Abstract
If the definition of eliminating of a health disparity were signified by the absence of any differences in incidence or mortality between a population's experiences with a health problem, then the only health disparity that has ever been eliminated is smallpox because with zero cases of smallpox in the world, no health disparities exist because of smallpox. The eradication of smallpox is perhaps the only historical example where the elimination of a health disparity has been achieved. Principles and lessons learned, particularly through the intersection of science and policy that could be applied to the elimination of other health disparities both domestically and internationally are proposed.
Keywords: Health disparities, Liver Cancer
Introduction
Pursuing a National Policy for Preventing Hepatitis B-induced Liver Cancer: Implications for Eliminating Health Disparities
As stated in Healthy People 2010, one of our national goals is “to eliminate health disparities” (US Department of Health and Human Services [DHHS], 2000). Operationalizing this goal has yielded many definitions of health disparities (Carter-Pokras, Baquet, 2002; DHHS, 2004), including a legal definition of a “health disparity population” as being mutually determined by the Director of the National Center on Minority Health and Health Disparities and the Director of the Agency for Healthcare Research and Quality based on the presence of a “significant disparity in the overall rate of disease incidence, prevalence, morbidity, mortality, or survival rates in the population as compared to the health status of the general population” (Public Law 106-525, 2000). Examples of health disparities abound between and among population groups in the US (DHHS, 1985; Haynes, Smedley, 1999; Smedley, Stith, Nelson, 2003; Williams, Jackson, 2005; Kawachi, Daniels, Robinson, 2005; Satcher, Fryer, McCann, 2005; Lurie, 2005; Miller, Chu, Hankey, Ries, 2007) and various methods have been proposed to measure health disparities (Harper, Lynch, 2005, National Research Council, 2005) Despite these efforts, there is a paucity of examples of health disparities that have been eliminated.
If the elimination of a health disparity were signified by the absence of any differences in incidence or mortality between any population's experience with a health problem or disease, then the only health disparity that has ever been eliminated was smallpox in 1980 (Fenner, Henderson, Arita, Jezek, Ladnyi, 1988). Smallpox is the first disease that the World Health Organization has ever certified as being “eradicated”, i.e., “reduction of the worldwide incidence of a disease to zero as a result of deliberate efforts, obviating the necessity for further control measures” (Recommendations of the International Task Force for Disease Eradication, 1993; Henderson, 1999) and thus, no disparities exist. Thus, pursuing a national policy to eliminate health disparities should consider the example of smallpox.
“Disparities” are not simply “differences” in health status (Adler, 2006; Braveman & Gruskin, 2003). Rather, in our context, disparities are differences that may be attributable to potentially amenable socio-cultural and socioeconomic factors (DHHS, 2000). (Differences related to immutable factors, e.g., inherited attributes are not disparities.) Eliminating health disparities must transcend eliminating barriers to health care since even the adoption and implementation of the Institute of Medicine's Unequal Treatment (Smedley, Stith, Nelson, 2003) would be principally contributing to the reduction of health care disparities, not their absolute elimination (Adler, 2006). With the elimination of smallpox as a health disparity condition as the model, the purposes of this paper are as follows: (1) justify the focus of hepatitis B-related liver cancer and its parallels with smallpox as a next opportunity for eliminating health disparities both on a national and a global basis; (2) recommend policies that would advance the elimination of health disparities; and (3) distill principles that appear to be key factors in eliminating health disparities.
Rationale for the focus on hepatitis B-related liver cancer and its parallels with smallpox
We chose to focus on hepatitis B-related liver cancer as the example for this paper for three major reasons:
The hepatitis B virus (HBV) is a documented human carcinogen.
HBV-induced liver cancer is a documented health disparity for U.S. racial/ethnic minority populations.
HBV-related liver cancer mortality is preventable and can potentially be eliminated as a cause of death and disparities.
