Abstract
Objective
Noncardiac chest pain (NCCP) is a common and persistent problem for children and adolescents; typically there is no clear medical cause. To date, no psychological intervention has been studied for chest pain in a pediatric sample.
Methods
a) We developed a brief psychological treatment for chest pain and associated worry in children and adolescents with NCCP. This program includes psychoeducation, breathing retraining, cognitive coping strategies, and one session of parent education and coaching regarding the impact of reinforcement on pain and coping behaviors. b) We treated nine youngsters with chronic NCCP, assessing pain, somatization, disability, anxiety and depressive symptoms, and coping before, after, and six months following treatment.
Results
Following treatment there was a significant decrease in chest pain and somatization. Benefits were maintained at six-month follow-up. There was no decrease in associated psychological symptoms.
Conclusions
A brief psychological treatment for pediatric NCCP is feasible to administer and may help alleviate symptoms of pediatric NCCP. Further study in a randomized trial is needed.
Keywords: Chest pain, pediatric, somatization, cognitive behavior therapy, coping
Chest pain is a common complaint in childhood and adolescence appearing in about ten percent of school-aged children.1 It is a common reason for medical visits accounting for over 600,000 annual visits in the United States.2 Prior to adulthood, positive cardiac findings are rare3-5 and most cases have no clear medical etiology.6 Nevertheless, pain often persists for years3,7 and is associated with a range of difficulties including school absence, sleep problems, restriction of activities3 and anxiety symptoms.7 In a large longitudinal study 54% of those with chest pain had missed school or work and 46% had been woken from their sleep due to chest pain.8 Despite concerns about the role of psychological factors in pediatric noncardiac chest pain (NCCP), no psychosocial intervention has been studied for its treatment.
We conceptualized pediatric NCCP within a cognitive behavioral framework, which sees symptoms as perpetuated through a cycle of cognitive, behavioral and physiological factors.9 Cognitive behavior therapy (CBT) aims to break this cycle by enhancing coping skills and a through a range of other strategies including contingency management, cognitive restructuring, and breathing retraining. A central feature of NCCP is catastrophic appraisal of chest pain as indicating serious illness.10,11 This appraisal is remedied through psychoeducation, cognitive monitoring and restructuring of catastrophic thoughts. Evidence from randomized trials supports the efficacy of CBT for NCCP.12
We developed a brief CBT intervention to alleviate pediatric NCCP by adapting components of treatments for other pediatric somatic syndromes such as recurrent abdominal pain.13 We also included techniques used for adult NCCP.14,10 To gather preliminary data regarding acceptability, feasibility, and potential efficacy of this intervention, we conducted a small open trial with nine youngsters with NCCP of at least six months duration. We hypothesized that this brief CBT would decrease chest pain (primary outcome) a) following treatment and b) at six month follow-up. We assessed a number of dimensions of NCCP severity (discomfort, worry, and frequency), pervasiveness of somatic symptoms, functional impact, and anxiety and depressive symptoms. Because one goal of CBT is to enhance coping skills, we measured changes in the child’s appraisal of problem-focused and emotion focused coping.
Methods
Participants
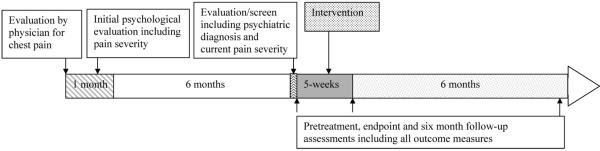
Participants were evaluated for complaints of chest pain in the pediatric cardiology clinic or emergency department of a large metropolitan US medical center. Children and adolescents (ages 8-18 years) whom the evaluating physician determined were free of cardiac or other acute medical illness based on minimum of history, physical examination and electrocardiogram were evaluated on a range of psychological dimensions including pain and associated features within one month of the medical visit and again six months later as part of a larger epidemiologic study (see Figure 1). Those who rated intensity of chest pain as at least moderate (≥3 on a pain scale of 0-5) at both time points, had no history of psychotic symptoms or mania, or recent drug or alcohol use diagnosis, and in which the child and a parent could communicate in English were eligible for participation. Because of the experimental stage of the intervention, the Institutional Review Board stipulated that children with severe psychopathology could not be included.
Figure 1.
Timeline for children and adolescents with chest pain treated with a brief intervention.
