Abstract
Burn injury initiates an enhanced inflammatory condition referred to as the systemic inflammatory response syndrome (SIRS) or the two-hit response phenotype. Prior reports indicated that macrophages respond to injury and demonstrate a heightened reactivity to Toll-like receptor (TLR) stimulation. Since we and others observed a significant increase in splenic GR-1+F4/80+CD11b+ macrophages in burn-injured mice, we wished to test if these macrophages might be the primary macrophage subset that shows heightened LPS reactivity. We report here that burn injury promoted higher level TNFα expression in GR-1+, but not GR-1− macrophages following LPS activation both in vivo and ex vivo. We next tested whether CD4+ T cells, which are known to suppress injury-induced inflammatory responses, might control the activation and expansion of GR-1+ macrophages. Interestingly, we found that GR-1+ macrophage expansion and LPS-induced TNFα expression was not significantly different between wild-type (WT) and CD4 T cell deficient (CD4−/−) mice. However, further investigations showed that LPS-induced TNFα production was significantly influenced by CD4 T cells. Taken together, these data indicate that GR-1+F4/80+CD11b+ macrophages represent the primary macrophage subset that expands in response to burn injury and that CD4 T cells do not influence the GR-1+ macrophage expansion process, but do suppress LPS-induced TNFα production. These data suggest that modulating GR-1+ macrophage activation as well as CD4 T cell responses following severe injury may help control the development of SIRS and the two-hit response phenotype.
Keywords: Trauma, innate immunity, inflammation, myeloid suppressor cells (MSCs), Tregs
INTRODUCTION
The host response to severe injury is a complex-type of an immune response that involves both innate and adaptive immune cells and networks (1, 2). In general, innate immune cell types demonstrate a progressive increase in inflammatory behavior, which may be an evolutionarily-conserved response to enhance anti-microbial immunity (3). On the other hand, adaptive immune cells show an increase in counter-inflammatory behavior that includes increases in CD4+ T helper 2 (Th2) cytokine producing cells and activation of CD4+ regulatory T cells (Tregs) (4–6). Several studies have reported that mice lacking an adaptive immune system (Rag1−/−) or CD4 T cell deficient mice (CD4−/−) develop higher innate immune cell inflammatory behavior following burn injury (7, 8). This suggests that the adaptive immune system and CD4 T cells play an active role in controlling the pro-inflammatory phenotype that develops after severe injury. One aim of these studies was to address whether CD4 T cells might control the inflammatory response to injury by affecting macrophage subset activation and expansion.
Our focus on studying the influence of injury on macrophages is based on the general observation that macrophages represent the primary immune cell type showing increased inflammatory reactivity following burn injury (9). For example, splenic macrophages showed a significant increase in responsiveness to Toll-like receptor (TLR) stimulation following burn injury in mice (10). In that report, F4/80+ macrophages, as compared to other innate immune cell subsets, were shown to express significantly higher levels of IL-1β, IL-6, and TNFα when stimulated with E. coli lipopolysaccharide (LPS) at 7 days after burn injury (10). The enhanced TLR responsiveness expressed by macrophages after burn injury was subsequently shown to be due to heightened TLR4-induced P38 MAPK signaling in macrophages (11). The experiments in this report were designed to further elucidate and characterize the macrophage response to injury. In particular, we were interested in confirming whether burn injury induces a unique macrophage sub-population called GR-1+ macrophages and whether this change in macrophage populations contributes to the development of the “two-hit” response phenotype (12, 13). This macrophage subset expresses the F4/80, GR-1, and CD11b cell-surface markers (14, 15). GR-1-expressing macrophages have a unique ring-shaped nucleus and because of this are thought to be early myeloid lineage cells with the capacity to undergo further differentiation (16). For this reason, GR-1+ macrophages have been shown to have pleotropic biologic activities including antigen presentation, tissue processing, wound healing, helminthes eradication, and tumor surveillance (17–19). Thus, it is unclear whether GR-1+ macrophages represent a subset or a precursor myeloid cell with the capacity to further differentiate. Because of their functional diversity, it is not surprising that GR-1+ macrophages have been implicated as mediators of many pathological host responses including burn injury and sepsis (12, 20).
The experiments reported here used a mouse burn injury model to characterize changes in the number and phenotype of GR-1+ and GR-1− macrophages at days 1 and 7 after injury. We confirm that burn injury leads to a significant increase in splenic GR-1+ macrophages by 7 days post injury. Further characterization of the burn-induced GR-1+ macrophages showed that this macrophage sub-population expresses higher levels of TNFα following LPS stimulation in vivo than GR-1− macrophages. We demonstrate that burn-injured CD4−/− mice showed GR-1+ macrophage expansion and LPS-induced TNFα expression that was indistinguishable from burn-injured WT mice. This result suggests that CD4 T cells do not directly suppress GR-1+ macrophage expansion or their pro-inflammatory phenotype. However, the results of additional experiments showed that CD4 T cells do affect the level of TNFα production by LPS-stimulated spleen cells. Taken together, these findings advance our current understanding of the cellular events that contribute to the development of post-injury inflammation, SIRS, and the two-hit response.
MATERIALS AND METHODS
Mice
All experiments used 6–9 week old male C57BL/6J mice purchased from Jackson Laboratory (Bar Harbor, ME) or CD4−/− mice bred to the C57BL/6J background that were maintained as breeders in our virus-AB-free (VAF) animal facility. Purchased mice were acclimatized for one week prior to use and allowed food and water ad libitum. Mice were housed in accordance with National Institute of Health and Harvard Medical School Standing Committee on Animal Research. Veterinary oversight was provided by the staff of the Harvard Center for Comparative Medicine.
