Abstract
Migraine is a common neurological disorder often treated with triptans. Triptan overuse can lead to increased frequency of headache in some patients, a phenomenon termed medication overuse headache. Previous preclinical studies have demonstrated that repeated or sustained triptan administration for several days can elicit persistent neural adaptations in trigeminal ganglion cells innervating the dura, prominently characterized by increased labelling of neuronal profiles for calcitonin gene related peptide. Additionally, triptan administration elicited a behavioural syndrome of enhanced sensitivity to surrogate triggers of migraine that was maintained for weeks following discontinuation of drug, a phenomenon termed ‘triptan-induced latent sensitization’. Here, we demonstrate that triptan administration elicits a long-lasting increase in identified rat trigeminal dural afferents labelled for neuronal nitric oxide synthase in the trigeminal ganglion. Cutaneous allodynia observed during the period of triptan administration was reversed by NXN-323, a selective inhibitor of neuronal nitric oxide synthase. Additionally, neuronal nitric oxide synthase inhibition prevented environmental stress-induced hypersensitivity in the post-triptan administration period. Co-administration of NXN-323 with sumatriptan over several days prevented the expression of allodynia and enhanced sensitivity to stress observed following latent sensitization, but not the triptan-induced increased labelling of neuronal nitric oxide synthase in dural afferents. Triptan administration thus promotes increased expression of neuronal nitric oxide synthase in dural afferents, which is critical for enhanced sensitivity to environmental stress. These data provide a biological basis for increased frequency of headache following triptans and highlight the potential clinical utility of neuronal nitric oxide synthase inhibition in preventing or treating medication overuse headache.
Keywords: triptans, migraine, medication overuse, cutaneous allodynia, nNOS, nitric oxide
Introduction
Migraine is a common and potentially debilitating neurological disorder that is characterized by a paroxysmal unilateral throbbing pain, which may be accompanied by nausea, vomiting, photophobia or phonophobia (Olesen et al., 2004). The underlying pathophysiology of this disorder remains unclear (Burstein, 2001; Bartsch and Goadsby, 2003; Arulmani et al., 2004; Bolay and Moskowitz, 2004; Boes et al., 2006; Dalkara et al., 2006). Regardless of the site or mechanism that underlies initiation of the headache attack, activation of primary afferent neurons are believed to mediate the pain and to initiate processes leading to the cephalic and extra-cephalic allodynia that is often observed in patients (Burstein et al., 2004, 2005; Olesen et al., 2009). Sensitization of primary afferents and especially of post-synaptic cells in the pain pathway may explain the occurrence of migraine-associated cutaneous allodynia that occurs at regions well outside the craniofacial area (Burstein et al., 2004; Burstein and Jakubowski, 2004; Landy et al., 2004). Recent evidence emerging from more detailed studies of migraineurs, employing quantitative sensory testing or detailed questionnaires indicates that there may be different populations of migraineurs with cephalic allodynia only or presenting also with extracephalic allodynia (Guy et al., 2009). In a recent study, ∼49% of individuals developing cutaneous allodynia indicated the presence of both cephalic and extra-cephalic allodynia, 49% indicated cephalic allodynia only and 2% indicated extra-cephalic allodynia only (Guy et al., 2009). Accordingly, in the present investigation it was decided to examine behavioural responses to stimuli applied to both cephalic and extracephalic sites. Triptans are often the acute treatment of choice and are recommended by the American Academy of Neurology for moderate or severe migraine headache, although reports of relief vary considerably from as low as 40% to as high as 75% of patients (Silberstein, 2000; Dahlof et al., 2002; Ferrari et al., 2002; Tfelt-Hansen, 2008). However, the frequent use of triptans over an extended period of time can lead to medication overuse headache, recently characterized as a global epidemic (Diener et al., 2004; Silberstein et al., 2005; Olesen et al., 2006; Ghiotto et al., 2009). The International Headache Society defines medication overuse headache as more than 15 headaches per month, during regular overuse (>15 days per month) of acute analgesics or symptomatic drugs for >3 months and specifies triptan-induced medication overuse headache as occurring with the intake of at least 10 doses of triptan per month for a period of 3 months (Silberstein et al., 2005; Olesen et al., 2006; Ghiotto et al., 2009). Migraine patients that overuse triptans generally characterize medication overuse headache as being identical to episodic migraine attacks, or simply report it as an increase in the frequency of migraine (Limmroth et al., 2002; Katsarava and Jensen, 2007). Patients with migraine also appear to be most susceptible to the development of medication overuse headache. A meta-analysis reviewing the treatment of 2612 patients in 29 studies revealed that 65% of patients with medication overuse headache reported migraine as the primary headache, 27% reported tension-type headache and the remaining 8% reported mixed headaches as the principal complaint (Diener and Dahlof, 1999; Katsarava and Jensen, 2007). Patients with a diagnosis of medication overuse headache rarely respond to prophylactic medications while overusing acute medications (Olesen et al., 2004). Some studies have shown that patients treated for cluster headaches do not develop an increase in their frequency, in spite of taking large doses of triptans (Ekbom et al., 1995; Dowson et al., 2005). However, others have demonstrated that overuse of triptans can in fact cause increased frequency of cluster headaches (Centonze et al., 2000; Paemeleire et al., 2006).
Although the mechanisms that underlie medication overuse headache are not known, growing evidence indicates that it may be associated with of the presence of central sensitization (Katsarava and Jensen, 2007; Ghiotto et al., 2009). For example, a recent study where pain-related cortical potentials were measured in patients with migraine revealed that medication overuse headache caused facilitation of trigeminal and somatic parameters (Ayzenberg et al., 2006). Cutaneous allodynia is thought to represent a clinical marker of central sensitization (Burstein et al., 2000a, b; Dodick and Freitag, 2006). Migraineurs present a much greater prevalence of cutaneous allodynia than do patients with non-migraine headaches (Bigal et al., 2008; Lipton et al., 2008). Moreoever, patients with medication overuse headache and chronic migraine show a greater prevalence of allodynia compared to those with episodic migraine (Bigal et al., 2008b; Bigal and Lipton, 2008, 2009). These data suggest that the transformation from episodic to chronic migraine involves the sensitization of trigeminal sensory pathways, and that the overuse of acute medications such as triptans is not only a risk factor for this transformation, but the mechanisms that drive medication overuse headache may be similar to those that drive the chronification of migraine (Bigal and Lipton, 2008, 2009). Establishing the nature of the mechanisms that underlie the pathobiology of medication overuse headache may assist in the development of new therapeutic strategies for improving the treatment of migraine and medication overuse headache.
