Abstract
Background
Indirect Acute Lung Injury (ALI) is a common manifestation in critically ill patients. Using a model of indirect ALI in mice, our lab has shown that local/pulmonary inhibition of extrinsic death receptor protein (Fas) leads to a decrease in lung inflammation and improved survival. However it is unknown if local, i.e., autocrine/paracrine inhibition of Fas ligand (FasL) affects on Fas expressing target cells itself or blockade of the actions of a more distal/endocrine source of FasL that accounts for these findings.
Methods
In order to examine this we used a model of indirect ALI in mice (dual insult of hemorrhagic shock followed 24hrs later by cecal ligation and puncture, in which animals received FasL siRNA intra-trachealy (IT; local silencing) or intravenously (IV; systemic/distal delivery) post-hemorrhage.
Results
After IT delivery of FasL siRNA there was a significant decrease in inflammatory cytokines, myeloperoxidase activity, and caspase-3 activity in lung tissue along with protein leak as compared to controls. There was no difference found in these various outcome markers between those treated with FasL siRNA IV versus controls.
Conclusions
The observation that local silencing of FasL, as opposed to distal/systemic silencing, ameliorates the effects of indirect ALI suggest not only that FasL produced in an autocrine/paracrine fashion in local tissues has pathological consequences within the lungs, but that FasL might be a valuable pulmonary therapeutic target.
Keywords: Fas Ligand, Fas, Acute Lung Injury, Acute Respiratory Distress Syndrome
Introduction
Acute Lung Injury (ALI) and the more severe condition Acute Respiratory Distress Syndrome (ARDS) are devastating conditions that have affected critically ill patients for many years. However, the American European Consensus Conference Committee did not reach a consensus definition until 1994. This includes the following four criteria; (1) acute onset; (2) Bilateral infiltrates on chest radiography; (3) the absence of left atrial hypertension; (4) a PaO2:FiO2 ratio of less than or equal to 300 (ALI) or a PaO2:FiO2 ratio of less than or equal to 200 (ARDS).[1] It is estimated that in the United States there are 190,600 cases a year, with 74,500 deaths and around 3.6 million hospital days attributed to acute lung injury.[2] Fortunately, the mortality rate of this destructive syndrome has been decreasing over the past couple decades from as high as 70% to around 30-40%.[2,3,4] One reason for this improvement may be due to advances in supportive care including methods of mechanical ventilation. However, even though these new treatment strategies are working to reduce mortality they are restricted to supportive measures that do not directly address the underlining pathological changes.
Acute Lung Injury is either caused by a direct insult to the lung (e.g. pneumonia, pulmonary contusion) or as a secondary insult (indirect) from such illness as acute pancreatitis. The pathophysiology behind acute lung injury remains poorly understood and is an area that remains under active research. Several studies have suggested that the difference between direct versus indirect acute lung injury is the activation of pulmonary epithelial cells in direct and endothelial cell activation in indirect acute lung injury.[5,6,7,8,9] However, from our previous studies in experimental models of indirect acute lung injury we have demonstrated that the pulmonary epithelial cells are activated/primed through Fas activation.[10,11,12]
Fas (CD95) is a member of the TNF death domain containing receptor super family and is a well studied death receptor. Fas Ligand (FasL) is its natural ligand and exists in membrane-bound as well as soluble forms, both of which are capable of inducing apoptosis. FasL is well known for its apoptotic role, but is has also been found to have a role in the production of inflammatory cytokines and in neutrophil recruitment.[13] Interestingly, soluble Fas and FasL have been found to be increased in the pulmonary edema fluid of patients with ALI or ARDS as compared to controls, along with greater concentration of sFasL in patients who died as a result of ARDS as oppose to those who did not.[14,15] Along these lines, lung tissues obtained at autopsy of patients who succumbed to ALI/ARDS have a greater cellular expression of Fas and FasL demonstrated by semiquantitative immunofluorescence.[14]
The local inhibition (pulmonary epithelial cell) of apoptotic targets Fas and caspase-3, by use of siRNA, has been shown to ameliorate the effects of acute lung injury in a murine model of indirect acute lung injury.[10,12] It appears that the activation of the apoptotic and/or inflammatory pathways in the pulmonary epithelial cells is antecedent to eventual macrophage activation and recruitment of neutrophils to the lung during the pathogenesis of acute lung injury.[10] However, the contribution of the vascular endothelial cells to this activation/pathogenesis is unclear. Using organ specific delivery of siRNA we set out to evaluate if local, i.e., autocrine/ paracrine inhibition of Fas ligand (FasL) affects on Fas expressing target cells, such as epithelial cells, or blockade of the actions from more distal/endocrine sources of FasL account for this decrease in inflammation, neutrophil influx, and apoptosis following the onset of extra-pulmonary ALI.
Materials and Methods
Validation of Fas Ligand siRNA Sequences in vitro
FasL and control GFP siRNA sequences were assembled, deprotected, duplexed and desalted by Dharmacon (Lafayette, CO, USA). The following sequences were tested in vitro: FasL duplex 1 sequence, sense: 5′-CUGCACUACUGGACAGAUAUU-3′, antisense: 5′-UAUCUGUCCAGUAGUGCAGUU-3′; FasL duplex 2 sequence, sense: 5′-CAACCAAAGCCUUAAAGUAUU-3′, antisense: 5′-UACUUUAAGGCUUUGGUUGUU-3′; Control (GFP)-sequence, sense: 5′-GGCUACGUCCAGGAGCGCACCUU-3′, antisense: 5′-GGUGCGCUCCUGGACGUAGCCUU-3′.
