Abstract
Background
Several susceptibility genes have been proposed for dyslexia (reading disability; RD) and specific language impairment (SLI). RD and SLI show comorbidity, but it is unclear whether a common genetic component is shared.
Methods
We have investigated whether candidate genes for RD and SLI affect specific cognitive traits or have broad effect on cognition. We have analyzed common risk variants within RD (MRPL19/C2ORF3, KIAA0319, and DCDC2) and language impairment (CMIP and ATP2C2) candidate loci in the Avon Longitudinal Study of Parents and Children cohort (n = 3725), representing children born in southwest England in the early 1990s.
Results
We detected associations between reading skills and KIAA0319, DCDC2, and CMIP. We show that DCDC2 is specifically associated with RD, whereas variants in CMIP and KIAA0319 are associated with reading skills across the ability range. The strongest associations were restricted to single-word reading and spelling measures, suggesting that these genes do not extend their effect to other reading and language-related skills. Inclusion of individuals with comorbidity tends to strengthen these associations. Our data do not support MRPL19/C2ORF3 as a locus involved in reading abilities nor CMIP/ATP2C2 as genes regulating language skills.
Conclusions
We provide further support for the role of KIAA0319 and DCDC2 in contributing to reading abilities and novel evidence that the language-disorder candidate gene CMIP is also implicated in reading processes. Additionally, we present novel data to evaluate the prevalence and comorbidity of RD and SLI, and we recommend not excluding individuals with comorbid RD and SLI when designing genetic association studies for RD.
Key Words: ALSPAC, association study, dyslexia, language, reading abilities, specific language impairment (SLI)
Dyslexia (or reading disability, RD) and SLI are common childhood disorders. RD is a specific deficit in learning to read, whereas SLI refers to an impairment in the acquisition of oral language (1). The biological cause of RD and SLI remains poorly understood, but it is clear that their manifestation is the result of multiple interacting factors, many of which have a genetic origin. Family studies have reported that, for both disorders, first-degree relatives of affected individuals also have a 30% to 50% chance of being affected, whereas the general population prevalence is approximately 5% (2,3). Comorbidity between RD and SLI has been consistently reported. Estimates indicate that 43% of children with SLI are later diagnosed with RD (4), and up to 55% of children with RD meet criteria for SLI (5). These figures have led to the hypothesis that SLI and RD may be manifestations of the same underlying deficit or may share etiologic factors, such as genetic determinants (1). Both SLI and RD show increased comorbidity with attention-deficit/hyperactivity disorder (ADHD), another common neurodevelopmental disorder, affecting 3% to 5% children (6). It is estimated that 25% to 40% of children with RD manifest symptoms of ADHD as well (7), and children with language disorder are at higher risk of developing ADHD (8).
Several genes have been proposed as susceptibility candidates for either RD or language-related skills and have been extensively reviewed (9−11). The RD candidates include the MRPL19/C2ORF3 locus, ROBO1, KIAA0319, DCDC2, and DYX1C1. With the exception of ROBO1 (12), these genes are supported by genetic associations with common single nucleotide polymorphisms (SNPs).
DYX1C1 was the first RD candidate to be identified following breakpoint mapping of a translocation cosegregating with RD (13). Association analysis in a cohort with RD implicated two putative coding variants: the -3A (rs3743205) and the 1249T (rs57809907) variants. A large number of replication studies have not reached consensus in supporting DYX1C1 RD susceptibility variants (14−22).
More consistent observations have been reported for the KIAA0319 and DCDC2 genes located at the chromosome 6 locus. Most of the associations with KIAA0319 cluster around the 5′ end of this gene and generally show the same allelic trend across independent studies (23−27). Functional studies showed that one particular RD-risk haplotype, effectively tagged by the minor allele of the rs2143340 SNP, is associated with reduced expression of KIAA0319 (28). This haplotype also harbors the minor allele of rs9461045, creating a binding site for a nuclear protein, which could explain the reduced gene expression and provides a functional mechanism underlying the genetic associations (29). Other studies did not find associations within KIAA0319 but identified the nearby DCDC2 gene as an RD candidate (30,31). Replication studies in samples selected for RD provided further support for DCDC2 but with modest associations (14,23,32,33). Rare variants located between these two genes have been found to be associated with speech perception in children with dyslexia (34).
The MRPL19 and C2ORF3 genes, which appear to be coregulated, are supported by single intergenic SNPs and overlapping haplotypes yielding significant associations in two independent samples of Finnish and German origin (35).
The candidate genes for language include CMIP, ATP2C2, and CNTNAP2. CMIP and ATP2C2 have been associated with nonword repetition, which is regarded as a measure of phonologic short-term memory, in samples of individuals with language impairment (36). Both genes were identified following high-density mapping at the chromosome 16 locus for SLI (37). The associations, originally identified in a cohort of individuals with SLI, were also seen in a subgroup of individuals selected on the basis of low language skills from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort (38), albeit with an opposite direction of trend for CMIP. Instead, no associations were detected with language traits in the entire ALSPAC cohort, which is representative of the general population. This suggests that the two genes have an effect on nonword repetition on a background of language impairment.
CNTNAP2 genetic variants were found to be associated with language-related phenotypes and a task of verbal short-term memory in the same language-impaired cohort used for CMIP and ATP2C2 (39). CNTNAP2 is a target of FOXP2 (39), which is implicated in severe and rare forms of language impairment (40).
The use of epidemiologic cohorts has proved to be a valid approach to investigate further genetic associations with some of these genes. The KIAA0319 RD-associated haplotype (26) was significantly associated with reading skills in both ALSPAC (41) and in a twin-based Australian sample (42), but with an opposite trend in the latter. The same Australian sample was also used to investigate DCDC2 (43) and DYX1C1 (44).
Phenotype definition is a key component when investigating the genetics of language and reading disorders. Tests of single-word reading are the most commonly used measures in genetic association studies of RD (45). Nonword repetition is a good marker for heritable SLI (46). However, an important issue is how far language problems should be identified solely by psychometric tests, which may miss key features of communication difficulties. Parental reports can be highly effective in identifying heritable communication problems (47,48), but they typically identify a different subset of children than those identified on direct language testing (49).
Bishop and Snowling (50) noted that RD and SLI were for a long time regarded as distinct disorders but in recent years have been reconceptualized as points on a continuum. This is an oversimplification, because different components of language and reading skills can fractionate, but it is possible that the same genetic components could contribute to both disorders and explain, at least partially, the observed comorbidity. Previous studies exploring the role of shared genes in contributing to both RD and SLI indicate KIAA0319 as a possible common risk factor supported by associations with language-related measures in samples selected for language impairment (51,52). One of these studies also showed that CMIP was associated with both reading and language-related measures in the same sample selected for language impairment (52). No association with language measures was reported for an investigation of DCDC2 and DYX1C1 in a sample of families ascertained for dyslexia (53). A genetic overlap has been suggested for RD and ADHD by a linkage study (54), and DCDC2 has been suggested to contribute to both RD and ADHD (55). ATP2C2 has also been found associated with ADHD (56).