Previous studies in our laboratory have demonstrated that persistent exposure of rats to sumatriptan or naratriptan over a period of 6–7 days leads to a state referred to as ‘triptan-induced latent sensitization’ (De Felice et al., 2010). This state is characterized by persistent increased labelling of neural profiles innervating the dura mater for calcitonin gene related peptide (CGRP) and to a lesser extent, substance P (De Felice et al., 2010). Additionally, exposure to triptans results in a state of generalized cutaneous allodynia during the period of triptan infusion, accompanied by hyper-responsiveness in the post-infusion state to challenge with a nitric oxide donor. Environmental stress is the most common reported trigger for migraine (Kelman, 2007) and studies with human volunteers showed that migraine can be reliably induced by administration of the nitric oxide donor nitroglycerin, and this condition is accompanied by an increase in blood levels of CGRP, which is directly linked to the severity of headache pain (Goadsby et al., 1990; Sarchielli et al., 2000; Juhasz et al., 2003). Similarly, our previous work also demonstrated that triptan-induced ‘latent sensitization’ is characterized by increased plasma CGRP levels following challenge of rats previously exposed to triptans with a nitric oxide donor (De Felice et al., 2010). In spite of these observed triptan-induced neural adaptations in dural afferents and enhanced sensitivity to presumed triggers of migraine, the mechanisms by which enhanced responsiveness occurred in these animals remain unknown.
Substantial evidence supports an important role of nitric oxide as a pivotal mediator contributing to the pathogenesis of migraine (Thomsen et al., 1997; Lassen et al., 1998; Christiansen et al., 1999). This sensitizing agent is formed by three different isoforms of nitric oxide synthase (NOS): neuronal NOS, endothelial NOS and inducible NOS (Freire et al., 2009). The presence of nitric oxide in peripheral and central nervous tissue is due primarily to neuronal NOS (Zhou and Zhu, 2009). Increased synthesis of nitric oxide by neuronal NOS in the spinal cord leads to spinal sensitization and enhancement of the spinal cord pain pathway activity (Meller and Gebhart, 1993; Gordh et al., 1995; Wu et al., 2000, 2001). Many studies also showed that inhibition of neuronal NOS reduces central sensitization in animal models of neurophatic and inflammatory pain (Coderre and Yashpal, 1994; Mao et al., 1997; Khalil and Khodr, 2001; Tanabe et al., 2009). For these reasons, the present study explored the possibility that triptan exposure might modulate the expression of neuronal NOS in dural afferents and that the activity of this enzyme might promote enhanced excitability that is observed in rats with triptan-induced latent sensitization. We used NXN-323, a potent and highly selective inhibitor of neuronal NOS, to demonstrate a critical role for this enzyme in promoting responses to stimuli hypothesized to be relevant to migraine attack in humans and perhaps in promoting the state of triptan-induced medication overuse headache.
Materials and methods
Animals
Adult male Sprague Dawley rats (175–250 g) were maintained in a climate-controlled room on a 12 h light/dark cycle with food and water ad libitum. All testing was performed in accordance with the policies and recommendations of the International Association for study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals, approval received from the Institutional Animal Care and Use Committee of the University of Arizona. Groups of 6–12 animals were used in all experiments.
Drug administration
Alzet osmotic mini-pumps (Alzet, Cupertino CA, USA; model 2001) with a nominal flow rate of 1 μl/h for 7 days were used for subcutaneous drug infusion. The mini-pumps were implanted subcutaneously in rats under anaesthesia with isoflurane. The day of the pump implant was considered as Day 0. Drugs administered by infusion were sumatriptan (0.6 mg/kg/day; gift of GSK, Philadelphia, PA, USA), NXN-323 (0.7 mg/kg, NeurAxon, Toronto, ON, USA). NXN-323 and sodium nitroprusside (3 mg/kg, i.p., Sigma-Aldrich, St Louis, MO, USA) were also given as a bolus injection. Inducible NOS inhibitor 1400W dihydrochloride (1 or 5 mg/kg/day; Tocris, Ellisville, MO); endothelial NOS inhibitor (l-iminoethyl) ornithine dihydrochloride (10 mg/kg/day Tocris, Ellisville, MO). NXN-323 was employed as a highly selective inhibitor of neuronal NOS. The pIC50 values for NXN-323 against neuronal NOS, endothelial NOS and inducible NOS were found to be 0.27 µM, 59 µM and >100 µM, respectively (Dr Shawn Maddaford, NeurAxon, personal communication). In contrast, the corresponding values at neuronal NOS, endothelial NOS and inducible NOS for (l-iminoethyl) ornithine are 0.95, 1.0 and 1.6 µM, respectively; the values for 1400 W at these enzymes are 3.2, 49 and 0.79 µM, respectively (Boer et al., 2000).
Evaluation of tactile sensitivity
Baseline withdrawal thresholds to von Frey filaments applied to the paraocular region of the face and the plantar surface of the hind paw were determined prior to osmotic mini-pump implantation. To determine tactile sensitivity, the rats were placed in cages and allowed to acclimatize in a quiet environment for 45 min. For the sensory threshold of the face or of the hind paw, the von Frey filament was applied perpendicularly to the paraocular region of the rat’s face or to the plantar surface of the hind paw until it buckled slightly, and was held for 3–6 s. A positive response was indicated by withdrawal of the face or hind paw from the von Frey filament. The withdrawal thresholds were determined by Dixon’s up-down method (Dixon, 1980; Chaplan et al., 1994). Maximum filament strengths were 8 g and 15 g for the face and hind paw, respectively.