The mouse-macrophage cell line J774 (American Type Culture Collection, Manassas, VA) was grown in Falcon® tissue culture flasks (Becton Dickinson, Franklin Lakes, NY) until around 50% confluence in Dulbeccos Modified Eagle Medium (DMEM) (Invitrogen, Gibco®, Carlsbad, CA) supplemented with 10% heat inactivated fetal bovine serum, 0.01% Gentamicin and 1% 100x 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffered solution, were then detached using Trypsin-EDTA 1x (Life Technologies, Rockville, MD). Cells were washed and resuspended in 5 ml DMEM (incl. 10% FCS, 1% 100x HEPES, but no Gentamicin) and counted using a Neubauer counting chamber and trypan blue exclusion for non-viable cells. Cells were divided into 2ml tubes (Eppendorf) at a concentration of 1.5×106 cells/ tube and centrifuged at 1000x g at 4°C for 10 minutes, and were then resuspendend in 100 μl Nucleofactor solution V (Amaxa, Gaithersburg, MD) containing either 300 pmol FasL silencing RNA sequences or GFP-siRNA serving as a control. Transfection protocol T-24 (Nucleofector, Amaxa) was chosen as it was found to provide excellent transfection results (> 90%) as determined using a Cy5-tagged-siRNA-sequence, visualized using Nikon TE-2000U inverted microscope (Nikon Corporation, Tokyo, Japan) combined with good cell survival (> 95%) as determined by trypan blue exclusion. Cells were resuspended in 2 ml of DMEM (incl. 10% FCS, 1% 100x HEPES, but no antibiotics) and seeded in six well cell culture plates (Corning Incorporated, Corning, NY). Non-attached cells were removed after a 24-hour incubation period and cells were stimulated with 1μg LPS/ml for 4 hours at 37°C, 5% CO2 to activate FasL.
Quantification of Fas Ligand/ 18s-ribosomal mRNA in vitro
RNA was isolated from J774 cells using TriPure Isolation Reagent (Boehringer, Mannheim, Germany) according to the manufacturers instruction as described previously. [10] cDNA was synthesized using iScript cDNA Synthesis Kit (BioRad, Hercules, CA) according to the manufacturers instruction as described previously.[10] Primer sequences for FasL (forward: 5′-TGCATCATGAGCCAGATGGAAGGA -3′; reverse: 5′-ATGAGCCTCCTTTCTCACCCTTGT -3′) and 18s ribosomal RNAs (forward: 5′-GGACACGGACAGGATTGACAGATT-3′; reverse: 5′-AATCGCTCCACCAACTAAGAACGG-3′) were assembled by Integrated DNA Technologies, Inc. (IDT, Coralville, IA). cDNA with forward and reverse primers (150nM final concentration) were combined with 25μl 2x PCR Mix. Conditions were set as follows: initial activation of the DNA polymerase at 95°C for 10 min, 40 cycles consisting of denaturation at 95°C for 30 sec, primer annealing at 60°C for 60 sec and extension at 72°C for 30 sec. All samples were then run in duplicate in 1% agarose gel for 40 minutes. Densitometry was performed using Chemilmanager Imaging System and FluorChem, Version 4.1.0 software (AlphaInnotech Corporation, San Leandro, CA, USA).
Acute Lung Injury
Male inbred C57BL/6 mice (Harlan Laboratories, South Easton, MA) 8-10 weeks old were used for this study. All experiments are in accordance with NIH guidelines and as approved by the Rhode Island Hospital Animal Use Committee.
Acute lung injury was induced as described previously and it should be noted that along with changes in a variety of inflammatory/apoptotic indices, this model under these conditions has been shown to concomitantly induce marked lung leak (protein leak), histological evidence of congestion and lung wet weight gain in our hands.[10,11] In brief, mice were anesthetized with isoflurane and PE 10 catheters were inserted into both femoral arteries. Anesthesia was discontinued and blood pressure was continuously monitored through one catheter attached to a blood pressure analyzer (BPA MicroMed, Louisville, KY). When fully awake, mice were bled over a 5 to 10 minute period to a mean blood pressure of 30 ±5 mmHg and maintained hypotensive for 90 minutes. Immediately after hemorrhage, mice were resuscitated through the femoral catheters with Lactated Ringer's at four times the drawn blood volume. Sham-operated controls had their femoral arteries ligated, but no catheters were inserted and no blood was shed. Polymicrobial sepsis as produced by cecal ligation and puncture, which was induced 24 hrs after hemorrhage as described previously.[10,11] Mice were anesthetized with isoflurane, the abdomen was shaved, and scrubbed with betadine. A 1 cm midline incision was made below the diaphragm to expose the cecum. The cecum was ligated, punctured twice with a 22-gauge needle and gently compressed to extrude a small amount of fecal contents through the punctured holes. The cecum was returned to the abdomen, and the incision was closed in layers with 6-0 Nylon suture (Ethicon, Somerville, NJ). The animals were then resuscitated with 0.8 ml of lactated Ringer's solution via subcutaneous injection. In sham-operated controls, laparotomy was performed in a similar fashion and the cecum was exposed, however it was neither ligated nor punctured.