Here, we investigated in the ALSPAC cohort genetic associations reported in the literature from samples selected for either RD or SLI. We conducted association analysis to 1) replicate associations with reading and nonword repetition measures, 2) dissect the phenotypic components of such associations by testing different but related quantitative phenotypes to pinpoint the underlying cognitive deficit(s), and 3) test for pleiotropic effects across reading- and language-related measures. We identified association between reading abilities and the DCDC2, KIAA0319, and CMIP genes and have shown that these associations follow different patterns; whereas DCDC2 is associated more specifically with dyslexia, CMIP and KIAA0319 are associated with reading abilities in the normal range. In addition, we show that these genes have a specific effect on a test of single-word reading rather than a more generalized impact. Lastly, we have evaluated the effect of individuals with comorbid RD and SLI in association analysis. Our results suggest that the inclusion of these individuals may increase power in genetic association studies for dyslexia.
Methods and Materials
We genotyped the ALSPAC children cohort (n ∼11,000) using either Sequenom iPLEX assays (San Diego, California) or the KBiosciences (Herts, United Kingdom) service using their in-house technology. Nineteen SNPs passed the quality control criteria of a call rate greater than 90%, error rate less than 1.5% (estimate derived from approximately 3% of samples blindly distributed in duplicates), minor allele frequency greater than .05, and genotype frequencies in Hardy−Weinberg equilibrium (p > .05). We had good quality data for SNPs within MRPL19/C2ORF3, KIAA0319, DCDC2, ATP2C2, and CMIP but not for DYX1C1 and CNTNAP2, which therefore were not included in this analysis.
Both quantitative and case−control analyses were performed within PLINK (57) testing for additive effects.
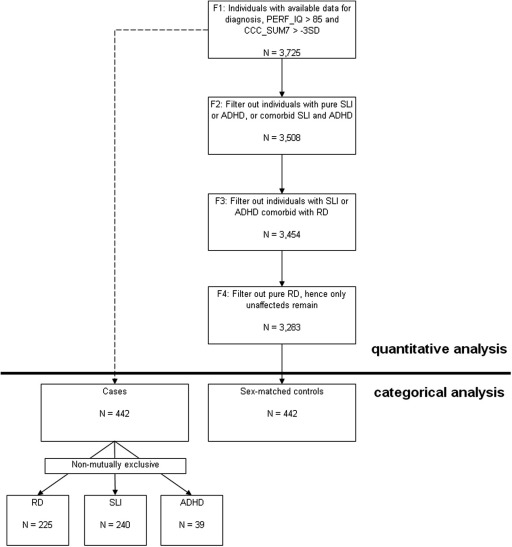
We based our initial analysis on the F1 sample (Figure 1; Methods in Supplement 1), which includes all available individuals after filtering for missing data, ethnicity, IQ, and autistic traits. Individuals were then assigned to the groups of RD, SLI, ADHD, any of the four comorbid combinations of these three disorders, or unaffected (Figure 1, Table 1). See Methods in Supplement 1 for full description of sample subgroups.
Figure 1.
Diagram illustrating how phenotypic subgroups were identified. The subgroups above the black horizontal lines were used for quantitative analysis while the ones below were used for case-control analysis. The extent of co-morbidity (hence the non-mutually exclusive definition of cases), can be seen in Figure 2. ADHD, attention-deficit/hyperactivity disorder; CCC_SUM7, sum of first seven scales from the Children's Communication Checklist; PERF_IQ, performance IQ; RD, reading disability; SLI, specific language impairment.
Table 1.
Affection Status Groups of All the Individuals from F1
| Affection Status | Frequency | % |
|---|---|---|
| Unaffected | 3283 | 88.13 |
| RD | 171 | 4.59 |
| SLI | 186 | 4.99 |
| ADHD | 26 | .70 |
| RD and SLI | 46 | 1.23 |
| RD and ADHD | 5 | .13 |
| SLI and ADHD | 5 | .13 |
| RD, SLI, and ADHD | 3 | .08 |
ADHD, attention-deficit hyperactivity disorder; RD, reading disability; SLI, specific language impairment.
Results
Observed Disorder Prevalence
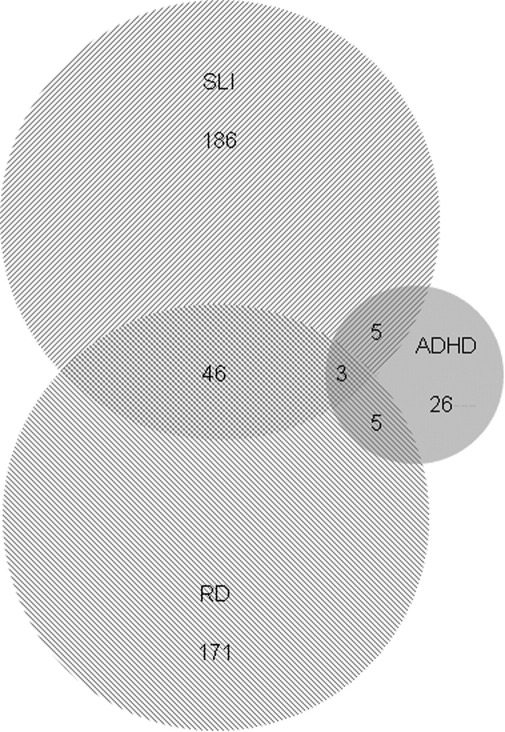
The ALSPAC children were assigned to one of eight affection status subgroups (Methods in Supplement 1); unaffected, RD, SLI, ADHD, or one of the four comorbid combinations (Figure 2, Table 1). From the initial sample, we filtered sequentially for missing data, ethnicity, performance IQ, and signs of autism, selecting 3725 individuals to calculate disorder prevalences. The prevalence of RD (6.04%) and SLI (6.44%) in this subgroup of the ALSPAC cohort is comparable to other studies (2,58), and the prevalence of ADHD (1.05%) is lower than previous reports of approximately 5% (59). The low prevalence of ADHD is explained primarily by a conservative assignment criterion but a specific dropout of children with ADHD from the ALSPAC study has also been suggested (60). Levels of comorbidity (Table 1) were comparable to other studies (1,9), but our conservative criterion for ADHD would have an impact on the rates of comorbidity with ADHD in this sample.
Figure 2.
A Venn diagram illustrating the distributions of the cases identified for reading disabilities (RD), specific language impairment (SLI), and attention-deficit/hyperactivity disorder (ADHD) from Sample F1. Circle size is proportional to sample size, and circle overlaps represent comorbidity.
