Abstract
Oxidative stress is involved in the pathogenesis of ischemic neuronal injury. A Chinese herbal formula composed of Poria cocos (Chinese name: Fu Ling), Atractylodes macrocephala (Chinese name: Bai Zhu) and Angelica sinensis (Chinese names: Danggui, Dong quai, Donggui; Korean name: Danggwi) (FBD), has been proved to be beneficial in the treatment of cerebral ischemia/reperfusion (I/R).This study was carried out to evaluate the protective effect of FBD against neuronal oxidative stress in vivo and in vitro. Rat I/R were established by middle cerebral artery occlusion (MCAO) for 1 h, followed by 24 h reperfusion. MCAO led to significant depletion in superoxide dismutase and glutathione and rise in lipid peroxidation (LPO) and nitric oxide in brain. The neurological deficit and brain infarction were also significantly elevated by MCAO as compared with sham-operated group. All the brain oxidative stress and damage were significantly attenuated by 7 days pretreatment with the aqueous extract of FBD (250 mg kg−1, p.o.). Moreover, cerebrospinal fluid sampled from FBD-pretreated rats protected PC12 cells against oxidative insult induced by 0.2 mM hydrogen peroxide, in a concentration and time-dependent manner (IC50 10.6%, ET50 1.2 h). However, aqueous extract of FBD just slightly scavenged superoxide anion radical generated in xanthine–xanthine oxidase system (IC50 2.4 mg ml−1) and hydroxyl radical generated in Fenton reaction system (IC50 3.6 mg ml−1). In conclusion, FBD was a distinct antioxidant phytotherapy to rescue neuronal oxidative stress, through blocking LPO, restoring endogenous antioxidant system, but not scavenging free radicals.
1. Introduction
Acute ischemic stroke is a leading cause of death in the majority of countries [1]. Evidence affords the involvement of oxidative stress in neuronal injury during brain ischemia/reperfusion (I/R) [2–4]. The lethal process was accompanied by elevated free radicals, including superoxide anion (O2 •−), hydroxyl radical (•OH) and hydrogen peroxide (H2O2), as well as progressive depletion in endogenous antioxidant system, including antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase, or antioxidants, glutathione (GSH), Vitamin (Vit) C and Vit E (α-tocopherol) [5]. Pathological free radicals directly damage neuronal proteins, lipids and DNA; generate toxic lipid peroxides and ultimately contribute to brain infarction and neurobehavioral symptoms. Although free radical scavengers, for example, edaravone [6] or extract of Ginkgo biloba (EGb761) [7], have been demonstrated to be antagonistic to brain I/R, the anti-I/R agents available were still far from sufficient [8].
In traditional Chinese medicine, Poria cocos (Chinese name: Fu Ling), the dried sclerotia of P. cocos (Schw.) Wolf (Polyporaceae), is used as a diuretic, sedative and tonic [9]. Triterpene acids and polysaccharides are the principal ingredients of P. cocos that are responsible for diverse bioactivities, including antitumor, anti-inflammatory, nematicidal, antioxidant, anti-rejection and anti-emetic effects, and act as inhibitors against DNA topoisomerases, phospholipase A2 and cholinesterase (see [9–16], Table 1). The dried rhizome of Atractylodes macrocephala Koidz. (Compositae) (Chinese name: Bai Zhu) is used as a digestive and a tonic, in which volatile oils, polysaccharides, sesquiterpenes and flavonoids were identified with anti-inflammatory, hypoglycemic and gastrointestinal inhibitory effects, and so forth. (see [10–12, 17–20], Table 1). The dried root of Angelica sinensis (Oliv.) Diels (Umbelliferae) (Chinese names: Danggui, Dong quai, Donggui; Korean name: Danggwi) is used as a vital blood tonic and especially to treat gynecological diseases. Due to its varied constituents, such as volatile oils, polysaccharides and coumarin derivatives, several pharmacological actions may be attributed to Danggui, including anticoagulation and antiplatelet activities, as well as hematopoiesis, immune support and uterine tonicity (see [12, 13, 21–33], Table 1). These three essential herbs have been used for thousands of years in Asia and first documented in Shen-nong-ben- cao-jing, the first Chinese medical pharmacopoeia written in the Han dynasty.
Table 1.
Active ingredients and physio-pharmacological functions of P. cocos, A. macrocephala and A. sinensis.