Intratracheal Instillation
Intratracheal (IT) delivery of small interfering RNA was performed as described previously.[10,11] One hour following resuscitation mice were anesthetized lightly with isoflurane and restrained in supine position with their head reclined. The tongue of the animals was pulled out gently to prevent swallow reflex and 75 μg of siRNA in PBS (75μl) was administered in the oral cavity.
Intravenous Delivery
Intravenous (IV) delivery of small interfering RNA was preformed by IV tail vein injection. One hour following resuscitation mice were anesthetized lightly with isoflurane and their tails were gently restrained. Seventy five μg of siRNA mixed with 50μg of N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methysulfate (DOTAP, Roche Applied Science, Indianapolis, IN) and 120μl of transfection buffer (HEPES-buffered saline, adjusted to pH 7.4) was then slowly injected into the mouse tail vein under low pressure.
Experimental Groups and Sample Acquisition for in vivo Experiments
Mice were subjected to hemorrhagic shock then received a single intratracheal or intravenous delivery of 75μg of FasL siRNA, control GFP siRNA, or PBS which was then followed by polymicrobial sepsis 24 hrs after hemorrhage. Blood and lungs were harvested 24 hrs after the induction of sepsis (i.e. 48 hrs after Hem). Plasma was obtained by centrifugation of blood for 10 minutes, 10,000x g at 4°C and frozen at −80°C until analysis was performed. Brochoalveolar lavage fluid was obtained by direct instillation of 0.8 ml × 2 of PBS into the trachea via a cut down method and then aspirated back sequentially. Lung lobes were excised separately and stored at −80°C until analyses were performed.
Cytometric Bead Array for Cytokine Measurements
Preparation of lung homogenates for cytokine measurements was performed as described previously.[10,11] Frozen lung samples were thawed on ice in phosphate buffered saline including 50μl/ml proteinase inhibitor cocktail for mammalian tissues (Sigma, Aldrich, St. Louis, MO). Samples were then homogenized on ice, centrifuged at 12,000x g at 4°C for 15 min and supernatants were obtained. Supernatants were again centrifuged at 12,000x g at 4°C for 15 min and supernatants were stored at −80°C until assays were performed. Plasma was obtained from mice after whole blood was collected and centrifuged at 10,000x g at 4°C for 10minutes. The plasma layer was then isolated and stored at −80°C until assays were performed.
The total amount of protein was quantified using the Bradford dye binding procedure, as described previously.[10] Protein concentration was determined and calculated against a standard curve.
Mouse TNF-α, IL-6, IL-10 and MCP-1 levels were established in lung tissue homogenates using the cytometric bead array technique (BD™ Cytometric Bead Array Mouse Inflammation Kit, BD Biosciences, San Jose, CA) according to the manufacturer's instruction as described previously.[10,11] In brief, 50 μl of Mouse Inflammation Capture Bead Suspension and 50 μl PE Detection Reagent were added to the equal amount of sample or standard dilution and incubated for 2 hrs at room temperature in the dark. Subsequently, samples were washed by adding 1 ml of wash buffer and centrifuging at 200x g at RT for 5 min. Samples were analyzed on a BD FACSArray™ bioanalyzer (BD Biosciences) according to the manufacturer's instruction.
Pulmonary Leak
As an index of pulmonary leak the protein concentration (μg/ml) for the BAL vs the plasma protein concentration were quantified using the Bradford dye binding procedure as described previously by Perl et al [11] and the ratio of BAL:plasma protein calculated.
Myeloperoxidase (MPO) Activity
Lung MPO activity was quantified as described previously.[10,11] In brief, lung tissue was homogenized in 50 mM potassium buffer pH 6.0 with 0.5% hexadecyltrimethylammonium bromide (Sigma), sonicated on ice, and then centrifuged at 12,000 x g at 4°C for 10 min. Supernatants were then assayed at a 1:20 dilution in reaction buffer (530 nmol/l o-dianisidine, 150 nmol/l H2O2 in 50 mM potassium phosphate buffer; (Sigma), and read at 450 nm.
Fas Ligand and β-Actin Western Blotting
FasL and β -Actin were semi-quantified in lung tissue via western blotting, as previously described.[11] In brief, 80μg of homogenized lung tissue were electrophoresed on a 12% Tris-Glycine Gel (Invitrogen, Carlsbad, CA) and transferred onto a Poly-Vinyliden-Di-Fluorid membrane (Amersham, Piscataway, NJ). Nonspecific binding was blocked with tris buffered saline with Tween-20 (TBST, 0.242%, TrisBase, 0.8% Sodiumchloride, pH 7.6, 0.1% Tween-20; (all Sigma) containing 5% non-fat dry milk (Nestle, Glendale, CA). Membranes were incubated with polyclonal rabbit anti-rat cleaved FasL antibody (Chemicon, Billerica, MA) at a concentration of 1:1000 at 4°C overnight. After washing, membranes were incubated with ECL Rabbit IgG, HRP-linked whole antibody from donkey (Amersham) at a concentration 1:2000 for 1 hour at room temperature. After washing, membranes were developed using enhanced chemiluminescence technique (Alpha Innotech) according to the manufacturer's recommendation. β-Actin was used as a loading control. Protein expression was quantified via densitometry using FluorChem, Version 4.1.0 (Alpha Innotech) and expressed as the ratio of the integrated density values of FasL and β -Actin, adjusted to background levels.