Reagents
Cells were prepared and cultured in complete-5 (C-5) medium (RPMI 1640 supplemented with 5% heat-inactivated FCS, 10 mM HEPES, 1 mM Glutamine, penicillin/streptomycin/fungizone, 100 μM nonessential amino acids and 2.5×10−5 M β-mercaptoethanol) all purchased from Life Technologies (Carlsbad, CA). The E. coli lipopolysaccharide (LPS) 026:B6 was purchased from Difco Laboratories (Detroit, MI). Liberase CI was purchased from Roche (Indianapolis, IN). The LPS was re-purified to reduce contaminants as described previously (21). Fluorescently-labeled antibodies used for FACS analysis included anti-GR-1 (Ly6C/G, clone RB6), anti-Ly6G (clone 1A8), anti-F4/80, anti-CD11b, and anti-TNFα antibodies all purchased from BioLegend (San Diego, CA). FC-receptor blocking antibody, CD16/32 (BioLegend, San Diego, CA) was used in FACS stains.
Mouse Burn Injury Model
The burn injury protocol used in these studies were approved by the Harvard Medical School Standing Committee on Animal Research and were performed as described previously (5, 8). Mice were anesthetized via intra-peritoneal injection of ketamine (125 mg/kg) and xylazine (20 mg/kg). The dorsal fur was shaved, and the animal was placed in an insulated plastic mold to expose 25% of its total body surface area. The exposed dorsum was then immersed in 90°C water for 9 seconds. This approach causes a well-demarcated, full-thickness burn injury. Due to nerve cell damage, this level of injury has been defined as being painless and analgesia is not required. Sham mice were treated exactly as burn mice, except that they were exposed to room temperature water. Immediately after the procedure, sham and burn mice were resuscitated by an intra-peritoneal injection of 1 ml of pyrogen-free normal saline. This is a non-lethal injury since mortality is less than 5 percent.
Ex vivo studies
Mice were killed by CO2 asphyxiation at either 1 day or 7 days after sham or burn injury. Spleens were harvested, and cell suspensions were prepared by mincing the tissues on a sterile wire mesh. Liberase CI (Roche Applied Science, Indianapolis, IN) was used to maximize release of splenic macrophages during cell expansion studies, but was omitted for intracellular cytokine staining experiments due to combined toxicity with Brefeldin A. Red blood cells were lysed with RBC lysis buffer, cells were washed, counted and resuspended in C-5 culture medium.
Preparation of CD4 T cell depleted spleen cells
Dynabeads (Dynal Biotech) were used according to the manufacturer’s instructions to deplete CD4+ T lymphocytes from WT splenocyte suspensions, so that splenocyte populations comparable to CD4−/− splenocytes were created for cell culture. Briefly, beads were washed twice in culture medium, added to total splenocyte suspensions at a 4:1 bead to target cell ratio, and incubated at 4°C with bi-directional rotation for 30 min. Beads with attached cells were then removed by placing the tube containing the treated splenocyte suspension against a strong magnet for 4 min. The depleted suspension, containing the negatively isolated cells, was then used for further studies. Flow cytometry was performed using a FACS Calibur instrument (BD Biosciences) and using anti-CD4 confirmed that 95% depletion was achieved for the relevant lymphocytes.
Intracellular TNFα staining protocol
For in vitro stains, spleen cells were plated at 2 × 106/well in 96-well plates (Corning, Corning, NY) without or with 100 ng/ml LPS. Cells were incubated at 37° C, 5% CO2 for 20 minutes. Brefeldin A (Sigma, St. Louis, MO) was then added at final concentration of 10μg/ml to block TNFα secretion, and cells were incubated for an additional 4 hours. For in vivo stains, spleens from individual mice were removed from mice at 30 minutes after LPS treatment (10 mg/kg) and placed in C-5 medium containing Brefeldin A (10 μg/ml), and then crushed to allow Brefeldin A perfusion. Cell suspensions were prepared by mincing the spleens and cells were plated at 2 × 106/well in 96-well plates (Corning, Corning, NY) in C-5 medium and cultured at 37° C. After3 hours incubation, cells were pelleted by centrifugation in 96-well plates. Cell pellets were disrupted by vortex and 20 μl of Tru-Stain FC receptor blocking reagent (anti-CD16/CD32 mAb, Biolegend, San Diego, CA) was added to each well. After 10 minutes, fluorescently-labeled anti-F4/80 and anti-GR-1 mAbs were added and plates were incubated for 20 minutes at 4° C in the dark. Cells were pelleted by centrifugation, vortexed to resuspend, and then fixed in Fix/Perm buffer (BioLegend, San Diego, CA). After 20 minutes fixation at 4° C, samples were then centrifuged and cells were resuspended in permeabilization buffer (BioLegend, San Diego, CA), containing rat and mouse immunoglobulin (at 10 μg/ml) for both) for 10 minutes at 4° C. Samples were centrifuged again and stained for 30 minutes with PE-labeled anti-TNFα mAb. After washing stained cells by centrifugation, cells were fixed in 0.3% paraformaldehyde in PBS. The protocol for intracellular TNFα staining was identical for in vitro and in vivo activated cells. Intracellular TNFα levels in GR-1+ or GR-1−, F4/80+ cells were measured by flow cytometry using a BD FACScaliber and analyzed by CellQuestPro software (BD Biosystems, San Jose, CA).
Spleen cell stimulation cultures
Splenocyte suspensions from groups of sham or burn C57BL/6J or CD4−/− mice were counted and plated in sterile Corning 96-well round bottom plates at a density of 1×106 cells per well with titrated concentrations of E. coli LPS or no LPS. After 48 hours incubation at 37°C in 5% CO2, supernatants were harvested and stored at −20°C until being tested for TNFα levels by a Luminex bead-based assay.
TNFα detection
TNFα levels in culture supernatants were measured by Luminex bead-based multiplex assays using a custom-made Luminex bead assay. In brief, supernatants were incubated with anti-TNFα antibody coated beads in incubation buffer (PBS + 0.1% Tween-20 + 0.05% sodium azide) at room temperature. After 2 hours incubation in the dark, the beads were washed by centrifugation at 600 × g for 5 minutes and biotinylated secondary anti-TNFα antibody was added for 1 hour incubation at room temperature. Plates were washed in PBS + 0.2% Tween-20 by centrifugation and PE-labeled streptavidin detection reagent was added. Samples were then run and analyzed on a Luminex-200 instrument (Applied Cytometry (Sheffield, UK). Cytokine levels were calculated by a standard curve from known concentrations of recombinant mouse TNFα (Peprotech, Rocky Hill, NJ) using the STarStation software program (Applied Cytometry, Sheffield, UK).