| Herbs | Active ingredients | Physio-pharmacological functions |
|---|---|---|
| P. cocos | Triterpene acids, Polysaccharides | Antitumor activity and DNA topoisomerases inhibitory activity, anti-inflammatory and anti-phospholipase A(2) activity, nematicidal, antioxidant, anti-cholinesterase, anti-rejection, anti-emetic and anti-nephritic effects |
| A. macrocephala | Volatile oils, Polysaccharides, Sesquiterpenes, Flavonoids | Anti-inflammatory, antitumor activity, gastrointestinal inhibitory effect, suppression of allergic diarrhea and uterine contraction, antioxidant and hypoglycemic effects, diuresis angiogenesis |
| A. sinensis | Volatile oils, Polysaccharides, Coumarin derivatives, Organic acids, Vitamins and minerals | Anticoagulation, antiplatelet activity, hematopoiesis, immune support, anti-inflammatory, antioxidant, antifibrotic and antispasmodic effects, uterine tonicity |
The beneficial effects of these plants on cerebrovascular disorders have drawn increasing attention in recent research. Clinically, a great deal of traditional herbal formulae comprising Fu Ling, Bai Zhu and Danggui (FBD) were applied to cure ischemia stroke and vascular dementia (VD), mostly with good efficacy. Statistical analysis showed that the three herbs are frequently used in formulae, notably anti-stroke/VD formula Toki-Shakuyaku-San or Yi-Gan San [34, 35]. To some extent, clinical neuroprotection of the three herbs was shown to be relevant to their antioxidant properties [10, 36, 37]. As traditional Chinese nourishing-tonifying drugs, crude extracts of Fu Ling, Bai Zhu and/or Danggui have the capacity to inhibit cellular lipid peroxidation (LPO) induced by free radicals, for example, H2O2 [21–23], as well as preserve tissue GSH status and GSH-Px activity [11, 24]. However, in vitro, their direct free radical scavenging activities are relatively weak due to high concentration in various biochemical reactions, including xanthine-xanthine oxidase (XO) system and Fenton reaction system [12, 13]. Therefore, it is hypothesized that FBD exerts its protective effects against I/R-induced neuronal oxidative stress, largely via inhibiting LPO and maintaining endogenous antioxidant system, instead of scavenging free radicals.
The primary aim of this study was to evaluate the herbal formula on neuronal oxidative stress induced by middle cerebral artery occlusion (MCAO) in vivo and by H2O2 in vitro. In addition, we evaluated scavenging activities of FBD against O2 •− generated in xanthine-XO system and •OH generated in Fenton reaction system to assess its antioxidant properties.
2. Methods
2.1. Preparation of Aqueous Extract of FBD
The three herbal materials used in this work were purchased from Nanjing herbal materials company (Nanjing, China) and authenticated by Prof. Boyang Yu, Department of Pharmacognosy, China Pharmaceutical University. Clinically, a single formula of FBD consisted of 10 g P. cocos, 5 g A. macrocephala and 3 g A. sinensis and the aqueous extract of FBD was prepared the three components were macerated for 30 min, decocted for 30 min with 8 times (v/w) double-distilled H2O and the filtrate obtained was concentrated and dried in vacuum at 60°C into a brown powder, with a yield of 12.5% (w/w).
2.2. Reagents and Chemicals
Vit E, Vit C, 2,3,5-triphenyltertrazolium chloride (TTC), 1,10-phenanthroline and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO). EGb761 was purchased from Schwarz Pharma AG (Monheim, Germany). Medical kits for malondialdehyde (MDA), nitric oxide (NO), GSH and SOD assays were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.3. Animals and Pretreatment
Male Sprague-Dawley rats weighting 250–350 g were randomized into four groups: rats in FBD-pretreated groups received FBD (250 mg kg−1, p.o.), while EGb761-pretreated rats were given EGb761 (24 mg kg−1, p.o.), as positive control. Sham-operated group and vehicle-pretreated group were given p.o. vehicle 0.5% carboxymenthylcellulose-saline. Vehicle or drugs were administrated once daily for 7 consecutive days. The animal handling procedures were in compliance with the China National Institutes of Healthy Guidelines for the Care and Use of Laboratory Animals.
2.4. Middle Cerebral Artery Occlusion
One hour after the seventh administration, rats were subject to 1 h right MCAO using the intraluminal filament technique [38]. Briefly, rats were anesthetized with chloral hydrate (400 mg kg−1, i.p.). The right common carotid artery was exposed at the level of the external and internal carotid artery (ECA and ICA) bifurcation. A 4-0 monofilament nylon suture was inserted into the ECA and advanced into the ICA for 17–20 mm until a slight resistance was felt, to block the origin of the middle cerebral artery. One hour after MCAO, the suture was slowly withdrawn. The sham-operated rats did not suffer MCAO, except with exposure to ECA and ICA. Animals were then returned to their cages for 24 h and closely monitored, with body temperature kept at 37 ± 0.5°C.
2.5. Neurological and Histological Examination
The neurological deficits in rats were assessed after 24 h reperfusion. Ten rats from each group were assigned a numerical score on a 5-point scale as described: no neurological deficit = 0; failure to fully extend right paw = 1; circling to right = 2; falling to right = 3; did not walk spontaneously and had depressed levels of consciousness = 4 [39]. Then, rats were killed and brain tissue was removed and sliced into 2.0 mm thick coronal sections. Brain slices were incubated in 2% TTC saline solution at 37°C for 30 min, then fixed in 10% phosphate-buffered formalin for 45 min. Infarct volume in brain slices, outlined in white, were captured with a digital camera and measured by image analysis system (Zeiss AxioVs 40, Oberkochen, Germany) and calculated using the following equation: % infarct volume = infarct volume/slice volume × 100%.