Caspase-3 Activity
Caspase-3 activity in lung tissue was assessed as described previously[10] with minor modifications. In brief, lung tissue was homogenized in buffer containing 25mM HEPES, 5mM MgCl2, 1mM EGTA (all Sigma), 1μg/ ml Aprotinin, 0.04 mg/ml Pefabloc, and 1μg/ml leupeptin (all Roche, Indianapolis, ID). Fifty μg of tissue protein in fifty μl of buffer were added to 50 μl of Caspase-3 reaction buffer (containing 100mM HEPES, 2% Sucrose, 0.2% CHAPS, 10mM Dithiothreitol (all Sigma) and 100μM Ac-DEVD-AFC fluorogenic substrate (Biomol, Plymouth Meeting, PA)). Extinction was read at 400 nm excitation, 505 nm emission on a microplate fluorescence Reader (FLx800, Bio-Tek Instruments Inc., Winooski, VT) in a 30-minute interval for 4 hours. Values were calculated against an AFC standard curve (Biomol) as μM cleavage of AFC/minute/mg total protein.
Statistical Analysis
Results are presented as mean ± standard error of the mean (SEM). One-Way Analysis of Variance (ANOVA) followed by the Student-Newman-Keuls test as a post hoc test for multiple comparisons was performed to determine significant differences between experimental means. A p-value < 0.05 was considered statistically significant.
Results
Validation of Fas Ligand siRNA
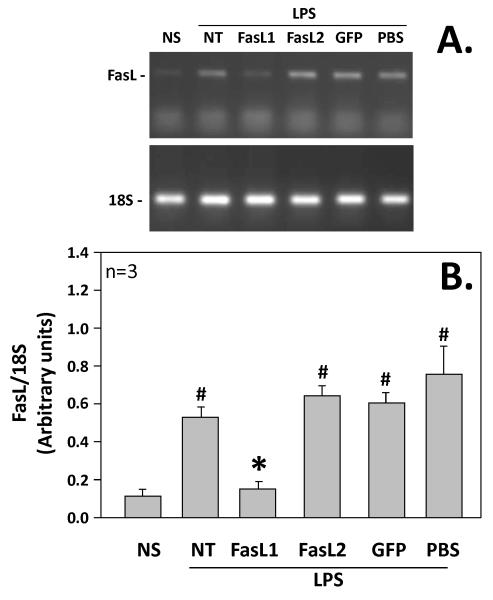
Transfection of J774 cells with FasL siRNA (sequence 1) produced a significant decrease in FasL mRNA expression following 4 hours stimulation with LPS. J774 cells non-stimulated (NS) had a base line expression of FasL of 0.13 arbitrary units (based on densitometry readings of FasL divided by control 18s expression). This was markedly elevated following four-hour LPS stimulation (NT) at 0.54 arbitrary units in cells that were not transfected. The FasL mRNA expression was significantly decreased after 4-hour stimulation with transfection of FasL siRNA (sequence 1) at 0.17 arbitrary units (FasL1), as compared to 4 hours stimulation (NT) and also those cells transfected with control GFP siRNA (GFP), which had an expression of 0.60 arbitrary units. Those cells transfected with FasL siRNA (sequence 2) had a FasL mRNA expression of 0.66 arbitrary units (FasL2) after LPS stimulation and did not seem to express any knockdown of the gene. Mock-transfection with PBS had a FasL mRNA expression after 4 hours stimulation of 0.87 arbitrary units (PBS). Given the significant knockdown of FasL mRNA expression in J774 cells following four-hour stimulation with LPS, sequence 1 was chosen for use in the subsequent in vivo studies. (Figure 1)
Figure 1.
Non-stimulated (NS) vs stimulated J774 cells (cells exposed to 1 μg LPS/ml [LPS] for 4 hours) which had been either not transfected (NT) or transfected with Fas Ligand siRNA sequence 1(FasL1); transfected with Fas Ligand siRNA sequence 2 (FasL2); transfected with GFP siRNA (GFP); or mock transfected with PBS (PBS), and then assessed for the extent Fas Ligand mRNA expression induced. (A.) Shows the results of a representative PCR-gel of lung homogenates for FasL (upper panel) and 18S (lower panel). (B.) Cumulative densitometric ratio data of lung homogenates for FasL:18S from n=9 independent samples/group. Data is shown as mean ± SEM; *, p<0.05 FasL1 group vs NT, GFP, FasL2 or PBS groups; N=3 repeat experiments.