Hepatitis B virus as a human carcinogen
First, the hepatitis B virus (HBV) is a documented human carcinogen. In 2004, the U.S. Department of Health and Human Services Report on Carcinogens listed the HBV and the hepatitis C virus (HCV) as substances known to be human carcinogens, making them the first viruses declared to be etiologically related to hepatocellular carcinoma (HCC) (DHHS, 2004) and is responsible for about 90% of the cases (London, McGlynn, 2006). In fact, one-third of the world's population or two billion people are infected with HBV (World Health Organization [WHO], 2004) and at least 350 to 400 million (6% of world's population) are chronically infected as carriers (WHO, 2004; Ocama, Opio, Lee, 2005), making it among the most common infections in the world and responsible for HCC being the third leading cause of cancer mortality in the world (Parkin, 2001). Second to tobacco use, the most preventable cause of cancer is infection (Kuper, Adami, 2000), and because of tobacco and infections, cancer is expected to become the world's leading cause of death in 2010 (International Agency on Research in Cancer, 2008).
Disparities associated with HBV in U.S. racial/ethnic minority populations
While the incidence and mortality rates for the great majority of cancer sites in the U.S. have been steadily decreasing, liver cancer is one of the few cancer sites with increases in incidence (second highest among all cancer sites) and mortality (highest among all cancer sites) (Jemal, Siegal, Ward, Xu, Thun, 2008). Between 2001-2005 the annual percentage change in male liver cancer incidence was 3.3 compared to a decrease of 3.2 for larynx cancer; the annual percentage change in liver cancer mortality was 2.1 compared to a decrease of 4.1 for prostate cancer. Among females, liver cancer incidence rose by 1.3 annually compared to a decrease of 3.7 for cervical cancer; the annual percentage change in liver cancer mortality was 1.1 compared to a decrease of 3.4 for cervical cancer. Increases in liver cancer may be attributable in great part to increasing infections by HBV and HCV and these increases have been disproportionally affecting all people of color (Jemal, Siegl, Ward, et al., 2008).
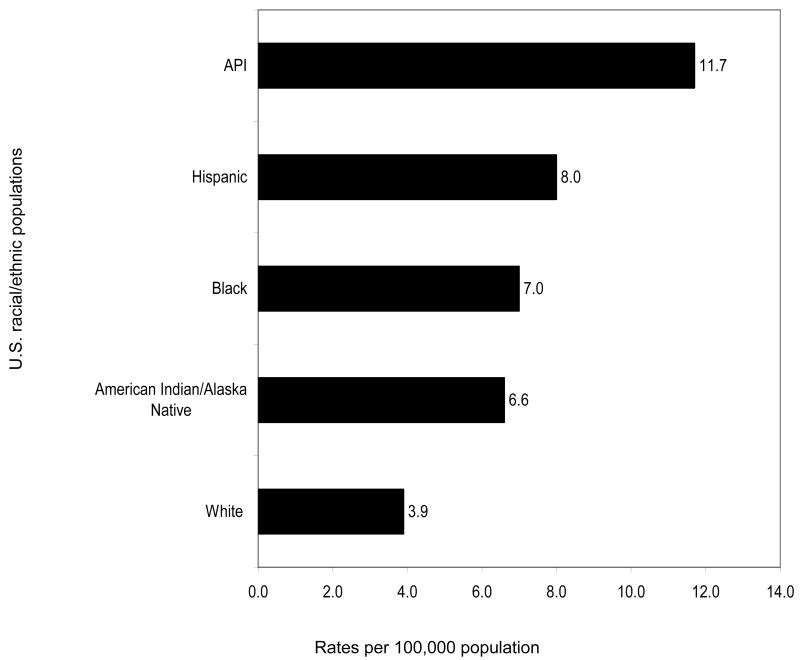
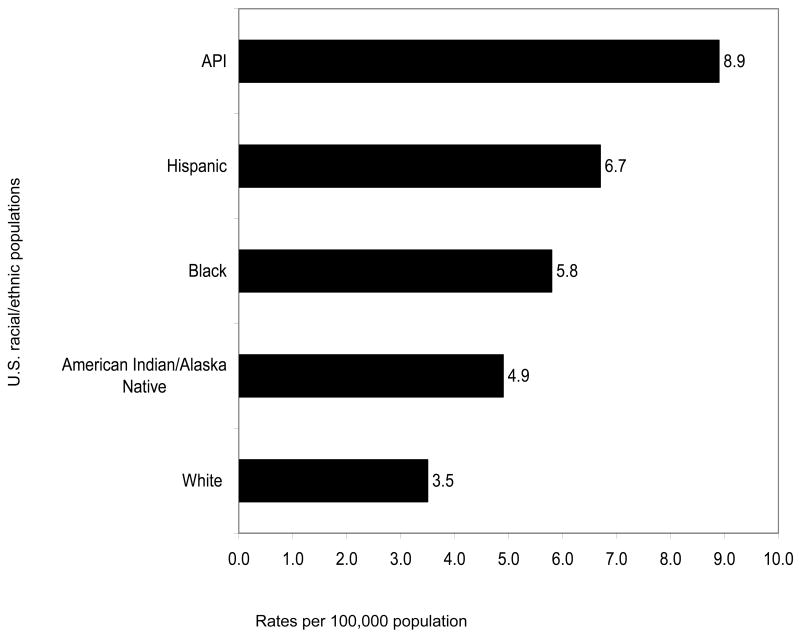
Figures 1 (incidence) and Figures 2 (mortality) graphically depict how the rates of HCC for each U.S. racial/ethnic minority population are all greater than those of Whites. Not shown in these graphs are analyses that indicate age-adjusted HCC incidence rates tripled with notable increases among Hispanic, Black, and White men between 1975 to 2005. At the same time, incidence rates are increasing with each successive birth cohort between 1900 and 1959 (Alterkruse, McGlynn, Reichman, 2009).
Figure 1. Age-adjusted HCC Incidence Rates Based on Diagnoses in 2003-2005 among U.S. Racial/Ethnic Populations.
