Abstract
Recent studies suggest that dementia in the most elderly (90 years of age and above) is only modestly related to Alzheimer’s disease pathology. This raises the possibility that other, as yet unknown, disease processes may underlie dementia in this rapidly growing demographic group, and that efforts designed to combat Alzheimer’s disease may not be appropriate for treating dementia in very elderly subjects. To study this question more closely, we examined the relationship between neocortical Alzheimer-type brain pathology and dementia in consecutive autopsies from 209 participants in the Baltimore Longitudinal Study of Ageing, a prospective longitudinal cohort study of the effect of ageing on cognition. Almost half of the cohort was older than 90 years of age at death. We found that several measures of neocortical Alzheimer’s pathology, including the Consortium to Establish a Registry of Alzheimer’s Disease neuritic plaque score and the Braak neurofibrillary tangle score, remained significant predictors of dementia, independent of age. In participants older than 90 years of age, intracranial atherosclerosis emerged as an important predictor of dementia in subjects with low Alzheimer’s pathology scores, but did not mitigate the importance or population attributable risk of high Alzheimer’s pathology scores on the odds of dementia. There was evidence that the threshold score for neurofibrillary pathology to cause dementia increased in the oldest subjects, but this was offset by an overall increase in neurofibrillary pathology in this age group. We conclude that neocortical Alzheimer’s disease pathology remains significantly correlated with dementia, independent of age. In the most elderly, atherosclerosis also emerged as a cause of dementia in subjects with low Alzheimer’s pathology scores. We found no evidence for a significant number of elderly subjects having dementia without an apparent cause.
Keywords: Alzheimer’s disease, pathology, ageing, natural history, neurodegenerative mechanisms
Introduction
Dementia is a growing problem in public health because the elderly are forming a higher proportion of the population (Kawas et al., 2000; Lobo et al., 2008). Amongst the causes of dementia, Alzheimer’s disease is most common (Clark and Trojanowski, 1999). Pathologically, the diagnosis of Alzheimer’s disease depends mainly on the presence of extracellular amyloid plaques and intracellular neurofibrillary tangles in the neocortex (Selkoe, 1996; Haroutunian et al., 2008; Kern and Behl, 2009; Mohajeri and Leuba, 2009). Previous research has illustrated the significant relationship between these pathologies and cognitive decline (Näslund, 2000; Jellinger, 2006). A recent cross-sectional study of autopsied brains suggests that Alzheimer-type brain pathology is more strongly related to dementia amongst younger subjects than it is in those in their nineties (Haroutunian et al., 2008). This is consistent with the recognition of elderly subjects with asymptomatic Alzheimer’s disease pathology (Bennett et al., 2006; Driscoll et al., 2006; Roe et al., 2007). A recent prospective longitudinal cohort study that assessed the prevalence of Alzheimer’s disease pathology amongst elderly subjects with and without dementia (Savva et al., 2009) suggests that Alzheimer’s disease pathology in the form of neuritic plaques does not differ significantly between demented and non-demented subjects in their mid-nineties and that the effect of neurofibrillary tangles on cognition in older subjects was significantly attenuated compared to younger subjects. Given that this age group is amongst the fastest growing age demographic worldwide (Christensen et al., 2009), the possibility that Alzheimer’s pathology does not explain dementia in the most elderly is unsettling.
We report results from the Baltimore Longitudinal Study of Ageing Autopsy Programme, a prospective longitudinal cohort study of the effects of ageing on cognition and dementia. The intensity of the evaluations of these subjects and the large number of subjects 90 years and older makes this cohort unique for elucidating the contribution of Alzheimer’s pathology to dementia in the most elderly. We report that neocortical Alzheimer’s pathology remains significantly correlated with dementia throughout the lifespan.
Materials and methods
Cohort
A total of 579 participants from the Baltimore Longitudinal Study of Ageing agreed to post-mortem brain exams. The rate of dementia in the autopsy cohort was similar to that in the Baltimore Longitudinal Study of Ageing cohort as a whole (Gamaldo et al., 2006). Thirty-four subjects withdrew from the autopsy study, the majority for issues of convenience. The age-adjusted dementia rate of the subjects who withdrew was identical to the remaining cohort. As of September 2009, 213 participants aged 70 and older at the time of death, who were cognitively and neurologically normal at entry into the study, died and underwent brain autopsy (89% autopsy rate). Of this group, we excluded one participant who had a primary brain tumour (aged 77 at death), one who had brain metastases and delirium (aged 81 at death) and another who had an inflammatory leukoencephalopathy (aged 84 at death). An additional participant was excluded because language deficits from a clinical stroke compromised assessment of cognition (aged 89 at death). After these exclusions, 209 individuals were included in the present analysis (140 male, 69 female). Participants were predominantly white (95%) with a mean of 17.0 ± 2.4 (SD) years of education. The mean age at death was 87.3 ± 7.6 years.