The quantitative measures selected for either ascertainment criteria or association analysis (Table 2 and Table S1 in Supplement 1) show various degrees of correlation (Table S2 in Supplement 1). A strong correlation (.514 ≤ r ≤ .814) was observed across the reading-related measures (excluding MEMSPAN). Low correlation was observed across the language-related measures (.099 ≤ r ≤ .197), and NW_REPT showed higher correlation with the reading measures. This is consistent with observations in our cohort of families with SLI (36). This also fits with the notion that the different language tests measure distinct language components and identify different groups of impaired individuals (49).
Table 2.
Description of Phenotypic Measures
| Measure | Assignment/Phenotypea | Summary Description | Target Age | Reference |
|---|---|---|---|---|
| READb | A/P | Single-word reading accuracy | 7.5 year | 67 |
| READ@9 | A | Single-word reading accuracy | 9.5 year | 68 |
| SPELL | P | Single-word spelling accuracy | 7.5 year | 68 |
| PHONEME | P | Phoneme awareness | 7.5 year | 69 |
| NW-READ | P | Single-non-word reading accuracy | 9.5 year | 68 |
| MEMSPAN | P | Working memory | 10.5 year | 70 |
| WOLD | A/P | Listening and comprehension test | 8.5 year | 71 |
| NW-REPTc | A/P | Phonological short-term memory test | 8.5 year | 72 |
| CCC-SUM7 | A/P | Sum of first seven scales from Children's Communication Checklist | 7.5 year | 73 |
| Speech/language therapy | A | Child has ever had speech/language therapy | 7.6 year | |
| DAWBA DSM-IV | A | Attention-deficit hyperactivity disorder diagnosis | 7.6 year−8.5 year | 74 |
| PERF_IQ | A | Performance IQ | 8.5 year | 75 |
See Table S1 in Supplement 1 for more details.
Specifies whether the measure was used for assignment (A) of case status or as phenotype (P) for quantitative analysis.
Core measure for RD.
Core measure for specific language impairment.
Quantitative Genetic Analysis
We analyzed 19 SNPs for association with READ and NW_REPT to replicate previous findings with RD and SLI, respectively (Table S3 in Supplement 1). This analysis was conducted in the F1 sample (Figure 1). DCDC2, KIAA0319, and CMIP showed associations with READ (Table 3 and Table S4 in Supplement 1). The association with rs2143340 (KIAA0319) was statistically significant (p < .0023) and in the same direction as previously reported. The only signal observed for NW_REPT was with the DCDC2 rs793862 marker (p = .03).
Table 3.
Associations Results of the 19 SNPs Tested in F1 with READ and NW_REPT
| READ |
NW-REPT |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr. | Gene Locus | SNP | n | β | SE | p | n | β | SE | p | Risk Allele |
| 2 | MRPL19/C2ORF3 | rs1000585 | 3,050 | .00 | .03 | .972 | 3,048 | .00 | .03 | .928 | |
| 2 | MRPL19/C2ORF3 | rs917235 | 3,165 | .00 | .03 | .949 | 3,163 | −.02 | .03 | .353 | |
| 2 | MRPL19/C2ORF3 | rs714939 | 3,041 | .02 | .03 | .427 | 3,039 | .01 | .03 | .646 | |
| 6 | DCDC2 | rs793862 | 3,117 | −.08 | .03 | .006 | 3,115 | −.06 | .03 | .031 | A (minor) |
| 6 | DCDC2 | rs807701 | 3,193 | −.05 | .03 | .033 | 3,191 | −.03 | .03 | .185 | G (minor) |
| 6 | DCDC2 | rs807724 | 3,085 | −.07 | .03 | .015 | 3,083 | −.03 | .03 | .257 | C (minor) |
| 6 | DCDC2 | rs1087266 | 3,198 | −.03 | .03 | .219 | 3,196 | .00 | .03 | .915 | |
| 6 | KIAA0319 | rs761100 | 3,190 | −.03 | .03 | .211 | 3,188 | −.01 | .03 | .603 | |
| 6 | KIAA0319 | rs6935076 | 3,006 | .07 | .03 | .011 | 3,004 | .02 | .03 | .482 | G (major)a |
| 6 | KIAA0319 | rs2038137 | 3,053 | −.02 | .03 | .374 | 3,051 | −.02 | .03 | .544 | |
| 6 | KIAA0319 | rs9461045 | 3,126 | −.08 | .03 | .024 | 3,124 | −.03 | .03 | .368 | T (minor) |
| 6 | KIAA0319b | rs2143340 | 3,042 | −.11 | .04 | .001 | 3,040 | −.04 | .04 | .242 | G (minor) |
| 16 | CMIP | rs12927866 | 3,055 | −.07 | .03 | .005 | 3,053 | −.04 | .03 | .136 | T (minor)a |
| 16 | CMIP | rs6564903 | 3,157 | −.08 | .02 | .002 | 3,155 | −.02 | .02 | .360 | T (minor)a |
| 16 | CMIP | rs4265801 | 3,052 | .02 | .03 | .449 | 3,050 | .03 | .03 | .289 | |
| 16 | CMIP | rs16955705 | 3,050 | −.06 | .03 | .029 | 3,048 | −.02 | .03 | .482 | C (minor)a |
| 16 | ATP2C2 | rs16973771 | 3,009 | .01 | .03 | .691 | 3,007 | .02 | .03 | .493 | |
| 16 | ATP2C2 | rs2875891 | 3,049 | .00 | .03 | .950 | 3,047 | .02 | .03 | .458 | |
| 16 | ATP2C2 | rs8045507 | 3,046 | .00 | .03 | .979 | 3,044 | .01 | .03 | .588 | |
Only one p value was statistically significant (< .0023; Methods in Supplement 1) and is highlighted in bold; β (beta) values are standardized and relative to the minor allele (as defined in Table S3 in Supplement 1). Risk allele is reported only for markers showing p values < .05.
SNP, single nucleotide polymorphism.
Opposite trend compared with original reports (24,36).
Within TTRAP.
To follow up the results observed with READ and NW_REPT, we analyzed the SNPs showing p values < .05 with the other available reading and language-related measures (Table S5 in Supplement 1). We detected the strongest associations with SPELL (DCDC2, KIAA0319, and CMIP; Table 4) and other weak signals with NW_READ (DCDC2 and KIAA0319; minimum p = .01) and MEMSPAN (CMIP; minimum p = .03). DCDC2 yielded slightly stronger associations with SPELL than READ, consistent with previous findings where DCDC2 was originally identified in a sample of individuals with spelling impairments (31). This analysis suggests that the KIAA0319, DCDC2, and CMIP genes contribute specifically to reading abilities and in particular to single-word reading and single-word spelling tests.
Table 4.
