Abstract
Proton magnetic resonance spectroscopy (1H MRS) has emerged as one of the most informative neuroimaging modalities for studying the effect of HIV infection in the brain, providing surrogate markers by which to assess disease progression and monitor treatment. Reductions in the level of N-Acetylaspartate (NAA) and NAA/creatine (NAA/Cr) are established markers of neuronal injury or loss. However, the biochemical basis of altered creatine levels in neuroAIDS is not well understood. This study used a rapid progression macaque model of neuroAIDS to elucidate the changes in creatine. As the disease progressed 1H MRS revealed a decrease in NAA, indicative of neuronal injury, and an increase in creatine yet to be elucidated. Subsequently, immunohistochemistry and stereology measures of decreased synaptophysin, microtubule-associated protein 2, and neuronal density confirmed neuronal injury. Furthermore, increases in ionized calcium binding adaptor molecule 1 and glial fibrillary acidic protein indicated microglial and astroglial activation, respectively. Given these data, elevated creatine may reflect enhanced high-energy phosphate turnover in highly metabolizing activated astrocytes and microglia.
Keywords: HIV, SIV, Macaques, MR Spectroscopy, Creatine, Gliosis
Introduction
Early in the AIDS epidemic, neurological symptoms produced by HIV (known as neuroAIDS) were recognized as an important clinical manifestation of the disease (1). With the advent of the antiretroviral therapy (ART) era, the incidence of HIV-associated dementia (HAD) decreased markedly. However, less severe manifestations of the disease persist in the infected population (2), and it is projected that as the virus gains resistance to ART, the incidence of severe HIV-associated neurodegeneration will again increase (3,4).
HIV enters the CNS during the early stages of infection (5), primarily through virally infected or activated monocytes (6,7). Macrophages and microglia are considered to play a key role in the pathogenesis of neuroAIDS, as the primary targets of productive infection in the brain. Once in the brain, infected macrophages or microglia emit viral proteins, cytokines, and chemokines, which activate macrophages and microglia, releasing neurotoxic substances that in turn induce neuronal injury and apoptosis (8,9). Although generally more resistant to HIV-1 infection than microglia, astrocytes have been found to express SIV nef during acute as well as terminal infection (10). Furthermore, macrophages and astrocytes have mutual feedback loops, by which cytokines stimulate astrocytosis.
In vivo proton magnetic resonance spectroscopy (1H MRS) has emerged as one of the most informative neuroimaging modalities for studying neuroAIDS, providing surrogate markers to assess disease progression and monitor therapeutic treatment. Neurochemical changes detectable by MRS include a decline in N-acetylaspartate (NAA) or NAA/Creatine (NAA/Cr), both established markers of neuronal injury (11,12) or loss (13,14).
The creatine (Cr) resonance observed by 1H MRS reflects the steady state levels of both brain creatine and phosphocreatine. Increased Cr concentrations in the frontal white matter and decreased Cr levels in the basal ganglia have been reported in chronic antiretroviral-naïve HIV patients in later stages of HAD (15). Increased levels of creatine in the white matter have also been observed in the SIV model of neuroAIDS during the acute phase of infection (16), as well as at later stages during chronic SIV infection (17). We hypothesize that in neuroAIDS, Cr may be an excellent noninvasive marker by which to monitor altered energy metabolism that may be associated with glial activation and inflammatory processes induced by viral infection of the brain. To examine this hypothesis, 1H MRS at 3 Tesla was used to study an accelerated nonhuman primate model of neuroAIDS.
Methods
Non-human Primates
Twelve 4- to 5-year-old male rhesus macaques (Macaca mulatta) were included in this study. The animals were housed according to standards set forth by the American Association for Accreditation of Laboratory Animal Care. Investigators adhered to the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The study was approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and by the Institutional Animal Care and Use Committee of Harvard University.
Eight of the twelve animals were inoculated with SIVmac251 virus (10 ng SIVp27, i.v.) and their CD8+ lymphocytes were depleted with antibody targeted against CD8 (cM-T807) at 6, 8 and 12 days post-inoculation (dpi) (18).
The eight SIV-infected animals were scanned two times preinoculation, and biweekly until sacrifice 4 weeks or 8 weeks post-inoculation (wpi); 4 animals were sacrificed at each time point. During MR imaging sessions, each animal was tranquilized with 15–20 mg/kg intramuscular ketamine hydrochloride and intubated to ensure a patent airway during the experiment. Intravenous injection of 0.4 mg/kg atropine was administered to prevent bradycardia, and continuous infusion of approximately 0.25 mg/kg/min propofol was maintained throughout the experiment, via catheter in a saphenous vein. Heart rate, oxygen saturation, end-tidal CO2 and respiratory rate were monitored continuously. A heated water blanket was used to prevent hypothermia.
For postmortem examination, a control cohort of four age-matched uninfected, CD8 T-lymphocyte-depleted animals was included. These animals were depleted of CD8 by the same three-dose regimen of anti-CD8 antibody injection, but were not inoculated with SIV. Animals were sacrificed 8 weeks post depletion. Data from these controls were compared to those from the infected animals sacrificed at 4 and 8 wpi.
MRI and MRS
All MRI/MRS experiments were performed on a 3 Tesla whole-body imager (Magnetom TIM Trio, Siemens AG, Erlangen, Germany), using a circularly polarized transmit-receive extremity coil. First, a three-plane localizer scan was acquired in order to position the monkey in the coil; using this approach, voxel placement is highly reproducible. To guide placement of the 1H-MRS volumes of interest (VOI), sagittal and axial turbo spin echo (TSE) images were obtained using the following parameters: 140×140 mm2 field of view (FOV), 512×512 matrix, TE of 16 ms; slice thickness was 2 mm for sagittal images and 1.2 mm for axial images; TR was 4500 ms for sagittal and 7430 ms for axial images resulting in an acquisition time of 3 minutes for the sagittal and 5 minutes for the axial TSE sequence.