Subjects were assessed at baseline, within 18 months of death (mean of 8.8 ± 6.4 months prior to death) and periodically in between. There is a possibility that in some subjects, the actual cognitive status at death was different from what was noted at the last visit. Evaluations included neuropsychological tests, neurological exam, interval medical history, medication review and a structured informant and subject interview as previously described (Troncoso et al., 2008). The majority were seen annually after age 70, although ∼25% of the cohort had gaps in their follow-up of several years. Studies of this cohort were conducted under the auspices of the Johns Hopkins and MedStar Research Institute Institutional Review Boards and all participants provided written informed consent.
Diagnosis of dementia
As previously described (Troncoso et al., 2008), all participants were reviewed at a consensus conference following death or, during life if their Blessed Information Memory Concentration score was 3 or above, their informant or subject Clinical Dementia Rating score was 0.5 or above or the dementia questionnaire was abnormal. Diagnoses of dementia were based on the Diagnostic and Statistical Manual-III-R criteria. The diagnosis of dementia required evidence of a progressive cognitive syndrome, including memory decline.
Brain pathology
Post-mortem examination of all brains was performed at the Johns Hopkins Division of Neuropathology. Neuritic plaques, neurofibrillary tangles and infarcts were scored in five brain regions (superior/middle temporal gyrus, medial frontal lobe, inferior parietal cortex, orbitofrontal cortex and occipital cortex) as described (Troncoso et al., 2008). Alzheimer’s disease pathology was examined on silver stains (Hirano’s modification of Bielschowsky’s technique) and graded according to the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD; neuritic plaques) and Braak (neurofibrillary tangles) criteria (Braak and Braak 1991; Mirra et al., 1991). For CERAD scoring we determined both the maximum neuritic plaque score seen in all cortical regions examined (peak CERAD score) and the mean of the CERAD scores in each of the cortical regions examined (mean CERAD score). In addition, we generated a composite Alzheimer’s disease pathology score by summing the CERAD and Braak scores in equal measure. We previously showed this scale to be a useful method for quantitating the combined effects of β-amyloid and tau pathology on cognition (Troncoso et al., 2008). CERAD scores were divided into three groups: 1 = zero or mild neuritic plaques; 2 = moderate neuritic plaques; 3 = frequent neuritic plaques. Braak scores were divided into three groups: 1 = Braak stages 0, I and II; 2 = Braak stages III and IV; 3 = Braak stages V and VI. The sum of the modified Braak and CERAD scores yielded a composite score ranging from 2 to 6. All brain specimens were also carefully examined for evidence of Parkinson’s disease by searching for neuronal loss in the substantia nigra, and for Lewy bodies in the substantia nigra, locus coeruleus and cerebral cortex on haematoxylin and eosin stains. Lewy body changes were also examined with alpha-synuclein immunostains in 62% (129/209) of the autopsies. Diagnoses of Lewy body-related dementia and frontotemporal dementia were made according to accepted criteria (McKhann et al., 2001; McKeith et al., 2005). All subjects had an examination of the intracranial circulation including the circle of Willis, carotid siphon, distal internal carotid arteries, intracranial vertebral arteries, basilar artery and the proximal portions of the middle, anterior and posterior cerebral arteries. All vessels were inspected visually; areas of atherosclerosis were identified and then sectioned to determine the degree of stenosis. Mild intracranial atherosclerosis was defined as having no stenotic lesions (20% of the luminal diameter or greater) in any vessel. Severe intracranial atherosclerosis was defined as having stenoses of 40% or greater in two vessels. Moderate intracranial atherosclerosis was the designation for intermediate lesions, which for the most part included single vessel disease or multiple low-grade stenoses. All pathological data were collected blinded to the clinical diagnosis.