For the evaluation of tactile sensitivity in response to exposure to a nitric oxide donor, rats were injected with sodium nitroprusside (3 mg/kg, i.p.) and sensory thresholds were measured at 1 h intervals for 6 h. For studies where the effects of environmental stress were measured, rats were placed in an open box (20 × 30 cm) and exposed to bright light for 1 h. The light was positioned to avoid temperature changes in the box. Periorbital and hind paw allodynia was measured at 1 h intervals for 6 h, starting 1 h after the end of the stress stimulus exposure. Environmental stress by exposure to the bright light was repeated a second time, 24 h after the first exposure.
Tracer injection
Four days prior to perfusion, animals were anaesthetized with a combination of ketamine and xylazine (80 mg/kg and 12 mg/kg; Sigma-Aldrich). In order to label the ophthalmic branch of the trigeminal ganglia, the skull of the rat was exposed and two holes were made, ∼–1 mm Anterior-Posterior (AP) and 1 mm Medial-Lateral (ML) from bregma and +1 mm AP and 1 mm right from lambda, without tearing the dura. A volume of 10 μl of the retrograde nerve tracer Fluorogold (4% in saline) was injected onto the dura through each hole, bone wax was applied to seal each hole and any solution that leaked out was wiped away with sterile cotton-tipped swabs. Control experiments were performed in which holes were made in the skull with the following exception; a thin layer of bone was left at the bottom of the holes for these control experiments so that solution added to the holes could not reach the dura. Fluorogold solution was added to the holes, which were then sealed with bone wax and any excess solution was wiped away. Using this procedure, no Fluorogold-positive cells were observed in the trigeminal ganglion, indicating that tracer spread was not a significant concern in these labelling experiments i.e. Fluorogold-positive cells represent dural afferents. At the time of tissue removal, only animals that showed no damage to the dura after Fluorogold was applied were used for immunohistochemistry analysis.
Immunohistochemistry
Anaesthetized rats were transcardially perfused with 0.1 M phosphate buffered saline followed by 4% formaldehyde/12.5% picric acid solution in 0.1 M phosphate buffered saline. Trigeminal ganglia were removed, cryoprotected (in 20% sucrose), frozen and sectioned (10 µm) on a cryostat. Sections were incubated with primary antibody against nNOS (host, rabbit or guinea pig, Chemicon, Temecula, CA, USA), CGRP (host, rabbit or guinea pig; Peninsula Laboratories, San Carlos, CA, USA), substance P (host, rabbit or guinea pig; Peninsula) or NF200 (host, mouse; Sigma) for 24 h. Secondary antibodies were goat anti-rabbit/guinea pig IgG conjugated with Alex fluor-488 (green) or 594 (red) (Molecular Probes, Portland, OR, USA) and goat anti-mouse IgG conjugated with Alex fluor-488 for 2 h. Analysis of co-localization of two neuromarkers in trigeminal ganglia was performed using dual-labelling immunofluorescence. The primary antibodies of both neuromakers were mixed, but raised from different species of host animals and the secondary antibodies were conjugated with either red or green fluorescent compound, as mentioned. For counts of the percentage of cells expressing specific neurochemicals, two sections from each trigeminal ganglion in each of three animals were randomly selected for each marker. In each section, the number of positively stained cells was counted directly from live images acquired off the microscope. If double or triple labelling was involved in the study, once one label was counted, the fluorescent filter was switched to view another label until all labels were counted. The sections were then incubated in ethidium bromide (5 μg/ml) or DAPI for 30 s to visualize all neuronal cells. Cells were counted by a technician who was unaware of the experimental protocols and groups.
Data analysis
Statistical analyses were performed with JFlashCalc (www.u.arizona.edu/∼michaelo). Behavioural studies among groups and across time were analysed by two-factor ANOVA. One-factor ANOVA followed by Fisher’s least significant difference post hoc test was used to detect behavioural changes from baseline values (Milligan et al., 2000). When allodynia was precipitated by nitric oxide donor or stress, the data were converted to percent allodynia as 100× response of treated/control group for purposes of illustration, and the means were compared by Student’s t-test. Counts of trigeminal profiles were collected per section, and transformed to the percentage of that seen in vehicle-treated control groups. Data among groups was analysed by ANOVA followed by Fisher’s least significant difference test.
Results
Sumatriptan treatment elicits long-lasting increases in neuronal NOS labelling in multiple classes of retrogradely-labelled dural afferents
Trigeminal profiles
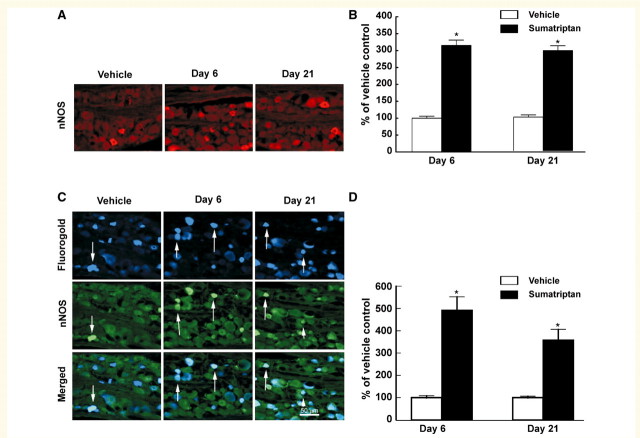
Approximately 5000 profiles overall were counted for each treatment group. On Day 6, the total number of neuronal NOS-positive profiles was 325 and 1034 for the saline and sumatriptan-treated groups, respectively. Thus, infusion of sumatriptan over 6 days produced a significant (P < 0.05), 3-fold increase in the numbers of neuronal profiles in the trigeminal ganglion immunolabelled for neuronal NOS (Fig. 1A and B). Immunofluorescent labelling for neuronal NOS was 315 ± 16% relative to saline treatment on Day 6 of sumatriptan infusion, remaining elevated at 297 ± 14% 15 days after termination of the infusion (i.e. Day 21).
Figure 1.