Figure 2. Age-adjusted HCC Mortality Rates Based on Diagnoses in 2003-2005 among U.S. Racial/Ethnic Populations.
Unfortunately, survival rates for liver cancer rank the second worst among all cancer sites. For liver cancer, the survival rate for Whites is only 10% but the survival rate for Blacks is lower, 7%. Racially-specific five-year survival rates from the National Cancer Institute's Surveillance Epidemiology and End Results Program are not available for other populations (Jermal, Siegel, Ward, et al., 2008). Clearly, HBV-induced liver cancer qualifies as a significant cancer health disparity for all U.S. racial/ethnic minority populations and is the most significant cancer health disparity affecting Asian Americans (Chen, 2005).
Potential for eliminating HBV-related liver cancer as a cause of death and disparities
With the exception of the strains of cervical cancer that could be prevented by new vaccines (Markowitz et al., 2007; Saslow et al., 2007), preventing hepatitis B-induced infections cancer offers us the potential for virtually eliminating transmission of this virus for the next generation. In fact, the American Cancer Society declared that the hepatitis B vaccine was the world's first effective “anti-cancer” vaccine (American Cancer Society, 2001; Aoki, Geller, Chen, 2009, p. 227-56).
The most cost-effective means of preventing HBV-related liver cancer and its precursor, chronic HBV infection, for those who never have been infected is by vaccination (Zanett, Van Damme, Shouval, 2008). Studies in Taiwan have demonstrated the effectiveness of HBV vaccination in reducing hepatitis B surface antigen positivity, the principal indicator of infection (Chang et al., 1997) and HBV carrier rates (Lee, 1997). Subsequently, the effectiveness of the HBV vaccination efforts in Taiwan have been documented by decreasing prevalence of the hepatitis B surface antigen positivity rates by birth cohort (Su et al., 2007; Ni et al., 2007)
In the United States, most states have instituted universal HBV vaccination of infants and requirements for HBV vaccination for school entry for older children. Thus, in the future, HBV vaccinations may eventually protect subsequent generations of youth from HBV infection (Aoki, Gella, Chen, 2009, p. 227-56).
However, vaccination does not protect those already infected with HBV; vaccination without serological testing would potentially miss many who are already infected providing a false sense of protection. An estimated two million people in the United States are chronically infected with HBV (Cohen, Evans, London, 2008), a large proportion of whom are unaware of their infection (Aoki, Gellar, Chen, 2009, p.227-56; Lin, Chang, So, 2007) and hence not under medical care. Despite the best intentions, serologic testing for HBV infection among Asian American adults is under-utilized (Nguyen, et al., 2007; Chang, et al., 2007).