Summary of Results Showing Association (p < .05) with Quantitative Measures
| Chr. | Gene Locus | SNP | F1 |
F2 |
F3 |
F4: Unaffected |
Risk Allele | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| READ | |||||||||||||||||||
| N | β | SE | P | N | β | SE | P | N | β | SE | P | N | β | SE | P | ||||
| 6 | DCDC2 | rs793862 | 3,117 | –.08 | .03 | .006 | 2,936 | –.09 | .03 | .004 | 2,890 | –.08 | .03 | .010 | 2,740 | –.06 | .03 | .042 | A (minor) |
| 6 | DCDC2 | rs807701 | 3,193 | –.05 | .03 | .033 | 3,003 | –.04 | .03 | .090 | 2,954 | –.03 | .03 | .276 | 2,803 | –.02 | .02 | .376 | G (minor) |
| 6 | DCDC2 | rs807724 | 3,085 | –.07 | .03 | .015 | 2,898 | –.07 | .03 | .018 | 2,850 | –.05 | .03 | .091 | 2,700 | –.02 | .03 | .422 | C (minor) |
| 6 | KIAA0319 | rs6935076 | 3,006 | .07 | .03 | .011 | 2,831 | .08 | .03 | .003 | 2,784 | .07 | .03 | .006 | 2,646 | .05 | .02 | .028 | G (major)a |
| 6 | KIAA0319 | rs9461045 | 3,126 | –.08 | .03 | .024 | 2,947 | –.08 | .03 | .026 | 2,901 | –.08 | .03 | .022 | 2,752 | –.05 | .03 | .162 | T (minor) |
| 6 | KIAA0319b | rs2143340 | 3,042 | –.11 | .04 | .001 | 2,864 | –.12 | .04 | .001 | 2,817 | –.12 | .04 | .001 | 2,677 | –.11 | .03 | .001 | G (minor) |
| 16 | CMIP | rs12927866 | 3,055 | –.07 | .03 | .005 | 2,874 | –.08 | .03 | .004 | 2,829 | –.07 | .03 | .005 | 2,690 | –.07 | .02 | .005 | T (minor)a |
| 16 | CMIP | rs6564903 | 3,157 | –.08 | .02 | .002 | 2,966 | –.08 | .03 | .002 | 2,919 | –.08 | .03 | .002 | 2,768 | –.07 | .02 | .002 | T (minor)a |
| 16 | CMIP | rs16955705 | 3,050 | –.06 | .03 | .029 | 2,869 | –.06 | .03 | .022 | 2,824 | –.06 | .03 | .019 | 2,684 | –.05 | .02 | .027 | C (minor)a |
| SPELL | |||||||||||||||||||
| 6 | DCDC2 | rs793862 | 3,094 | –.09 | .03 | .003 | 2,913 | –.09 | .03 | .003 | 2,871 | –.08 | .03 | .009 | 2,729 | –.06 | .03 | .030 | A (minor) |
| 6 | DCDC2 | rs807724 | 3,065 | –.08 | .03 | .007 | 2,878 | –.08 | .03 | .011 | 2,834 | –.06 | .03 | .050 | 2,691 | –.04 | .03 | .204 | C (minor) |
| 6 | KIAA0319b | rs2143340 | 3,023 | –.10 | .04 | .004 | 2,845 | –.10 | .04 | .005 | 2,802 | –.11 | .04 | .004 | 2,669 | –.10 | .04 | .006 | G (minor) |
| 16 | CMIP | rs12927866 | 3,036 | –.06 | .03 | .014 | 2,855 | –.07 | .03 | .009 | 2,814 | –.07 | .03 | .011 | 2,682 | –.06 | .03 | .014 | T (minor)a |
| 16 | CMIP | rs6564903 | 3,136 | –.07 | .02 | .008 | 2,945 | –.07 | .03 | .003 | 2,901 | –.07 | .03 | .004 | 2,758 | –.07 | .02 | .008 | T (minor)a |
| 16 | CMIP | rs16955705 | 3,030 | –.06 | .03 | .026 | 2,849 | –.06 | .03 | .019 | 2,808 | –.06 | .03 | .017 | 2,675 | –.06 | .03 | .027 | C (minor)a |
Only SNPs showing p values < .05 in any group tested are reported; p values statistically significant (< .0023; Methods in Supplement 1) are in bold; β (beta) values are standardized and relative to the minor allele (as defined in Table S3 in Supplement 1).
SNP, single nucleotide polymorphism.
Opposite trend compared with original reports (24,36).
Within TTRAP.
We then tested whether the associations with READ and SPELL in this population cohort were driven by the inclusion of impaired individuals (Figure 1; Methods in Supplement 1). First we tested a sample that retained the unaffected and RD cases but excluded any cases with pure SLI and/or pure ADHD (F2). Then we removed the cases of RD that had comorbidity with SLI, ADHD, or both (F3). Finally, we tested for association in the unaffected group only (F4). This analysis revealed different patterns of association underlying the results detected in F1 (Table 4 and Table S4 and S6 in Supplement 1). Specifically, the data show that the DCDC2 associations are indeed driven by the small proportion of individuals with RD. For example, rs793862 is associated with READ (p = .004) and SPELL (p = .003) in the subgroup including all cases with RD (F2) and showing similar signal strength to F1 (p = .006, READ; p = .003, SPELL). The associations become progressively weaker when removing the approximately 50 RD cases comorbid with SLI or ADHD (p ∼.01; F3) and in the unaffected group (F4; minimum p = .03). Conversely, the SNPs that showed the strongest associations at the KIAA0319 locus (rs2143340) and in CMIP (rs6564903) had similar effect sizes in the different subgroups with little variation from F1 to F4. Two other SNPs in CMIP (rs12927866 and rs16955705) showed the same pattern. However, two of the other four SNPs tested in KIAA0319 (rs6935076 and rs9461045) showed modest associations with a pattern similar to DCDC2, where association disappeared in the unaffected subgroup (F4).
The associations with DCDC2 show the same allelic trends as previously reported (Table 3 and Table S3 in Supplement 1). This is also the case for KIAA0319, with the exception of rs6935076, which showed the opposite trend from the original report in a UK sample of individuals with RD (24). This is surprising because the major allele of rs6935076, which we found to be associated with poor reading, is in high linkage disequilibrium with all the other associated alleles at this locus in populations of European descendent (ALSPAC and our cohort of dyslexic individuals). Regarding CMIP, the original study was conducted in two samples: a cohort of individuals with SLI and a subgroup derived from ALSPAC for being language impaired. The associations showed opposite trend between these two samples (36). Our present associations show a trend consistent with the original report in that ASLPAC subgroup.
In summary, our data suggest that DCDC2 has a specific effect on RD, while CMIP and one variant at the KIAA0319 locus (rs2143340) are significantly associated with general reading abilities. The actual location of rs2143340 is within the gene TTRAP, but it is in linkage disequilibrium with KIAA0319 variants and is tagging the risk haplotype that originally refined the association to KIAA0319 (26). The other two KIAA0319 markers, showing an association pattern suggestive of a more specific role in RD, are instead located in the first intron (rs6935076) or regulatory sequences (rs9461045) of KIAA0319.