Statistics
The PRISM 3.0 software program (Graph Pad, San Diego, CA) was used for all statistical calculations. One-Way ANOVA with Tukey’s multiple comparison test or one-tailed Student’s t tests were used to analyze these data as indicated in the figure legends. P<0.05 was considered significant.
RESULTS
Burn injury induces increased GR-1+ F4/80+ CD11b splenic macrophages by 7 days after injury
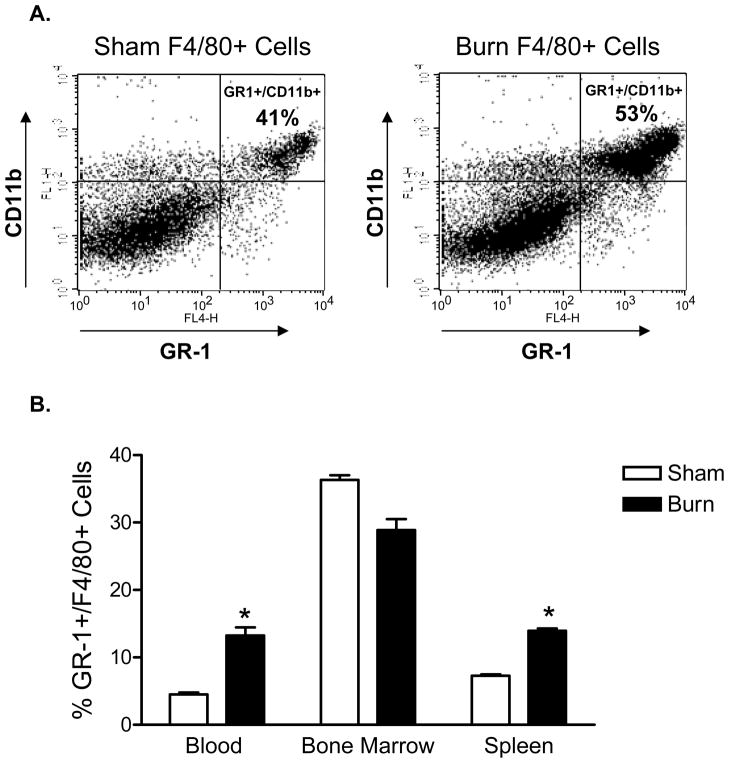
These experiments were initiated to test whether burn injury may cause a change in macrophage numbers or subsets. Groups of male, C57BL/6J mice underwent sham or burn injury and at day 7, splenic macrophages were prepared from individual mice for flow cytometry analysis. We were primarily interested in measuring changes in the percentages of GR-1+ versus GR-1− macrophage subsets. To accomplish this, a simple three-color flow cytometry staining approach was used to stain splenocytes with fluorescently-labeled antibodies specific for F4/80, CD11b, and GR-1. The FACS plots shown in Figure 1A represent the level of staining for GR-1+F4/80+CD11b+ cells seen in the spleen at day 7 after burn injury. It is apparent from these FACS plots that macrophages and in particular, GR-1+ macrophages were increased in the spleens of burn as compared to sham mice. We then evaluated changes in GR-1+ macrophages in the bone marrow, blood, and spleen at 7 days post-injury. The data plotted in Figure 1B show that burn injury induced a significant increase in GR-1+F4/80+CD11b+ cells in the blood and spleen, but not the bone marrow, at 7 days after injury. These findings confirm that burn injury induces a significant expansion or recruitment of GR-1+ macrophages in the blood and spleen by 7 days post injury and also documents that GR-1+ macrophage levels are lower reduced in the in the bone marrow at this same time point (22).
Figure 1. Burn injury induces an increase in the percentage of F4/80+ CD11b+ GR-1+ splenic macrophages.
Spleens from WT sham or burn-injured mice were harvested at day 7 and splenocyte suspensions were prepared. Cells were stained with fluorescently-labeled anti-GR-1, -F4/80, and -CD11b specific antibodies. The dot plots shown in (A) represent the GR-1 and CD11b staining profiles of FACS-gated live and F4/80+ spleen cells. Cells were prepared from the blood, bone marrow, and spleens of WT sham or burn mice at day 7 post-injury and stained for cell-surface GR-1+/F4/80+/CD11b+. The combined FACS data from all mice is plotted in Figure 1B. There was a significant increase in GR-1+/F4/80+/CD11b+ blood and spleen macrophages that were harvested from burn as compared to sham mice (* p<0.05, Student’s t test). Data expressed as mean ± SEM of n=4 mice per group and are representative of 3 independent experiments.