Single voxel 1H MR spectroscopy was performed in the parietal cortex (PC), frontal cortex (FC), basal ganglia (BG) and white matter semiovale (WM) using a point-resolved spectroscopy sequence (PRESS) with WET water suppression, and the following parameters: TE = 30 ms, TR = 2500 ms, and 192 acquisitions resulting in an acquisition time of 8 minutes for each spectrum. All spectra were processed offline using the LCModel software package (19) to determine the quantities of the brain metabolites N-acetylaspartate and N-acetylaspartylglutamate (collectively referred to as NAA), creatine-containing compounds (referred to as Cr), choline-containing compounds (referred to as Cho) and myo-Inositol (MI). Absolute metabolite concentrations in institutional units were derived from the same voxel using localized water signals (without water suppression) obtained under fully relaxed conditions (TR=10 s).
Viral Load Analysis
Plasma and cerebral spinal fluid SIV RNA was quantified using real-time reverse transcription-PCR, as previously described (20). Blood was centrifuged at 20,000 g for 1 hour, and CSF samples were stored at −80 °C until study endpoint. The threshold sensitivity was 100 copy eq./mL. Results are averages of duplicate determinations.
Flow Cytometry
CD8+ lymphocyte depletion was monitored by flow cytometry prior to antibody treatment and weekly post-inoculation in the SIV-infected animals. Flow cytometric analyses were performed with 100 μl aliquots of blood incubated with fluorochrome-conjugated antibodies including anti–CD3-APC (clone FN18; BioSource International), anti–CD4-FITC (OKT4; Ortho Diagnostic Systems), anti–CD8-PE (DK25; DakoCytomation), and anti–CD20–PE–Texas Red (B1; Beckman Coulter), using a FACSCalibur flow cytometer (BD). The absolute number of CD8+ lymphocytes was determined by multiplying the percentage of CD8+/CD3+ cells by absolute lymphocyte counts obtained using a standard veterinary 3-point WBC differential, CBC Hematology Analyzer (Hema-True, HESKA).
Neuropathology
On the day of sacrifice, all animals were anesthetized with ketamine-HCl, sacrificed by intravenous pentobarbital overdose and exsanguinated. CNS tissues were collected in 10% neutral buffered formalin, embedded in paraffin and sectioned at 5 μm for routine histology and quantitative neuropathology examination. All brain slides were stained with hematoxylin and eosin (H&E) and evaluated by a neuropathologist (SVW).
Microglial activation was assessed by quantifying calcium binding adaptor protein 1 (IBA-1). For this purpose, frontal and parietal cortex brain sections of the four SIV+/CD8− animals sacrificed at 8 wpi and the four uninfected CD8-depleted control animals were stained with rabbit anti-IBA-1 (Wako Corp. Japan). Images of tissue sections were captured without manipulation using an Olympus 3-CCD T60C color video camera mounted on an Olympus Vanox-SI microscope, and analyzed using NIH Image J software (http://rsbweb.nih.gov/ij/).
The degree of reactive astrogliosis was assessed with monoclonal anti-glial fibrillary acidic protein (1:1000; Boehringer Mannheim, Indianapolis, IN). Five-μm-thick paraffin sections from the frontal cortex and parietal cortex were immunolabeled overnight with these monoclonal antibodies, followed by biotinylated horse anti-mouse immunoglobulin G and avidin-horseradish peroxidase (Vectastain Elite kit; Vector, Burlingame, CA), and reacted with diaminobenzidine tetrahydrochloride and peroxide (0.03%). Integrity of the synapses was evaluated with the monoclonal antibody against synaptophysin (SYN) (1:10) (Boehringer Mannheim, Indianapolis, IN) and the status of neuronal dendrites with monoclonal antibody against microtubule-associated protein 2 (MAP2) (Boehringer Mannheim).
Levels of GFAP, synaptophysin and MAP2 were estimated by computer-aided image analysis, as previously described (21). Immunoreactivity was semiquantitatively assessed as corrected optical density by using a microdensitometer (Quantimet 570C; Leica, Microsystem Cambridge, UK). For this purpose, three immunolabeled sections were analyzed from each animal, e.g. the SIV+/CD8− (4wpi), the SIV+/CD8− (8wpi) and the uninfected CD8-depleted controls. As previously described (11,21,22), the system was first calibrated with a set of filters of various densities, and 10 images for each section at ×100 magnification were obtained. After delineating the area of interest (layers 2–5) with the cursor, the optical density within that area was obtained. The optical density was then averaged in each image and expressed as the mean per case. All measurements for SYN, MAP2 and GFAP are in arbitrary optical density units, and range from 0 to 500 (i.e., 0 indicates all light is allowed to pass through the sample, while 500 indicates no light is allowed to pass through the sample. All values are expressed as mean ± standard error of the mean.
Cortical neuronal populations were determined by stereology, as previously described (23,24). Blind-coded vibratome sections were immunolabeled with antibodies against calbindin (CB, mouse monoclonal, Sigma) (25), and free-floating sections were incubated with biotinylated horse anti-mouse IgG followed by Avidin D-HRP (ABC Elite, Vector), reacted with DAB and counter-stained with cresyl violet. Anti-CB immunostained cells were analyzed by optical dissector, and cells were sampled using an Olympus BH2 microscope (Olympus America Inc., Center Valley, PA) attached to a DataCell computer-assisted image analysis system (Image-Pro Plus) for stereology. From each case, at least three random sections from each brain region were analyzed.