Procedures
All procedures were approved by the Institutional Review Board (IRB) of the investigating university and affiliated medical center. Eligible families were contacted by telephone and offered participation in treatment free of charge. We obtained written informed consent from the parent and written assent from the child/adolescent before beginning the evaluation. We interviewed each youngster and a parent (about the youngster) using the Anxiety Disorders Interview Schedule for Children, Fourth Edition, Child and Parent Versions (ADIS-C-IV).15 An ADIS-C-IV clinical severity rating (CSR) of four (on 0-8 scale) represents diagnostic threshold. Six (severe) was the threshold for exclusion due to severe psychopathology. Clinical assessments of pain and other outcome variables were conducted: 1) prior to initiating treatment, 2) at post-treatment (week five), and 3) at six-month follow-up (see Figure 1).
A doctoral level clinical psychologist with experience treating children provided therapy in an outpatient clinic. Treatment consisted of four individual visits, three of which included single 50-minute sessions with the child or adolescent and one which included an additional 50-minute session with one or both parents. Participants were reimbursed for public transportation. All sessions were taped and reviewed to ensure fidelity to the manual. Clinician ratings were made by specially trained, masters level independent evaluators (IE) with ≥ two years training in assessment of child psychopathology who were established as reliable based on matching on two independently rated cases and group ratings from audiotapes.
Manual development
To address the challenge of limited motivation for psychological treatment in many families presenting with somatic complaints we required an intervention which was brief in duration and which focused explicitly on alleviating chest pain rather than general distress. We adapted key components of efficacious CBT interventions for pediatric pain, including coping skills training and parent contingency training.13 We included specific techniques used for adult NCCP including re-attribution and expanded breathing retraining.14 We did not include distraction strategies used in some pain interventions 1) in order to streamline and 2) because it is unclear whether distraction might impede exposure-relevant mechanisms involved in overcoming anxiety,16 which is prominent in NCCP. As outlined in Table 1, each session focused on specific goals, procedures, and intervention targets which aim to alleviate chest pain and related distress. Developmentally appropriate handouts were adapted for two age levels, adolescents and children.
Table 1.
Outline of Goals and Procedures for Psychological Intervention for pediatric chest pain
| Visit | Goals | Procedures |
|---|---|---|
| 1. Psychoeducation |
|
|
| 2a. (Child) Breathing retraining |
|
|
| 2b. (Parent/s) “How you can help” |
|
|
| 3. “Taking charge of your thoughts about pain” |
|
|
| 4. “Pulling it all together” |
|
|
Assessments
Pain, somatization, and disability
The Faces Pain Scale-Revised (FPS-R)17 is a self rating of intensity of pain using faces depicting pain levels from 0 to 5. We asked the child to rate a) greatest intensity of pain for the past week and b) greatest level of discomfort. Using the same 0-5 scaling, the child rated degree of worry from pain, and frequency of episodes for the past week. The Clinical Global Impression (CGI)18 includes two global clinician ratings frequently used in treatment trials. a) Overall severity of condition is rated from 1-absent to 7-extremely severe and b) Change from baseline is rated from 1-very much improved to 4-unchanged to 7-very much worse; scores of 1 or 2 (much improved) are thought to indicate “response” or clinically significant change. The CGI is used to measure improvement in psychopathology in pediatric samples19 and has good reliability and validity when clear time frames and specific anchors are used as in this study.20 The Children’s Somatization Inventory (CSI) child version1 assesses overall somatization based on how much the child was bothered by any of 35 symptoms in the past 2 weeks (0-4). Symptoms include an array of complaints such as headaches, sore muscles, back pain, and chest pain. The CSI has good reliability and validity. The Functional Disability Inventory (FDI;Child version)21 is a 15-item self-report scale designed to assess impairment from abdominal pain. We adapted the scale to address chest pain. Each of 15 items reflects an activity (e.g., doing homework) which is rated on a 5-point scale (0-4). The child version has good internal consistency and validity.21
Psychological symptoms and coping
The Child Depression Inventory (CDI 22) is a widely used 27-item self-report scale of depressive symptoms with statement choices for each item rated from 0-2. The Multidimensional Anxiety Scale for Children23 is a widely used 39-item self-report scale designed to measure anxiety symptoms in children and adolescents. Items are rated from 0-never true about me to 3-often true about me. The Pain Beliefs Questionnaire (PBQ, Child version, PBQ)24 is a 32-item self-report scale which assess the child’s appraisal of her a) problem focused (or active) and b) emotion focused (or passive) coping potential. Items are rated from 0-not at all true to 4 very true.
Data Analysis
We first compared those who entered treatment with those who declined treatment on demographic and baseline features using chi square and t tests. To assess change from baseline to endpoint and baseline to follow-up, we used paired t tests. For (two) of the nine participants who dropped out before the final session, last observations were carried forward. Effect sizes were computed using a Hedges g. All tests were two-sided. Significance was set at p <.05.