GR-1+ macrophages are the primary producers of TLR4-induced TNFα
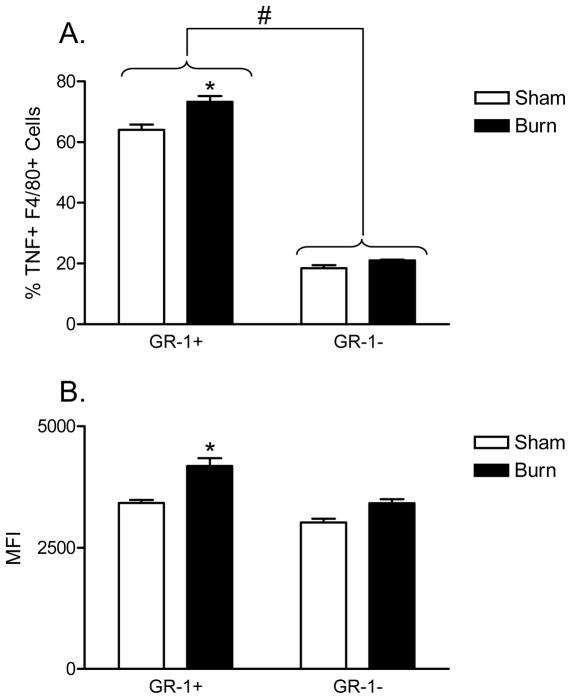
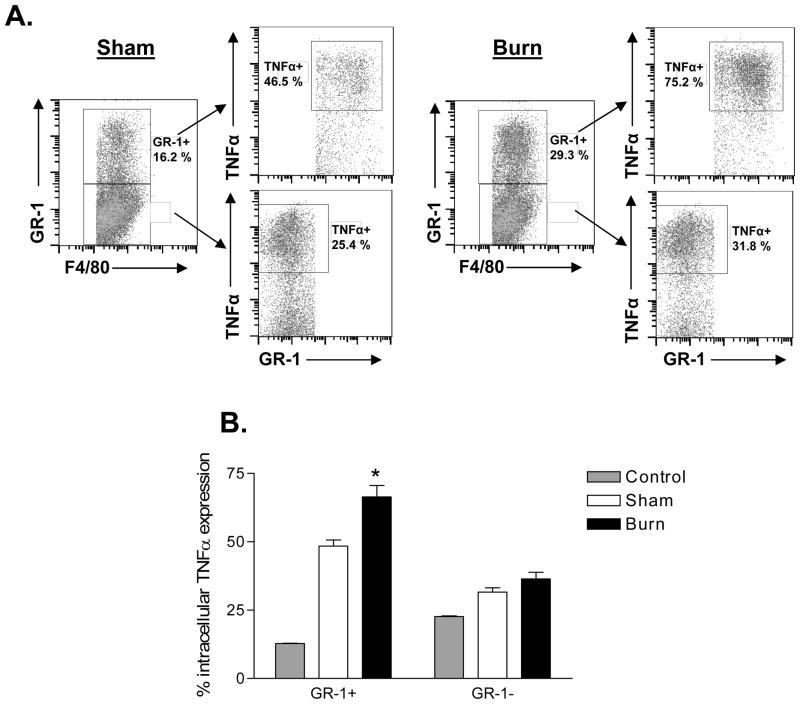
Since we observed a burn-induced increase in GR-1+ macrophages, we wanted to determine if GR-1+ macrophages might contribute to the well-described injury-dependent increase in TLR4-induced TNFα production by macrophages (10). To accomplish this, we performed intracellular TNFα staining in LPS-stimulated FACS-gated GR-1+ versus GR-1− macrophages prepared from sham and burn mice at 7 days post-injury. In brief, the cells were stained with anti-F4/80, anti-GR-1, and anti-TNFα antibody to measure which macrophage cell subset produced TNFα in response to LPS stimulation. The results shown in Figure 2A demonstrate that a higher percentage of GR-1+/F4/80+ expressed TNFα. We also show by mean fluorescence intensity (MFI) FACS analysis that GR-1+ macrophages expressed significantly higher levels of LPS-induced TNFα than GR-1− macrophages (Figure 2B). Importantly, these ex vivo observations were confirmed in vivo by challenging sham- or burn-injured mice with LPS and measuring intracellular TNFα expression in GR-1+ and GR-1− macrophages (Figure 3). As shown, burn mice that were challenged with LPS showed significantly higher TNFα expression in GR-1+ macrophages than in GR-1− macrophages. Collectively, these data demonstrate that GR-1+ macrophages are the primary source for LPS-induced TNFα production and that burn injury primes GR-1+ macrophages for higher LPS/TLR4-induced TNFα expression than GR-1− macrophages.
Figure 2. GR-1+ F4/80+ macrophages are principal producers of TNFα following burn injury with LPS stimulation at day 7 following injury.
Spleens from WT sham or burn injured mice were harvested at day 7 and splenocyte suspensions prepared. LPS, followed by Brefeldin A, was added to cultures to induce an in vitro two-hit effect. Cells were stained with fluorescently-labeled anti-GR-1, -F4/80, and intracellular -TNFα specific antibodies. The data plotted in (A) shows the percentage TNFα-expressing cells in GR-1+ versus GR-1− macrophages. The data plotted in (B) shows the MFI for TNFα staining in GR-1+ and GR-1− macrophages. There is a significantly higher percentage TNFα expression and expression levels (MFI) in GR-1+ F4/80+ macrophages from burn-injured mice as compared to GR-1+ F4/80+ sham (*p<0.01). There was no significant difference observed between sham or burn-injured mice in GR-1 F4/80+ macrophages (# p>0.05 ns). Overall, GR-1+ F4/80+ macrophages expressed higher levels of TNFα as compared withGR-1− F4/80+ macrophages (** p<0.001, One-Way ANOVA with Tukey’s multiple comparison). Data expressed as mean ± SEM of n=4 mice per group and are representative of 3 independent experiments.
Figure 3. GR-1+ macrophages from burn-injured mice express significantly higher TNFα following in vivo challenge with LPS.
Groups of mice underwent sham or burn injury. Seven days later, mice were challenged with LPS (10 mg/kg) by i.p. injection. After 30 minutes, spleens were harvested into medium containing Brefeldin A (10 μg/ml) to prevent cytokine release. Cells were plated in C-5 medium and cultured at 37° C with Brefeldin A (10 μg/ml) for an additional 3 hours. Cells were stained with fluorescently-labeled anti-GR-1, -F4/80, and intracellular -TNFα specific antibodies. The FACS plots in (A) illustrate the intracellular TNFα staining profile in GR-1+ and GR-1− macrophages for day 7 sham versus burn mice. The data plotted in (B) represents combined TNFα expression staining in GR-1+ versus GR-1− macrophages from the spleens of sham versus burn mice at day 7 after injuries. Data are expressed as mean ± SEM of n=8 mice per group. GR-1+ macrophages from burn mice showed a significant increase (*, p<0.05) in intracellular TNFα expression as compared to sham mice.