Five to eight regions within the H&E section of frontal cortex were examined and scored by a neuropathologist at 10 × magnification using the following criteria for satellitosis, neurodegeneration and gliosis:
Satellitosis scoring: Neurons surrounded by 3 or more glial cells are characterized as mild (occasionally occurring), moderate (frequently) and severe (numerous, in addition to clusters of 3 or more glial cells at sites of neuronal drop-out).
Neurodegeneration scoring: Mild - occasional angular, shrunken neurons; Moderate -frequent angular, shrunken neurons; Severe - numerous angular, shrunken neurons and neuronal drop-out.
Gliosis scoring: Mild - increased cellularity in white matter in one brain region; Moderate - increased cellularity in white matter in two or three regions plus occasional microglial nodules; Severe - increased cellularity in white matter in all regions with frequent microglial nodules.
Statistical Methods
All analyses were conducted using JMP 7.0 (SAS, Cary, NC). For the serial in vivo MR spectroscopy data, analysis of variance with repeated measures (RM-ANOVA) was used to detect temporal metabolic changes during disease progression. If significant by RM-ANOVA (p < 0.05), Holm’s t-test was used, which corrects for multiple comparisons, to isolate significant differences between time points. For the postmortem measures ANOVA was performed between the cohorts; if ANOVA was significant, least square means Student’s t-tests were used to isolate the cohort that was significantly different. To assess potential correlations between immunohistochemical findings and metabolic markers, Spearman Rank correlation coefficients were used.
Results
Following SIV inoculation and CD8+ lymphocyte depletion, the amount of virus in the peripheral blood plasma increased rapidly, reaching levels greater than 107 copy eq./mL by 12 dpi (p < 0.001). The plasma viral load continued to increase to a maximum of 4.9 × 108 copy eq./mL. CSF virus levels increased significantly to a mean level of 105 copy eq./mL by 12 dpi (p < 0.001). Flow cytometry revealed that all SIV+/CD8− animals in this study were persistently CD8 T lymphocyte-depleted (> 28 dpi). Table 1 summarizes the clinical CNS pathological findings of the eight SIV+/CD8− animals sacrificed at 4 and 8 wpi as well as the four CD8-depleted uninfected controls. Non-infected animals that were CD8-depleted for 8 weeks did not show any significant pathological findings. The effect of CD8 depletion without SIV infection on brain metabolism and neuropathology has been described elsewhere (26). Infected animals sacrificed at 4 wpi exhibited minor neuronal CNS pathological findings including mild gliosis and minimal perivascular infiltrates. Animals sacrificed at 8 wpi exhibited signs of gliosis, cortical neuronal degeneration, and satellitosis. Three animals had perivascular infiltrates and one had multinucleated giant cells (MNGC) indicative of SIV encephalitis (SIVE).
Table 1.
Clinical Findings of CNS pathology
| Animal | CD8 depleted | SIV infected | Survival (WPI) | CNS Pathology |
|---|---|---|---|---|
| M4606 | Y | N | NA | NSF |
| M4706 | Y | N | NA | NSF |
| M9706 | Y | N | NA | NSF |
| M9506 | Y | N | NA | NSF |
| M4007 | Y | Y | 4 | No satellitosis and neurodegeneration; Mild gliosis; minimal perivascular infiltrates |
| M4107 | Y | Y | 4 | NSF |
| M4207 | Y | Y | 4 | No satellitosis and neurodegeneration; Mild gliosis; minimal perivascular infiltrates |
| M4307 | Y | Y | 4 | No satellitosis and neurodegeneration; Mild gliosis; minimal perivascular infiltrates |
| M5207 | Y | Y | 8 | Severe satellitosis and neurodegeneration; Moderate gliosis; Perivascular infiltrates in one brain region |
| M5407 | Y | Y | 8 | Moderate satellitosis and neurodegeneration; Moderate gliosis; Perivascular infiltrates in one brain region |
| M7207 | Y | Y | 8 | Moderate satellitosis and neurodegeneration; Mild gliosis; No Perivascular infiltrates |
| M1308 | Y | Y | 8 | Severe satellitosis and neurodegeneration; Mild gliosis; Perivascular infiltrates in two or more brain region plus rare MNGCs. |
WPI: week post-inoculation, NSF: no significant findings, MNGC: multinucleated giant cells
In vivo Magnetic Resonance Spectroscopy
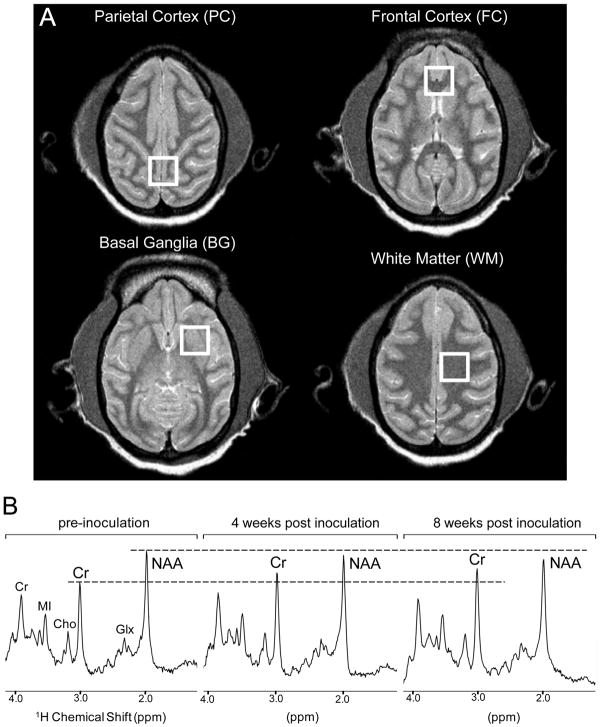
In vivo 1H MR spectra were acquired in the PC, FC, BG and WM. Figure 1 shows the voxel locations (Figure 1A) and representative spectra acquired prior to infection, at 4 wpi and at 8 wpi (Figure 1B). It can be seen that the NAA resonance decreases and creatine resonance increases throughout disease progression. The normalized changes in the mean concentrations of NAA/Cr with respect to time after SIV infection and CD8 depletion in each brain region are displayed in Figure 2. SIV infection resulted in a rapid decline in NAA/Cr levels in all four brain regions measured. Analysis of the MR spectra acquired from all 8 SIV+/CD8− animals revealed that decreases in NAA/Cr in the PC, FC, and WM were significantly different from baseline at 4 wpi. The four SIV+/CD8− animals studied until 8 wpi exhibited a 16% decrease in this ratio in the PC over the 8 weeks following inoculation, as well as decreases of 19% in the FC, 13% in the BG and 17% in the WM. The declines in NAA/Cr were significant at both 6 and 8 wpi when compared to pre-infection scans. It is important to note that each animal serves as its own control; pre-infection metabolite levels are measured and serve as a baseline for individual animals. Specific details regarding MRS of uninfected, CD8-depleted controls will be published elsewhere.