Results
Sample
Of 25 cases screened for treatment, six denied moderate chest pain in the week of the screening and two had psychopathology rated as six or higher on the ADIS-C-IV. Of 17 eligible youngsters, nine (53%) agreed to participate. Seven of nine (78%) participants completed all five visits. Of the two who dropped out, one moved residence and one could not be reached.
Characteristics
There were no differences in demographic characteristics, pain or somatization between those who entered treatment and those who declined. Youngsters ranged in age from 10 to 18 years (M = 15.0; SD = 3.1). Seven of nine participants (78%) were male. Three were Hispanic, three were African American, two were Caucasian, and one was of mixed ethnicity.
Duration of chest pain at time of initiation of treatment ranged from six months to five years (M = 2.1; SD = 1.8). Disability as measured by FDI ranged from 0-17 (M=7.0; SD; 5.9) with commonly reported problems being participating in sports or gym (n=5), sleep problems (n=4), school problems (n=3), and climbing stairs (n=3). Somatization (CSI) ranged from 10 to 50 (M =22.6; SD=13.2). Five of nine (55%) participants were diagnosed with one or more current DSM-IV disorders including (overlapping as some had > 1 diagnosis) panic disorder (n=2), specific phobia (n=2), generalized anxiety disorder (n=1), social phobia (n=1), post traumatic stress disorder (n=1), separation anxiety disorder (n=1), and major depression (n=1).
Outcome: pain, somatization and disability
Self-rated pain intensity was decreased at post-treatment as was discomfort, worry, and frequency of pain episodes (Table 2). Clinician rating of overall clinical severity (CGI) also decreased significantly as did somatization. At six month follow-up, decreases remained significant relative to pretreatment. Effect sizes were generally large. Decrease in disability (FDI) was not significant. Patterns of improvement were identical at post-treatment and follow-up when only completers (n=7) were analyzed. Six of nine participants (67%) reported a decrease of 50% in pain intensity at post treatment. Five (55%) retained this level of decrease at follow-up. The same six (67%) were rated much improved or very much improved on the CGI by the IE; five were also rated this way at follow-up.
Table 2.
Pain and Associated Features at Baseline, Post-treatment, and Follow up for (N-9) participants with NCCP
| Treated Group (N=9) | Effects sizesb (95% confidence intervals) | ||||
|---|---|---|---|---|---|
| Measure | Pretreatment | Post- treatmenta |
Six monthsa |
Pre-treatment to Post-treatment |
Pre-treatment to six months |
| M (SD) | M (SD) | M (SD) | |||
| Pain, somatization and disability | |||||
| Faces – Pain intensity | 3.3 (0.9) | 1.7 (1.8)** | 1.8 (1.9)* | 1.78 ( 0.60 2.87) | 1.67 ( 0.60-2.74) |
| Discomfort | 3.1 (1.2) | 2.0 (2.0)* | 1.9 (2.1)* | 0.92 (−0.05 1.89) | 1.00 ( 0.02-1.98) |
| Worry | 3.0 (0.7) | 0.8 (1.3)** | 1.0 (1.2)* | 3.14 (1.76 4.52) | 2.86 (1.55 4.17) |
| Frequency | 2.2 (1.5) | 1.4 (1.7)* | 1.4 (1.7)* | 0.53 (−0.41 1.47) | .53 (−0.41 1.47) |
| Clinical Global Impression | 4.7 (0.9) | 3.3 (1.7)** | 3.2 (1.6)** | 1.56 (0.50 2.62) | 1.67 ( 0.60-2.74) |
| Child Somatization Inventory |
22.6 (13.2) | 14.1 (8.4)* | 14.7 (8.7)* | 0.64 (−0.31 1.59) | 0.60 (−0.34-1.54) |
| Functional Disability Index | 7.0 (5.9) | 3.8 (2.5) | 5.5 (4.3) | 0.54 (−0.40 1.48) | 0.25 (−0.68-1.18) |
| Associated psychological symptoms | |||||
| Child Depression Inventory | 7.9 (4.2) | 6.9 (5.5) | 7.2 (2.5) | 0.24 (−0.69 1.17) | 0.17(−0.76 1.10) |
| MASC – Total | 33.5 (13.5) | 26.9 (11.5) | 26.1 (8.5) | 0.49 (−0.45 1.43) | 0.55(−0.39 1.49) |
| MASC- Physical Symptoms | 10.3 (4.4) | 7.1 (2.7)* | 7.8 (3.5)* | −0.73 (−0.22 1.68) | 0.57 (−0.37 1.51) |
| Pain Beliefs | |||||
| Problem focused coping | 11.2(3.9) | 16.1(5.2)* | 15.4(6.9) | 1.26 (0.25 2.27) | 1.08(0.09 2.07) |
| Emotion focused coping | 16.2 (5.7) | 17.6(5.7) | 17.7(5.8) | 0.25 (−0.68 1.18) | 0.26(−0.67 1.19) |
Last observation carried forward for two children who dropped out of treatment and did not complete later assessments
Hedges g
Paired t-test compared to pretreatment significant at P <.05, two tailed test
Paired t-test compared to pretreatment significant p<.01, two tailed test
Outcome: Associated symptoms and coping
There was no change in depressive or anxiety symptoms at post-treatment or follow-up (Table 2). Participants reported a significant increase in problem focused coping potential following treatment. This increase was no longer significant at follow-up. There was no change in emotion focused coping.