Sustained infusion of sumatriptan (0.6 mg/kg/day, s.c.) for 6 days promoted increased and persistent expression of neuronal NOS (nNOS) in the trigeminal ganglia of rats. Immunofluorescence labelling neuronal NOS in the trigeminal ganglion is shown for sections obtained 6 and 21 days after vehicle or sumatriptan pump was implanted (A). Sumatriptan exposure resulted in a significant (P < 0.05) increase in numbers of neuronal NOS-labelled profiles in the trigeminal ganglia, relative to vehicle-infused animals, at both Day 6 and 21 (B). Additionally, dural afferents were identified by application of Fluorogold to the dura and profiles expressing labelling for neuronal NOS (C) were evaluated in the trigeminal ganglia 6 and 21 days after sumatriptan exposure. The relative proportion of profiles obtained from sumatriptan-treated animals and expressing both the retrograde label and label for neuronal NOS in the trigeminal ganglia showed a significant and persistent (Day 6 and Day 21) increase relative to vehicle-infused animals (D). Asterisk indicates P < 0.05 relative to vehicle.
Retrogradely labelled dural afferents
In order to explore possible sumatriptan-induced effects in identified dural afferents, Flurogold was applied to the dura 4 days prior to perfusion. Tissue was taken for immunohistochemical analysis and neuronal NOS labelling was evaluated in Flurogold-positive trigeminal ganglion cells. Control studies were performed for possible Fluorogold tracer spread by creating a hole in the skull that left the last layer of bone intact therefore not allowing the tracer to reach the dura. Following application of the Fluorogold to this hole, virtually no labelling in trigeminal ganglia neurons was observed. Sumatriptan treatment produced a significant (P < 0.05) increase in labelling for neuronal NOS in the dural afferent subpopulation of the trigeminal neurons that was greater than that observed in the overall trigeminal ganglion population of cells (Fig. 1C and D). This increase was 490 ± 62% and 357 ± 50% relative to saline-infused animals on Day 6 of sumatriptan treatment as well as 15 days after termination of sumatriptan treatment (i.e. Day 21) (Fig. 1C and D).
Changes in neuromarkers in myelinated and unmyelinated profiles
This pattern of marked sumatriptan-induced up-regulation of neuronal NOS was consistent when labelled dural afferents were also co-labelled with immunofluorescent markers for IB4 and NF200 in order to differentiate unmyelinated peptide-poor or myelinated fibres, respectively (Silverman and Kruger, 1988, 1990; Lawson and Waddell, 1991). Sumatriptan exposure led to an increase in expression of neuronal NOS in retrogradely-labelled trigeminal profiles also labelled with IB4 on Day 6 of sumatriptan treatment that was sustained after termination of drug administration (i.e. Day 21) (Fig. 2A). The proportion of retrogradely-labelled profiles expressing neuronal NOS and labelled for IB4 were increased to 176 ± 17.7% and 192 ± 21.6% on Days 6 and 21, respectively, relative to the saline-infused groups (Fig. 2B). Similarly, sumatriptan exposure led to an increase in expression of neuronal NOS in retrogradely-labelled NF200 positive myelinated (i.e. labelled with NF200) trigeminal profiles on treatment Day 6 and post-treatment Day 21 (Fig. 2B). The proportions of retrogradely-labelled, myelinated dural afferents expressing neuronal NOS were increased to 171 ± 32.4% and 126 ± 13.5% on Days 6 and 21, respectively (Fig. 2C and D).
Figure 2.
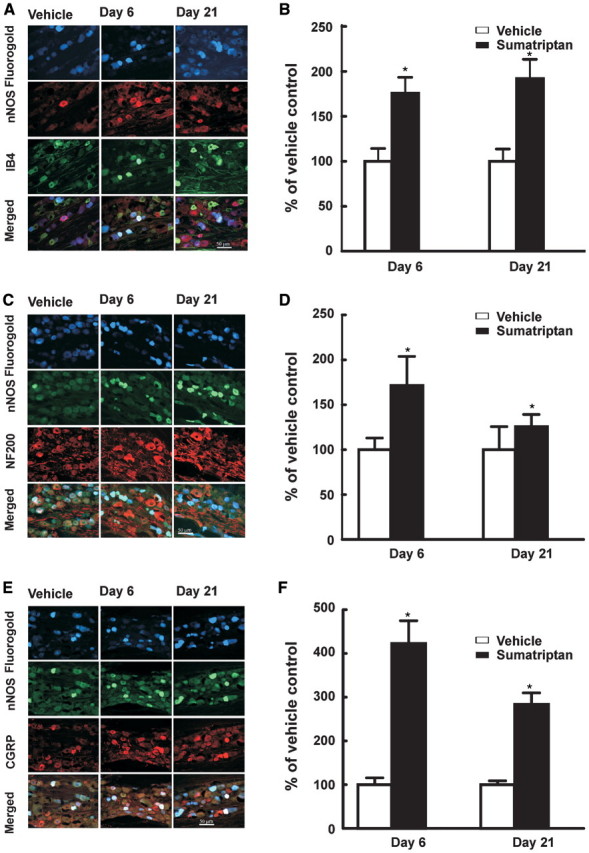
Sustained infusion of sumatriptan lead to an increased expression of neuronal NOS (nNOS) in normally ‘peptide-poor’ unmyelinated fibres and in myelinated fibres. Dural afferents were identified by administration of Fluorogold to the dura 4 days prior to collecting trigeminal tissue for immunofluorescent imaging. Trigeminal ganglion sections were obtained from rats 6 and 21 days after initiation of sumatriptan infusion (0.6 mg/kg/day, s.c.) and labelled for neuronal NOS. The sections were also labelled for reactivity to IB4 (A, B) or NF200 (C, D). The proportion of retrogradely-labelled profiles also showing label for neuronal NOS and for either IB4 or NF200 were determined relative to that shown by sections obtained from saline-treated rats. Sumatriptan infusion resulted in significant (P < 0.05) increase in retrogradely-labelled trigeminal profiles expressing label for neuronal NOS and either IB4 (B) or NF200 (D) 6 and 21 days after initiation of infusion. In dural afferents of the trigeminal ganglia, exposure to sumatriptan increased the co-expression of neuronal NOS with CGRP in retrogradely-labelled trigeminal profiles. After retrolabelling the dural afferent of the trigeminal ganglia, sections were prepared for fluorescent staining to visualize CGRP and neuronal NOS at Day 6 and 21 after sumatriptan exposure (E). The numbers of profiles expressing neuronal NOS and CGRP were counted (F). Sumatriptan exposure induced a marked significant (P < 0.05) increase in co-expression, relative to vehicle-infused animals, 6 and 21 days after sumatriptan pump implantation. Asterisk indicates P < 0.05 relative to vehicle.