Statistics and comparisons
Participants were divided into either two or four age groups. The four age groups were created with the goals of keeping at least a 5-year spread in the mean age difference between the groups and retaining relatively equal numbers of subjects in each group. We also divided subjects into those 90 and above and those below 90, which were also groups with relatively equal numbers. Standard error plots were generated for Alzheimer’s disease pathology scores as a function of age and cognitive status (demented versus non-demented). Lines were fitted to the standard error plots using the method of least squares. The role of each type of Alzheimer’s disease pathology on the odds of becoming demented was determined in each of the different age groups using standard logistic regression, adjusting for sex. We also used logistic regression to examine threshold effects of Alzheimer’s disease pathology on cognition; for this analysis we created categorical variables for each of the Alzheimer’s disease pathology scores and ran them simultaneously. Sex was included as a covariate. Statistical software used for these analyses included Stata and Statistical Package for the Social Sciences (SPSS).
Results
The relationship between Alzheimer’s disease pathology, dementia and age
The characteristics of subjects in our cohort are shown in Table 1. To examine the effect of age on the role of Alzheimer’s disease pathology in the development of dementia, we generated standard error plots depicting the relationship between four different measures of cortical Alzheimer’s disease pathology and dementia as a function of age (Fig. 1). The 209 consecutive autopsies from the Baltimore Longitudinal Study of Ageing are divided into either four age groups (Fig. 1A) or two age groups (Fig. 1B). Although there was some minor convergence of the Braak and composite Alzheimer’s disease pathology scores of demented and non-demented subjects in the oldest groups, the lines fitted to the data describing the role of Alzheimer’s disease pathology in the development of dementia as a function of increasing age remained significantly different throughout the age span of our study. Moreover, tests of interactions between age group and Alzheimer’s disease pathology scores in predicting dementia (logistic regression), or age group and dementia diagnosis in predicting Alzheimer’s disease pathology score (analysis of variance, ordinal regression) were all non-significant for the interaction variable, suggesting that age does not influence the relationship between Alzheimer’s disease pathology and dementia or that our sample size was too small to detect it. Finally, in linear regression analyses of age as a continuous variable, Alzheimer’s disease pathology scores and dementia status showed that the slope of the relationship between age and Alzheimer’s disease pathology score did not differ between demented and non-demented subjects (data not shown).
Table 1.
Characteristics of Baltimore Longitudinal Study of Ageing autopsy participants
| Total subjects (n = 209) | Subjects who were demented at death (n = 104) | Subjects who were non-demented at death (n = 105) | ||
|---|---|---|---|---|
| Panel A | ||||
| Age at death | 87.3 ± 7.6 (70–102) | 88.5 ± 6.3 (71–102) | 86.4 ± 8.1 (70–101) | |
| Sex | 140 male, 69 female | 65 male, 39 female | 75 male, 30 female | |
| Baseline Mini-Mental State Exam | 29 (25–30) | 29 (25–30) | 29 (25–30) | |
| Final Mini-Mental State Exam | 25 (0–30) | 14 (0–30) | 29 (25–30) | |
| Length of follow-up (years) | 17.3 ± 5.4 (8–28) | 17.7 ± 5.6 (8–28) | 17.0 ± 5.1 (9–26) | |
| Time between last evaluation and death (months) | 8.8 ± 6.4 (1–18) | 9.1 ± 6.4 (1–18) | 8.5 ± 6.1 (1–18) | |
| Years of education | 17.0 ± 2.4 (9–22) | 16.9 ± 2.3 (10–22) | 17.0 ± 2.5 (9–22) | |
| Mini-Mental State Exam scores across age groups |
||||
| Panel B | ||||
| Age at death (years) | 70–82 (n = 51) | 83–87 (n = 53) | 88–93 (n = 55) | 94–101 (n = 50) |
| Initial Mini-Mental State Exam | ||||
| Demented at death | 29 (27–30) | 29 (25–30) | 28 (26–30) | 28 (26–30) |
| Non-demented at death | 29 (26–30) | 29 (27–30) | 29 (27–30) | 28 (25–30) |
| Final Mini-Mental State Exam | ||||
| Demented at death | 15 (0–30) | 14 (0–28) | 14 (0–25) | 13 (0–24) |
| Non-demented at death | 29 (25–30) | 29 (26–30) | 28 (25–30) | 28 (25–30) |
The data are presented as mean, standard deviation and range except for the baseline and final Mini-Mental State Exam values, which are presented as median and range. Using analysis of variation, none of the characteristics are different at the 0.05 level between the groups except for the initial and final Mini-Mental State Exam values of subjects who became demented.