As well as positive findings, we also report lack of replications. We could not detect associations between NW_REPT and the language candidates CMIP and ATP2C2 in the general population. This is consistent with our previous study, which found associations with nonword repetition for CMIP and ATP2C2 only in a subgroup of individuals with language impairment (36). Our data do not support the role of the MRPL19/C2ORF3 locus in influencing reading abilities. MRPL19/C2ORF3 was tested using both single markers and haplotypes according to previous reports (35), but none showed any associations (haplotype analysis not shown).
Case−Control Analysis
To test directly for association between the candidate genes and RD or SLI, we analyzed the 19 SNPs in a case−control setting. We used four subgroups of cases against a unique control group (see Methods and Materials; Figure 1). The different subgroups of cases included individuals with SLI only, RD only, SLI including cases showing comorbidity for RD and/or ADHD, and RD including cases showing comorbidity for SLI and/or ADHD. The strongest associations were observed for DCDC2 (Table 5 and Table S7 in Supplement 1) in the cases selected for RD and including individuals with comorbidity with SLI and ADHD (minimum p = .003). Other association signals were observed for KIAA0319 in the RD cases regardless of comorbidity with SLI and ADHD.
Table 5.
Summary of the Results of the Case−Control Analysis
| Chr. | Gene Locus | SNP | No. of Controls | SLI Only |
SLI and Comorbid Cases |
RD Only |
RD and Comorbid Cases |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | p | Odds Ratio | n | p | Odds Ratio | n | p | Odds Ratio | n | p | Odds Ratio | Risk Allele | ||||
| 2 | MRPL19/C2ORF3 | rs917235 | 375 | 162 | .033 | 1.33 | 211 | .103 | 1.22 | 148 | .610 | 1.07 | 197 | .672 | 1.06 | G (minor) |
| 6 | DCDC2 | rs793862 | 375 | 155 | .418 | 1.13 | 201 | .101 | 1.26 | 150 | .021 | 1.42 | 196 | .005 | 1.47 | a (minor) |
| 6 | DCDC2 | rs807701 | 379 | 161 | .173 | 1.21 | 210 | .016 | 1.36 | 151 | .173 | 1.21 | 200 | .018 | 1.36 | G (minor) |
| 6 | DCDC2 | rs807724 | 371 | 158 | .754 | 1.05 | 206 | .146 | 1.24 | 150 | .035 | 1.40 | 198 | .003 | 1.52 | C (minor) |
| 6 | KIAA0319 | rs6935076 | 363 | 149 | .993 | 1.00 | 196 | .661 | .95 | 138 | .026 | .72 | 185 | .011 | .71 | G (major)a |
| 6 | KIAA0319 | rs9461045 | 375 | 153 | .692 | 1.08 | 199 | .561 | 1.10 | 149 | .026 | 1.47 | 195 | .035 | 1.40 | T (minor) |
RD, reading disability; SLI, specific language impairment; SNP, single nucleotide polymorphism.
Only SNPs showing p values < .05 in any of group tested are reported.
Opposite trend compared with original report (24).
These results complement the findings observed in the quantitative analysis and support the idea that DCDC2 is associated with RD. The SNPs rs793862 and rs807724 consistently showed the strongest associations for DCDC2 in both the quantitative and the case−control analysis. This also agrees with our recent case−control analysis of these candidate genes in samples of individuals with RD where the DCDC2 rs807724 marker showed the strongest association (rs793862 was not tested) (52). The case−control analysis of KIAA0319 also agrees with the quantitative analysis, but the associations were of modest size. Associations in the case−control analysis were detected for rs6935076 and rs9461045 in the RD samples; interestingly, both these SNPs showed an association pattern in the quantitative analysis suggestive of a specific effect on RD. Conversely, the rs2143340 marker, which showed the strongest signal in the quantitative analysis and was associated with variation in the normal range, was not associated with RD in the case−control analysis.
The only other observed signal was for the MRPL19/C2ORF3 locus showing a weak association with SLI. Our analysis found no role of MRPL19/C2ORF3 in contributing to RD, nor was there any evidence that CMIP or ATP2C2 influenced SLI.
In summary, our case−control analysis provides support for DCDC2 and suggestive evidence for KIAA0319 as candidate genes for RD. Inclusion of cases showing comorbidity between RD and SLI or ADHD contributed to the association signals.
Discussion
We have described a genetic association analysis of candidate genes for RD and SLI based on the ALSPAC children cohort. The large sample size made it possible to conduct the analysis in different sample subgroups to answer specific questions. First, we sought to replicate associations reported in clinical samples, and then we tested whether these associations are detectable with specific or multiple measures to understand whether shared genetic effects contribute to the comorbidity observed between RD and SLI. Our findings support association between DCDC2, KIAA0319, and CMIP specifically with reading measures, but not for associations of MRPL19/C2ORF3 with RD nor of ATP2C2 or CMIP with language measures. We did not detect any pleiotropic effect, which could partly explain the comorbidity between RD and SLI, although CMIP, selected as a candidate for language disorder, showed association with reading.
Our strategy tested whether associations were driven by the most severe individuals. We assessed the contribution to associations of individuals that meet criteria for disorder diagnosis by removing them from the quantitative analysis (Table 4) or evaluating them directly in case−control tests (Table 5). To the best of our knowledge this is the first study that used such a strategy. The association signals we detected were supported by complementary results obtained in the two types of analysis. We show that rs2143340, the most strongly associated marker at the KIAA0319 locus, and CMIP variants are significantly associated with reading and spelling skills regardless of the inclusion of the RD individuals. Consistently, these SNPs did not show associations in the case−control analysis, supporting the hypothesis that these variants contribute to reading ability variation in the normal range. Conversely, the associations detected for DCDC2 are driven by the most impaired individuals; the associations disappear from the quantitative analysis when RD cases are removed and DCDC2 showed the strongest associations in case−control analysis. These findings suggest that DCDC2 is associated specifically with RD. A pattern similar to DCDC2 is observed for two KIAA0319 markers. Interestingly, rs9461045, one of these two markers, has a functional effect on the expression of KIAA0319 (29). One could speculate that different genetic variants at the KIAA0319 locus have different effects with some variants involved in the general reading processes and other directly involved in RD.
Association between reading abilities in the general population and KIAA0319 and DCDC2 have been reported in previous studies (42,43), including our own analysis of KIAA0319 in ALSPAC (41). It would be interesting to see whether similar patterns will be observed in the Australian sample (42,43) when removing the most severely impaired individuals.
This is the first study reporting an effect of CMIP on the reading abilities of the general population. We previously analyzed CMIP and ATP2C2 in the ALSPAC sample and reported an association with nonword repetition for both genes but only in a specific subgroup of language impaired individuals (36). Both quantitative and case−control analyses were carried out within that specific subgroup, the latter by comparing the two tails of the phenotypic distribution. In that study, we also failed to detect any effect of CMIP and ATP2C2 on language skills in the entire ALSPAC cohort. Therefore, it is possible that the associations between nonword repetition and CMIP and ATP2C2 can only be detected on a background of language impairment. This is consistent with our current findings suggesting that these two genes cannot be considered as general susceptibility factors for SLI. The association between CMIP and reading instead represents a direct replication of our recent findings showing that CMIP is associated with reading measures in the same SLI cohort where it was originally found associated with nonword repetition (52).