The influence of CD4+ T cells on LPS-induced TNFα expression by GR-1+ macrophages and TNFα production by splenocytes
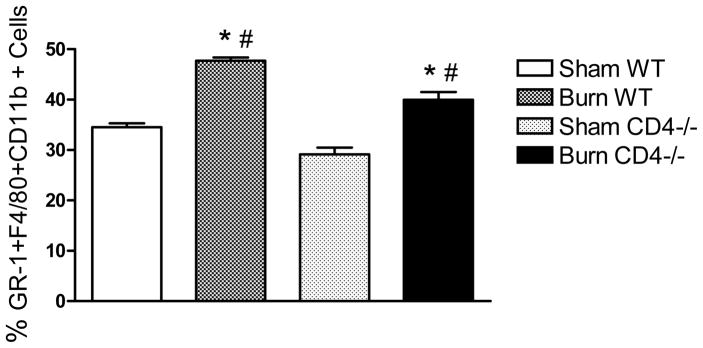
We previously reported that mice lacking CD4+ T cells exhibit a more pro-inflammatory phenotype following burn injury (7). Therefore, we wished to test if the burn-induced expansion of GR-1+ macrophages or increase in TLR4-induced TNFα expression by GR-1+ macrophages might account for this phenotypic change. To accomplish this aim, C57BL/6J (WT) or CD4−/− mice underwent sham or burn injury and at days 1 or 7, spleen cells were prepared. Changes in GR-1+ macrophages were measured by staining spleen cells with anti-GR-1 and anti-F4/80 antibodies for FACS analysis and intracellular TNFα staining was used to measure the effect of CD4 T cell deficiency on TNFα expression by GR-1+ or GR-1− F4/80+ macrophages. An addition control to test for in vitro effects of CD4 T cells on TNFα expression was included by using CD4 T cell depleted spleen cells from sham or burn WT mice. The data in Figure 4 show that GR-1+ cells were significantly increased in the spleens of burn-injured WT as well as CD4 T cell deficient mice at day 7 post-injury. Since we did not observe a difference in GR-1+ macrophage expansion between burn-injured WT and CD4−/− mice, we conclude that CD4 T cells did not influence the injury-induced GR-1+ macrophage expansion process.
Figure 4. CD4+ T cells do not influence the burn-induced increase in GR-1+ macrophages.
Spleen cells were prepared from sham- or burn-injured WT or CD4 −/− mice at 7 days after injury. Cells were stained with fluorescently-labeled anti-GR-1, anti-F4/80, and anti-CD11b antibodies. After staining, cells were fixed and stained for FACS analysis. Burn injury induced a significant increased in GR-1+F4/80+CD11b+ macrophages in WT and CD4−/− (*p<0.05 by One-Way ANOVA). The percentage of GR-1+F4/80+CD11b+ macrophages was significantly lower in burn-injured CD4−/− mice than burn WT mice (#, p<0.05 by One-Way ANOVA). Data are expressed as mean ± SEM of n=3 mice per group from 2 independent experiments.
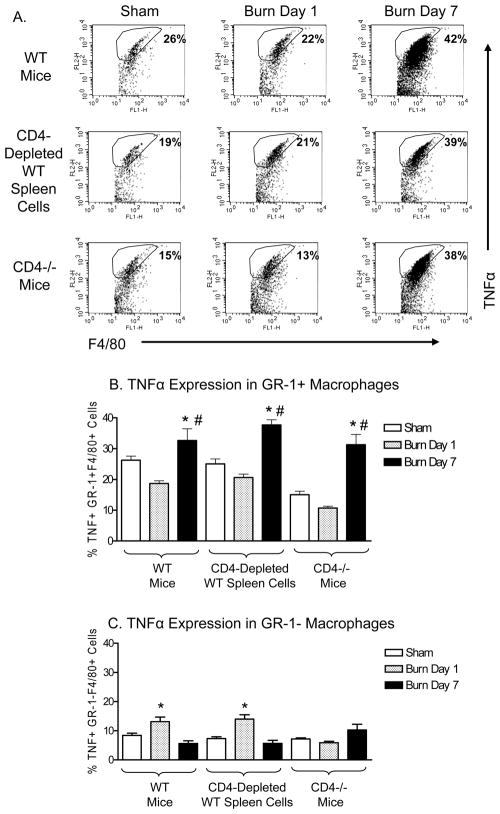
The results of experiments testing the affect of CD4 T cells on LPS-induced TNFα expression by GR-1+ macrophages are shown in Figure 5. The representative FACS plots illustrate that the burn-induced increase in TNFα expression by LPS-stimulated GR-1+ macrophages occurred in CD4−/− mice to a similar level as was observed in WT mice. We also found that depleting WT spleen cells of CD4 T cells in vitro prior to LPS stimulation did not alter the burn-induced increase in TNFα expression by GR-1+ macrophages. In general, GR-1− macrophages expressed low levels of TNFα, and did not demonstrate a burn-induced increase in TNFα expression at day 7. Taken together, these data indicate that CD4 T cells did not influence the injury-induced increase in TNFα expression by GR-1+ macrophages.
Figure 5. CD4+ T cells do not influence the injury-induced increase in TNF α expression by LPS-stimulated GR-1+ macrophages.
Spleen cells were prepared from WT or CD4 −/− sham or burn-injured mice at days 1 and 7. As a control, splenocytes from sham or burn-injured WT mice were depleted of CD4 T cells. Following in vitro LPS stimulation, we measured TNFα expression in FACS-gated F4/80+ GR-1+ or GR-1− macrophages. Panel (A) shows representative FACS plots of gated GR-1+F4/80+ spleen cells stimulated with LPS from sham or burn injured WT, CD4 depleted WT spleen cells or CD4−/− mice at day 1 and day 7 after injury. Panel (B) shows TNFα expression in live, F4/80+ GR-1+ macrophages, while (C) shows TNFα expression in F4/80+ GR-1− macrophages. Our results demonstrate that LPS-stimulated GR-1+ macrophages from day 7 burn mice expressed significantly higher levels of TNFα as compared with GR-1+ macrophages from day 7 sham or day 1 burn mice. The in vivo or in vitro absence of CD4 T cells did not influence GR-1+ macrophage TNFα expression levels, as confirmed by results in CD4−/− and CD4 T cell depleted splenocytes (#p>0.05 ns, one way ANOVA with Tukey’s multiple comparison). In contrast, GR-1− macrophages were more inflammatory at day 1 as compared to day 7 after burn injury (**p<0.001). Data are expressed as mean ± SEM of n=3 mice per group and are representative of 3 independent experiments.