Figure 1.
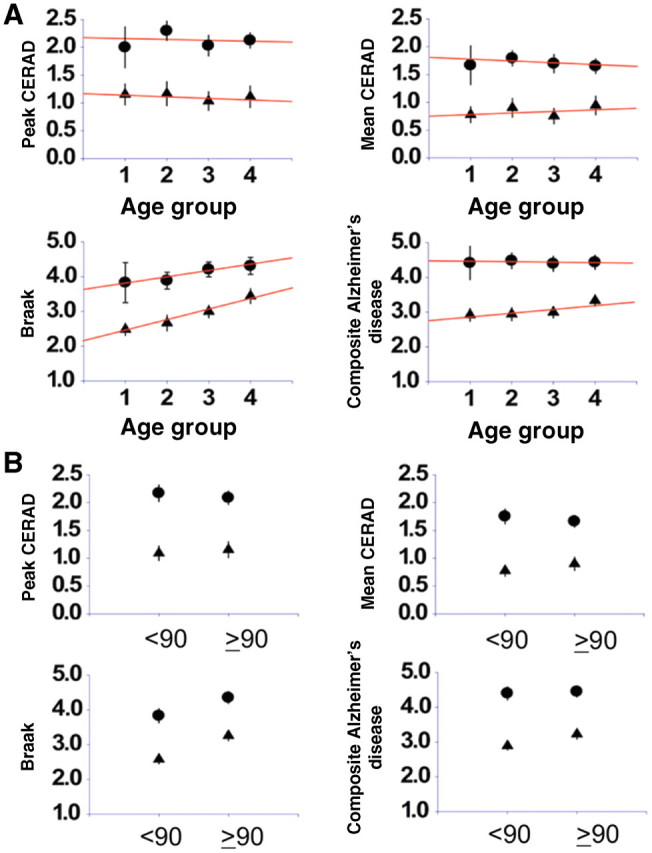
Cortical Alzheimer’s disease pathology scores as a function of age. Mean neocortical Alzheimer’s disease pathology scores for demented (circles) and non-demented (triangles) subjects in each age group (±SE) are plotted. (A) The cohort is divided into four groups. (B) The cohort is divided into those 90 and above (n = 96) and those below 90 (n = 113). A line is fitted to the data for demented and non-demented subjects for each pathology measure using the method of least squares. The composite pathology score is a combined weighting of the Braak and CERAD scores as defined in Materials and methods section.
The odds ratio for dementia conferred by a step-wise increase in each of the indicated Alzheimer’s disease pathology scores as a function of age are shown in univariate and multivariate (corrected for sex and brain weight) analyses in Table 2. For all age groups, Alzheimer’s disease pathology remained significantly related to cognition. In examining the role of Alzheimer’s disease pathology for subjects 90 and above (mean age 93.8) compared to those less than 90 (mean age 81.7), the odds of dementia related to a step-wise increase in Alzheimer’s disease pathology score were nearly identical (Fig. 1B and Table 2).
Table 2.