Consistent with previous observations (Edvinsson et al., 2001), our data showed that the total numbers of trigeminal ganglia dural profiles expressing neuronal NOS relative to the whole population of counted profiles is rather low. In the vehicle-treated group, of a total of 232 retrogradely labelled neuronal profiles counted in five sections, only 13 expressed label for neuronal NOS. However, such expression was significantly increased in this population by sumatriptan treatment. On Day 6 following sumatriptan exposure, out of a total of 312 labelled dural afferents that were examined, 101 expressed label for neuronal NOS. When examined by co-labelling with CGRP, under basal conditions, eight neuronal NOS profiles were observed with CGRP co-labelling and five expressed label for neuronal NOS in the absence of label for CGRP (of 232 labelled profiles). The same analysis on Day 6 after sumatriptan exposure showed 44 expressed label for neuronal NOS in the presence of label for CGRP and 61 expressed label for neuronal NOS in the absence of CGRP (of 312 labelled profiles). On Day 21 after sumatriptan exposure, 37 profiles expressed CGRP and neuronal NOS whereas 32 profiles expressed neuronal NOS in the absence of CGRP of a total of 272 retrogradely labelled profiles (Fig. 2E and F). The proportion of trigeminal profiles expressing both CGRP and neuronal NOS was increased to 422 ± 51.8 and 234 ± 26.1%, relative to the saline-infused rats, on Days 6 and 21 after initiation of sumatriptan infusion (Fig. 2E and F). Thus, on a proportional basis, CGRP-positive profiles show the greatest increase in numbers of profiles also expressing neuronal NOS.
NXN-323, a highly selective neuronal NOS inhibitor, reverses and prevents sumatriptan-induced allodynia
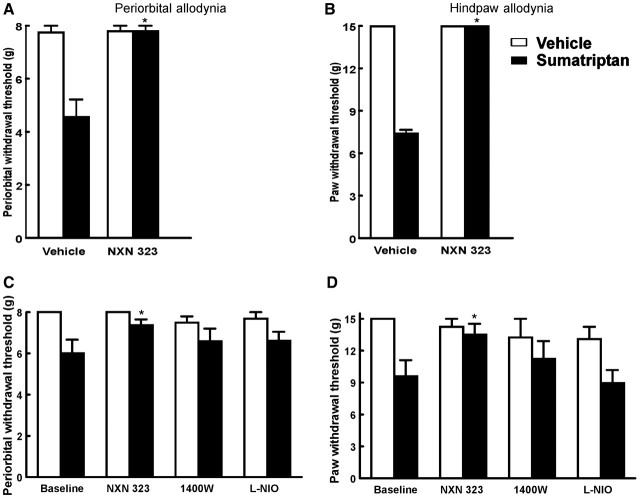
On the sixth day of sumatriptan infusion, when tactile allodynia of the periorbital region and of the hind paw was well established, rats received a bolus injection of NXN-323 (0.7 mg/kg, i.p.). Withdrawal thresholds to tactile stimuli applied to the periorbital area and the hind paw were significantly (P < 0.05) elevated to pre-sumatriptan baseline values within 0.5 h of administration of NXN-323 and remained elevated for 2 h before returning to the allodynic state after a further 60 min (Fig. 3A and B). NXN-323 had no effect on sensory thresholds in saline-treated rats.
Figure 3.
The effects of triptan exposure are mediated by neuronal NOS, and not inducible NOS or endothelial NOS. Rats infused with sumatriptan for 6 days were challenged with a single bolus injection of NXN-323 (A, B), which significantly (P < 0.05) elevated withdrawal thresholds, indicating a reversal of triptan-induced allodynia. Asterisk indicates P < 0.05 relative to vehicle. Additionally, co-infusion of sumatriptan (0.6 mg/kg/day, s.c.) with selective inhibitors of neuronal NOS (NXN-323), inducible NOS (1400W) or endothelial NOS [(l-iminoethyl) ornithine] resulted in significant (P < 0.05) reduction in tactile allodynia of the periorbital area (C) or hindpaws (D) with NXN-323. Neither 1400W nor (l-iminoethyl) ornithine prevented the development of tactile allodynia in rats infused with sumatriptan.
In order to evaluate if inhibition of neuronal NOS activity prevented development of cutaneous allodynia, rats received infusions of sumatriptan (0.6 mg/kg/day) and of NXN-323 (0.7 mg/kg/day) or vehicle delivered by separate subcutaneous implanted mini-pumps. The sumatriptan-treated animals with vehicle co-infusion demonstrated decreased periorbital and hind paw withdrawal thresholds, indicative of cutaneous allodynia. In contrast, co-infusion of NXN-323 along with sumatriptan prevented the development of these behavioural signs of allodynia in either the periorbital region or the hind paw. The withdrawal thresholds over the entire observation period of the NXN-323/sumatriptan treated rats were significantly greater (P < 0.05) than those receiving vehicle/sumatriptan. The mean periorbital response threshold of sumatriptan-infused animals receiving vehicle on Day 6 was significantly reduced (P < 0.05) to 6.5 ± 0.42 g whereas that of rats receiving NXN-323 along with sumatriptan was 7.37 ± 0.28 g, relative to the baseline value of 8 g. The mean hind paw response threshold of the sumatriptan-treated rats also receiving vehicle infusion was 9.71 ± 0.41 g whereas that of the rats receiving NXN-323 infusion along with sumatriptan was 15 ± 0 g. The infusion of NXN-323 did not elicit any changes in periorbital or hind paw withdrawal thresholds in the saline-infused animals (Fig. 3C and D).