Discussion
We developed a four-visit CBT intervention for pediatric NCCP drawing on strategies used for other pediatric pain syndromes and for adult NCCP. Results of a small open trial suggest that this treatment is feasible to administer and is acceptable to many families of children with chest pain. It is important to note that we offered treatment to all potentially eligible participants and not to patients who were seeking treatment or to those who were referred or encouraged to seek treatment by their physicians. However, the somewhat low proportion who accepted treatment (53%) may be a function of our offering this intervention six months after the index medical evaluation rather than at the time of the visit when motivation might be higher. Seven of nine participants (78%) completed all sessions, a rate comparable with completion rates of some other psychological interventions for pediatric pain.25 Significant decreases in pain and somatization and high rates of clinically significant change at post treatment and at six-month follow-up suggests that this brief intervention may help alleviate recurrent chest pain. Increase in problem focused coping appraisal, but no change in emotion focused coping highlights a possible mechanism for treatment effect.
Lack of change in disability, anxiety and depressive symptoms may be due to a floor effect as severe psychopathology was excluded and pretreatment symptom levels were not high. However, disability at baseline was similar to the level reported in a sample of youngsters with recurrent abdominal pain in primary care.26 Increasing the length and breadth of this intervention may broaden benefits. On the other hand, this could potentially decrease compliance. For adult NCCP, Mayou10 proposed a two-tier system in which all patients are offered a brief pain-focused intervention and some then opt to continue a more extensive psychological treatment. If this brief intervention demonstrates efficacy in a controlled trial, it could be used as the initial stage of such a two stage model.
This was a small uncontrolled trial. Although moderate chest pain was reported six months prior to treatment and again at pre-treatment, persistence of pain during the interim period was not assessed. Furthermore, ratings were based on child or adolescent report or by non-blinded clinician, based primarily on the child’s own descriptions and limited observation. Inclusion of parent reports of pain and disability would strengthen validity. Finally, youngsters with severe psychological symptoms were excluded from this first pilot testing and this restricted the range for some symptoms.
A growing body of literature supports the efficacy of targeted CBT interventions for a range of pediatric somatic syndromes including headache and recurrent abdominal pain26 and for adults with NCCP.12 The current study represents a first step in developing such an approach for pediatric NCCP. A randomized controlled trial could recruit children at the time of medical evaluation and news of cardiac health. Assessments should include a range of measures of pain and functioning as well as longer term follow up to determine durability of benefits from this brief intervention in developing youngsters.
