Acids Res. 2005;33:e62. doi: 10.1093/nar/gni061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ledda F, Bravo AI, Adris S, Bover L, Mordoh J, Podhajcer OL. The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol. 1997;108:210–214. doi: 10.1111/1523-1747.ep12334263. [DOI] [PubMed] [Google Scholar]
- 46.Rempel SA, Golembieski WA, Ge S, Lemke N, Elisevich K, Mikkelsen T, Gutierrez JA. SPARC: a signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J Neuropathol Exp Neurol. 1998;57:1112–1121. doi: 10.1097/00005072-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Porter PL, Sage EH, Lane TF, Funk SE, Gown AM. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem. 1995;43:791–800. doi: 10.1177/43.8.7622842. [DOI] [PubMed] [Google Scholar]
- 48.Thomas R, True LD, Bassuk JA, Lange PH, Vessella RL. Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res. 2000;6 :1140–1149. [PubMed] [Google Scholar]
- 49.Wang Y, Yu Q, Cho A, Rondeau G, Welsh J, Adamson E, et al. Survey of differentially methylated promoters in prostate cancer cell lines. Neoplasia (New York, NY) 2005;7:748–760. doi: 10.1593/neo.05289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Jimenez F, Caldes T, Iniesta P, Vidart J, Lopez G. Overexpression of SPARC protein contrasts with its transcriptional silencing by aberrant hypermethylation of SPARC CpG-rich region in endometrial carcinoma. Oncology reports. 2007;17:1301–1307. [PubMed] [Google Scholar]
- 51.Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su G, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor–stromal interactions. Oncogene. 2003;22:5021–5030. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 52.Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT. SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2(prime)deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer. 2008;98:1810–1819. doi: 10.1038/sj.bjc.6604377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DiMartino J, Lacayo N, Varadi M, Li L, Saraiya C, Ravindranath Y, et al. Low or absent SPARC expression in acute myeloid leukemia with MLL rearrangements is associated with sensitivity to growth inhibition by exogenous SPARC protein. Leukemia. 2006;20:426–432. doi: 10.1038/sj.leu.2404102. [DOI] [PubMed] [Google Scholar]
- 54.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 55.Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 56.Lee S, Hwang K, Lee H, Kim J, Kang G. Aberrant CpG island hypermethylation of multiple genes in colorectal neoplasia. Laboratory invest. 2004;84:884–893. doi: 10.1038/labinvest.3700108. [DOI] [PubMed] [Google Scholar]
- 57.Toyota M, Ahuja N, Ohe-Toyota M, Herman J, Baylin S, Issa J. CpG island methylator phenotype in colorectal cancer. Proc Nat Acad Sci USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki M, Hao C, Takahashi T, Shigematsu H, Shivapurkar N, Sathyanarayana U, et al. Aberrant methylation of SPARC in human lung cancers. Br J Cancer. 2005;92:942–948. doi: 10.1038/sj.bjc.6602376. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin J, Fraumeni J, Adami H. A prospective study of obesity and cancer risk (Sweden) Cancer Causes and Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 60.Larsson S, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 61.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine Reviews. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 62.Scherer P, Williams S, Fogliano M, Baldini G, Lodish H. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 63.Gregoire F, Smas C, Sul H. Understanding adipocyte differentiation. Physiological Reviews. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 64.Rosen E, Spiegelman B. Molecular regulation of adipogenesis. Annual review of cell and Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 65.Liu J, DeYoung S, Zhang M, Cheng A, Saltiel A. Changes in integrin expression during adipocyte differentiation. Cell Metabol. 2005;2:165–177. doi: 10.1016/j.cmet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Nie J, Sage E. SPARC inhibits adipogenesis by its enhancement of -catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver A. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 68.O'Connor K, Song H, Rosenzweig N, Jansen D. Extracellular matrix substrata alter adipocyte yield and lipogenesis in primary cultures of stromal-vascular cells from human adipose. Biotechnol lett. 2003;25:1967–1972. doi: 10.1023/b:bile.0000004386.08923.ab. [DOI] [PubMed] [Google Scholar]