Figure 1.
Representative 1H MR spectroscopy voxel locations from the parietal cortex (PC), frontal cortex (FC), basal ganglia (BG) and white matter semiovale (WM) using a PRESS sequence (A). MR spectra obtained from one representative SIV-infected CD8-depleted animal at the following time points: before SIV inoculation, 4 weeks post-inoculation (wpi) and at 8 wpi (B). It can be seen that the NAA resonance decreases and creatine resonance increases with disease progression.
Figure 2.
Normalized %-changes from baseline of NAA/Cr mean concentrations of in all four brain regions analyzed during SIV infection. Analyzing NAA/Cr from all 8 animals up to 4 wpi revealed significant decreases in the PC (p = 0.019), FC (p < 0.050) and WM (p = 0.024). Using RM-ANOVA for the four animals studied until 8 wpi, changes in NAA/Cr are significant (PC, FC and WM p < 0.001, BG p=0.004), and Holm’s t-tests revealed significant differences at both 6 and 8 wpi when compared to pre-infection scans for all regions.
In an attempt to identify whether changes in NAA and/or Cr are responsible for the changes observed in the ratio, NAA and Cr concentrations were estimated using tissue water as the internal standard. Metabolite-water ratios have much higher variance than metabolite ratios, due to the greater than four-order-of-magnitude difference between metabolite and water concentrations. Consequently, obtaining statistical significance for comparisons of metabolite-water concentrations is more difficult than for those involving metabolite ratios.
In the four SIV+/CD8− animals studied until 8 wpi, the mean NAA concentrations decreased relative to baseline measurements in the FC, BG and WM; the differences were statistically significant in the WM (PC −4% p = 0.68, FC −11% p = 0.068, BG −8% p = 0.16, WM −9% p = 0.03). When analyzing all 8 animals studied until 4 wpi, NAA changes were significantly different from baseline in the BG and WM at 2 wpi, although not at 4 wpi. The mean brain concentrations of Cr in the four SIV+/CD8− animals studied until 8 wpi were elevated in all four regions, and statistical significance was found in the parietal cortex and white matter (PC +14% p=0.01, FC +11% p = 0.43, BG + 6% p=0.07 and WM +9% p=0.01). Figure 3 illustrates the changes in NAA and Cr in the individual brain regions.
Figure 3.
NAA and total creatine (i.e. phosphocreatine and creatine) concentrations over 8 wpi in all four brain regions. Analyzing the four animals studied until 8 wpi, WM NAA showed a significant decrease by RM-ANOVA (p = 0.03). Evaluating all eight animals until 4 wpi using RM-ANOVA revealed significant decreases in the BG and WM (p < 0.048 and p = 0.024, respectively).
In the four animals studied until 8 wpi, creatine in the PC and WM increased significantly (RM-ANOVA p = 0.01 and p = 0.013, respectively).
Both choline and myo-Inositol concentrations show complex variations over time post infection (Figure 4). Significant changes in choline (Cho) were observed in all eight animals during the first four weeks of SIV infection (RM-ANOVA p < 0.001 for PC, FC, WM and RM-ANOVA p < 0.025 for BG). Subsequent Holm’s t-tests in the parietal and frontal cortex indicated large elevations (20 – 30%) from pre-infection levels at 2 wpi (p < 0.001) and large reductions between 2 wpi and 4 wpi (p < 0.001 and p = 0.002, for PC and FC respectively). In the BG and WM elevations in Cho at 2 weeks were generally smaller (7 – 8%, p = 0.039 and p = 0.05, respectively) but also showed decreases between two weeks and four weeks (p = 0.01 and p < 0.001, respectively). Reductions between pre-infection and 4 wpi were significant only in the WM (p=0.016). With further disease progression, Cho increases above baseline levels in the PC (p = 0.003), FC (p = 0.045) and WM (p = 0.01) at 8 wpi.
Figure 4.