Recommendations for policies to eliminate hepatitis B-induced liver cancer
Based on the rationale presented above, we propose a three-tiered U.S. national policy that emphasizes primary prevention of HBV infections---i.e., measures intended to avoid infection; secondary prevention---screening for those considered to be at high-risk for HBV-induced liver cancer; and tertiary prevention---aggressive treatment of those who have been infected to improve their quality of life and to reduce transmission to others. In some respects, these three tiers are already operational; however, we have not articulated a clear national policy that links these tiers together synergistically.
Primary Prevention
Prevention of HBV infections through vaccination had its origins through pioneering epidemiological studies by Beasley (1982; 1988) documenting the etiological relationships between HBV infections and hepatocellular carcinoma, followed by the initiation of the world's first nationwide HBV vaccination program in Taiwan, first for infants born to hepatitis B surface antigen carrier mothers in 1984 and subsequently to all infants in 1986 (Chang, et al., 1997; Chen, et al., 1987). Two decades after nation-wide implementation of universal birth-dose HBV vaccination, Taiwan has seen dramatic reductions in seropositive rates for hepatitis B surface antigens and other markers of HBV infections and increases in immunity against HBV. These indicators of reduced HBV infections have been consistent with the longitudinal tracking of the same birth cohorts in surveys previously conducted in 1984, 1989, 1994, and 1999 (Ni, et al., 2007). Primary prevention prior to their onset through HBV vaccinations is unquestionably the most preferred, most effective (Chang, et al., 1997; Ni et al., 2007; Chen et al., 1987; Poland, Jacobson, 2004; Chen et al., 2004; Chang et al., 2005) and the most cost-effective approach (Margolis et al., 1995; Zhou et al., 2003; Fontham, 2009; Lang, Danchenko, Gondek, Shah, Thompson, 2009). The estimate of the financial burden of hepatocellular carcinoma in the United States is $32,907 per patient or $454.9 million. Hence, interventions to reduce the prevalence of hepatocellular carcinoma would be economically beneficial (Lang, Danchenko, Gondek, Shah, Thompson, 2009).
The incidence of HBV transmission has declined due to the adoption of a Centers for Disease Control and Prevention recommended comprehensive strategy (Centers for Disease Control and Prevention [CDC], 1991; 2002; 2004; Weinbaum, et al., 2008). For example, data from Hawaii, the state with the largest proportion of at-risk Asian Pacific Islanders, exemplify the effectiveness of the first three of CDC's recommended strategies among elementary school-aged children (Dilraj et al., 2003; Perez et al., 2006), namely, 1) Universal vaccination of infants beginning at birth; 2) prevention of perinatal HBV infection through routine screening of all pregnant women for HBV infection and provision of immunoprophylaxis to infants born to infected women or to women of unknown infection status; 3) routine vaccination of previously unvaccinated children and adolescents. The adoption of these three strategies has been facilitated by professional endorsement from various organizations such as the ACIP and the passage of various state laws requiring evidence of HBV vaccination as prerequisites for school entry and the provision of free or low-cost HBV vaccinations through the Federally subsidized Vaccines for Children's program.