In Vivo Silencing of Fas Ligand
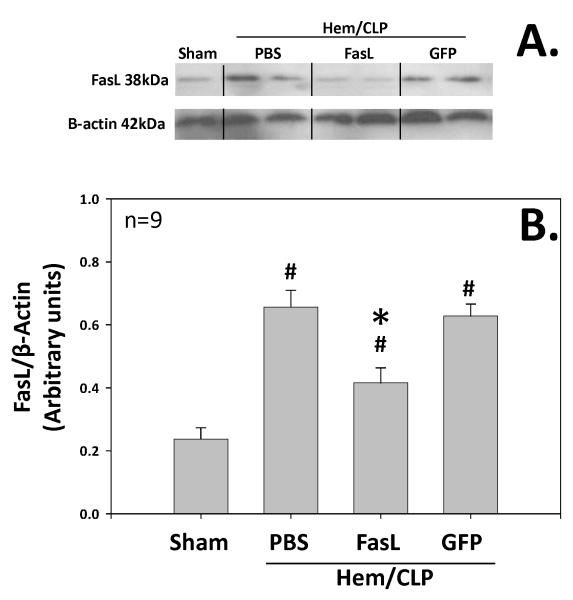
Pulmonary FasL was significantly increased (p<.05) in animals subjected to our model of extra-pulmonary acute lung injury as compared to sham animals. FasL protein expression (as measured by densitometry of FasL divided by control β-actin expression) was 0.23 arbitrary units in sham animals (Sham; n=4) as compared to 0.65 arbitrary units in animals subjected to extra-pulmonary acute lung injury (PBS; n=9). Animals treated with FasL siRNA administered intratracheally had significantly less FasL pulmonary protein expression at 0.31 arbitrary units (FasL; n=9) as compared to those animals treated with control GFP siRNA (0.62 arbitrary units; GFP; n=9). There was no statistical significant difference between those in the PBS group vs. those in the GFP group. (Figure 2)
Figure 2.
Fas Ligand protein expression as detected by Western blot for pulmonary/whole lung tissue homogenates taken from animals subjected to either Sham surgery or mice subjected to hemorrhage followed by cecal ligation and puncture 24hrs later (Hem/CLP) and either no siRNA (PBS), anti-Fas Ligand siRNA intratracheal (FasL), or anti-GFP siRNA intratracheal (GFP) treatment. (A.) Shows the results of a representative Western blot of lung homogenates for FasL (upper panel) and β-Actin (lower panel). (B.) Cumulative densitometric ratio data of lung homogenates for FasL:β-Actin from n=9 independent samples/group. Data is shown as mean ± SEM; *, p<0.05 for FasL vs PBS or GFP groups; #, p<0.05 Sham vs all other groups; n=9.
Inflammatory Markers
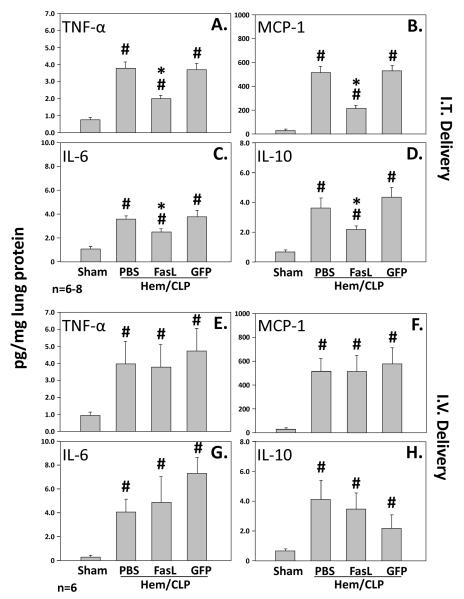
Following intratracheal administration of FasL siRNA, pulmonary homogenate levels of TNF-α, MCP-1, IL-6, and IL-10 where all significantly decreased as compared to those animals treated with control GFP siRNA as well as those animals which were exposed to extra-pulmonary acute lung injury and no siRNA treatment (PBS). All respective cytokines measured (TNF-α, MCP-1, IL-6, and IL-10) in all three groups (FasL, GFP, and PBS) were elevated with respect to sham surgery animals. (Figure 3)
Figure 3.
The upper panel illustrates lung TNF-α (A.), MCP-1 (B.), IL-6 (C.) and IL-10 (D.) concentrations [pg/mg lung protein] in mice (n=6-8 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP) that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intratracheally (I.T. Delivery). Alternatively, the lower panel shows lung TNF-α (E.), MCP-1 (F.), IL-6 (G.) and IL-10 (H.) concentrations [pg/mg lung protein] in mice (n=6 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP) that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intravenously (I.V. Delivery). Data are shown as mean ± SEM; *, p<0.05 for FasL vs PBS or GFP groups; #, p<0.05 Sham vs all other groups.
With respect to those animals treated with intravenous FasL siRNA as compared to intravenous GFP siRNA as well as those animals which were exposed to extra-pulmonary acute lung injury and no siRNA treatment (PBS), there was no significant difference in the measured lung homogenate cytokines (TNF-α, MCP-1, IL-6, and IL-10) (Figure 3)
Lung Myeloperoxidase Activity
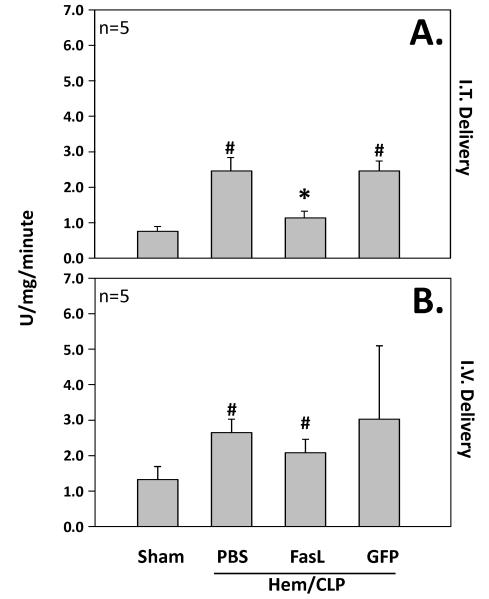
The animals treated with intra-tracheal delivery of FasL siRNA had significantly lower levels of myeloperoxidase activity as compared to intra-tracheal delivery of GFP siRNA along with those animals exposed to hemorrhage and cecal ligation and puncture alone without siRNA delivery (PBS). All three groups had higher levels of MPO activity as compared to sham surgery animals. (Figure 4)
Figure 4.