2.6. Neurochemical Assays
Twenty-four hours after reperfusion, rats were sacrificed and cortical cortexes were collected. A 10% (w/v) homogenate was prepared in ice-cold saline and the supernatant was obtained after centrifugation at 3000 r.p.m. for 15 min. Neurochemical assays were conducted in accordance with the specification of medical kits. When unsaturated fatty acids undergo LPO, MDA is formed. Thiobarbituric acid reaction was used to determine MDA (expressed as μmol g−1 protein) [40]. Nitrite in cortical supernatant was measured after reaction with Griess reagent (sulfanilamide 1%, naphthylethylene diamine 0.01%, H3PO4 5%) with sodium nitrite as a standard, by which NO production might be assessed as micromole per gram of protein [41]. The assay for SOD was based on its ability to inhibit the oxidation of oxymine by the xanthine-XO system (expressed as U mg−1 protein) [42]. GSH (expressed as μmol g−1 protein) was measured through a reaction using dithiobisnitrobenzoic acid, as described by Ball [43]. Protein concentration was measured by Lowry method with bovine serum albumin as standard.
2.7. Sampling of Cerebrospinal Fluid
Fresh cerebrospinal fluid (CSF) was sampled 0, 0.5, 1, 1.5, 2.0 and 2.5 h after the seventh administration, from FBD-pretreated rats free of MCAO, using three rats per time point. In short, rats were anesthetized with chloral hydrate (400 mg kg−1, i.p.), 30–50 μl CSF was carefully pricked from bulbus medullae pool using a sterile injection syringe [44]. After centrifugation at 3000 r.p.m. for 10 min, the CSF containing FBD (CSF-FBD) was stored at −20°C.
2.8. Oxidative Insult in PC12 Cells Induced by H2O2
Neuron-like pheochromocytoma (PC12) cells were provided by Institute of Cells Biology (Shanghai, China). The cells were suspended in Dulbecco Modified Eagle's Medium supplemented with 10% heat-inactivated newborn calf serum, benzylpenicillin (100 kU l−1) and streptomycin (100 mg l−1) and incubated at 37°C in 5% CO2. PC12 cells were exposed to H2O2 (200 μM) for 1 h to induce oxidative insult, then treated with CSF-FBD (v/v), Vit E (10 μM) or blank CSF. Twenty hours later, MTT assay was performed to observe the cell viability in PC12 cells [37]. Briefly, MTT solution (0.5 mg ml−1) was added to each culture well. After incubation for 4 h, the formazan crystals were dissolved by addition of 50 μl dimethyl sulfoxide and read at dual wavelength, 570 nm/650 nm.
2.9. Superoxide Radical Generated in Xanthine-Xanthine Oxidase System
According to Link and Riley [45], the xanthine-XO system of a final volume of 2.0 ml contained 375 μmol l−1 xanthine, 6.25 U l−1 XO, 500 μmol l−1 hydroxylamine, 100 mmol l−1 Na2HPO4 ·12H2O–NaH2PO4 ·2H2O buffer (pH 7.8) and FBD or Vit C at different concentrations (0.05–5 mg ml−1). Reaction was initiated by adding XO and the tubes were incubated at 37°C for 40 min and then terminated by placing in an ice bath. The absorbance of nitrate from hydroxylamine was measured at 550 nm after reaction with Griess reagent. O2 •− scavenging by FBD was calculated by the following equation: %Scavenging rate = [1−A 1/A 0] × 100% [A 0: Control; A 1: Drug].
2.10. Hydroxyl Radical Generated in Fenton Reaction System
The Fenton reaction system of a final volume of 2 ml contained 0.75 mmol l−1 FeSO4, 0.75 mmol l−1 1,10-phenanthroline, 0.8 mmol l−1 H2O2, 150 mmol l−1 PBS buffer and FBD or Vit C at different concentrations (0.05–5 mg ml−1). Reaction was initiated by adding H2O2 and the tubes were incubated for 60 min in a water bath at 37°C. The absorbance of the Fe2+-phenanthroline complex was measured at 510 nm [46]. All values represent the average of triplicate experiments. •OH scavenging by FBD was calculated by the following equation: %Scavenging rate = [1−(A 2−A 1)/(A 2−A 0)] × 100% [A 0: Control; A 1: Drug; A 2: Blank without drug and H2O2].
2.11. Statistical Analysis
SPSS 12.0 software and Origin 7.0 software were applied to analyze experimental data and results were expressed as mean ± SD. All data were evaluated with analysis of variance (ANOVA) following by Dunnett's t-test for multiple comparisons and P < .05 indicates that the difference was statistically significant.
3. Results
3.1. Neuroprotective Effects of FBD In Vivo
Rats surviving more than 24 h awakened from anesthesia with a moderately severe left hemiparesis and circling movements. TTC staining indicated that infarction zone existed in right lobus temporalis cortical and striatal tissues. The neurological score and infarct size in the vehicle-pretreated MCAO rats rose up to 2.6 ± 0.7 and 19.7% ± 2.2%, respectively, indicating that I/R resulted in neuronal injury. In comparison to the vehicle-pretreated group, FBD (250 mg kg−1) significantly reduced the neurological score by 28.4% (P < .05) and infarct size by 20.1% (P < .01). Its actions were to some extent stronger than those of 24 mg kg−1 EGb761 (by 27.6%, P < .05 and by 18.9%, P < .01, resp., Figure 1).
Figure 1.