It has been shown that SLI and RD share a common high heritability if the child had poor nonword repetition abilities (50,61). Therefore, we might expect to see evidence of overlapping genetic associations for RD and SLI that might only become apparent in samples with specific deficits. It might be possible that the same CMIP variants have an effect on both reading and language problems depending on the presence of other risk factors. Our data do not support a pleiotropic effect of KIAA0319 on reading and language-related measures as reported previously (51,52). One possible explanation is that the previously reported associations between KIAA0319 and language skills were confined to individuals selected as language-impaired. Another explanation could be the use of psychometric tests not available in ALSPAC. These included the Omnibus language test (62) reported by Rice et al. (51) and measures of expressive and receptive language based on the scales of the Clinical Evaluation of Language Fundamentals (CELF-R) (63) reported by Newbury et al. (52).
It is striking how the associations detected in this study are specific to the single-word reading and spelling tests and not to other reading or language-related measures, despite the correlation across measures. This observation does not exclude that KIAA0319, DCDC2, and CMIP affect additional cognitive functions not tested here. Nevertheless this is interesting in relation to the biological function proposed for some of these genes. KIAA0319 and DCDC2 have been shown to play a role during the development of the cerebral cortex by regulating neuronal migration, a critical step of cortex development (64). Defects in neuronal migration lead to several human syndromes with various degrees of symptoms from epilepsy to mental retardation (65). It is therefore notable that genes involved in such a general process can lead to specific disorders rather than have a broad impact on cognition or behavior. Subtle neuronal migration defects have been suggested to be causative of RD (66). With the data reported here, we reinforce the idea that KIAA0319 and DCDC2, with proven roles in neuronal migration, affect specific phenotypes.
Another important observation stems from our ability to test the effect of comorbidity on association analysis. We were able to show for the first time that inclusion of individuals with comorbid RD and SLI or ADHD do not weaken the association but rather can strengthen it, as in the case−control analysis of DCDC2 (Table 5). This may result from an increase in sample size by including comorbid cases. Given previous reports of associations between DCDC2 and ADHD (55), it is also possible that the associations we observe here for this gene are the combined effects of this gene on RD and ADHD separately. We could not test this hypothesis here because our ADHD sample was small (n = 39). In either case, these findings have an important implication. It is common practice to exclude individuals with SLI and ADHD when designing RD genetic studies to obtain samples as homogeneous as possible and to avoid confounding effects. Our data suggest that the same genes contribute to reading impairment even in the background of different disorders. This would imply also that the same cognitive deficit is at the basis of reading problems regardless of other clinical diagnoses. Providing that our observations are valid for other RD susceptibility genes, we suggest that individuals with RD comorbid for SLI or ADHD should not be excluded when designing genetic studies of RD, and their inclusion could improve sample power.
Acknowledgments
This study was funded by the United Kingdom Medical Research Council (Grant No. G0800523/86473) and a Wellcome Trust core grant (Grant No. 075491/Z/04). For the Avon Longitudinal Study of Parents and Children (ALSPAC) study, we thank the midwives for their help in recruiting the families, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The UK Medical Research Council (Grant No. 74882), the Wellcome Trust (Grant no. 076467) and the University of Bristol provided core support for ALSPAC. This publication is the work of the authors, and Silvia Paracchini will serve as guarantor for the contents of this article.
All authors reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online.
Supplementary data
References
- 1.Pennington B.F., Bishop D.V. Relations among speech, language, and reading disorders. Annu Rev Psychol. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- 2.Barry J.G., Yasin I., Bishop D.V. Heritable risk factors associated with language impairments. Genes Brain Behav. 2007;6:66–76. doi: 10.1111/j.1601-183X.2006.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher S.E., DeFries J.C. Developmental dyslexia: Genetic dissection of a complex cognitive trait. Nat Rev Neurosci. 2002;3:767–780. doi: 10.1038/nrn936. [DOI] [PubMed] [Google Scholar]
- 4.Snowling M., Bishop D.V., Stothard S.E. Is preschool language impairment a risk factor for dyslexia in adolescence? J Child Psychol Psychiatry. 2000;41:587–600. doi: 10.1111/1469-7610.00651. [DOI] [PubMed] [Google Scholar]
- 5.McArthur G.M., Hogben J.H., Edwards V.T., Heath S.M., Mengler E.D. On the “specifics” of specific reading disability and specific language impairment. J Child Psychol Psychiatry. 2000;41:869–874. [PubMed] [Google Scholar]
- 6.Burd L., Klug M.G., Coumbe M.J., Kerbeshian J. Children and adolescents with attention deficit-hyperactivity disorder: 1: Prevalence and cost of care. J Child Neurol. 2003;18:555–561. doi: 10.1177/08830738030180080101. [DOI] [PubMed] [Google Scholar]
- 7.Pennington B.F. From single to multiple deficit models of developmental disorders. Cognition. 2006;101:385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Beitchman J.H., Nair R., Clegg M., Ferguson B., Patel P.G. Prevalence of Psychiatric Disorders in Children with Speech and Language Disorders. J Am Academy Child Psychiatry. 1986;25:528–535. doi: 10.1016/s0002-7138(10)60013-1. [DOI] [PubMed] [Google Scholar]
- 9.Scerri T.S., Schulte-Korne G. Genetics of developmental dyslexia. Eur Child Adolesc Psychiatry. 2010;19:179–197. doi: 10.1007/s00787-009-0081-0. [DOI] [PubMed] [Google Scholar]
- 10.Newbury D.F., Fisher S.E., Monaco A.P. Recent advances in the genetics of language impairment. Genome Med. 2010;2:6. doi: 10.1186/gm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paracchini S., Scerri T., Monaco A.P. The genetic lexicon of dyslexia. Annu Rev Genomics Hum Genet. 2007;8:57–79. doi: 10.1146/annurev.genom.8.080706.092312. [DOI] [PubMed] [Google Scholar]
- 12.Hannula-Jouppi K., Kaminen-Ahola N., Taipale M., Eklund R., Nopola-Hemmi J., Kaariainen H., Kere J. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taipale M., Kaminen N., Nopola-Hemmi J., Haltia T., Myllyluoma B., Lyytinen H. A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proc Natl Acad Sci U S A. 2003;100:11553–11558. doi: 10.1073/pnas.1833911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brkanac Z., Chapman N.H., Matsushita M.M., Chun L., Nielsen K., Cochrane E. Evaluation of candidate genes for DYX1 and DYX2 in families with dyslexia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:556–560. doi: 10.1002/ajmg.b.30471. [DOI] [PubMed] [Google Scholar]