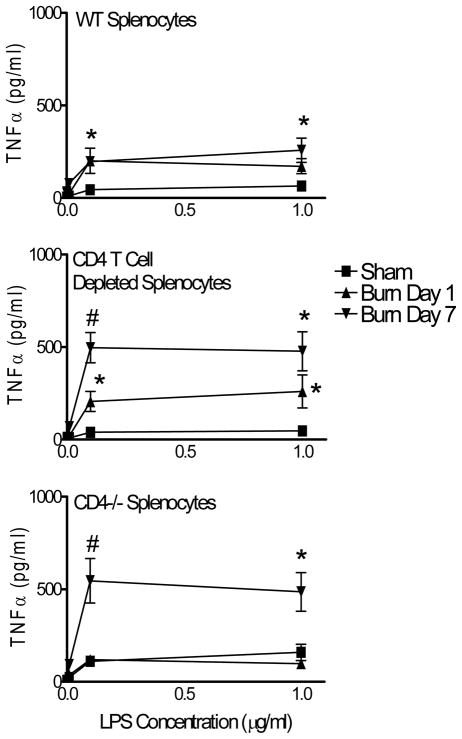
We next measured TNFα production and release by spleen cells from sham or burn CD4−/− and WT mice. In particular, we wished to investigate the in vivo impact of CD4 T cells on LPS-induced TNFα production. These experiments were needed because intracellular TNFα expression does not necessarily translate to TNFα production and release. Splenocytes from sham or burn injured WT or CD4−/− mice were prepared at days 1 or 7 after burn injury. We also prepared CD4 T cell depleted spleen cell cultures from sham or burn WT mice to test if CD4 T cells might influence LPS-stimulated TNFα production in vitro. Spleen cell preparations were stimulated with LPS doses (0.001, 0.01, 0.1, 1 μg/ml) or no LPS and culture supernatants were tested for TNFα levels at 48 hours after stimulation. As anticipated from prior reports, spleen cells from burn-injured mice produced significantly higher levels of TNFα (Figure 6) (10). Our results also show that TNFα production was significantly greater in spleen cell cultures from day 7 burn-injured CD4−/− mice or from CD4 T cell depleted WT spleen cells. These findings support the idea that CD4 T cells play an active role in suppressing TNFα production or release, however, they do not appear to affect TNFα expression by macrophages.
Figure 6. Mice lacking CD4 T cells produce higher levels of TNFα following injury.
WT or CD4−/− mice were randomized to sham or burn injury. At day 7 after injury, mice were killed and splenocyte suspensions were prepared. Spleen cells were cultured with increasing doses of LPS (0–1μg/ml, five concentrations) for 48 hours and supernatants were tested for TNFα levels using a Luminex assay. The plots show LPS-induced TNFα levels in supernatants from (A) WT, (B) WT/CD4 T cell depleted, and (C) CD4−/− spleen cells. Spleen cells from all groups of burn mice produced significantly higher TNFα at 7 days as compared to spleen cells from sham mice; *p<0.05 by One-Way ANOVA. In addition, splenocytes from CD4−/− mice and CD4 T cell depleted spleen cells exhibited higher TNFα production at day 7 after burn injury as compared to spleen cells from burn WT mice; #p<0.001 by One-Way ANOVA. Data are expressed as mean ± SEM of n=4 mice per group and are representative of 2 independent experiments.
DISCUSSION
The development of a pro-inflammatory state following severe injury has been well documented and is referred to clinically as the systemic inflammatory response syndrome (SIRS) (23). Moreover, injury has been shown to prime the host for an enhanced secondary response to infection, which can lead to an overwhelming inflammatory response and organ damage to the host. This immune system priming and enhanced secondary response to infection was defined by Moore et al as the “two-hit” response (13, 24). We and other investigators are tremendously interested in defining the cellular and molecular mechanisms responsible for the development of SIRS and the “two-hit” response phenotype following burn, trauma, and sepsis because this pro-inflammatory condition predisposes the patient to developing complications such as opportunistic infection and organ damage. This study focused primarily on how injury affects macrophages. Our interest in studying the macrophage response to burn injury stems from the observation that injury primes splenic macrophages for high level TNFα expression in response to TLR2 or TLR4 stimulation as compared to other innate immune cell types (10). This increased macrophage responsiveness to TLR stimulation suggested to us that macrophages may be the primary cell mediator of the two-hit response phenotype following severe injury. It was subsequently shown by other groups that burn injury or sepsis causes a marked increase in GR-1− expressing macrophages, which suggests that the increase prevalence of GR-1+ macrophages may be central to the development of SIRS and the two-hit response phenotype (12, 20, 22). The results of studies presented here further support that burn injury induces a significant increase in splenic and circulating GR-1+ macrophages/monocytes. We also show that GR-1+ macrophages represent the dominant macrophage source for LPS-induced TNFα expression in vitro and in vivo. The observation that GR-1+ F4/80+ macrophages express high levels of TNFα following LPS challenge in injured mice directly supports that idea that GR-1+ macrophages represent the macrophage subset that is primed by injury for enhanced LPS reactivity.