Normalized mean concentrations of choline (Cho) and myo-Inositol (MI) in all four brain regions analyzed during SIV infection. RM ANOVA showed significant changes in Cho in all four regions during the first four weeks of infection (p < 0.001 for PC, FC, WM and RM-ANOVA p < 0.025 for BG), as Cho levels increase at 2 wpi before returning to near-baseline levels after 4 wpi. With further disease progression, Cho increases once more above baseline levels in the PC (p = 0.003), FC (p = 0.045) and WM (p = 0.01) at 8 wpi (A).
Myo-Inositol (MI) shows complex regional variations during SIV infection. RM ANOVA reveals significant changes in MI levels in all regions (RM-ANOVA p = 0.024 for PC, p < 0.001 for FC, p = 0.06 for BG and p = 0.002 for WM), as MI tends to be elevated during early infection (B).
Myo-Inositol (MI) levels show very complex regional variations during the first two months of SIV-infection and CD8-depletion (RM-ANOVA p = 0.024 for PC, p < 0.001 for FC, p = 0.06 for BG and p = 0.002 for WM) with increases at 2 weeks in the PC (p = 0.01), FC (p < 0.0001) and BG (p = 0.028) and increases at 4 weeks in the FC (p < 0.0001) and WM (p = 0.002). At 6 wpi MI is indistinguishable from baseline levels but at later time points (8 wpi) MI becomes elevated in the PC (p = 0.038) and FC (p = 0.02).
Postmortem Neuropathology
Neuronal injury was confirmed postmortem by stereology and quantitative immunohistochemistry (IHC) assessment of synaptic (synaptophysin, SYN) and dendritic (microtubule-associated protein 2, MAP2) integrity. Figure 5 depicts IHC and stereology data acquired in the frontal and parietal cortices of the four uninfected CD8-depleted control animals, four SIV+/CD8− animals sacrificed at 4 wpi, and four SIV+/CD8− animals sacrificed at 8 wpi. Levels of synaptophysin (SYN), an established marker of pre-synaptic neuronal integrity, were significantly different among the three cohorts in the FC (ANOVA, p = 0.0059) and PC (ANOVA p = 0.011). SYN levels in both the SIV+/CD8− groups were significantly lower compared to control animals when evaluated with least square means t-test.
Figure 5.
Quantification of neuronal markers SYN (A), MAP2 (B) and neuronal numbers (C) in the frontal and parietal cortex comparing the three cohorts (four uninfected CD8-depleted controls, four SIV+/CD8− macaques sacrificed at 4 wpi, and four SIV+/CD8− sacrificed 8 wpi). SYN levels were significantly lower in infected animals at 4 wpi (FC p = 0.0057; PC p = 0.0099) and 8 wpi (FC p = 0.0034; PC p = 0.0073) compared to control animals when evaluated with least-means squared t-tests. MAP-2 showed significant changes in the frontal cortex and parietal cortex, decreasing in infected animals by 4 wpi (FC p = 0.059; PC p = 0.0073) and 8 wpi (FC p = 0.004; PC p = 0.029) compared to control animals. Parietal cortex neuronal counts were significantly lowers at both 4 wpi (p = 0.01) and 8 wpi (p = 0.015).
In the same animals MAP-2, a marker for dendritic neuronal integrity, was also evaluated. Frontal cortex MAP2 (ANOVA, p = 0.022) was found to be significantly lower in the SIV+/CD8− animals sacrificed at 8 wpi, and showed a trend toward decreased levels 4 wpi. In the PC, neuronal density was significant different among the three cohorts (ANOVA, p = 0.017), with lower levels at both 4 wpi and 8 wpi. While neuronal counts measured in the frontal cortex were also lower in infected animals compared to controls, these differences were not statistically significant (ANOVA p = 0.1).
Glial fibrillary acidic protein (GFAP), a pathologic marker of astrogliosis, was quantified in the same animals (FC ANOVA, p = 0.08; PC ANOVA p = 0.016); levels were higher in both groups of SIV+/CD8− animals than in control animals (Figure 6A). Microglial activation was assessed using calcium binding adaptor protein, IBA-1. IBA-1 is expressed by resting microglia and is upregulated when the cells are activated. Compared to the uninfected control cohort, the SIV+/CD8− animals sacrificed at 8 wpi exhibited widespread microglial activation accompanied by intense IBA-1 staining in the frontal cortex of (p = 0.0021, Figure 6B). Computer-aided image analysis of IBA-1 was not performed for the four animals sacrificed at 4 wpi.
Figure 6.
Quantification of astrogliosis glial fibrillary acidic protein, GFAP (A) and microglial activation by ionized calcium binding adaptor molecule 1, IBA1 (B) in the frontal and parietal cortex comparing four uninfected CD8-depleted controls, (four SIV+/CD8− macaques sacrificed at 4 wpi for GFAP only) and four SIV+/CD8− sacrificed 8 wpi. GFAP levels were elevated in infected animals compared to controls in the frontal cortex at 8 weeks post-inoculation (p = 0.04) and in the parietal cortex at 4 and 8 wpi (p = 0.024 and p = 0.007), respectively. Widespread microglial activation accompanied by intense staining of IBA-1 was observed in the frontal cortex from the SIV+/CD8− animals 8 weeks after SIV infection compared to the uninfected control cohort (p = 0.0021). IHC stains from the frontal cortex revealed higher IBA-1 expression in the SIV+/CD8− animals sacrificed at 8 wpi compared to the uninfected control cohort (p = 0.0021) suggesting increased microgliosis.