Secondary prevention of hepatitis B infections
However, HBV continues to be an important cause of mortality, particularly among foreign-born residents (Kim et al., 2004) with attribution also to horizontal transmission (Franks et al., 1989). For these adults, secondary prevention or early detection and screening is highly recommended because early detection of HCC improves survival and alerts family members and friends to screening. Patients with HCC diagnosed at an early stage who are able to undergo liver transplant or resection have a five year mortality rate of 60%. While those who have been diagnosed with HCC diagnosed at a late stage, their survival rate is less than 10%. Due to the improved survival with early detection, the American Association for the Study of Liver Disease (AASLD) produced guidelines of the patients and methods of screening for liver cancer (Lok, McMahon, 2007). The AASLD recommends screening for HCC, by testing an alpha-fetoprotein (AFP) and abdominal ultrasound every 6 to 12 months, among HBV carriers who were born in Asia, starting at age 40 in men and women starting at age 50. This is based on prospective data from Asia that showed that among HBV carriers the risks of developing HCC increases to 0.2% per year starting at age 40 in men. In addition to age, HBV carriers who have a family history of HCC are at a high risk of developing HCC (Yu et al., 2000) and thus anyone with a family history of HCC should also be screened for HCC regardless of development of cirrhosis, though the age at which this should start is unknown. HBV carriers born in Africa, also appear to have a high risk of HCC which can occur at a young age. Therefore the AASLD recommends screening at age 20 in this group (Kew, Macerollo, 1988). Finally anyone with cirrhosis due to HBV should be screened for HCC as these patients have a 2.5% annual incidence of HCC (Velazquez et al., 2003). Controversy regarding these guidelines has been seen in the U.S., largely because HBV induced HCC is a relatively small contributor to the rise in HCC in the U.S. In Asia, where hepatitis B is the main contributor to HCC, screening using AFP and abdominal ultrasound has been shown to be beneficial to reducing mortality from HCC. This has been shown in numerous retrospective studies, and one randomized controlled trial showed that despite only 60% adherence, the surveillance group had a 37% reduction in mortality compared to the control group (Zhang, Yang, Tang, 2004). Thus it is important that adults born in HBV endemic areas, such as Africa or Asia, need to be tested for the hepatitis B surface antigen before vaccination to determine if they are chronic carriers.
Tertiary prevention
Unfortunately, tertiary prevention, i.e., aggressive treatment of those who have been infected to avoid relapse or death, lags far behind the state-of-the-science in primary prevention and secondary prevention. While there have been promising treatments (Dienstag, 2008) and liver transplants that have prolonged survival (Lok, McMahon, 2007; Taketomi et al., 2008), the prognosis for hepatitis B-induced liver cancer patients remains dismal. Certainly, increased funding for research should be a part of our national policy. In the meanwhile, a national policy that continues to emphasize primary prevention (vaccination) and secondary prevention (early detection of HBV) are key.
At the same time, we recognize that simply increasing HBV vaccination and early detection efforts will not entirely eliminate the occurrence of liver cancer. HCC may also be attributable to HCV infections for which no vaccination exist and to other risk factors or conditions such as tobacco use (Chen, Yu, Liaw, 1997), being overweight (Calle, Rodriguez, Walker-Thurmond, Thun, 2003; Cadwell, Crespo, Kang, Al-Osaimi, 2004), alcohol abuse (Chen et al., 1991), diabetes (Davida, Morgan, Shib, McGlynn, El-Serag, 2005; El-Serag, Hampel, Javadi, 2006), aflatotoxin consumption (Qian, et al., 1994), and other factors, both behavioral and environmental (Yu, Yuan, 2004). Unquestionably, each of these factors being both behavioral or environmental in origin are much more difficult to alter. By contrast, primary and secondary prevention of HBV are relatively easier to implement. Preventing HBV infections and treating those infections should eventually eliminate HBV as a causative agent and significant reduce rates of HCC.
Conclusion
Lessons learned and principles for eliminating health disparities
Using the example of smallpox as a model, we derived the factors displayed in Table 1 that contribute to elimination of a health disparity. With respect to a disease worth the effort, we postulate that just as smallpox was a highly prevalent and devastating disease worthy of sparing humanity, so is HBV-induced liver cancer for the same reasons. The elimination of hepatitis B-induced liver cancer through prevention (vaccination), early detection, and treatment is a most worthwhile national policy and priority. Eliminating hepatitis B-induced liver cancer is feasible; eliminating this known carcinogen through prevention and earlier detection will save lives and money.