- 69.Hohenester E, Sasaki T, Giudici C, Farndale R, Bächinger H. Structural basis of sequence-specific collagen recognition by SPARC. Proc Nat Acad Sci USA. 2008;105:18273–18277. doi: 10.1073/pnas.0808452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi M, Nagaretani H, Funahashi T, Nishizawa H, Maeda N, Kishida K, et al. The expression of SPARC in adipose tissue and its increased plasma concentration in patients with coronary artery disease. Obesity. 2001;9:388–393. doi: 10.1038/oby.2001.50. [DOI] [PubMed] [Google Scholar]
- 71.Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23:11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bradshaw A, Reed M, Sage E. SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem. 2002;50:1–10. doi: 10.1177/002215540205000101. [DOI] [PubMed] [Google Scholar]
- 73.Shi Q, Bao S, Song L, Wu Q, Bigner D, Hjelmeland A, Rich J. Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene. 2007;26:4084–4094. doi: 10.1038/sj.onc.1210181. [DOI] [PubMed] [Google Scholar]
- 74.Delany A, Kalajzic I, Bradshaw A, Sage E, Canalis E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology. 2003;144:2588–2596. doi: 10.1210/en.2002-221044. [DOI] [PubMed] [Google Scholar]
- 75.Kang S, Bennett C, Gerin I, Rapp L, Hankenson K, MacDougald O. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein and peroxisome proliferator-activated receptor. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 76.Blobe G, Schiemann W, Lodish H. Role of transforming growth factor {beta} in human disease. The New Eng J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 77.Motamed K, Funk S, Koyama H, Ross R, Raines E, Sage E. Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cellular Biochem. 2002;84:759–771. doi: 10.1002/jcb.10095. [DOI] [PubMed] [Google Scholar]
- 78.Bassuk J, Pichler R, Rothmier J, Pippen J, Gordon K, Meek R, Bradshaw A, Lombardi D, Strandjord T, Reed M, et al. Induction of TGF-beta1 by the matricellular protein SPARC in a rat model of glomerulonephritis. Kidney Int. 2000;57:117–128. doi: 10.1046/j.1523-1755.2000.00811.x. [DOI] [PubMed] [Google Scholar]
- 79.Reed M, Vernon R, Abrass I, Sage E. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cellular Physiol. 1994;158:169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- 80.Shanker Y, Shetty U, Rao A. Regulation of low density lipoprotein receptor mRNA levels by estradiol 17 beta and chorionic gonadotropin in human placenta. Mol Cellular Biochem. 1998;187:133–139. doi: 10.1023/a:1006843812495. [DOI] [PubMed] [Google Scholar]
- 81.Shiba H, Fujita T, Doi N, Nakamura S, Nakanishi K, Takemoto T, et al. Differential effects of various growth factors and cytokines on the syntheses of DNA, type I collagen, laminin, fibronectin, osteonectin/secreted protein, acidic and rich in cysteine (SPARC), and alkaline phosphatase by human pulp cells in culture. J Cellular Physiol. 1998;174:194–205. doi: 10.1002/(SICI)1097-4652(199802)174:2<194::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 82.Schiemann B, Neil J, Schiemann W. SPARC Inhibits Epithelial Cell Proliferation in Part through Stimulation of the Transforming Growth Factor-{beta}-Signaling System. Mol Biol Cell. 2003;14:3977–3988. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhanesuan N, Sharp J, Blick T, Price J, Thompson E. Doxycycline-inducible expression of SPARC/Osteonectin/BM40 in MDA-MB-231 human breast cancer cells results in growth inhibition. Breast Cancer Res Treat. 2002;75:73–85. doi: 10.1023/a:1016536725958. [DOI] [PubMed] [Google Scholar]
- 84.Yunker C, Golembieski W, Lemke N, Schultz C, Cazacu S, Brodie C, Rempel S. SPARC-induced increase in glioma matrix and decrease in vascularity are associated with reduced VEGF expression and secretion. Int J Cancer. 2008;122:2735–2743. doi: 10.1002/ijc.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haber C, Gottifredi V, Llera A, Salvatierra E, Prada F, Alonso L, et al. SPARC modulates the proliferation of stromal but not melanoma cells unless endogenous SPARC expression is downregulated. Int J Cancer. 2007;122:1465–1475. doi: 10.1002/ijc.23216. [DOI] [PubMed] [Google Scholar]
- 86.Said N, Socha M, Olearczyk J, Elmarakby A, Imig J, Motamed K. Normalization of the ovarian cancer microenvironment by SPARC. Mol Cancer Res. 2007;5:1015–1030. doi: 10.1158/1541-7786.MCR-07-0001. [DOI] [PubMed] [Google Scholar]