The effect of neocortical Alzheimer pathology on the odds of dementia in different age groups
| Group | Age range | Mean age | Peak CERAD | Mean CERAD | Braak score | Composite score | |
|---|---|---|---|---|---|---|---|
| Panel A | |||||||
| Univariate analysis | |||||||
| 1 | 70–82 | n = 51 | 76.4 ± 4.3 | 2.2 (1.2–4.0) | 2.1 (1.2–4.5) | 2.0 (1.2–3.4) | 2.2 (1.3–3.9) |
| 2 | 83–87 | n = 53 | 85.2 ± 1.7 | 3.2 (1.6–6.6) | 3.5 (1.6–7.5) | 2.6 (1.4–4.7) | 3.7 (1.8–7.6) |
| 3 | 88–93 | n = 55 | 90.6 ± 1.6 | 2.8 (1.6–4.9) | 3.0 (1.6–5.5) | 2.8 (1.6–5.2) | 3.1 (1.7–5.4) |
| 4 | 94–101 | n = 50 | 96.0 ± 2.1 | 2.6 (1.4–5.3) | 2.7 (1.2–5.8) | 1.7 (1.1–2.9) | 2.4 (1.3–4.6) |
| <90 | 70–89 | n = 113 | 81.7 ± 5.8 | 2.7 (1.8–3.9) | 3.1 (1.9–4.8) | 2.3 (1.6–3.4) | 2.9 (1.9–4.4) |
| ≥90 | 90–101 | n = 96 | 93.8 ± 2.9 | 2.5 (1.6–3.9) | 2.8 (1.6–4.5) | 2.1 (1.4–3.2) | 2.6 (1.7–3.8) |
| Panel B | |||||||
| Multivariate analysis | |||||||
| 1 | 70–82 | n = 51 | 76.4 ± 4.3 | 2.1 (1.1–4.3) | 2.3 (1.1–4.9) | 2.1 (1.2–3.9) | 2.2 (1.2–4.1) |
| 2 | 83–87 | n = 53 | 85.2 ± 1.7 | 3.3 (1.6–6.9) | 3.5 (1.6–7.9) | 2.5 (1.3–4.9) | 3.5 (1.7–7.5) |
| 3 | 88–93 | n = 55 | 90.6 ± 1.6 | 2.7 (1.5–5.0) | 3.0 (1.6–6.1) | 2.8 (1.5–5.0) | 2.9 (1.7–5.1) |
| 4 | 94–101 | n = 50 | 96.0 ± 2.1 | 2.7 (1.6–4.7) | 3.1 (1.3–8.0) | 1.8 (1.1–3.1) | 2.9 (1.4–6.0) |
| <90 | 70–89 | n = 113 | 81.7 ± 5.8 | 2.5 (1.7–3.7) | 3.2 (2.0–5.3) | 2.3 (1.6–3.5) | 2.7 (1.9–4.4) |
| ≥90 | 90–101 | n = 96 | 93.8 ± 2.9 | 2.7 (1.6–4.6) | 2.9 (1.6–5.2) | 2.0 (1.4–3.2) | 2.7 (1.7–4.3) |
The 209 autopsies in the Baltimore Longitudinal Study of Ageing autopsy cohort were divided into four or two groups based on age. Logistic regression analysis of the effects of a step-wise increase in each of the indicated neocortical Alzheimer’s disease pathologies on the odds of dementia are shown as a function of age group along with the 95% confidence intervals. A univariate analysis is presented in panel A, and a multivariate analysis corrected for sex and the weight of the brain at death is presented in panel B. The composite score is a combined weighting of the Braak and CERAD scores as defined in Materials and methods section.
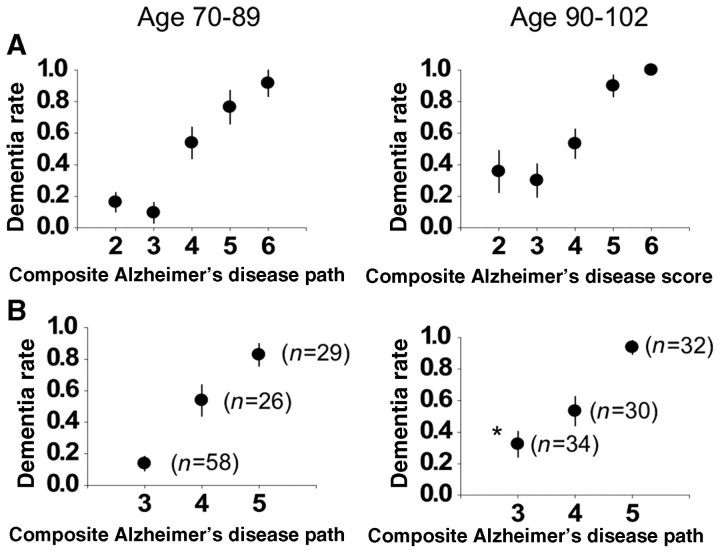
We next examined the relationship between composite Alzheimer’s disease pathology scores and dementia in subjects less than 90 years of age and those 90 and above. We chose to look at the composite Alzheimer’s disease pathology score as it is the most predictive score of dementia outcome in our cohort (Troncoso et al., 2008). As shown in Fig. 2A, for participants younger than 90 there was a strong relationship between the composite Alzheimer’s disease pathology score and dementia, with those whose scores were below four almost never being demented, while those whose scores were above four almost always being demented. Participants with an intermediate score of four (a substantial fraction of our cohort) had more variable outcomes. We collapsed the composite Alzheimer’s disease pathology scale in Fig. 2B, combining groups two and three and groups five and six for greater statistical power. For subjects aged 90 and above, the relationship between the composite Alzheimer’s disease pathology score and dementia was identical to those under 90 years of age if their composite Alzheimer’s disease pathology score was 4 or above [dementia rate 38/55 (69%) for subjects under 90 versus 62/96 (65%) for subjects 90 and above]. However, in subjects with low composite Alzheimer’s disease pathology scores (2 and 3), there were a significant number, aged 90 and above, who were demented (11/34) compared to those below age 90 where dementia was very uncommon with a low composite Alzheimer’s disease pathology score (7/58; P = 0.02; Fisher's exact test).