Table 1. Factors Leading to the Elimination of a Health Disparity Based on the Smallpox Model.
| Disease worth the effort |
| Evidence base to prevent and control the disease |
| Professional endorsement |
| Universal implementation |
| Legal backing and social acceptance |
| Economic feasibility |
| Commitment and tracking |
Acknowledgments
This work was supported in part by research awards from the National Cancer Institute (U01 CA114640) and joint funding by the National Cancer Institute and the National Center on Minority Health and Health Disparities (P01 CA109091-01A1 and P01 CA109091-02S1). However, the contents of this commentary are solely the responsibility of the author and do not necessarily represent the official views of the funders. The author extends his appreciation to Christopher A. Aoki, M.D., M.S., Adjunct Assistant Professor, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of California, Davis for sharing his clinical insights and expertise in hepatology.
References
- Adler NE. Overview of health disparities. In: Thomson GE, Mitchell F, Williams MB, editors. Examining the health disparities research plan of the National Institutes of Health: Unfinished business. Washington, DC: Institute of Medicine. The National Academy Press; 2006. pp. 121–174. [PubMed] [Google Scholar]
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. JCO. 2009 doi: 10.1200/JCO.2008.20.7753. http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed]
- American Cancer Society endorses Hepatitis B “catch-up” immunization for children: urges use of first cancer prevention vaccine. [Accessed April 26, 2001];American Cancer Society. http://www.cancer.org.
- Aoki C, Geller A, Chen MS., Jr . Melanoma and primary hepatocellular carcinoma (Chapter 10) In: Koh HK, editor. Toward the Elimination of Cancer Disparities. New York: Springer Science and Business Media; 2009. pp. 227–56. Howard K. Koh, Editor. Springer 2009. [Google Scholar]
- Beasley RP. Hepatitis B virus as the etiologic agent in hepatocellular carcinoma: epidemiological considerations. Hepatology. 1982;2:218–268. [Google Scholar]
- Beasley RP. Hepatitis B Virus: The major etiology of hepatocellular carcinoma. Cancer. 1988;10:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus: A prospective study of 22,707 men in Taiwan. Lancet. 1981;2(1):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Braveman P, Gruskin S. Defining equality in health. J Epidemiol Comm Health. 2003;54:254–258. doi: 10.1136/jech.57.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell SH, Crespo D, Kang HS, Al-Osaimi MS. Obesity and hepatocellular carcinoma. Gastrolenterol. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Carter-Pokras O, Baquet C. What is a “health disparity”? Public Health Rep. 2002;117:426–434. doi: 10.1093/phr/117.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Achievements in public health: hepatitis B vaccination, United States, 1982-2002. MMWR. 2002;51:549–563. [Google Scholar]
- Centers for Disease Control and Prevention. Acute hepatitis B among children and adolescents, United States, 1990-2002. MMWR. 2004;53:1015–1018. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR. 1991;40(RR-13):1–19. [PubMed] [Google Scholar]
- Chang ET, Keegan THM, Gomez SL, et al. The burden of liver cancer in Asians and Pacific Islanders in the Greater San Francisco Bay Area, 1990 through 2004. Cancer. 2007;109:2100–8. doi: 10.1002/cncr.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. New Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- Chang MH, Chen TH, Hsu HM, Wu TC, Kong MS, Liang DC, Ni YH, Chen CJ, Chen DS. Taiwan Childhood HCC Study Group. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B: the effect and problems. Clin Ca Res. 2005;11:7953–7957. doi: 10.1158/1078-0432.CCR-05-1095. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liang KY, Chang AS, Chang YC, Lu SN, Liaw YF, Chang WY, Sheen MC, Lin TM. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13(3):398–406. [PubMed] [Google Scholar]