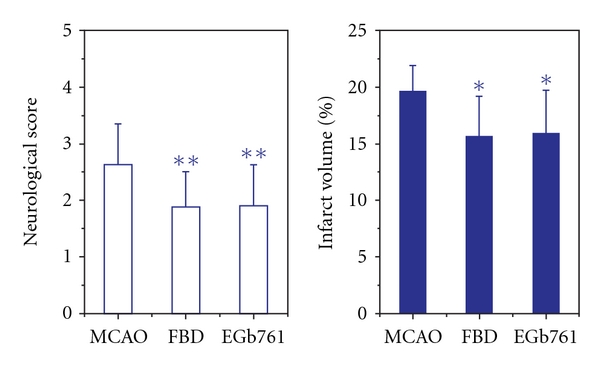
Effects of aqueous extract of FBD on neurological score and brain infarction in rats subject to MCAO. Each column represents the mean ± SD of 10–12 rats. Significance was evaluated with one-way ANOVA following by two-sided Dunnett's t-test. * P < .05, ** P < .01 versus the vehicle-pretreated MCAO group.
3.2. Antioxidant Effects of FBD In Vivo
MCAO-induced neurochemical changes are shown in Table 2. After 24 h reperfusion, MDA and NO contents in vehicle-pretreated group rose significantly (P < .01); in contrast, GSH content and SOD activity reduced significantly (P < .01), which implied that oxidative stress occurred. With respect to the vehicle-pretreated group, FBD (250 mg kg−1) significantly reduced MDA and NO production (P < .01) and restored SOD activity (P < .01) and GSH content (P < .05); likewise, EGb761 significantly suppressed oxidative stress to a similar extent.
Table 2.
Effects of the aqueous extract of FBD on the contents of MDA, NO and GSH, and SOD activity in rat brain subject to MCAO.
| Group | Dose (mg/kg) | NO (μmol/g prot) | MDA (μmol/g prot) | GSH (μmol/g prot) | SOD (U/mg prot) |
|---|---|---|---|---|---|
| Sham | 4.58 ± 0.75 | 3.77 ± 0.83 | 45.33 ± 5.54 | 143.07 ± 26.65 | |
| MCAO | 7.94 ± 0.74## | 6.68 ± 0.54## | 27.08 ± 3.92## | 96.57 ± 22.78## | |
| EGb761 | 24 | 6.01 ± 0.96** | 4.56 ± 1.72** | 35.03 ± 6.06** | 124.43 ± 15.77** |
| FBD | 250 | 6.25 ± 0.80** | 4.64 ± 0.96** | 32.51 ± 5.00* | 131.73 ± 22.40** |
All the data were shown as the mean ± SD, n = 10−12. Significance was evaluated with one-way analysis of variance (ANOVA) following by two-sided Dunnett's t-test. ## P < .01 versus the sham-operated group; *P < .05, **P < .01 versus the vehicle-pretreated MCAO group.
3.3. Antioxidant Activity of FBD Ex Vivo
Incubation with H2O2 for 3 h significantly reduced cell viability. However, when the cells were treated with rat CSF-FBD, the observed cell toxicity was significantly attenuated. As illustrated in Figure 2, CSF-FBD markedly reduced H2O2 injury within 1.5 h in a time-dependent manner (ET50 1.2 h) and concentration-dependant manner within 20% (IC50 10.6%). Meanwhile, blank CSF had no obvious influence on the control PC12 cells and Vit E (10 mM) protected PC12 cells by only 25.2%.
Figure 2.
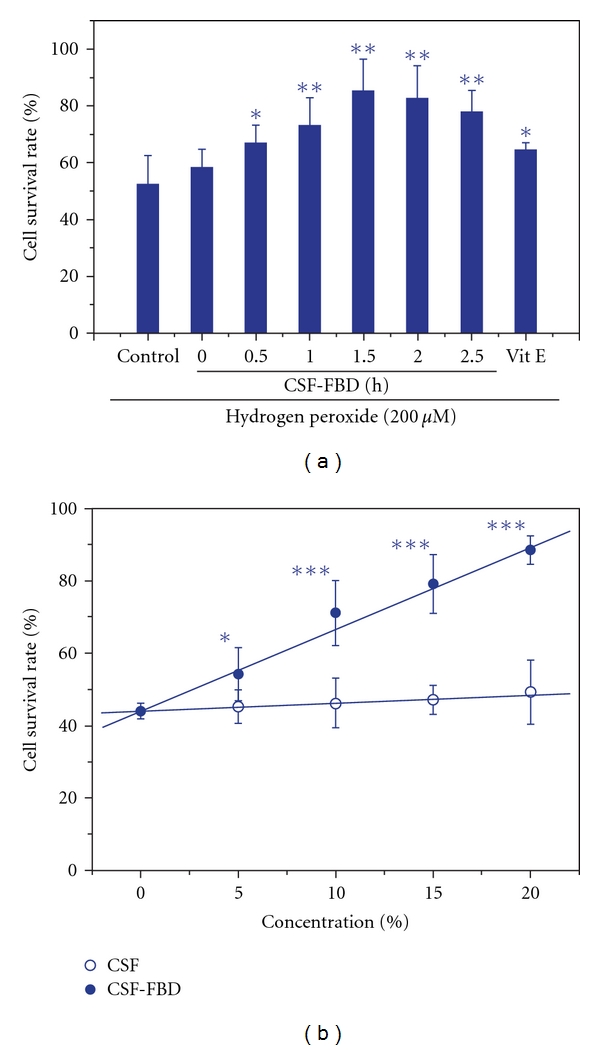
Effect of rat CSF-FBD on PC12 cells induced by hydrogen peroxide. All the data were shown as the mean ± SD, n = 6. Significance was evaluated with one-way ANOVA following by two-sided Dunnett's t-test. *P < .05, ** P < .1, *** P < .001 versus blank CSF.