Figure 2.
The role of increasing Alzheimer’s disease pathology in dementia as a function of age. (A) Prevalence of dementia (referred to as the dementia rate) at the indicated composite Alzheimer’s disease pathology scores are plotted (±SE) for subjects 90 and above at time of death and those <90 at time of death. (B) Data are collapsed so that composite Alzheimer’s disease pathology scores of 2 and 3 are combined (and designated as 3) and scores of 5 and 6 are combined (and designated as five). The dementia rate for a composite Alzheimer’s disease pathology score of 3 differs significantly (P < 0.05) between the younger subjects (left graph) and the older subjects (right graph).
The increase in dementia in the most elderly subjects with low Alzheimer’s disease pathology scores suggested that the threshold for Alzheimer’s disease pathology to cause dementia may change with age. To examine this, we performed logistic regression in subjects aged 90 and above or under 90 using Alzheimer’s disease pathology scores as categorical variables (Table 3). For the CERAD score there was no evidence for a change in the amount of neuritic plaques needed to cause dementia (the threshold) in the two age groups. Moreover there was no change in the mean CERAD score between the young and old groups. Therefore, the risk of dementia attributable to CEARD pathology was unchanged in the two age groups. For the Braak neurofibrillary tangle score, the amount of neurofibrillary pathology needed to cause dementia (the threshold) was higher in the older age group. This was accompanied by an increase in the mean Braak score in older subjects, partially offsetting the threshold effect. The net effect of these two trends is that the risk attributable to neurofibrillary pathology is somewhat less in the older subjects. Finally, the composite Alzheimer’s disease pathology score (derived from the Braak and CERAD scores) showed an intermediate effect.
Table 3.
Threshold effects of Alzheimer’s disease pathology on the odds of dementia in the most elderly
| Age under 90 (n = 113) | Age 90 and over (n = 96) | |
|---|---|---|
| CERAD score and odds of dementia (reference CERAD 0) | ||
| CERAD 1 | 0.9 (0.2–4.8) | 0.8 (0.2–3.6) |
| CERAD 2 | 3.6 (1.3–11.9) | 3.4 (1.2–12.4) |
| CERAD 3 | 15.2 (4.3–50) | 29.1 (4.2–163) |
| Mean CERAD score | 1.6 ± 1.1 | 1.7 ± 1.0 |
| Attributable risk of dementia for CERAD score >1 | 55% | 53% |
| Braak score and odds of dementia (reference Braak 1 and 2) | ||
| Braak 3 | 3.8 (1.3–11.9) | 1.5 (0.4–5.5) |
| Braak 4 | 5.3 (1.9–14.7) | 2.7 (1.1–12.2) |
| Braak 5 or 6 | 65 (7.4–569) | 41 (5.2–373) |
| Mean Braak score | 3.1 ± 1.3 | 3.9 ± 1.2* |
| Attributable risk of dementia for Braak score >2 | 59% | 42% |
| Composite Alzheimer’s disease pathology score and odds of dementia (reference score of 2) | ||
| Score 3 | 0.5 (0.1–2.4) | 0.7 (0.2–1.7) |
| Score 4 | 5.0 (1.6–15) | 2.8 (1.1–8.3) |
| Scores 5 or 6 | 21.6 (5.8–73) | 26.8 (5.5–148) |
| Mean composite score | 3.5 ± 1.3 | 4.0 ± 1.2* |
| Attributable risk of dementia for Alzheimer’s disease pathology score >3 | 63% | 54% |
Subjects were divided into two age groups. Individual Alzheimer’s disease pathology scores were transformed into categorical variables and were run simultaneously against the indicated reference with dementia as the outcome. Sex was included as a covariate. Braak and composite Alzheimer’s disease pathology scores of five and six were combined for the analysis to avoid an infinite odds ratio seen in subjects with scores of six.