- 87.Chlenski A, Liu S, Crawford S, Volpert O, DeVries G, Evangelista A, et al. SPARC is a key Schwannian-derived inhibitor controlling neuroblastoma tumor angiogenesis. Cancer Res. 2002;62:7357–7363. [PubMed] [Google Scholar]
- 88.Holland P, Harper S, McVey J, Hogan B. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987;105:473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sage H, Vernon R, Funk S, Everitt E, Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+ 2-dependent binding to the extracellular matrix. J Cell Biol. 1989;109:341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Funk S, Sage E. The Ca2 (+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Nat Acad Sci USA. 1991;88:2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi Q, Bao S, Maxwell J, Reese E, Friedman H, Bigner D, et al. Secreted protein acidic, rich in cysteine (SPARC), mediates cellular survival of gliomas through AKT activation. J Biol Chem. 2004;279:52200–52209. doi: 10.1074/jbc.M409630200. [DOI] [PubMed] [Google Scholar]
- 92.Arnold S, Mira E, Muneer S, Korpanty G, Beck A, Holloway S, et al. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp Biol Med. 2008;233:860–873. doi: 10.3181/0801-RM-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iruela-Arispe M, Lane T, Redmond D, Reilly M, Bolender R, Kavanagh T, Sage E. Expression of SPARC during development of the chicken chorioallantoic membrane: evidence for regulated proteolysis in vivo. Mol Biol Cell. 1995;6:327–343. doi: 10.1091/mbc.6.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lane T, Sage E. Functional mapping of SPARC. peptides from two distinct Ca+ (+)binding sites modulate cell shape. J Cell Biol. 1990;111:3065–3076. doi: 10.1083/jcb.111.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golembieski W, Thomas S, Schultz C, Yunker C, McClung H, Lemke N, et al. HSP27 mediates SPARC-induced changes in glioma morphology, migration, and invasion. Glia. 2008;56:1061–1075. doi: 10.1002/glia.20679. [DOI] [PubMed] [Google Scholar]
- 96.Seno T, Harada H, Kohno S, Teraoka M, Inoue A, Ohnishi T. Downregulation of SPARC expression inhibits cell migration and invasion in malignant gliomas. Int J Oncol. 2009;34:707–715. doi: 10.3892/ijo_00000197. [DOI] [PubMed] [Google Scholar]
- 97.Everitt E, Sage E. Expression of SPARC is correlated with altered morphologies in transfected F9 embryonal carcinoma cells. Exp Cell Res. 1992;199:134–146. doi: 10.1016/0014-4827(92)90471-j. [DOI] [PubMed] [Google Scholar]
- 98.Ledda M, Adris S, Bravo A, Kairiyama C, Bover L, Chernajovsky Y, et al. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nature Med. 1997;3:171–176. doi: 10.1038/nm0297-171. [DOI] [PubMed] [Google Scholar]
- 99.Alvarez M, Prada F, Salvatierra E, Bravo A, Lutzky V, Carbone C, et al. Secreted protein acidic and rich in cysteine produced by human melanoma cells modulates polymorphonuclear leukocyte recruitment and antitumor cytotoxic capacity. Cancer Res. 2005;65:5123–5132. doi: 10.1158/0008-5472.CAN-04-1102. [DOI] [PubMed] [Google Scholar]
- 100.Robert G, Gaggioli C, Bailet O, Chavey C, Abbe P, Aberdam E, et al. SPARC represses E–cadherin and induces–mesenchymal transition during melanoma development. Cancer Res. 2006;66:7516–7523. doi: 10.1158/0008-5472.CAN-05-3189. [DOI] [PubMed] [Google Scholar]
- 101.Carmeliet P, Jain R. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 102.Jendraschak E, Helene Sage E. Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Elsevier; 1996. pp. 139–146. [DOI] [PubMed] [Google Scholar]
- 103.Chen L, Yang Z, Wang G, Wang C. Expression of angiopoietin-2 gene and its receptor Tie2 in hepatocellular carcinoma. J Tongji Med Univer Tong ji yi ke da xue xue bao. 2001;21:228–230. doi: 10.1007/BF02886437. [DOI] [PubMed] [Google Scholar]
- 104.Shankavaram U, DeWitt D, Funk S, Sage E, Wahl L. Regulation of human monocyte matrix metalloproteinases by SPARC. J Cellular Physiol. 1998;173:327–334. doi: 10.1002/(SICI)1097-4652(199712)173:3<327::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 105.Schaeffer H, Weber M. Mitogen-activated protein kinases. specific messages from ubiquitous messengers. Mol Cellular Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chlenski A, Liu S, Guerrero L, Yang Q, Tian Y, Salwen H, et al. SPARC expression is associated with impaired tumor growth, inhibited angiogenesis and changes in the extracellular matrix. Int J Cancer. 2005;118:310–316. doi: 10.1002/ijc.21357. [DOI] [PubMed] [Google Scholar]