In order to evaluate the possible role of other NOS isoforms in sumatriptan-induced allodynia separate groups of rats were treated with sumatriptan and inducible NOS or sumatriptan and endothelial NOS inhibitors. The co-infusion of the inducible NOS inhibitor 1400 W (1 and 5 mg/kg/day) (Hesslinger et al., 2009) or of the endothelial NOS inhibitor (l-iminoethyl) ornithine (10 mg/kg/day) (Rees et al., 1990) did not produce any significant change in periorbital or hind paw withdrawal thresholds induced by sumatriptan infusion (Fig. 3C and D). There were no significant differences in periorbital or hind paw sensory thresholds of rats receiving sumatriptan with either 1400W or (l-iminoethyl) ornithine when compared to the vehicle-treated groups. Furthermore, (l-iminoethyl) ornithine or 1400W did not alter sensory thresholds when given alone.
NXN-323, a selective neuronal NOS inhibitor, reverses and prevents stress-induced allodynia in sumatriptan pre-exposed rats
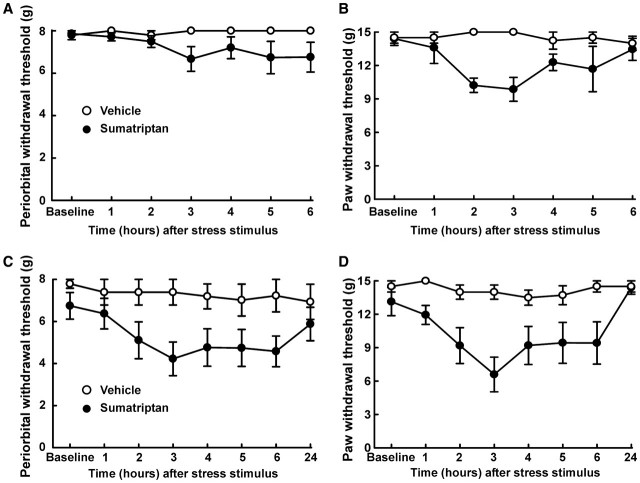
Our previous work showed that following triptan treatment, animals demonstrate enhanced behavioural responses to stimuli proposed as migraine triggers. For example, the administration of the nitric oxide donor sodium nitroprusside to rats pretreated with sumatriptan evoked behavioural signs of periorbital and hind paw allodynia (De Felice et al., 2010). Similarly, exposure to environmental stress in the form of bright light for 1 h, 14 days after termination of sumatriptan exposure also produced behavioural signs of cutaneous allodynia (Fig. 4A and B). After light exposure, the response thresholds of the sumatriptan-treated rats to periorbital tactile stimuli were significantly reduced (P < 0.05) from the baseline value of 8 g to 6.67 ± 0.58 g and paw withdrawal thresholds were reduced from the mean baseline value of 15 g to 9.86 ± 1.07 g (Fig. 4A and B). The rats received a second exposure to bright light stress the following day (i.e. 15 days after termination of sumatriptan infusion), which again provoked behavioural signs of cutaneous allodynia (Fig. 4C and D). The mean periorbital withdrawal threshold was significantly reduced (P < 0.05) to 4.22 ± 0.80 g and the mean paw withdrawal threshold was significantly reduced (P < 0.05) to 6.59 ± 1.56 g by the second exposure to the stressor (Fig. 4C and D). Exposing saline-treated control animals to bright light did not alter mean withdrawal threshold of either the periorbital area or the hind paws on any of the days tested.
Figure 4.
Sumatriptan-induced latent sensitization. Twenty days after pump implant, sumatriptan-exposed rats showed sensitivity to environmental stress. On Day 20, rats were exposed to bright light for 1 h, which caused a significant (P < 0.05) reduction in periorbital (A) or hind paw (B) thresholds in sumatriptan-exposed rats. More over on Day 21 a second exposure to bright light produced an even more robust facial (C) and hind paw (D) allodynia in sumatriptan pre-exposed animals. Two-factor ANOVA indicated significant (P < 0.05) differences in periorbital and hindpaw withdrawal thresholds between the vehicle-treated and sumatriptan-treated groups on both days.
A single bolus injection of NXN-323 (0.7 mg/kg, i.p.) given 30 min after the exposure to bright light blocked stress-induced cutaneous allodynia of both the periorbital region and the hind paws. Compared to the vehicle-challenged group, the response thresholds to periorbital stimuli and to hind paw stimuli were significantly (P < 0.05) elevated by the injection of NXN-323 (Fig. 5A–D). The periorbital withdrawal thresholds 1 h after environmental stress were 8 ± 0 g with both the first and second stress challenge (Fig. 5A and C). Likewise, the response thresholds to hind paw stimulation were 15 ± 0 g with both the first and second challenge with bright light (Fig. 5B and D).
Figure 5.