*P < 0.01 (ANOVA) compared with subjects <90 years.
The aetiology of dementia in subjects with low Alzheimer’s disease pathology scores
We examined the medical records of the 34 subjects aged 90 and above with low Alzheimer’s disease pathology scores, 11 of whom were demented. Of the 11 demented subjects, two were diagnosed with Lewy body-related dementias, one had a diagnosis of frontotemporal dementia and eight had severe cerebral atherosclerosis—six of whom had at least one cortical infarct. The number of subjects aged 90 and above in the demented group with low Alzheimer’s disease pathology scores who had severe intracranial atherosclerosis (8/11) was significantly different from the rate of severe intracranial atherosclerosis in subjects aged 90 and above with low Alzheimer’s disease pathology scores who were not demented (6/23; P = 0.01 Fisher's exact test, one-tailed). The significance of intracranial atherosclerosis as a predictor of dementia in this cohort [odds ratio = 2.3 (1.4 − 3.7) per step increase in intracranial pathology score] was confirmed using logistic regression with age group, sex and composite Alzheimer’s disease pathology score as covariates.
Discussion
Our results suggest that neocortical Alzheimer’s disease pathology remains significantly correlated with dementia throughout the lifespan. In the oldest subjects in our cohort the relationship between dementia and Alzheimer’s disease pathology changes somewhat, as dementia appears in subjects with low Alzheimer’s disease pathology scores—something that is uncommon in subjects under 90. Moreover, the amount of neurofibrillary tangle pathology needed to cause dementia increases with age. This difference between younger and older subjects, however, does not lessen the impact of Alzheimer’s disease pathology on cognition in elderly subjects with high Alzheimer’s disease pathology scores. Moreover, elderly subjects with low Alzheimer’s disease pathology scores have additional, well described, pathologies to account for their cognitive status, suggesting that dementia in elderly subjects is not dependent on new, unknown, processes. Importantly, atherosclerosis, with and without cerebral infarction, emerges as an important aetiology of dementia in this group, a finding that has recently been explored by our group (Troncoso et al., 2008). Other pathologies that we have not explored, such as Lewy body pathology, may also play an important role in dementia in the elderly.
Our results are based strictly on neocortical Alzheimer’s disease pathology and not hippocampal pathology. The importance of neocortical Alzheimer’s disease pathology in the clinical manifestations of Alzheimer’s disease was codified by the seminal publications of Braak and Braak (1991) and Mirra et al. (1991), which defined neocortical neurofibrillary tangles and neocortical neuritic amyloid plaques as the key elements in correlating Alzheimer’s disease pathology with dementia. These techniques for scoring Alzheimer’s disease pathology are semi-quantitative and it is hoped that more formal quantitative analyses of Alzheimer’s disease pathology will be even more informative. While hippocampal pathology and atrophy are clearly important in early disease pathogenesis (Reitz et al., 2009), dementia is a complex clinical phenomenon, dependent on multiple components of the cerebral cortex (Buckner et al., 2005). Indeed our recent work looking at the importance of infarcts in dementia showed that only neocortical infarcts played a role in cognitive decline (Troncoso et al., 2008).