One aim of this study was to examine if these injury-induced GR-1+ macrophages resemble the GR-1-expressing myeloid suppressor cells (MSC) that have been shown to increase in the spleens of tumor-bearing, septic, and burn mice (19, 20, 25, 26). This was accomplished by simply staining spleen cells from sham- or burn-injured mice with anti-GR-1 antibody (clone RB6, anti-Ly6C/Ly6G) and F4/80 and CD11b specific antibodies at days 1 versus 7 post injury. We chose F4/80 as a monocyte/macrophage marker since it is a highly specific cell-surface marker for mouse monocytes and macrophages(27). Even though CD11b has a wider cell type expression pattern than F4/80, we stained for its co-expression with F4/80 and GR-1 since it commonly used to characterize MSCs and GR-1+ macrophages (22, 28). We report here that burn injury induced a significant increased in GR-1+F4/80+CD11b+ cells in the blood and spleen by 7 days, but not 1 day, post-injury. This finding agrees with data from prior reports showing that burn-injured mice have higher relative levels of GR-1+ macrophages in the spleen by 7 days after injury (12, 22, 29, 30).
Since CD4 T cells and Tregs are known to control the inflammatory response to burn injury by suppressing LPS-induced TNFα, we hypothesized that burn-injured CD4−/− mice would show an augmented expansion of GR-1+ macrophages (8). Instead, we did not observe a burn-induced increase in GR-1+ macrophages in the spleens of CD4−/− mice beyond what occurred in WT mice. This suggests that GR-1+ macrophage expansion is not controlled by CD4 T cells. This conclusion is also supported by the observation that LPS-induced TNFα expression in GR-1+ macrophages was not significantly different between WT and CD4−/− burn mice as judged by both in vivo and in vitro experimental approaches. However, when we examined the effects of CD4 T cells on TNFα production, we observed significantly higher levels of TNFα in the supernatants from LPS-stimulated spleen cells that were prepared from burn-injured CD4−/− mice as compared to WT mice. We also found that CD4 T cell depleted spleen cells prepared from burn-injured mice showed higher levels of TNFα in supernatants than total spleen cells from burn mice. These were mixed splenocyte cultures so it is possible that other cells might influence LPS-induced TNFα production. However, these data do demonstrate that the absence of CD4 T cells in vivo or in vitro can markedly influence LPS-induced TNFα production and further confirm that CD4 T cells control the inflammatory response to burn injury (7, 8). Importantly, these TNFα production findings contradict the observation that CD4 T cells do not appear to affect TNFα expression by LPS-stimulated GR-1+ macrophages. Thus, we speculate that CD4 T cells may influence TNFα production, release, or extracellular presence by mechanisms that are downstream of LPS-induced TNFα induction and expression. These may include blocking TNFα secretion by soluble or cell contact mechanisms, degrading released TNFα, or CD4 T cell dependent absorption of TNFα by TNF receptors or binding proteins. Some examples of these potential mechanisms include IL-10 or TGFβ production by Tregs or Th2-type CD4 T cells (31), cell-contact inhibition of TNFα release by Tregs, degradation or binding of TNFα by unknown factors produced by CD4 T cells, or sequestration of TNFα by TNFR2, which is a decoy TNF receptor that is expressed on CD4 Tregs, and can also be produced as a soluble receptor (32). These possible mechanisms are currently being tested in our laboratory. To our knowledge, this report provides new data to suggest a disconnect between LPS-induced TNFα expression by macrophages and extracellular TNFα.
Specific in vivo depletion of GR-1+ F4/80+ macrophages remains a challenge since there are currently no specific reagents available to selectively identify and deplete these cells. Numerous studies have been conducted using the GR-1 antibody clone RB6-8C5 to deplete GR-1+ macrophages and neutrophils in a variety of conditions, however it has been well described that GR-1 (Ly6C and Ly6G) is expressed on other immune cell types including neutrophils, dendritic cells, CD8 T cells and memory cells (1, 14, 17, 33). Nevertheless, we attempted to use the anti-GR-1 antibody clone RB6-8C5, which binds to Ly6C and Ly6G, to deplete burn-injured mice of GR-1+ macrophages. We found that while it had effectively depleted F4/80+GR-1+ macrophages, it’s use in in vivo studies was confounded by the fact that it depleted mature granulocytes, showed signs of toxicity, and caused high mortality in LPS-treated sham mice (data not shown). We also tested whether another anti-GR-1 antibody clone that recognizes Ly6G (clone 1A8) could effectively deplete burn-injured mice of GR-1+ macrophages. We found that we were able the deplete mice of GR-1+ cells in the bone marrow, blood, and spleen by 3 days after antibody treatment (10 mg/kg). Although this approach has limitations with regard to specifically depleting GR-1+ macrophages, we believe that this method provided us the best available method for testing the impact of GR-1+ cell depletion on injury-induced two-hit response mortality to LPS challenge. We found that depleting burn-injured mice of GR-1+ cells did not significantly alter the two-hit LPS response mortality (data not shown). Moreover, it was recently reported that the 1A8 antibody can stimulate a STAT1 signaling response, which further confounds accurate interpretation of results generated using the 1A8 antibody to deplete GR-1+ cells from mice (34). However, another report that was recently published by Noel et. al. used an anti-proliferative drug called gemcitabine, a ribonucleotide reductase inhibitor that kills proliferating cells, to test whether the burn-induced increase in GR-1+ myeloid cells was harmful or protective in a mouse burn injury model (35). They showed that eliminating these inflammatory cells by drug treatment reduced the inflammatory behavior of spleen cells from burn mice and protected burn mice from LPS-induced mortality. They also showed that gemcitabine-treated mice were not able to control Pseudomonas aeruginosa infection as well as untreated burn mice. Therefore, although their experimental approach had the same limitations with regards to specifically depleting GR-1+ macrophages, their findings support our original hypothesis that GR-1+ macrophages may be central mediators of the two-hit inflammatory response. It remains to be determined if GR-1+ macrophages serve a beneficial or detrimental function following severe injury.