Correlations between Immunohistochemistry (IHC) and MRS metabolic markers
We also investigated potential correlations between immunohistochemical markers GFAP and IBA-1 and the changes in creatine between the animals’ baseline scan and last scan prior to necropsy using Spearman Rank analysis. Due to the small sample size of only 8 points for GFAP vs. creatine-changes (parietal cortex Rs = 0.29, p = 0.55, frontal cortex Rs = 0.35, p = 0.39) and 4 points for IBA-1 vs. creatine changes (parietal cortex Rs = 0.20, p = 0.80, frontal cortex Rs = 0.40, p = 0.20) no significant associations were found between these markers.
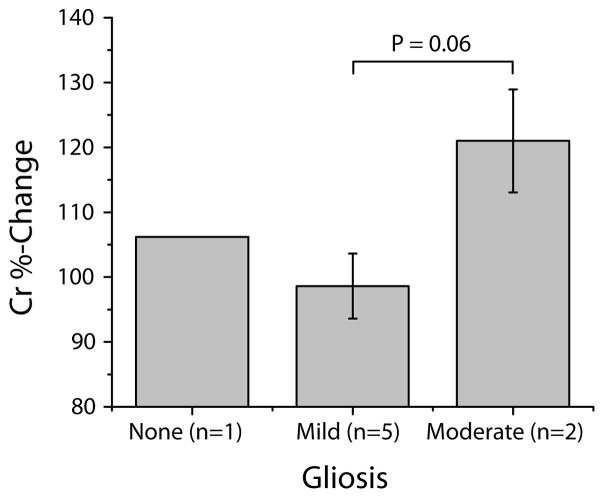
Furthermore, we correlated the creatine %-change with the gliosis rankings provided by a neuropathologist (Table 1). Five animals had mild gliosis and two had moderate gliosis. One animal had no gliosis and was eliminated from the statistical analyses. A Student’s t-test revealed higher creatine levels in animals with moderate compared to mild gliosis (p = 0.06. Figure 7, new). Based on the small sample size, however, we consider these results highly preliminary.
Figure 7.
Histopathological findings showing gliosis ranking (moderate, mild or none) and corresponding creatine levels for the eight animals included in the study. Average creatine levels were significantly higher in the two animals that exhibited moderate gliosis than in animals with mild gliosis (n=5). One animal did not exhibit gliosis and was excluded from statistical analysis.
Discussion
To determine whether the creatine resonance may be used as an energy marker for enhanced glial activation and inflammatory processes, 1H MRS was used to examine an accelerated nonhuman primate model of neuroAIDS. The SIV-infected, CD8+ lymphocyte-depleted (SIV+/CD8−) macaque model produces brain encephalitis that mimics characteristics of HIV encephalitis including accumulation of viral-laden perivascular macrophages and multinucleated giant cells (MNGCs), astrogliosis, microgliosis and neuronal injury (18,27). SIV infection and CD8 depletion in these animals resulted in CNS injury manifested by neuronal degeneration, astrogliosis and microgliosis. These findings were more severe in animals examined postmortem 8 weeks post-inoculation versus animals examined 4 weeks post-inoculation. In addition, quantitative immunohistochemistry of cortical tissue showed evidence of pre- and post-synaptic neuronal damage, as evidenced by significant decreases in synaptophysin and microtubule-associated protein 2 as well as neuronal counts at 4 and 8 wpi. GFAP and IBA-1 were significantly elevated in animals sacrificed 8 weeks post-inoculation, indicating astrogliosis and microglial activation.
As previously reported, SIV infection and CD8 depletion resulted in a rapid decline in NAA/Cr ratios in all brain regions examined, indicating neuronal injury (18). In diseases with severe irreversible neuronal injury, such as Pick’s and Alzheimer’s disease (13,28), it has been found that neuronal density (as determined by stereology) correlates well with the concentration of NAA/Cr measured in neighboring tissue by high-resolution magic angle spinning 1H MRS. However, transient in vivo MRS changes discovered in acute SIV infection (29) led us to speculate that NAA/Cr could also be a marker of reversible neuronal injury (30–32). Furthermore, it was found that NAA/Cr correlated best with synaptophysin during early, reversible neuronal injury (11).
Changes in total Cr (i.e., phosphocreatine and creatine) are associated with altered energy metabolism. Elevated Cr levels have been reported in the frontal white matter and basal ganglia of chronic HIV patients (15,30), in the white matter during the acute phase of SIV infection (16), and also at later stages of chronic SIV (17). It is believed that the virus enters the brain through activated and infected monocytes that later differentiate into macrophages (7,9). During this process of monocytic cell infiltration and subsequent astrocytic and microglial activation and proliferation, high metabolic demand may explain the increase in Cr. At the same time, NAA decreases resulting from neuronal cell injury lead to a concomitant decline in the energy demand of neurons, and thus, a decline in Cr would be expected in these cells. However, in the MR spectrum, this change is overwhelmed by Cr elevations within the more metabolically active immune and glial cells that occupy the majority of the brain. An in vitro study of neural cell types demonstrated that the creatine content of glial cell is two to four times that of neurons (33).
The postulation of a creatine increase as a surrogate marker for inflammation or active gliosis is supported by the fact that elevated Cr levels are abated in HIV+ patients during treatment with antiretroviral therapy (30). Furthermore, when measuring NAA and Cr concentrations in a previously reported study of eight SIV+/CD8− animals - in which four had been treated with CART (34) - a significant increase in creatine in the frontal cortex of untreated animals 8/10 wpi (RM ANOVA p = 0.019) was found, while CART-treated animals did not reveal these changes (RM ANOVA p = 0.14) (unpublished data). In addition, our group has shown that SIV-infected animals treated with minocycline (an anti-inflammatory/neuroprotective antibiotic), exhibited higher NAA and lower Cr levels compared to the untreated control group (35). These findings suggest that Cr elevations were ameliorated by anti-retroviral and minocycline treatment. It is suspected that both treatments result in less trafficking of monocytes and accumulation of macrophages in the CNS (18,34). An increase in creatine has also been detected in other neurodegenerative diseases such as adrenoleukodystrophy (ALD). For instance, Eichler et al. have reported an increase in Cr levels at the edge of the demyelinating lesion in ALD, likely reflecting gliosis (36).