- 15.Dahdouh F., Anthoni H., Tapia-Paez I., Peyrard-Janvid M., Schulte-Korne G., Warnke A. Further evidence for DYX1C1 as a susceptibility factor for dyslexia. Psychiatr Genet. 2009;19:59–63. doi: 10.1097/YPG.0b013e32832080e1. [DOI] [PubMed] [Google Scholar]
- 16.Scerri T.S., Fisher S.E., Francks C., MacPhie I.L., Paracchini S., Richardson A.J. Putative functional alleles of DYX1C1 are not associated with dyslexia susceptibility in a large sample of sibling pairs from the UK. J Med Genet. 2004;41:853–857. doi: 10.1136/jmg.2004.018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wigg K.G., Couto J.M., Feng Y., Anderson B., Cate-Carter T.D., Macciardi F. Support for EKN1 as the susceptibility locus for dyslexia on 15q21. Mol Psychiatry. 2004;9:1111–1121. doi: 10.1038/sj.mp.4001543. [DOI] [PubMed] [Google Scholar]
- 18.Marino C., Citterio A., Giorda R., Facoetti A., Menozzi G., Vanzin L. Association of short-term memory with a variant within DYX1C1 in developmental dyslexia. Genes Brain Behav. 2007;6:640–646. doi: 10.1111/j.1601-183X.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 19.Cope N.A., Hill G., van den Bree M., Harold D., Moskvina V., Green E.K. No support for association between dyslexia susceptibility 1 candidate 1 and developmental dyslexia. Mol Psychiatry. 2005;10:237–238. doi: 10.1038/sj.mp.4001596. [DOI] [PubMed] [Google Scholar]
- 20.Bellini G., Bravaccio C., Calamoneri F., Donatella Cocuzza M., Fiorillo P., Gagliano A. No evidence for association between dyslexia and DYX1C1 functional variants in a group of children and adolescents from southern Italy. J Mol Neurosci. 2005;27:311–314. doi: 10.1385/jmn:27:3:311. [DOI] [PubMed] [Google Scholar]
- 21.Meng H., Hager K., Held M., Page G.P., Olson R.K., Pennington B.F. TDT-association analysis of EKN1 and dyslexia in a Colorado twin cohort. Hum Genet. 2005;118:87–90. doi: 10.1007/s00439-005-0017-9. [DOI] [PubMed] [Google Scholar]
- 22.Paracchini S., Ang Q.W., Stanley F.J., Monaco A.P., Pennell C.E., Whitehouse A.J. Analysis of dyslexia candidate genes in the Raine cohort representing the general Australian population. Genes Brain Behav. 2011;10:158–165. doi: 10.1111/j.1601-183X.2010.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harold D., Paracchini S., Scerri T., Dennis M., Cope N., Hill G. Further evidence that the KIAA0319 gene confers susceptibility to developmental dyslexia. Mol Psychiatry. 2006;11:1085–1091. doi: 10.1038/sj.mp.4001904. 1061. [DOI] [PubMed] [Google Scholar]
- 24.Cope N., Harold D., Hill G., Moskvina V., Stevenson J., Holmans P. Strong evidence that KIAA0319 on chromosome 6p Is a Susceptibility Gene for Developmental Dyslexia. Am J Hum Genet. 2005;76:581–591. doi: 10.1086/429131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deffenbacher K.E., Kenyon J.B., Hoover D.M., Olson R.K., Pennington B.F., DeFries J.C., Smith S.D. Refinement of the 6p21.3 quantitative trait locus influencing dyslexia: Linkage and association analyses. Hum Genet. 2004;115:128–138. doi: 10.1007/s00439-004-1126-6. [DOI] [PubMed] [Google Scholar]
- 26.Francks C., Paracchini S., Smith S.D., Richardson A.J., Scerri T.S., Cardon L.R. A 77-kilobase region of chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. Am J Hum Genet. 2004;75:1046–1058. doi: 10.1086/426404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan D.E., Gayan J., Ahn J., Won T.W., Pauls D., Olson R.K. Evidence for linkage and association with reading disability on 6p21.3-22. Am J Hum Genet. 2002;70:1287–1298. doi: 10.1086/340449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paracchini S., Thomas A., Castro S., Lai C., Paramasivam M., Wang Y. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- 29.Dennis M.Y., Paracchini S., Scerri T.S., Prokunina-Olsson L., Knight J.C., Wade-Martins R. A common variant associated with dyslexia reduces expression of the KIAA0319 gene. PLoS Genet. 2009;5:e1000436. doi: 10.1371/journal.pgen.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng H., Smith S.D., Hager K., Held M., Liu J., Olson R.K. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proceedings of the Natl Academy Sci U S A. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher J., Anthoni H., Dahdouh F., Konig I.R., Hillmer A.M., Kluck N. Strong genetic evidence of DCDC2 as a susceptibility gene for dyslexia. Am J Hum Genet. 2006;78:52–62. doi: 10.1086/498992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcke A., Weissfuss J., Kirsten H., Wolfram G., Boltze J., Ahnert P. The role of gene DCDC2 in German dyslexics. Ann Dyslexia. 2009;59:1–11. doi: 10.1007/s11881-008-0020-7. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig K.U., Schumacher J., Schulte-Korne G., Konig I.R., Warnke A., Plume E. Investigation of the DCDC2 intron 2 deletion/compound short tandem repeat polymorphism in a large German dyslexia sample. Psychiatr Genet. 2008;18:310–312. doi: 10.1097/YPG.0b013e3283063a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czamara D., Bruder J., Becker J., Bartling J., Hoffmann P., Ludwig K.U. Association of a rare variant with mismatch negativity in a region between KIAA0319 and DCDC2 in dyslexia. Behav Genet. 2011;41:110–119. doi: 10.1007/s10519-010-9413-6. [DOI] [PubMed] [Google Scholar]
- 35.Anthoni H., Zucchelli M., Matsson H., Muller-Myhsok B., Fransson I., Schumacher J. A locus on 2p12 containing the co-regulated MRPL19 and C2ORF3 genes is associated to dyslexia. Hum Mol Genet. 2007;16:667–677. doi: 10.1093/hmg/ddm009. [DOI] [PubMed] [Google Scholar]
- 36.Newbury D.F., Winchester L., Addis L., Paracchini S., Buckingham L.L., Clark A. CMIP and ATP2C2 modulate phonological short-term memory in language impairment. Am J Hum Genet. 2009;85:264–272. doi: 10.1016/j.ajhg.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SLI Consortium (SLIC) Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am J Hum Genet. 2004;74:1225–1238. doi: 10.1086/421529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golding J., Pembrey M., Jones R. ALSPAC—The Avon Longitudinal Study of Parents and Children: I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 39.Vernes S.C., Newbury D.F., Abrahams B.S., Winchester L., Nicod J., Groszer M. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai C.S., Fisher S.E., Hurst J.A., Vargha-Khadem F., Monaco A.P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 41.Paracchini S., Steer C.D., Buckingham L.L., Morris A.P., Ring S., Scerri T. Association of the KIAA0319 dyslexia susceptibility gene with reading skills in the general population. Am J Psychiatry. 2008;165:1576–1584. doi: 10.1176/appi.ajp.2008.07121872. [DOI] [PubMed] [Google Scholar]