- Chen CJ, Yu MW, Liaw YF. Epidemiological characteristics and risk factors of hepatocellular carcinoma. J Gastroenerol Hepatol. 1997;12(9-10):S294–308. doi: 10.1111/j.1440-1746.1997.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Chen DS, Hsu NH, Sung JL, Hsu TC, Hsu ST, Kup YT, Lo KJ, Shih YT. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. JAMA. 1987;257:2597–2603. [PubMed] [Google Scholar]
- Chen HL, Chang CJ, Kong MS, Huang FC, Lee HC, Lin CC, Lee IH, Wu TC, Wu SF, Ni YH, Hsu HY, Chen DS, Chang MH. Pediatric fulminant hepatic failure in endemic areas of hepatitis B infection: 15 years after universal hepatitis B vaccination. Hepatology. 2004;39:58–63. doi: 10.1002/hep.20006. [DOI] [PubMed] [Google Scholar]
- Chen MS., Jr Cancer health disparities: what we know and what we need to do. Cancer. 2005;104(Suppl 12):2895–2902. doi: 10.1002/cncr.21501. [DOI] [PubMed] [Google Scholar]
- Cohen C, Evans AA, London WT, Block J, Conti M, Block T. Underestimation of chronic hepatitis B virus infection in the United States of America. Letter to the Editor. J Viral Hepatitis. 2008;15:12–13. doi: 10.1111/j.1365-2893.2007.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davida JA, Morgan RO, Shib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- Dilraj A, Strait-Jones J, Nagao M, Cui K, Terrell-Perica S, Effler PV. A statewide hepatitis B vaccination program for school children in Hawaii: vaccination series completion and participation rates over consecutive school years. Public Health Rep. 2003;118(2):127–133. doi: 10.1016/S0033-3549(04)50227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma : A systematic review of epidemiologic evidence. Clin Gastroenterol Hepatology. 2006;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization; 1988. [Google Scholar]
- Fontham ETH. Infectious diseases and global cancer control. CA Cancer J Clin. 2009;59:5–7. doi: 10.3322/caac.20000. http://caonline.amcancersoc.org/cgi/content/full/59/1/5. [DOI] [PubMed]
- Franks AL, Berg CJ, Kane MA, Browne BB, Sikes RK, Elsea WR, Burton AH. Hepatitis B virus infection among children born in the United States to Southeast Asian refugees. New Engl J Med. 1989;321:201–1305. doi: 10.1056/NEJM198911093211905. [DOI] [PubMed] [Google Scholar]
- Harper S, Lynch J. Methods for Measuring Cancer Disparities: Using Data Relevant to Healthy People 2010 Cancer-Related Objectives. Bethesda, MD: National Cancer Institute; 2005. (NCI Cancer Surveillance Monograph Series, Number 6). NIH Publication No. 05-5777. [Google Scholar]
- Haynes A, Smedley BD, editors. Unequal Burden of Cancer. Washington, DC: The National Academy Press; 1999. [PubMed] [Google Scholar]
- Henderson DA. Eradication: Lessons from the past. MMWR. 1999;48(SU01):16–22. [Google Scholar]
- International Agency on Research in Cancer. World Cancer Report. 2008 [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Affairs. 2005;24(2):343–352. doi: 10.1377/hlthaff.24.2.343. [DOI] [PubMed] [Google Scholar]
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology. 1988;94(2):439–406. doi: 10.1016/0016-5085(88)90434-9. [DOI] [PubMed] [Google Scholar]
- Kim WR, Benson JR, Therneau TM, Torgerson HA, Yawn BP, Melton LJ. Changing epidemiology of hepatitis B in a U.S. community. Hepatology. 2004;39:811–816. doi: 10.1002/hep.20098. [DOI] [PubMed] [Google Scholar]
- Kuper H, Adami HO. Trichopoulous D. Infections as a major preventable cause of human cancer. J Int Med. 2000;248:171–181. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatology. 2009;50:89–99. doi: 10.1016/j.jhep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: A cross-sectional study of Asians in California. Hepatology. 2007;46:1034–1040. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- London WT, McGlynn KA. Liver cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3. New York, NY: Oxford University Press; 2006. pp. 763–786. [Google Scholar]
- Lurie N. Health disparities—less talk, more action. New Engl J Med. 2005;353:727–729. doi: 10.1056/NEJMe058143. [DOI] [PubMed] [Google Scholar]
- Margolis HS, Coleman PJ, Brown RE, Mast EE, Steingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization: An economic analysis of current recommendations. JAMA. 1995;274(15):1201–1208. [PubMed] [Google Scholar]
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER Centers for Disease Control and Prevention; Advisory Committee on Immunization Practicces. ACIP Recommendations on quadrivalent HPV vaccination. MMWR. 2007;56:1–24. [PubMed] [Google Scholar]