The upper panel (A.) illustrates lung MPO activity (U/mg/min) in mice (n=5 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intratracheally (I.T. Delivery). Alternatively, the lower panel (B.) shows lung MPO activity (U/mg/min) in mice (n=5 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP) that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intravenously (I.V. Delivery). Data are shown as mean ± SEM; *, p<0.05 for FasL vs PBS or GFP groups; #, p<0.05 Sham vs all other groups.
Although there was no significant difference in levels of lung myeloperoxidase activity of animals treated with intravenous FasL siRNA as compared to those treated with intravenous control GFP siRNA and those subjected to hemorrhage and cecal ligation and puncture without siRNA (PBS), there was a trend toward lower levels in the intravenous FasL siRNA group. All groups, intravenous FasL siRNA, GFP siRNA, and PBS had higher levels then sham surgery animals. (Figure 4)
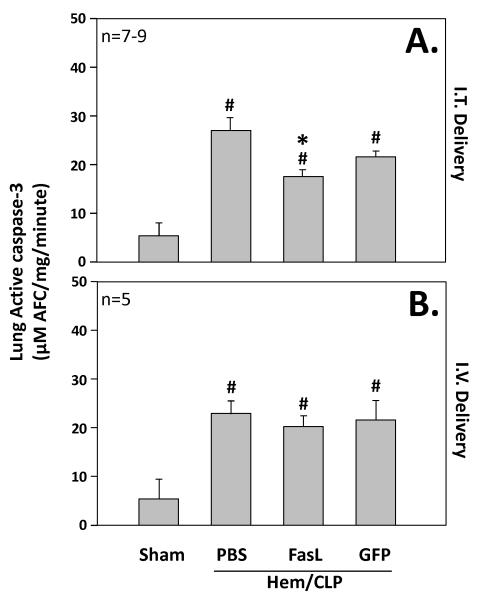
Lung Caspase-3 Activity
The animals treated with intratracheal delivery of FasL siRNA had significantly lower levels of caspase-3 activity as compared to intratracheal delivery of GFP siRNA along with those animals exposed to hemorrhage and cecal ligation and puncture alone without siRNA delivery (PBS). All three groups had higher levels of caspase-3 activity as compared to sham surgery animals. (Figure 5)
Figure 5.
The upper panel (A.) illustrates lung Caspase-3 activity (μM AFC/mg/min) in mice (n=5 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP) that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intratracheally (I.T. Delivery). Alternatively, the lower panel (B.) shows lung Caspase-3 activity (μM AFC/mg/min) in mice (n=5 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP) that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intravenously (I.V. Delivery). Data are shown as mean ± SEM; *, p<0.05 for FasL vs PBS or GFP groups; #, p<0.05 Sham vs all other groups.
There was no significant difference in levels of lung caspase-3 activity observed in animals treated with intravenous FasL siRNA as compared to those treated with intravenous control GFP siRNA or animals exposed to hemorrhage and cecal ligation and puncture without siRNA delivery (PBS). Again all groups, intravenous FasL siRNA, GFP siRNA, and PBS had higher levels of caspase-3 activity as compared to sham surgery animals. (Figure 5)
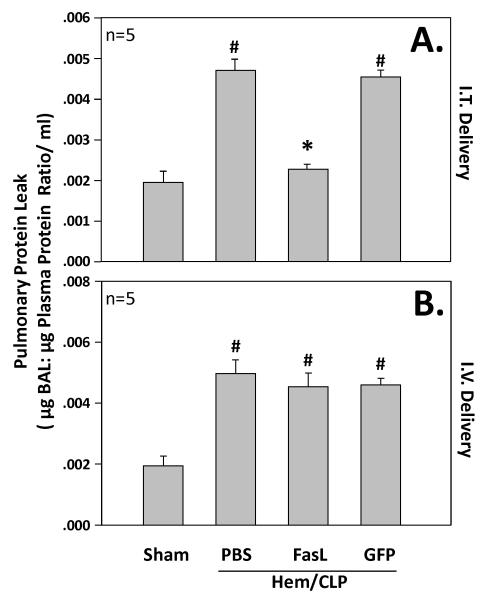
Bronchoalveolar Lavage Protein
Bronchoalveolar lavage protein was quantified and presented as a ratio to plasma protein. Animals treated with intratracheal delivery of FasL siRNA has significantly lower levels of BAL/plasma protein as compared to those treated with GFP siRNA along with those exposed to hemorrhage and cecal ligation and puncture alone (PBS)(Figure 6). There was no significant difference in the levels of BAL/plasma protein ratio of those treated with intravenous delivery of FasL siRNA compared to intravenous GFP siRNA as well as to those subjected to hemorrhage and cecal ligation and puncture alone (PBS)(Figure 6).