Our study contrasts with a previous retrospective study using brain bank specimens suggesting that Alzheimer-type pathology has a stronger relationship with dementia in the younger-old compared to the oldest-old (Haroutunian et al., 2008). However in this study, the authors still find significant differences in neocortical Alzheimer’s disease pathology between demented and non-demented subjects even into very advanced age. A limited number of cognitively normal subjects in the older age groups and a reliance on clinical dementia rating scores rather than consensus conferencing for diagnostic determination of dementia also limit this analysis. More recently, Savva et al. (2009), in a study from the Medical Research Council Cognitive Function and Ageing Study (MRC-CFAS), found that the association between Alzheimer’s disease pathology and dementia was stronger in younger participants than older participants. Specifically, their data suggested that Alzheimer’s disease pathology in the form of neuritic plaques did not differ significantly between demented and non-demented subjects in their mid-nineties and that the effect of neurofibrillary tangles on cognition was significantly attenuated compared to younger subjects. There are several important differences between our study and the MRC-CFAS. First, hippocampal Alzheimer’s disease pathology in the MRC-CFAS shows little difference between demented and non-demented elderly subjects, especially the amount of neurofibrillary tangles, suggesting a potential ceiling effect in the hippocampus. The MRC-CFAS Alzheimer’s disease pathology scores in neocortical areas of subjects in their early nineties show clear differences between those who are demented and those who are not. Neocortical pathology scores in demented and non-demented MRC-CFAS subjects in their late nineties, in contrast, do show overlap. Importantly, the MRC-CFAS investigators analysed the relationship between dementia and CERAD or Braak Alzheimer’s disease pathology scores by dividing subjects into just two groups (those with high and those with low Alzheimer’s disease pathology scores), rather than analysing the full range of scores, so that their analysis could not detect changes in the amount (threshold) of each of these pathologies which correlated with dementia as a function of age. The characteristics of subjects enrolled in our study and in the MRC-CFAS are also different. While the mean ages in the two studies are similar, all of our subjects were cognitively normal at entry into the study and have high education levels. In contrast, the MRC-CFAS is a population-based sample of urban and rural areas of England and Wales that contain institutions that care for elderly subjects. Many subjects were demented on entry into the MRC-CFAS and the final median Mini-Mental State Examination score of the normal subjects in the MRC-CFAS is 25, while in our study it is 29 (Table 1). We feel that the higher educational level and cognitive functioning of our cohort at baseline is more likely to emphasize the effects of Alzheimer’s disease pathology and may be more representative of the future US and European populations (National Centre for Education Statistics, 2008; Christensen et al., 2009) that will be better educated and have greater access to health care than generations born in the early 20th century; our cohort does not reflect the demographics of populations at risk for dementia throughout the developing world however (Prince and Jackson, 2009).
In conclusion, our findings indicate that Alzheimer’s disease pathology remains a significant predictor of dementia throughout the lifespan. Although other factors contribute to the cognitive decline of patients suffering from Alzheimer’s disease and dementia, including atherosclerosis, Alzheimer-type brain pathology continues to play an important role even in the most elderly.
Funding
National Institute on Ageing (grant P50 AG05146); The Burroughs Wellcome Fund (grant #1005227) for Translational Research; The Intramural Research Program, National Institute on Ageing, National Institutes of Health.
Glossary
Abbreviations
- CERAD
Consortium to Establish a Registry of Alzheimer’s Disease
- MRC-CFAS
Medical Research Council Cognitive Function and Ageing Study
References
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Trojanowski J. Neurodegenerative dementias. New York, NY: McGraw-Hill; 1999. [Google Scholar]
- Driscoll I, Resnick SM, Troncoso JC, An Y, O'Brien R, Zonderman AB. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–95. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O'Brien R. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006;67:1363–9. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Schnaider-Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP, et al. Role of the neuropathology of Alzheimer disease in dementia in the oldest-old. Arch Neurol. 2008;65:1211–7. doi: 10.1001/archneur.65.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly. J Alzheim Dis. 2006;9:61–70. doi: 10.3233/jad-2006-9s308. [DOI] [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–7. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Kern A, Behl C. The unsolved relationship of brain aging and late-onset Alzheimer disease. Biochim Biophys Acta. 2009;1790:1124–32. doi: 10.1016/j.bbagen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Lobo A, Launer LJ, Fratiglioni, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: a collaborate study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology. 2008;54(Suppl 5):S4–9. [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mohajeri MH, Leuba G. Prevention of age-associated dementia. Brain Res Bull. 2009;80:315–25. doi: 10.1016/j.brainresbull.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Näslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, et al. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–7. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- National Center for Education Statistics; Digest of Educational Statistics. 2008. [(Advance Access published March 2010)]. http://nces.ed.gov/programs/digest/d08/ [Google Scholar]
- Prince M, Jackson J. 2009. [(Advance Access published March 2010)]. Alzheimer’s Disease International. World Alzheimer Report. http://www.alz.co.uk/research/worldreport/ [Google Scholar]
- Reitz C, Brickman AM, Brown TR, Manly J, DeCarli C, Small SA, et al. Linking hippocampal structure and function to memory performance in an aging population. Arch Neurol. 2009;66:1385–92. doi: 10.1001/archneurol.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68:223–8. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. New Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Amyloid β-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–8. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]