- 42.Luciano M., Lind P.A., Duffy D.L., Castles A., Wright M.J., Montgomery G.W. A haplotype spanning KIAA0319 and TTRAP is associated with normal variation in reading and spelling ability. Biol Psychiatry. 2007;62:811–817. doi: 10.1016/j.biopsych.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Lind P.A., Luciano M., Wright M.J., Montgomery G.W., Martin N.G., Bates T.C. Dyslexia and DCDC2: Normal variation in reading and spelling is associated with DCDC2 polymorphisms in an Australian population sample. Eur J Hum Genet. 2010;18:668–673. doi: 10.1038/ejhg.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bates T.C., Lind P.A., Luciano M., Montgomery G.W., Martin N.G., Wright M.J. Dyslexia and DYX1C1: Deficits in reading and spelling associated with a missense mutation. Mol Psychiatry. 2009;15:1190–1196. doi: 10.1038/mp.2009.120. [DOI] [PubMed] [Google Scholar]
- 45.Shaywitz S.E. Dyslexia. N Engl J Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- 46.Bishop D.V., North T., Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: Evidence from a twin study. J Child Psychol Psychiatry. 1996;37:391–403. doi: 10.1111/j.1469-7610.1996.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 47.Bishop D.V., Laws G., Adams C., Norbury C.F. High heritability of speech and language impairments in 6-year-old twins demonstrated using parent and teacher report. Behav Genet. 2006;36:173–184. doi: 10.1007/s10519-005-9020-0. [DOI] [PubMed] [Google Scholar]
- 48.Bishop D.V., Hayiou-Thomas M.E. Heritability of specific language impairment depends on diagnostic criteria. Genes Brain Behav. 2008;7:365–372. doi: 10.1111/j.1601-183X.2007.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bishop D.V., McDonald D. Identifying language impairment in children: Combining language test scores with parental report. Int J Lang Commun Disord. 2009;44:600–615. doi: 10.1080/13682820802259662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bishop D.V., Snowling M.J. Developmental dyslexia and specific language impairment: Same or different? Psychol Bull. 2004;130:858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- 51.Rice M.L., Smith S.D., Gayan J. Convergent genetic linkage and associations to language, speech and reading measures in families of probands with specific language Impairment. J Neurodev Disord. 2009;1:264–282. doi: 10.1007/s11689-009-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newbury D.F., Paracchini S., Scerri T.S., Winchester L., Addis L., Walter J. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behav Genet. 2011;41:90–104. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marino C., Mascheretti S., Riva V., Cattaneo F., Rigoletto C., Rusconi M. Pleiotropic effects of DCDC2 and DYX1C1 genes on language and mathematics traits in nuclear families of developmental dyslexia. Behav Genet. 2011;41:67–76. doi: 10.1007/s10519-010-9412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willcutt E.G., Pennington B.F., Smith S.D., Cardon L.R., Gayan J., Knopik V.S. Quantitative trait locus for reading disability on chromosome 6p is pleiotropic for attention-deficit/hyperactivity disorder. Am J Med Genet. 2002;114:260–268. doi: 10.1002/ajmg.10205. [DOI] [PubMed] [Google Scholar]
- 55.Couto J.M., Gomez L., Wigg K., Ickowicz A., Pathare T., Malone M. Association of attention-deficit/hyperactivity disorder with a candidate region for reading disabilities on chromosome 6p. Biol Psychiatry. 2009;66:368–375. doi: 10.1016/j.biopsych.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lesch K.P., Timmesfeld N., Renner T.J., Halperin R., Roser C., Nguyen T.T. Molecular genetics of adult ADHD: Converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115:1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- 57.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snowling M.J., Muter V., Carroll J. Children at family risk of dyslexia: A follow-up in early adolescence. J Child Psychol Psychiatry. 2007;48:609–618. doi: 10.1111/j.1469-7610.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 59.Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 60.Wolke D., Waylen A., Samara M., Steer C., Goodman R., Ford T., Lamberts K. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. Br J Psychiatry. 2009;195:249–256. doi: 10.1192/bjp.bp.108.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bishop D.V. Genetic influences on language impairment and literacy problems in children: Same or different? J Child Psychol Psychiatry. 2001;42:189–198. [PubMed] [Google Scholar]
- 62.Zimmerman I.L., Steiner V.G., Pond R.E. Psychological Corporation; San Antonio, TX: 1992. Preschool Language Scale—3. [Google Scholar]
- 63.Semel Em W.E.H., Secord W. Psychological Corporation; San Antonio, TX: 1992. Clinical Evaluation of Language Fundamentals—Revised. [Google Scholar]
- 64.Galaburda A.M., LoTurco J., Ramus F., Fitch R.H., Rosen G.D. From genes to behavior in developmental dyslexia. Nat Neurosci. 2006;9:1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- 65.McManus M.F., Golden J.A. Neuronal migration in developmental disorders. J Child Neurol. 2005;20:280–286. doi: 10.1177/08830738050200040301. [DOI] [PubMed] [Google Scholar]
- 66.Galaburda A.M., Sherman G.F., Rosen G.D., Aboitiz F., Geschwind N. Developmental dyslexia: Four consecutive patients with cortical anomalies. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 67.Rust J., Golombok S., Trickey G. Psychological Corporation; Sidcup, UK: 1993. WORD: Wechsler Objective Reading Dimensional Manual. [Google Scholar]
- 68.Nunes T., Bryant P., Olsson J. Learning morphological and phonological spelling rules: An intervention study. Sci Stud Reading. 2003;7:298–307. [Google Scholar]
- 69.Rosner J., Simon D.P. The auditory analysis test: An initial report. J Learn Disabilities. 1971;4:40–48. [Google Scholar]
- 70.Case R., Kurland D.M., Goldberg J. Operational efficiency and the growth of short-term memory span. J Exp Child Psychol. 1982;33:386–404. [Google Scholar]
- 71.Rust J. Psychological Corporation; London UK: 1996. WOLD Wechsler Objective Language Dimensions Manual. [Google Scholar]
- 72.Gathercole S.E., Willis C.S., Baddeley A.D., Emslie H. The Children's Test of Nonword Repetition: A test of phonological working memory. Memory. 1994;2:103–127. doi: 10.1080/09658219408258940. [DOI] [PubMed] [Google Scholar]
- 73.Bishop D.V. Development of the Children's Communication Checklist CCC: A method for assessing qualitative aspects of communicative impairment in children. J Child Psychol Psychiatry. 1998;39:879–891. [PubMed] [Google Scholar]
- 74.Goodman R., Ford T., Richards H., Gatward R., Meltzer H. The development and well-being assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- 75.Wechsler D., Golombok S., Rust J. Psycological Corporation; Sidcup, UK: 1992. WISC-IIIUK: Wechsler Intelligence Scale for Children. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.