Figure 6.
The upper panel (A.) illustrates BAL/plasma protein (in μg/ml) ratio in mice (n=5 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP) that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intratracheally (I.T. Delivery). Alternatively, the lower panel (B.) shows BAL/plasma protein ratio in mice (n=5 mice/group) subjected to sham surgery compared to those subjected to hemorrhage followed 24 hrs later by cecal ligation and puncture (Hem/CLP) that were either given no siRNA (PBS), anti-Fas Ligand siRNA, or anti-GFP siRNA treatment intravenously (I.V. Delivery). Data are shown as mean ± SEM; *, p<0.05 for FasL vs PBS or GFP groups; #, p<0.05 Sham vs all other groups.
Discussion
There is much support in the literature for the role of pulmonary epithelial cell apoptosis in indirect acute lung injury. However the activation and triggering of this process in indirect acute lung injury remains poorly understood. Our goal here was to evaluate the role of the pulmonary endothelial cells in initiating the pulmonary epithelial cell apoptosis that occurs.
Using the modified tongue-pull technique of intratracheal delivery of naked siRNA [16,17] which appears to be primarily taken up by pulmonary epithelial cells [10], we have demonstrated that local inhibition of Fas Ligand leads to improved outcomes in our model of indirect acute lung injury. This includes the pulmonary cytokine response, mainly in the form of TNF-α, MCP-1, IL-10, and IL-6. Also with pulmonary epithelial cell inhibition of Fas ligand we can see a significant decrease in neutrophil influx as measured by myeloperoxidase activity. We have also demonstrated a significant decrease in apoptosis as measured by active caspase-3 activity. Finally, we see a significant decrease in the BAL protein to plasma protein ratio of those animals treated with local inhibition of FasL with intratracheal delivery of siRNA. These findings are consistent with previous studies in which mice expressing nonfunctional Fas ligand (B6Smn.C3-Faslgld/J [gld]) have had similar outcomes following hemorrhagic shock and polymicrobial sepsis.[11] However those mice have global FasL dysfunction and this study adds further significance as it is the first to demonstrate that local inhibition (pulmonary epithelial cells) of FasL ameliorates the effect in our mouse model of indirect acute lung injury. In other words; although the insults of hemorrhagic shock and secondary peritoneal sepsis are in a sense distal/systemic events that have their nidus outside the lungs, the critical aspect of death receptor ligand, eg, FasL, and death receptor, eg, Fas, ligation that precipitate the early aspects of pulmonary injury must be initiated locally, most likely by autocrine/paracrine interactions between epithelial cells. And while the stimulus for this pulmonary response produced by the combined insults of shock and sepsis is still unclear, it seems unlikely that it is due to the exocrine release of FasL from the vascular endothelium and/or transmigrating leukocytes. These findings add to the body of knowledge as to the involvement of Fas-FasL system and pulmonary epithelial cells in acute lung injury.
To the extent that the pulmonary endothelial cells were not involved, we choose to access the extent to which siRNA via intravenous delivery altered the development of indirect acute lung injury. As it has been shown that the delivery of liposomally encapsulated siRNA intravenously is taken up primarily by vascular endothelial cells and to a lesser extent circulating leukocytes, as opposed to pulmonary epithelial cells.[18,19,20,21,22,23] When FasL siRNA was administered intravenously we observed no significant change in the pulmonary cytokine response to this model of indirect acute lung injury. There was also no significant change in the rate of pulmonary apoptosis as measured by active caspase-3 activity. Although not significant there was a trend toward a decrease in the rate of neutrophil influx as measured by myeloperoxidase activity after intravenous delivery of FasL siRNA. Finally, we found no difference in protein leak as measured by BAL protein to plasma protein ratio.
This study is the first attempt to help delineate the relationship between the endothelial cell Fas activation and the Fas activation of pulmonary epithelial cells that occurs during indirect acute lung injury consisting of hemorrhagic shock followed by cecal ligation and puncture in a mouse model. The findings of this study, which are no change in the inflammatory profile, myeloperoxidase activity, caspase-3 activity and protein leak after endothelial cell inhibition of FasL leads us to believe that the Fas activation of pulmonary epithelial cells is due to a autocrine/paracrine feedback of neighboring epithelial cells.
There are limitations to this study, which include the inability to solely localize the effect of intravenous delivery of FasL siRNA to the vascular endothelial cell. In order to further investigate the relationship between endothelial cell activation of Fas-FasL system of pulmonary epithelial cells during indirect acute lung injury perhaps one can repeat this study in mice with a lineage specific deletion of FasL receptor, Fas on endothelial cells.
Also as alluded to earlier, since hemorrhagic shock and peritoneal sepsis are distal (non-lung) insults, it is likely that the stimulus for the Fas-FasL autocrine/paracrine changes that we have seen here in the epithelium of the lungs still must in some way be derived from the vascular endothelial:leukocyte interface. One possible such agent/mediator is TNF. TNF is a well known stimulant of Fas and FasL expression.[24] We have preliminary data that suggest that intravenous but not intratracheal delivery of siRNA against TNF suppresses the development of indirect acute lung injury.[25] Thus, it is tempting to speculate that the upstream stimuli for pulmonary epithelial cell autocrine/paracrine expression of FasL here could be mediated by TNF derived from the activated pulmonary endothelial cell in response to shock and/or peritoneal sepsis.