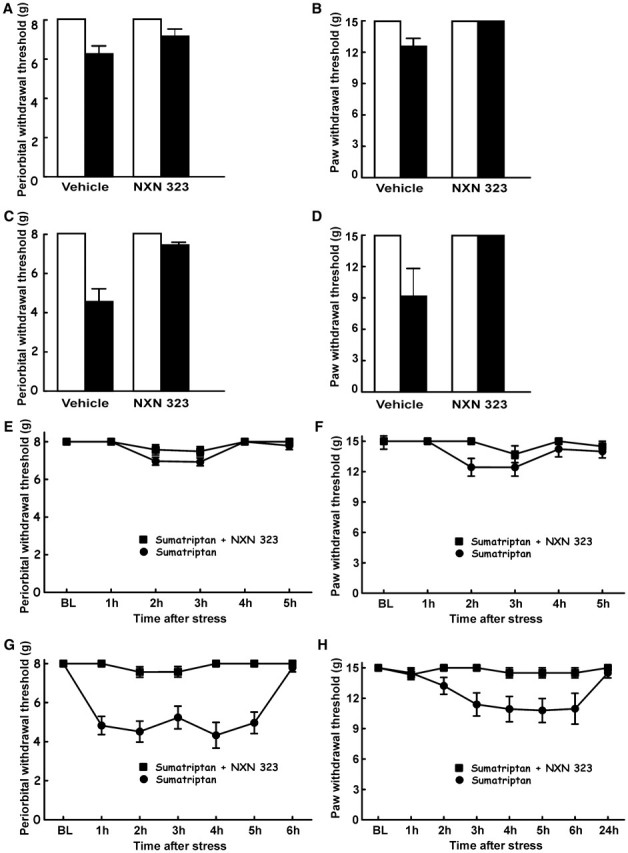
Stress-induced allodynia is blocked by selective neuronal NOS inhibitor. (A–D) Rats received sumatriptan infusion and were challenged on Days 20 and 21 with a single injection of NXN-323 prior to exposure to the bright light environmental stressor. NXN-323 blocked the expression of periorbital (A: Day 20, C: Day 21) and hind paw (B: Day 20, D: Day 21) tactile allodynia in sumatriptan-exposed rats. Two-factor ANOVA indicated significant (P50.05) differences in periorbital and hind paw withdrawal thresholds between the groups receiving vehicle and that receiving NXN-323 on both days. (E–H) On Days 20 and 21 after pump implantation, rats were exposed to bright light for 1 h, which caused a significant reduction in periorbital or hind paw thresholds in sumatriptan-exposed rats. However, co-infusion of sumatriptan and NXN-323 prevented the expression of periorbital (E: Day 20, G: Day 21) or hind paw (F: Day 20, H: Day 21) allodynia.
Co-infusion of sumatriptan and neuronal NOS inhibitor together not only resulted in prevention of sumatriptan-induced allodynia, but additionally prevented stress-induced allodynia in animals with latent sensitization. Animals that received co-infusion of both sumatriptan (0.6 mg/kg/day) and NXN-323 (0.7 mg/kg/day) did not develop periorbital or hind paw allodynia after exposure to stress on Days 20 and 21. Response thresholds to periorbital and hind paws tactile stimuli were not reduced from the baseline value of 8 g or 15 g (Fig. 5E–H).
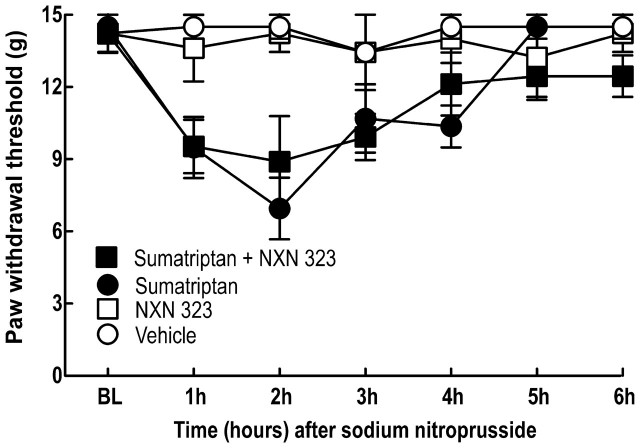
In contrast, co-infusion of the neuronal NOS inhibitor did not block nitric oxide donor-induced allodynia in sumatriptan-exposed animals. Rats were implanted with two mini-pumps delivering saline or sumatriptan and NXN-323 for 6 days. On Day 20 animals were challenged with the nitric oxide donor sodium nitroprusside (3 mg/kg, i.p.). Response thresholds to tactile stimuli applied to the hind paws of rats co-infused with saline and NXN-323 and then challenged with either saline or sodium nitroprusside were unchanged (Fig. 6). Rats that were infused with sumatriptan over 6 days showed normalized response thresholds at Day 20 and a significant (P < 0.05) reduction in mean paw withdrawal threshold to 6.9 ± 1.28 g 2 h after challenge with the nitric oxide donor sodium nitropruside (3 mg/kg, i.p.) (Fig. 6). Likewise, rats that were co-infused with NXN-323 along with sumatriptan for 6 days demonstrated normalized withdrawal responses at Day 20, but a significant (P < 0.05) reduction in mean paw withdrawal threshold 8.9 ± 1.89 g 2 h after challenge with sodium nitropruside (Fig. 6). These results indicate that unlike stress, challenge with a treatment that bypasses the neuronal NOS enzyme induces allodynia in triptan pre-exposed rats regardless of prior co-infusion with the neuronal NOS inhibitor.
Figure 6.
Sumatriptan-induced latent sensitization. Rats were pre-exposed to sumatriptan infusion and co-infusion of vehicle or NXN-323 for 6 days. The rats were challenged with sodium nitroprusside, which produced significant (P < 0.05) reductions in paw withdrawal thresholds in both vehicle-exposed and NXN-323-treated rats that also received sumatriptan infusion. Two-factor ANOVA indicated no significant (P > 0.05) differences withdrawal thresholds between the sumatriptan-infused groups receiving vehicle and that receiving NXN-323.
Co-infusion of NXN-323 with sumatriptan did not prevent sumatriptan-induced CGRP up-regulation in dural afferents. Exposure to sumatriptan produced an increase in retrogradely-labelled trigeminal dural afferent profiles expressing CGRP 298 ± 5% and 246 ± 7% of baseline values on Days 6 and 20, respectively (data not shown). These values were similar to those obtained from animals exposed to sumatriptan and receiving a co-infusion of NXN323, which were 280 ± 20% and 243 ± 6% of baseline on Days 6 and 20, respectively.
Discussion
The present investigation explored the basis for increased sensitivity of rats with triptan-induced latent sensitization to hypothesized triggers of migraine as a possible basis for development of medication overuse headache, and possibly to gain insight into mechanisms relevant to migraine pathophysiology (De Felice et al., 2010). Exposure to sumatriptan markedly increased the numbers of trigeminal neuronal profiles expressing neuronal NOS that was particularly evident in retrogradely-labelled dural afferents, and especially pronounced among those also co-expressing CGRP. The up-regulation of neuronal NOS in trigeminal dural afferents endured beyond the period of triptan-treatment, suggesting that these persistent neuroplastic changes might promote increased neural excitability. This concept was supported by the reversal and prevention of hypersensitivity to stress by inhibition of neuronal NOS, but not of endothelial or inducible NOS. This observation is consistent with the nitric oxide hypothesis of migraine headache proposed by Olesen et al. (1993) that suggests a causative role for nitric oxide in initiating and maintaining migraine headache.