In summary, the findings presented here show that burn injury induces a time-dependent phenotypic change in macrophages. We report that burn injury increases the prevalence of GR-1+ macrophages in the spleens of burn mice by day 7 and that GR-1+ macrophages appear to be the primary cell source for LPS/TLR4-induced TNFα. We confirmed their enhanced reactivity to LPS stimulation by both in vitro and in vivo approaches. Because of our interest in better understanding the control of the injury response by CD4 T cells, we tested the impact of CD4 T cell deficiency on the activation and expansion of GR-1+ macrophages and showed that CD4 T cells had no detectable influence on GR-1+ macrophage expansion or activation. However, CD4 T cells did influence LPS-induced TNFα production by spleen cells prepared from burn-injured WT versus CD4−/− mice. Thus, CD4 T cells may control TNFα production by a mechanism that is independent of cellular TNFα expression by innate immune cells. We believe that the future development of specific cell-surface markers for identifying and depleting injury-induced GR-1+ macrophages will clearly help us better understand the biological functions that may be mediated by this unique injury-reactive immune cell subset.
Acknowledgments
This study was supported by grant funding from the National Institutes of Health 5R01GM035633-22 and 2RO1GM57664-09.
Abbreviations
- SIRS
systemic inflammatory response syndrome
- TBSA
total body surface area
- WT
wild type
- MSCs
myeloid suppressor cells
- TLR
Toll-like receptor
- Tregs
CD4+ regulatory T cells
- GR-1
granulocyte receptor 1
- TNF
tumor necrosis factor
- FACS
fluorescence-activated cell sorting
- LPS
lipopolysaccharide
- i.p
intraperitoneal
Footnotes
Potential conflict of interest: Nothing to report
References
- 1.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11(3):153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Zedler S, Faist E. The impact of endogenous triggers on trauma-associated inflammation. Curr Opin Crit Care. 2006;12(6):595–601. doi: 10.1097/MCC.0b013e3280106806. [DOI] [PubMed] [Google Scholar]
- 3.Maung AA, Fujimi S, MacConmara MP, Tajima G, McKenna AM, Delisle AJ, Stallwood C, Onderdonk AB, Mannick JA, Lederer JA. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J Immunol. 2008;180(4):2450–2458. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–490. doi: 10.1097/00000658-199522240-00006. discussion 490-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176(1):225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 6.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244(4):514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20(2):123–129. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 8.Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174(5):2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 9.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 10.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171(3):1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 11.Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78(2):565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 12.Noel JG, Guo X, Wells-Byrum D, Schwemberger S, Caldwell CC, Ogle CK. Effect of thermal injury on splenic myelopoiesis. Shock. 2005;23(2):115–122. doi: 10.1097/01.shk.0000154239.00887.18. [DOI] [PubMed] [Google Scholar]
- 13.Moore FA, Moore EE, Read RA. Postinjury multiple organ failure: role of extrathoracic injury and sepsis in adult respiratory distress syndrome. New Horiz. 1993;1(4):538–549. [PubMed] [Google Scholar]
- 14.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86(5):398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 15.Angulo I, de las Heras FG, Garcia-Bustos JF, Gargallo D, Munoz-Fernandez MA, Fresno M. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000;95(1):212–220. [PubMed] [Google Scholar]
- 16.Biermann H, Pietz B, Dreier R, Schmid KW, Sorg C, Sunderkotter C. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J Leukoc Biol. 1999;65(2):217–231. doi: 10.1002/jlb.65.2.217. [DOI] [PubMed] [Google Scholar]
- 17.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 18.Voisin MB, Buzoni-Gatel D, Bout D, Velge-Roussel F. Both expansion of regulatory GR-1+ CD11b+ myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect Immun. 2004;72(9):5487–5492. doi: 10.1128/IAI.72.9.5487-5492.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 20.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165(2):618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 22.Noel JG, Osterburg A, Wang Q, Guo X, Byrum D, Schwemberger S, Goetzman H, Caldwell CC, Ogle CK. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock. 2007;28(6):684–693. doi: 10.1097/shk.0b013e31805362ed. [DOI] [PubMed] [Google Scholar]
- 23.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24(7):1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75(2):257–277. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Yoshida T, Takeuchi D, Jones VC, Shigematsu K, Herndon DN, Suzuki F. Gr-1(+)CD11b(+) cells as an accelerator of sepsis stemming from Pseudomonas aeruginosa wound infection in thermally injured mice. J Leukoc Biol. 2008;83(6):1354–1362. doi: 10.1189/jlb.0807541. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181(5):3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 27.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 28.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176(1):284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 29.Ogle CK, Valente JF, Guo X, Li BG, Ogle JD, Alexander JW. Thermal injury induces the development of inflammatory macrophages from nonadherent bone marrow cells. Inflammation. 1997;21(6):569–582. doi: 10.1023/a:1027377904641. [DOI] [PubMed] [Google Scholar]
- 30.Noel JG, Valente JF, Ogle JD, Cornelius J, Custer DA, Li BG, Alexander JW, Ogle CK. Changes in bone marrow-derived myeloid cells from thermally injured rats reflect changes in the progenitor cell population. J Burn Care Rehabil. 2002;23(2):75–86. doi: 10.1097/00004630-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Zissel G, Schlaak J, Schlaak M, Muller-Quernheim J. Regulation of cytokine release by alveolar macrophages treated with interleukin-4, interleukin-10, or transforming growth factor beta. Eur Cytokine Netw. 1996;7(1):59–66. [PubMed] [Google Scholar]
- 32.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180(10):6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortega N, Caro MR, Buendia AJ, Gallego MC, Del Rio L, Martinez CM, Nicolas L, Cuello F, Salinas J. Role of polymorphonuclear neutrophils (PMNs) and NK cells in the protection conferred by different vaccines against Chlamydophila abortus infection. Res Vet Sci. 2007;82(3):314–322. doi: 10.1016/j.rvsc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur J Immunol. 2009;39(12):3538–3551. doi: 10.1002/eji.200939530. [DOI] [PubMed] [Google Scholar]
- 35.Noel G, Wang Q, Osterburg A, Schwemberger S, James L, Haar L, Giacalone N, Thomas I, Ogle C. A Ribonucleotide Reductase Inhibitor Reverses Burn Induced Inflammatory Defects. Shock. doi: 10.1097/SHK.0b013e3181e14f78. [DOI] [PubMed] [Google Scholar]