3.4. Free Radical Scavenging Activity of FBD In Vitro
Direct free radical scavenging activity by FBD is shown in Figure 3. At concentrations of 0.05–5.0 mg ml−1, FBD exhibited concentration-dependent scavenging activities against O2 •− generated in xanthine-XO system and •OH generated in a Fenton reaction system, with IC50 2.4 mg ml−1 and 3.6 mg kg−1, respectively, higher than those of Vit C (IC50 0.01 mg ml−1 and 0.25 mg ml−1, resp.).
Figure 3.
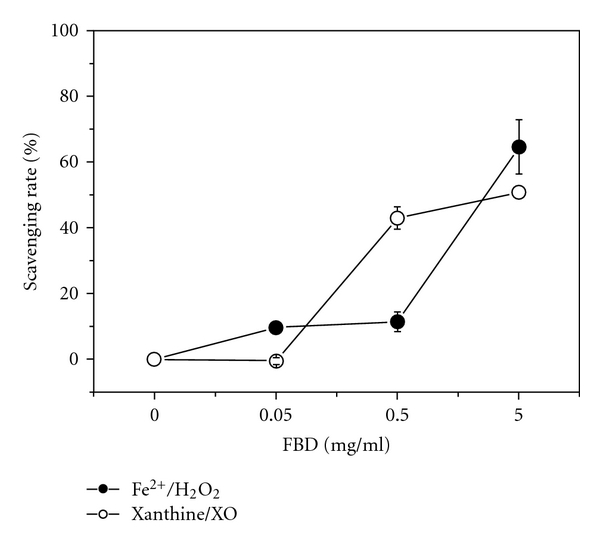
Scavenging activities of aqueous extract of FBD against superoxide anion radical generated in xanthine-XO system and hydroxyl radical generated in Fe2+/H2O2 Fenton reaction system with ascorbic acid as a positive control. Values were means ± SD, n = 6.
4. Discussion
This study demonstrates the neuroprotective potential of FBD against MCAO-induced oxidative stress in rats, as well as H2O2-induced oxidative stress in neuron-like PC12 cells. Its neuroprotection appears to reduce LPO and restore endogenous antioxidant system but not scavenge free radicals.
It is well documented that transient focal MCAO results in neurological and histological abnormality. Our results indicated that pretreatment with FBD offered protection against cortical and striatal neuronal damage induced by MCAO, as FBD reduced the neurological score and infarct size (Figure 1), in harmony with other studies [47, 48].
Free radical involvement in the development of I/R-induced brain injury is well investigated [3, 4], among which, O2 •− and •OH are potent by inducing LPO [49]. The highly reactive •OH is formed from H2O2 in the presence of divalent metal ions, especially Fe2+ and Cu2+, via the Fenton reaction. In addition, during ischemia, xanthine dehydrogenase undergoes irreversible proteolytic conversion to XO, producing O2 •− and H2O2 in the presence of oxygen [3]. O2 •− does not directly induce LPO but can react with •NO to form cytotoxic peroxynitrite (ONOO−) [50]. We found that focal MCAO induced increases of LPO and NO (Table 2), in agreement with recent studies [51, 52]. FBD inhibited NO production but its scavenging activity against either •OH or O2 •− was feeble compared to that of Vit C (Figure 3), supporting previous findings that P. cocos and A. sinensis were relatively weak natural free radical scavengers [13].
The overproduction of free radicals can be detoxified by endogenous antioxidants, causing their cellular stores to be depleted [52, 53]. Physiologically, SOD reacts with O2 •− to form H2O2; Catalase and GSH-Px are involved in the detoxification of H2O2; GSH, which is considered the most prevalent and important intracellular non-protein thiol, has a crucial role as a free radical scavenger. Here, GSH content and SOD activity were significantly reduced (Table 2). Similar to EGb761, FBD significantly prevented SOD activity and GSH content decline caused by MCAO.
In addition to restoring the endogenous antioxidant system, anti-LPO activity was also implicated in the antioxidant properties of FBD. In 1996, Taylor et al. observed the inhibition of T cells by human CSF, and in 2000, Nakagawa et al. found human CSF altered intracellular calcium regulation in endothelial cells [54, 55]. Since CSF is the natural vehicle for CNS agents, both reports enlightened us to design a novel experimental method to evaluate neuroeffectiveness of FBD ex vivo. PC12 cells injured by H2O2 are a typical model used to evaluate anti-LPO activity of drug on neuronal oxidative stress [56, 57]. In this work, CSF-FBD attenuated oxidative insult affects PC12 cells in both time- and concentration-dependent manner (Figure 2), in accordance with in vivo finding that MDA level in MCAO-subjected rats was depressed by FBD extract.