When creatine levels in the frontal cortex were compared to histopathological findings, a t-test revealed a trend toward higher creatine in animals with moderate compared to mild gliosis, underling our hypothesis of creatine as a surrogate marker for inflammation or active gliosis. Spearman Rank analyses revealed no significant correlations between GFAP and IBA-1 and the changes in creatine. The lack of significant correlation for these comparisons may be due to the small sample size. Including additional SIV-infected CD8-depleted animals [ten untreated and seven minocycline-treated, an anti-inflammatory/neuroprotective drug (35)], reveals a positive correlation between creatine and astroglial marker GFAP in the PC (Rs=0.65, p=0.005) but not in the FC (unpublished data). Further investigations are currently ongoing.
The major focus of this study was to reveal the increases in creatine due to viral infection. However, in addition to Cr and NAA, other metabolite concentrations such as choline and myo-Inositol were also obtained. Elevations of Cho and MI have been documented in a variety of neuroinflammatory and neurodegenerative diseases and are considered markers of ongoing central nervous system (CNS) inflammation and gliosis (22,27). Elevations in both markers have been reported in HIV+ subjects (37–39). In this study, we found a temporal increase in Cho at 2 wpi and a subsequent decrease to baseline and below at 4 wpi consistent with the acute phase of SIV infection (16,29). At later stages (8 wpi) Cho levels increase again suggesting a second inflammatory response.
MI is elevated at 2 and 4 wpi and decreases back to baseline levels at 6 wpi, again consistent with the non-accelerated macaque model of neuroAIDS (16,29,40). However, MI tends to increase again at 8 wpi in cortical tissue. The detailed findings will be presented in a separate manuscript.
In conclusion, decreases in NAA/Cr levels are consistent with increased neuronal injury. Decreases in NAA are directly related to neuronal distress; increases in Cr during disease progression may be related to increased energy demand due to astrocytosis and glial activation induced by the entrance of SIV-infected monocytes into the brain. This dual effect of decreased NAA and increased Cr make the NAA/Cr ratio a sensitive marker for brain disease status. A more thorough investigation of the relationships among metabolites, viral load levels, neuronal damage and gliosis is crucial for better understanding metabolic changes in this accelerated animal model of neuroAIDS.
Acknowledgments
We thank Drs. Joanne Morris and Elisabeth Moeller, Ms. Shannon Luboyeski and staff from the Center of Comparative Medicine at Massachusetts General Hospital, as well as Dr. Angela Carville from the New England Primate Research Center (NEPRC) for animal veterinary care. We also wish to thank Michael O’Connell for pathology support for this study, Dr. Ronald Desrosier for providing us the inoculum, SIVmac251, Drs. Mike Piatak and Jeffrey Lifson for viral load analyses (SAIC Frederick, Inc.), and Dr. Keith Reimann for the depleting anti-CD8 (human recombinant, cM-807) antibodies. Reagents used in these studies were provided by the NIH Nonhuman Primate Reagent Resource (R24 RR016001, N01 AI040101). Finally, we wish to thank Nichole Eusemann for editing this manuscript. This work was supported by NIH grants R21NS059331 (EMR), R01NS050041 (RGG), R01NS040237 (KW), R01NS37654 (KW), R01MH62962 (EM), HNRC MH59754 (EM), HNRC MH62512 (EM), NIH-NS051129 (MRL) and RR00168 (NEPRC Base Grant). The Massachusetts General Hospital Athinoula A. Martinos Center for Biomedical Imaging is also supported by the National Center for Research Resources, grant number P41RR14075.