- Miller BA, Chu KC, Hankey BF, Ries LAG. Cancer incidence and mortality among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2007 doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Eliminating Health Disparities: Measurement and Data Needs Panel on DHHS Collection of Race and Ethnicity Data. In: Ver Ploeg M, Perrin E, editors. Committee on National Statistics, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- Nguyen TT, Taylor V, Chen MS, Jr, Bastani R, Maxwell AE, McPhee SJ. Hepatitis B awareness, knowledge, and screening among Asian Americans. J Cancer Educ. 2007;22(4):266–72. doi: 10.1007/BF03174128. [DOI] [PubMed] [Google Scholar]
- Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, Kao JH, Lin YC, Chen HL, Hsu HY, Chen DS. Two decades of universal hepatitis B vaccination in Taiwan: impact and implication for future strategies. Gastroenterol. 2007;132(4):1287–1293. doi: 10.1053/j.gastro.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Ocama P, Opio CK, Lee WM. Hepatitis B virus infection: current status. Am J Med. 2005;118(12):1413. doi: 10.1016/j.amjmed.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- Perez JF, Elm JL, Jr, Fiore AE, Huggler JI, Kuhnert WL, Effler PV. Near elimination of hepatitis B virus infections among Hawaii's elementary school children after universal infant hepatitis B vaccination. Pediatrics. 2006;118:1403–1408. doi: 10.1542/peds.2006-0724. [DOI] [PubMed] [Google Scholar]
- Poland GA, Jacobson RM. Prevention of hepatitis B with hepatitis B vaccine. New Engl J Med. 2004;351:2832–2838. doi: 10.1056/NEJMcp041507. [DOI] [PubMed] [Google Scholar]
- Public Law 106-525. Minority Health and Health Disparities Research and Education Act of 2000.
- Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, Wogan GN, Groopman JD. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3(1):3–10. [PubMed] [Google Scholar]
- Recommendations of the International Task Force for Disease Eradication. MMWR. 1993;42(RR16):1–25. [PubMed] [Google Scholar]
- Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscickim AB, Noller KL, Wheeler CM, Ades T, Andrews KS, Doroshenk MK, Kahn KG, Schmidt C, Shafey O, Smith RA, Partridge EE, Gynecologic Cancer Advisory Group. Garcia F. American Cancer Society Guideline for Human Papillomavirus (HPV) vaccine to prevent cervical cancer and its precursors. CA: A Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- Satcher D, Fryer GE, McCann J, Troutman A, Woolf SH, Rust G. What If We Were Equal? A Comparison of the Black-White Mortality Gap in 1960 and 2000. Health Affairs. 2005;24(2):459–464. doi: 10.1377/hlthaff.24.2.459. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academy Press; 2003. [PubMed] [Google Scholar]
- Su FH, Chen JD, Cheng SH, Lin CH, Liu YH, Chu FY. Seroprevalence of hepatitis B infection amongst Taiwanese university students 18 years following the commencement of a national hepatitis B vaccination program. J Med Virol. 2007;79:138–143. doi: 10.1002/jmv.20771. [DOI] [PubMed] [Google Scholar]
- Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Yamashita Y, Maehara Y. Liver transplantation for hepatocellular carcinoma. J Hepatobilary Pancreat Surg. 2008;15:124–130. doi: 10.1007/s00534-007-1296-4. [DOI] [PubMed] [Google Scholar]
- Trans-HHS Cancer Health Disparities Progress Review Group. Making Cancer Health Disparities History. Washington, DC: U.S. Department of Health and Human Services; 2004. [Google Scholar]
- U.S. Department of Health and Human Services. Report on Carcinogens, 11th ed. Washington, DC: U.S. Department of Health and Human Services; 2004. [Google Scholar]
- US Department of Health and Human Services. Secretary's Task Force on Black and Minority Health. U.S. Government Printing Office; Washington, DC: 1985. [Google Scholar]
- US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health, 2nd ed. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- Velazquez RF, Rodriguez M, Navascués CA, Linares A, Perez R, Sotorrios NG, Martinez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37(3):520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]