The exact mechanism by which FBD abated oxidative stress is not yet clear but it is strongly believed that recently identified active compounds may be responsible. Triterpenes from Fu Ling inhibited FeCl2-ascorbic acid induced LPO and lysis of red blood cells [14]. Atractylon from Bai Zhu inhibited LPO by CCl4 in liver lesion and its acetylene compound (6E,12E)-tetradecadiene-8,10-diyne-1,3-diol diacetate suppressed gastric lesions induced by I/R, via inhibition of XO [17, 18]. Z-ligustilide from Danggui protected against H2O2-induced cytotoxicity in PC12 cells and forebrain I/R by enhancing antioxidant defense [25, 26]. Coniferyl ferulate is the main antioxidant from essential oil of Danggui [27] and ferulic acid could reduce neuronal damage from exposure to iron, hydroxyl and peroxyl radicals [28, 29]. In addition, Danggui polysaccharides protected macrophages against tert-butylhydroperoxide-induced oxidative injury [30, 31].
In conclusion, our present findings suggested that FBD might exert protection against neuronal oxidative stress, induced by either MCAO in vivo or H2O2 in vitro. It is a distinct botanical antioxidant agent to reduce LPO and restore the endogenous antioxidant system, without the activity of free radical scavengers. This research expands and elaborates the biological model underlying one complementary and alternative medicine treatment for neuronal oxidative stress.
Acknowledgments
We thank Wen Liu (M.S.) and Min Chen (M.S.) from the Department of Pharmacology and Pharmaceutical Sciences, University of Southern California, for reviewing the manuscript. This work was supported by the National Natural Science Foundation of China (No. 30500683).
References
- 1.Ikeda Y, Long DM. The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery. 1990;27(1):1–11. doi: 10.1097/00006123-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ozkul A, Akyol A, Yenisey C, Arpaci E, Kiylioglu N, Tataroglu C. Oxidative stress in acute ischemic stroke. Journal of Clinical Neuroscience. 2007;14(11):1062–1066. doi: 10.1016/j.jocn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. International Review of Neurobiology. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- 4.Taylor JM, Crack PJ. Impact of oxidative stress on neuronal survival. Clinical and Experimental Pharmacology and Physiology. 2004;31(7):397–406. doi: 10.1111/j.1440-1681.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- 5.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxidants & Redox Signaling. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Reviews. 2006;12(1):9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg RK, Nag D, Agrawal A. A double blind placebo controlled trial of ginkgo biloba extract in acute cerebral ischemia. Journal of Association of Physicians of India. 1995;43:760–763. [PubMed] [Google Scholar]
- 8.Wang CX, Shuaib A. Neuroprotective effects of free radical scavengers in stroke. Drugs and Aging. 2007;24(7):537–546. doi: 10.2165/00002512-200724070-00002. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Chang H. Antiproliferative and differentiating effects of polysaccharide fraction from Fu-Ling (Poria cocos) on human leukemic U937 and HL-60 cells. Food and Chemical Toxicology. 2004;42:759–769. doi: 10.1016/j.fct.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Ichikawa H, Konishi T. Antioxidant potential of Qizhu Tang, a Chinese herbal medicine, and the effect on cerebral oxidative damage after ischemia reperfusion in rats. Biological & Pharmaceutical Bulletin. 2001;24:558–563. doi: 10.1248/bpb.24.558. [DOI] [PubMed] [Google Scholar]
- 11.Wang X-J, Feng P. Antioxidant activity of Qizhu Tang. Acta Pharmacologica Sinica. 2000;21(12):1141–1144. [PubMed] [Google Scholar]
- 12.Schinella GR, Tournier HA, Prieto JM, Mordujovich De Buschiazzo P, Ríos JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sciences. 2002;70(9):1023–1033. doi: 10.1016/s0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 13.Wu S-J, Ng L-T, Lin C-C. Antioxidant activities of some common ingredients of traditional Chinese medicine, Angelica sinensis, Lycium barbarum and Poria cocos . Phytotherapy Research. 2004;18(12):1008–1012. doi: 10.1002/ptr.1617. [DOI] [PubMed] [Google Scholar]
- 14.Sekiya N, Goto H, Shimada Y, Endo Y, Sakakibara I, Terasawa K. Inhibitory effects of triterpenes isolated from hoelen on free radical-induced lysis of red blood cells. Phytotherapy Research. 2003;17(2):160–162. doi: 10.1002/ptr.1097. [DOI] [PubMed] [Google Scholar]
- 15.Huang Q, Jin Y, Zhang L, Cheung PCK, Kennedy JF. Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohydrate Polymers. 2007;70(3):324–333. [Google Scholar]