Conclusions
Acute lung injury and its more severe from acute respiratory distress syndrome continue to cause significant mortality in our intensive care units. There is clear evidence for the role of Fas-FasL activation on pulmonary epithelial cells, however little is known as to what triggers this event. This study is the first to demonstrate that local inhibition (pulmonary epithelial cell) of FasL ameliorates the effects of indirect acute lung injury in our murine model of hemorrhagic shock followed by polymicrobial sepsis. We are also able to conclude that the Fas-FasL pulmonary epithelial cell activation appears to be stimulated through an autocrine/ paracrine feedback mechanism as opposed to a more distal (endocrine) vascular endothelial source of Fas-FasL activation.
Acknowledgments
We would like to thank the support of the Carter family for their endowment of the Armand D. Versaci research scholarship to R.K.T. and S.F.M. and funds from NIH grant HL73525 (to A.A.).
Support: This work was funded in part by NIH HL 73525 (A.A.) as well as the Armand D. Versaci Research Scholar in Surgical Sciences Award (R.K.T, S.F.M.).
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am.J.Respir.Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N.Engl.J.Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Abel SJ, Finney SJ, Brett SJ, Keogh BF, Morgan CJ, Evans TW. Reduced mortality in association with the acute respiratory distress syndrome (ARDS) Thorax. 1998;53:292–294. doi: 10.1136/thx.53.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983-1993. JAMA. 1995;273:306–309. [PubMed] [Google Scholar]
- 5.Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J.Clin.Invest. 2003;112:1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am.J.Physiol Lung Cell Mol.Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Matute-Bello G, Liles WC, Hayashi S, Kajikawa O, Lin SM, Frevert CW, Martin TR. Differential response of human lung epithelial cells to fas-induced apoptosis. Am.J.Pathol. 2004;164:1949–1958. doi: 10.1016/S0002-9440(10)63755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, Barbas CS, Chiaranda M, Gattinoni L. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur.Respir.J.Suppl. 2003;42:48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 9.Takeda S, Ishizaka A, Fujino Y, Fukuoka T, Nagano O, Yamada Y, Takezawa J. Time to change diagnostic criteria of ARDS: towards the disease entity-based subgrouping. Pulm.Pharmacol.Ther. 2005;18:115–119. doi: 10.1016/j.pupt.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am.J.Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perl M, Chung CS, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, Ayala A. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am.J.Respir.Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl M, Chung CS, Perl U, Thakkar R, Lomas-Neira J, Ayala A. Therapeutic accessibility of caspase-mediated cell death as a key pathomechanism in indirect acute lung injury. Crit Care Med. 2010;38:1179–1186. doi: 10.1097/CCM.0b013e3181d4563f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lettau M, Paulsen M, Kabelitz D, Janssen O. Storage, expression and function of Fas ligand, the key death factor of immune cells. Curr.Med.Chem. 2008;15:1684–1696. doi: 10.2174/092986708784872384. [DOI] [PubMed] [Google Scholar]
- 14.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am.J.Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J.Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 16.Lomas-Neira J, Chung CS, Wesche DE, Perl M, Ayala A. In vivo silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage induced neutrophil mediated septic acute lung injury. JLB. 2005 Jun;77(6):846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG. Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. Journal of Immun. 2001;167:2808–2815. doi: 10.4049/jimmunol.167.5.2808. [DOI] [PubMed] [Google Scholar]
- 18.Aleku M, Fisch G, Mopert K, Keil O, Arnold W, Kaufmann J, Santel A. Intracellular localization of lipoplexed siRNA in vascular endothelial cells of different mouse tissues. Microvasc.Res. 2008;76:31–41. doi: 10.1016/j.mvr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 19.McLean JW, Fox EA, Baluk P, Bolton PB, Haskell A, Pearlman R, Thurston G, Umemoto EY, McDonald DM. Organ-specific endothelial cell uptake of cationic liposome-DNA complexes in mice. Am.J.Physiol. 1997;273:H387–H404. doi: 10.1152/ajpheart.1997.273.1.H387. [DOI] [PubMed] [Google Scholar]
- 20.Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am.J.Physiol Lung Cell Mol.Physiol. 2006;290:L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 21.Moschos SA, Williams AE, Lindsay MA. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochem.Soc.Trans. 2007;35:807–810. doi: 10.1042/BST0350807. [DOI] [PubMed] [Google Scholar]
- 22.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 23.Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, Hanahan D, McDonald DM. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J.Clin.Invest. 1998;101:1401–1413. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouaaz F, Li M, Beg AA. A critical role for the RelA subunit of nuclear factor kappaB in regulation of multiple immune-response genes and in Fas-induced cell death. J.Exp.Med. 1999;189:999–1004. doi: 10.1084/jem.189.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomas-Neira J, Perl M, Soldato D, Venet F, Chung CS, Ayala A. Endothelial Not Epithelial-Cell Expression of TNF-alpha is Critical for the Development of Shock Induced Acute Lung Injury (ALI): IT vs. IV. FASEB J. 2008;22:47. [Google Scholar]