- 107.Lane T, Sage E. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. [PubMed] [Google Scholar]
- 108.Said N, Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Amer J Pathol. 2005;167:1739–1752. doi: 10.1016/S0002-9440(10)61255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krammer P, Galle P, Moller P, Debatin K. CD 95 (APO-1/Fas)-Mediated Apoptosis in Normal and Malignant Liver, Colon, and Hematopoietic Cells. Advan in Cancer Res. 1998;75:251–273. doi: 10.1016/s0065-230x(08)60744-7. [DOI] [PubMed] [Google Scholar]
- 110.Schmitz I, Kirchhoff S, Krammer P. Regulation of death receptor-mediated apoptosis pathways. Int J Biochem Cell Biol. 2000;32:1123–1136. doi: 10.1016/s1357-2725(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 111.Yiu G, Chan W, Ng S, Chan P, Cheung K, Berkowitz R, Mok S. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Amer J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang M, Tai I. A novel interaction between procaspase 8 and SPARC enhances apoptosis and potentiates chemotherapy sensitivity in colorectal cancers. J Biol Chem. 2007;282:34457–34457. doi: 10.1074/jbc.M704459200. [DOI] [PubMed] [Google Scholar]
- 113.Tai IT, Tang MJ. SPARC in cancer biology. its role in cancer progression and potential for therapy. Drug Resist Updat. 2008;11:231–246. doi: 10.1016/j.drup.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Wong S, Crowley D, Bronson R, Hynes R. Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer. Clin Exp Metast. 2008;25:109–118. doi: 10.1007/s10585-007-9126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhoopathi P, Chetty C, Gujrati M, Dinh D, Rao J, Lakka S. Cathepsin B facilitates autophagy-mediated apoptosis in SPARC overexpressed primitive neuroectodermal tumor cells. Cell Death Differ. 2010:1–10. doi: 10.1038/cdd.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koblinski J, Kaplan-Singer B, VanOsdol S, Wu M, Engbring J, Wang S, et al. Endogenous osteonectin/SPARC/BM-40 expression inhibits MDA-MB-231 breast cancer cell metastasis. Cancer Res. 2005;65:7370–7377. doi: 10.1158/0008-5472.CAN-05-0807. [DOI] [PubMed] [Google Scholar]
- 117.Briggs J, Chamboredon S, Castellazzi M, Kerry J, Bos T. Transcriptional upregulation of SPARC, in response to c-Jun overexpression, contributes to increased motility and invasion of MCF7 breast cancer cells. Oncogene. 2002;21:7077–7091. doi: 10.1038/sj.onc.1205857. [DOI] [PubMed] [Google Scholar]
- 118.Lien H, Hsiao Y, Lin Y, Yao Y, Juan H, Kuo W, et al. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: identification of genes potentially related to epithelial mesenchymal transition. Oncogene. 2007;26:7859–7871. doi: 10.1038/sj.onc.1210593. [DOI] [PubMed] [Google Scholar]
- 119.Prada F, Benedetti L, Bravo A, Alvarez M, Carbone C, Podhajcer O. SPARC endogenous level, rather than fibroblast-produced SPARC or stroma reorganization induced by SPARC, is responsible for melanoma cell growth. J Invest Dermatol. 2007;127:2618–2628. doi: 10.1038/sj.jid.5700962. [DOI] [PubMed] [Google Scholar]
- 120.Chan S, Griffith O, Tai I, Jones S. Meta-analysis of colorectal cancer gene expression profiling studies identifies consistently reported candidate biomarkers. Cancer Epidemiol Biomark Prevent. 2008;17:543–552. doi: 10.1158/1055-9965.EPI-07-2615. [DOI] [PubMed] [Google Scholar]
- 121.Liang J, Wang H, Xiao H, Li N, Cheng C, Zhao Y, et al. Relationship and prognostic significance of SPARC and VEGF protein expression in colon cancer. J Exp Clini Cancer Res. 2010;29:71–75. doi: 10.1186/1756-9966-29-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rempel S, Golembieski W, Fisher J, Maile M, Nakeff A. SPARC modulates cell growth, attachment and migration of U87 glioma cells on brain extracellular matrix proteins. J Neuro-Oncol. 2001;53:149–160. doi: 10.1023/a:1012201300188. [DOI] [PubMed] [Google Scholar]
- 123.Schultz C, Lemke N, Ge S, Golembieski W, Rempel S. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002;62:6270–6277. [PubMed] [Google Scholar]
- 124.Huang S, Robinson J, DeGuzman A, Bucana C, Fidler I. Blockade of Nuclear Factor-{kappa} B Signaling Inhibits Angiogenesis and Tumorigenicity of Human Ovarian Cancer Cells by Suppressing Expression of Vascular Endothelial Growth Factor and Interleukin 8. Cancer Res. 2000;60:5334–5339. [PubMed] [Google Scholar]