- 16.Kang H-M, Lee S-K, Shin D-S, et al. Dehydrotrametenolic acid selectively inhibits the growth of H-ras transformed rat2 cells and induces apoptosis through caspase-3 pathway. Life Sciences. 2006;78(6):607–613. doi: 10.1016/j.lfs.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 17.Kiso Y, Tohkin M, Hikino H. Mechanism of antihepatotoxic activity of atractylon, I: effect on free radical generation and lipid peroxidation. Planta Medica. 1985;51:97–100. doi: 10.1055/s-2007-969416. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai T, Sugawara H, Saito K, Kano Y. Effects of the acetylene compound from Atractylodes rhizome on experimental gastric ulcers induced by active oxygen species. Biological & Pharmaceutical Bulletin. 1994;17:1364–1368. doi: 10.1248/bpb.17.1364. [DOI] [PubMed] [Google Scholar]
- 19.Li C-Q, He L-C, Dong H-Y, Jin J-Q. Screening for the anti-inflammatory activity of fractions and compounds from Atractylodes macrocephala koidz. Journal of Ethnopharmacology. 2007;114(2):212–217. doi: 10.1016/j.jep.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Guo F-Q, Huang L-F, Zhou S-Y, Zhang T-M, Liang Y-Z. Comparison of the volatile compounds of Atractylodes medicinal plants by headspace solid-phase microextraction-gas chromatography-mass spectrometry. Analytica Chimica Acta. 2006;570(1):73–78. [Google Scholar]
- 21.Hou YZ, Zhao GR, Yang J, Yuan YJ, Zhu GG, Hiltunen R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sciences. 2004;75(14):1775–1786. doi: 10.1016/j.lfs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Kong L, Wu M, Xi P. Effects of different processed products of radix Angelica sinensis on clearing out oxygen free radicals and anti-lipid peroxidation. Zhongguo Zhong Yao Za Zhi. 1996;21:599–601, 639. [PubMed] [Google Scholar]
- 23.Qiu Y, Du G, Qu Z. Comparison of the anti-lipid peroxidative effects of some traditional Chinese nourishing-tonifying drugs in vitro . Zhongguo Yao Xue Za Zhi. 1996;31:83–86. [Google Scholar]
- 24.Mak DH, Chiu PY, Dong TT, Tsim KW, Ko KM. Dang-Gui Buxue Tang produces a more potent cardioprotective effect than its component herb extracts and enhances glutathione status in rat heart mitochondria and erythrocytes. Phytotherapy Research. 2006;20:561–567. doi: 10.1002/ptr.1904. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y, Du J-R, Wang C-Y, Qian Z-M. Protection against hydrogen peroxide-induced injury by Z-ligustilide in PC12 cells. Experimental Brain Research. 2008;184(3):307–312. doi: 10.1007/s00221-007-1100-3. [DOI] [PubMed] [Google Scholar]
- 26.Kuang X, Yao Y, Du JR, Liu YX, Wang CY, Qian ZM. Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Research. 2006;1102(1):145–153. doi: 10.1016/j.brainres.2006.04.110. [DOI] [PubMed] [Google Scholar]
- 27.Li S-Y, Yu Y, Li S-P. Identification of antioxidants in essential oil of radix Angelicae sinensis using HPLC coupled with DAD-MS and ABTS-based assay. Journal of Agricultural and Food Chemistry. 2007;55(9):3358–3362. doi: 10.1021/jf070140t. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Wei T, Hou J, Li G, Yu S, Xin W. Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. European Journal of Pharmacology. 2003;467:41–47. doi: 10.1016/s0014-2999(03)01597-8. [DOI] [PubMed] [Google Scholar]
- 29.Kanski J, Aksenova M, Stoyanova A, Butterfield DA. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. Journal of Nutritional Biochemistry. 2002;13(5):273–281. doi: 10.1016/s0955-2863(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Zhao Y, Lv Y, Yang Y, Ruan Y. Protective effect of polysaccharide fractions from Radix A. sinensis against tert-butylhydroperoxide induced oxidative injury in murine peritoneal macrophages. Journal of Biochemistry and Molecular Biology. 2007;40:928–935. doi: 10.5483/bmbrep.2007.40.6.928. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Zhao Y, Zhou Y, Lv Y, Mao J, Zhao P. Component and antioxidant properties of polysaccharide fractions isolated from Angelica sinensis (Oliv.) Diels. Biological and Pharmaceutical Bulletin. 2007;30(10):1884–1890. doi: 10.1248/bpb.30.1884. [DOI] [PubMed] [Google Scholar]
- 32.Sarker SD, Nahar L. Natural medicine: the genus Angelica. Current Medicinal Chemistry. 2004;11(11):1479–1500. doi: 10.2174/0929867043365189. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Ma H-Q, Sun Y-J, Qiao C-D, Shao S-J, Jiang S-X. Fingerprint quality control of Angelica sinensis (Oliv.) Diels by high-performance liquid chromatography coupled with discriminant analysis. Talanta. 2007;72(2):434–436. doi: 10.1016/j.talanta.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Pu F, Mishima K, Egashira N, et al. Post-ischemic treatment with Toki-shakuyaku-san (Tang-Gui-Shao-Yao-San) prevents the impairment of spatial memory induced by repeated cerebral ischemia in rats. American Journal of Chinese Medicine. 2005;33(3):475–489. doi: 10.1142/S0192415X05003077. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, Tomita N, et al. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. Journal of Clinical Psychiatry. 2005;66:248–252. doi: 10.4088/jcp.v66n0214. [DOI] [PubMed] [Google Scholar]