References
- 1.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19(6):525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 2.Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. Int Rev Psychiatry. 2008;20(1):25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- 3.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157(1–2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8 (Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 5.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42(9):1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 6.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 7.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 8.Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74(5):691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- 9.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 10.Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, Clements JE. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol. 2003;77(12):6855–6866. doi: 10.1128/JVI.77.12.6855-6866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, He J, Sehgal PK, Halpern EF, Lackner AA, Masliah E, Gonzalez RG. Quantative neuropathological correlates of changes in macque brain NAA/Cr. Radiology. 2005;235(2):461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- 12.Menon DK, Ainsworth JG, Cox IJ, Coker RC, Sargentoni J, Coutts GA, Baudouin CJ, Kocsis AE, Harris JR. Proton MR spectroscopy of the brain in AIDS dementia complex. J Comput Assist Tomogr. 1992;16(4):538–542. doi: 10.1097/00004728-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Cheng LL, Newell K, Mallory AE, Hyman BT, Gonzalez RG. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging. 2002;20(7):527–533. doi: 10.1016/s0730-725x(02)00512-x. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhoff DJ, MacKay S, Bachman L, Poole N, Dillon WP, Weiner MW, Fein G. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993;43(3 Pt 1):509–515. doi: 10.1212/wnl.43.3_part_1.509. [DOI] [PubMed] [Google Scholar]
- 15.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17(3):1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- 16.Ratai EM, Pilkenton SJ, Greco JB, Lentz MR, Bombardier JP, Turk KW, He J, Joo CG, Lee V, Westmoreland S, Halpern E, Lackner AA, Gonzalez RG. In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci. 2009;10:63. doi: 10.1186/1471-2202-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lentz MR, Lee V, Westmoreland SV, Ratai EM, Halpern EF, Gonzalez RG. Factor analysis reveals differences in brain metabolism in macaques with SIV/AIDS and those with SIV-induced encephalitis. NMR Biomed. 2008;21(8):878–887. doi: 10.1002/nbm.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115(9):2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 20.Lifson JD, Rossio JL, Piatak M, Jr, Parks T, Li L, Kiser R, Coalter V, Fisher B, Flynn BM, Czajak S, Hirsch VM, Reimann KA, Schmitz JE, Ghrayeb J, Bischofberger N, Nowak MA, Desrosiers RC, Wodarz D. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. 2001;75(21):10187–10199. doi: 10.1128/JVI.75.21.10187-10199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32(3):321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- 22.Kim JP, Lentz MR, Westmoreland SV, Greco JB, Ratai EM, Halpern EF, Lackner AA, Masliah E, Gonzalez RG. Relationship between astrogliosis and 1H MRS measures of brain Cho/Cr and MI/Cr in a primate model. AJNR Am J Neuroradiol. 2005;6(4):752–759. [PMC free article] [PubMed] [Google Scholar]
- 23.Masliah E, Ge N, Achim CL, Wiley CA. Differential vulnerability of calbindin-immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1995;54(3):350–357. doi: 10.1097/00005072-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Everall IP, DeTeresa R, Terry R, Masliah E. Comparison of two quantitative methods for the evaluation of neuronal number in the frontal cortex in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(11):1202–1206. doi: 10.1097/00005072-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 26.Ratai EM, Pilkenton SJ, Bombardier JP, Joo CG, Turk KW, Lentz MR, He J, Annamalai L, O’Neil S, Westmoreland SV, Burdo TH, Campbell J, Soulas C, Autissier P, Kim WK, Williams KC, González RG. 7 Tesla MR Spectroscopy reveals that CD8 T lymphocyte depletion has no effect on brain metabolite concentration confirming the accelerated rhesus macaque model of neuroAIDS. 2009 [Google Scholar]
- 27.Gonzalez RG, Cheng LL, Westmoreland SV, Sakaie KE, Becerra LR, Lee PL, Masliah E, Lackner AA. Early brain injury in the SIV-macaque model of AIDS. Aids. 2000;14(18):2841–2849. doi: 10.1097/00002030-200012220-00005. [DOI] [PubMed] [Google Scholar]
- 28.Cheng LL, Ma MJ, Becerra L, Ptak T, Tracey I, Lackner A, Gonzalez RG. Quantitative Neuropathology by High Resolution Magic Angle Spinning Proton Magnetic Resonance Spectroscopy. Proc Natl Acad USA. 1997;94:6408–6413. doi: 10.1073/pnas.94.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greco JB, Westmoreland SV, Ratai EM, Lentz MR, Sakaie K, He J, Sehgal PK, Masliah E, Lackner AA, Gonzalez RG. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51(6):1108–1114. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, DeSilva M, Trivedi N, Speck O, Miller EN. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther. 2003;8(1):17–26. [PubMed] [Google Scholar]
- 31.Vion-Dury J, Nicoli F, Salvan AM, Confort-Gouny S, Dhiver C, Cozzone PJ. Reversal of brain metabolic alterations with zidovudine detected by proton localised magnetic resonance spectroscopy. Lancet. 1995;345(8941):60–61. doi: 10.1016/s0140-6736(95)91184-7. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson ID, Lunn S, Miszkiel KA, Miller RF, Paley MN, Williams I, Chinn RJ, Hall-Craggs MA, Newman SP, Kendall BE, Harrison MJ. Proton MRS and quantitative MRI assessment of the short term neurological response to antiretroviral therapy in AIDS. J Neurol Neurosurg Psychiatry. 1997;63(4):477–482. doi: 10.1136/jnnp.63.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13(3):981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez RG, Greco JB, He J, Lentz MR, O’Neil S, Pilkenton S, Ratai EM, Westmoreland SV. New Insights into the Neuroimmunity of SIV Infection by Magnetic Resonance Spectroscopy. Journal of NeuroImmune Pharmacology. 2006;1(2):152–159. doi: 10.1007/s11481-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 35.Ratai EM, Bombardier JP, Joo CG, Annamalai L, Burdo TH, Campbell J, Fell R, Hakimelahi R, He J, Autissier P, Lentz MR, Halpern EF, Masliah E, Williams KC, Westmoreland SV, Gonzalez RG. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS One. 2010;5(5):e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eichler FS, Itoh R, Barker PB, Mori S, Garrett ES, van Zijl PC, Moser HW, Raymond GV, Melhem ER. Proton MR spectroscopic and diffusion tensor brain MR imaging in X-linked adrenoleukodystrophy: initial experience. Radiology. 2002;225(1):245–252. doi: 10.1148/radiol.2251011040. [DOI] [PubMed] [Google Scholar]
- 37.Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195(1):58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- 38.Tracey I, Carr CA, Guimaraes AR, Worth JL, Navia BA, Gonzalez RG. Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: A proton magnetic resonance spectroscopic study. Neurology. 1996;46(3):783–788. doi: 10.1212/wnl.46.3.783. [DOI] [PubMed] [Google Scholar]
- 39.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52(1):100–108. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- 40.Fuller RA, Westmoreland SV, Ratai E, Greco JB, Kim JP, Lentz MR, He J, Sehgal PK, Masliah E, Halpern E, Lackner AA, Gonzalez RG. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004;5(1):10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]