Acknowledgments
This work was supported by a pilot award from the Child Psychiatry Research Center of New York State Psychiatric Institute NIMH 5P30MH060570 NIMH (Dr. Gur) and NIMH R01MH067912 (Dr. Lipsitz)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garber J, Walker LS, Zeman J. Somatization Symptoms in a Community Sample of Children and Adolescents: Further Validation of the Children’s Somatization Inventory. Psychological Assessment. 1991;3(4):588–595. [Google Scholar]
- 2.NHAMCS: National Hospital Ambulatory Medical Care Survey. National Center for Health Statistics; Hyattsville MD: [Google Scholar]
- 3.Selbst SM, Ruddy RM, Clark BJ, et al. Pediatric Chest Pain: A Prospective Study. Pediatrics. 1988;82(3):319–323. [PubMed] [Google Scholar]
- 4.Pantell RH, Goodman BW. Adolescent Chest Pain: A Prospective Study. Pediatrics. 1983;71(6):881–887. [PubMed] [Google Scholar]
- 5.Tunaoglu FS, Olguntürk R, Akcabay S, et al. Chest pain in children referred to a cardiology clinic. Pediatr Cardiol. 1995;16(2):69–72. doi: 10.1007/BF00796820. [DOI] [PubMed] [Google Scholar]
- 6.Kocis KC. Chest pain in pediatrics. Pediatr. Clin. North Am. 1999;46(2):189–203. doi: 10.1016/s0031-3955(05)70112-7. [DOI] [PubMed] [Google Scholar]
- 7.Lipsitz JD, Masia-Warner C, Apfel H, et al. Anxiety and Depressive Symptoms and Anxiety Sensitivity in Youngsters With Noncardiac Chest Pain and Benign Heart Murmurs. J. Pediatr. Psychol. 2004;29(8):607–612. doi: 10.1093/jpepsy/jsh062. [DOI] [PubMed] [Google Scholar]
- 8.Selbst SM, Ruddy RM, Clark BJ. Chest pain in children: Follow-up of patients previously reported. Clin Pediatrics. 1990;29(7):374–377. doi: 10.1177/000992289002900702. [DOI] [PubMed] [Google Scholar]
- 9.Deary V, Chalder T, Sharpe M. The cognitive behavioural model of medically unexplained symptoms: A theoretical and empirical review. Clin Psychol Review. 2007;27(7):781–797. doi: 10.1016/j.cpr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Mayou R. Chest pain, palpitations and panic. J Psychosom Res. 1998;44(1):53–70. doi: 10.1016/s0022-3999(97)00209-2. [DOI] [PubMed] [Google Scholar]
- 11.Salkovskis PM. Psychological treatment of noncardiac chest pain: The cognitive approach. Am J Med. 1992;92(5, Supplement 1):S114–S121. doi: 10.1016/0002-9343(92)80066-9. [DOI] [PubMed] [Google Scholar]
- 12.van Peski-Oosterbaan AS, Spinhoven P, van Rood Y, et al. Cognitive-behavioral therapy for noncardiac chest pain: a randomized trial. Am J Med. 1999;106(4):424–429. doi: 10.1016/s0002-9343(99)00049-2. [DOI] [PubMed] [Google Scholar]
- 13.Sanders MR, Shepherd RW, Cleghorn G, et al. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol. 1994;62(2):306–314. doi: 10.1037//0022-006x.62.2.306. [DOI] [PubMed] [Google Scholar]
- 14.DeGuire S, Gevirtz R, Kawahara Y, et al. Hyperventilation syndrome and the assessment of treatment for functional cardiac symptoms. Am. J. Cardiol. 1992;70(6):673–677. doi: 10.1016/0002-9149(92)90211-g. [DOI] [PubMed] [Google Scholar]
- 15.Silverman W, Albano A. Anxiety Disorders Interview for DSM-IV-Child Version. Psychological Corporation; 1996. [Google Scholar]
- 16.Parrish CL, Radomsky AS, Dugas MJ. Anxiety-control strategies: is there room for neutralization in successful exposure treatment? Clin Psychol Rev. 2008;28(8):1400–1412. doi: 10.1016/j.cpr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Hicks CL, von Baeyer CL, Spafford PA, et al. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 18.Guy W. ECDEU assessment manual for psychopharmacology. US Dept Health, Education, and Welfare publication (ADM); 1976. pp. 76–338. [Google Scholar]
- 19.Walkup JT, Labellarte MJ, Riddle MA, et al. Fluvoxamine for the Treatment of Anxiety Disorders in Children and Adolescents. N Engl J Med. 2001;344(17):1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 20.Zaider TI, Heimberg RG, Fresco DM, et al. Evaluation of the Clinical Global Impression Scale Among Individuals with Social Anxiety Disorder. Psychol Med. 2003;33(04):611–622. doi: 10.1017/s0033291703007414. [DOI] [PubMed] [Google Scholar]
- 21.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs M. Children’s Depression Inventory Manual. Multihealth; Tonawanda: 1992. [Google Scholar]
- 23.March JS, Parker JD, Sullivan K, et al. The Multidimensional Anxiety Scale for Children (MASC) J Am Acad Child Adolesc Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Walker LS, Smith CA, Garber J, et al. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24(4):364–374. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degotardi PJ, Klass ES, Rosenberg BS, et al. Development and Evaluation of a Cognitive-Behavioral Intervention for Juvenile Fibromyalgia. J. Pediatr. Psychol. 2006;31(7):714–723. doi: 10.1093/jpepsy/jsj064. [DOI] [PubMed] [Google Scholar]
- 26.Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113(4):817–824. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]