- 36.Chung T-W, Koo B-S, Choi E-G, Kim M-G, Lee I-S, Kim C-H. Neuroprotective effect of a Chuk-Me-Sun-Dan on neurons from ischemic damage and neuronal cell toxicity. Neurochemical Research. 2006;31(1):1–9. doi: 10.1007/s11064-005-9006-6. [DOI] [PubMed] [Google Scholar]
- 37.Lin Z, Yan Y, Zhu D, Yu B, Wang Q. Protective effects of FBD—an experimental Chinese traditional medicinal formula on memory dysfunction in mice induced by cerebral ischemia-reperfusion. Journal of Ethnopharmacology. 2005;97(3):477–483. doi: 10.1016/j.jep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 39.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion, evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 40.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 41.Escobales N, Rivera-Correa M, Altieri PI, Rodriguez JF. Relationship between NO synthesis, arginine transport, and intracellular arginine levels in vascular smooth muscle cells. Amino Acids. 2000;19:451–468. doi: 10.1007/s007260070023. [DOI] [PubMed] [Google Scholar]
- 42.Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Analytical Biochemistry. 1984;142(2):290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- 43.Ball CR. Estimation and identification of thiols in rat spleen after cysteine or glutathione treatment: Relevance to protection against nitrogen mustards. Biochemical Pharmacology. 1966;15(7):809–816. doi: 10.1016/0006-2952(66)90157-2. [DOI] [PubMed] [Google Scholar]
- 44.Sharma AK, Schultze AE, Cooper DM, Reams RY, Jordan WH, Snyder PW. Development of a percutaneous cerebrospinal fluid collection technique in F-344 rats and evaluation of cell counts and total protein concentrations. Toxicologic Pathology. 2006;34:393–395. doi: 10.1080/01926230600798609. [DOI] [PubMed] [Google Scholar]
- 45.Link EM, Riley PA. Role of hydrogen peroxide in the cytotoxicity of the xanthine/xanthine oxidase system. Biochemical Journal. 1988;249(2):391–399. doi: 10.1042/bj2490391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz-Larrea B, Leal A, Martín C, Martínez R, Lacort M. Effects of estrogens on the redox chemistry of iron: a possible mechanism of the antioxidant action of estrogens. Steroids. 1995;60(11):780–783. doi: 10.1016/0039-128x(95)00119-b. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka Y, Koizumi C, Marumo T, Omura T, Yoshida S. Serum S100B indicates brain edema formation and predicts long-term neurological outcomes in rat transient middle cerebral artery occlusion model. Brain Research. 2007;1137(1):140–145. doi: 10.1016/j.brainres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Walberer M, Stolz E, Müller C, Friedrich C, Rottger C, Blaes F, et al. Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI) Laboratory Animals. 2006;40:1–8. doi: 10.1258/002367706775404426. [DOI] [PubMed] [Google Scholar]
- 49.Bromont C, Marie C, Bralet J. Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke. 1989;20:918–924. doi: 10.1161/01.str.20.7.918. [DOI] [PubMed] [Google Scholar]
- 50.Hirabayashi H, Takizawa S, Fukuyama N, Nakazawa H, Shinohara Y. 7-Nitroindazole attenuates nitrotyrosine formation in the early phase of cerebral ischemia-reperfusion in mice. Neuroscience Letters. 1999;268(2):111–113. doi: 10.1016/s0304-3940(99)00368-7. [DOI] [PubMed] [Google Scholar]
- 51.Shah ZA, Gilani RA, Sharma P, Vohora SB. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. Journal of Ethnopharmacology. 2005;101(1–3):299–307. doi: 10.1016/j.jep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad S, Yousuf S, Ishrat T, et al. Effect of dietary sesame oil as antioxidant on brain hippocampus of rat in focal cerebral ischemia. Life Sciences. 2006;79(20):1921–1928. doi: 10.1016/j.lfs.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK. Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Experimental Neurology. 2007;204(2):819–827. doi: 10.1016/j.expneurol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor AW, Streilein JW. Inhibition of antigen-stimulated effector T cells by human cerebrospinal fluid. NeuroImmunoModulation. 1996;3(2-3):112–118. doi: 10.1159/000097235. [DOI] [PubMed] [Google Scholar]
- 55.Nakagawa K, Hirai K, Aoyagi M, Yamamoto K, Hirakawa K, Katayama Y. Bloody cerebrospinal fluid from patients with subarachnoid hemorrhage alters intracellular calcium regulation in cultured human vascular endothelial cells. Neurological Research. 2000;22:588–596. doi: 10.1080/01616412.2000.11740724. [DOI] [PubMed] [Google Scholar]
- 56.Liu J-W, Yu Y-J, Zheng P-Y, et al. Synergistic protective effect of picroside II and NGF on PC12 cells against oxidative stress induced by H2O2 . Pharmacological Reports. 2007;59(5):573–579. [PubMed] [Google Scholar]
- 57.Shimazawa M, Chikamatsu S, Morimoto N, Mishima S, Nagai H, Hara H. Neuroprotection by Brazilian green propolis against in vitro and in vivo ischemic neuronal damage. Evidence-based Complementary and Alternative Medicine. 2005;2(2):201–207. doi: 10.1093/ecam/neh078. [DOI] [PMC free article] [PubMed] [Google Scholar]