There is mounting evidence that sensitivity to nitric oxide could trigger headache in migraineurs, perhaps as a consequence of elevated nitric oxide levels or of neuronal NOS activity (Pardutz et al., 2000; Olesen, 2008). Clinical studies have clearly shown that administration of nitroglycerin and other nitric oxide donors can precipitate a full-blown migraine attack in migraineurs, and that non-selective NOS inhibitors can abolish migraine (Olesen, 2008). The infusion of nitroglycerin produces a remarkably reproducible syndrome, beginning with an immediate headache of short duration in all subjects, followed by a migraine headache only in migraineurs after a delay of several hours (Olesen et al., 1993; Christiansen et al., 1999; Afridi et al., 2004; Offenhauser et al., 2005; Olesen, 2008). The migraine thus elicited is indistinguishable from spontaneous migraine episodes by the patients (Thomsen et al., 1994), and nitric oxide-induced migraine is an accepted clinical model of migraine (Iversen et al., 1989; Olesen et al., 1993; Thomsen et al., 1994). Furthermore, patients that develop medication overuse headache also show evidence of increased nitric oxide and nitric oxide-dependent cyclic guanosine monophosphate production (Sarchielli et al., 1999). Clinical trials strongly suggested that inhibition of nitric oxide synthase abolished migraine headache and also attenuated photophobia and phonophobia when compared to a placebo-treated group from a similar, but separate study (Lassen et al., 1998).
Previous studies have shown that in the basal state, expression of neuronal NOS in the trigeminal ganglion is very low. In one study examining neurons retrogradely labelled from the middle cerebral artery of the rat, there was virtually no expression of NOS in the rat trigeminal ganglion (Edvinsson et al., 2001), a finding that is consistent with our observations of low levels of neuronal NOS expression in this region. Here, we showed that sumatriptan exposure produced a substantial up-regulation of trigeminal neuronal profiles that express reactivity for neuronal NOS. Remarkably, the change in expression of neuronal NOS within the whole trigeminal population was magnified by changes observed in identified dural afferents. These observations suggest that primary afferents innervating the dura may respond differently than trigeminal afferents overall to triptan exposure, and this difference may have implications regarding the manifestation of medication overuse headache and of migraine headache, since the headaches that occur in migraine patients who overuse triptans are indistinguishable from their spontaneous attacks (Limmroth et al., 2002). The up-regulation of neuronal NOS positive profiles was observed in multiple classes of dural afferents. Increased neuronal NOS labelling was prominent in profiles labelled with IB4, generally described as primary afferents that do not normally substantially express peptidic excitatory transmitters. Furthermore, myelinated trigeminal afferents labelled with NF200 also demonstrated a strong increase in neuronal NOS labelling following triptan treatment. Previous work from our laboratory has shown that triptans increase CGRP labelling in dural afferents and that both IB4 and NF200 positive cells show increased co-labelling for CGRP. Perhaps most importantly, the triptan-induced increase in neuronal NOS was highly co-localized with fibres expressing CGRP, suggesting a potential functional link between CGRP and neuronal NOS activity in labelled dural afferents.
It should be noted that triptan-induced neuroplastic changes were present not only at the time of termination of triptan treatment (i.e. Day 6) but additionally, persisted for at least 2 weeks following termination of triptan administration, and at a time when hypersensitivity to purported migraine triggers was observed. For this reason, although small in numbers relative to the overall proportion of cells innervating the dura, such changes could be of physiological significance. This conclusion is supported by the efficacy of a highly selective neuronal NOS inhibitor, NXN-323. In our studies, we showed that blockade of neuronal NOS activity with an acute administration of NXN-323 reversed cutaneous allodynia induced by 6-day infusion with sumatriptan. Additionally, co-administration of NXN-323 with sumatriptan prevented the development of cutaneous allodynia. Co-administration of the neuronal NOS inhibitor NXN-323 also prevented the cutaneous allodynia elicited by environmental stress in the latent stage following termination of sumatriptan administration, i.e. at Day 20 or 21. However, co-administration of NXN-323 with sumatriptan did not prevent rats from demonstrating cutaneous allodynia following a nitric oxide-donor challenge. This result would be expected as NXN-323 inhibits enzyme activity and the nitric oxide donor would bypass the enzyme to result in nitric oxide production. The co-infusion of rats with sumatriptan and NXN-323 additionally did not alter the number of neuronal NOS or CGRP expressing profiles in dural afferents.
These observations suggest that sumatriptan treatment may elicit long-lasting changes that result in enhanced functional activity of neuronal NOS to promote nitric oxide-dependent behavioural hypersensitivity. Such hypersensitivity is likely to be related to CGRP release although this is not yet established. However, numerous reports support this idea. Studies performed in cultured trigeminal neurons revealed that nitric oxide promotes the release of CGRP from neurons (Bellamy et al., 2006) and these effects are blocked by sumatriptan (Bellamy et al., 2006), which is consistent with the known co-expression of 5-HT1B/D receptors with NOS and CGRP (Hou et al., 2001). Animal studies revealed that nitric oxide promotes the release of CGRP from capsaicin-sensitive primary afferent fibres (Brain et al., 1993; Hughes and Brain, 1994; Edvinsson et al., 2001). Inhibitors of NOS have blocked meningeal vasodilation elicitied by CGRP or electrical stimulation in rats (Akerman et al., 2002). Systemic administration of the nitric oxide donor nitroglycerin facilitated nociofensive face-grooming behaviour caused by systemic CGRP in rats (Yao and Sessle, 2008). Elevated NOS levels may promote nocioception since nitric oxide promotes central sensitization of second-order neurons (Meller and Gebhart, 1993; Vetter et al., 2001; Neeb and Reuter, 2007), and increased levels of CGRP released from primary afferent fibres interact with the sensitized second-order neurons to yield a markedly enhanced response. We recently demonstrated that systemic administration of a nitric oxide donor to rats previously exposed to triptans, increased blood levels of CGRP (De Felice et al., 2010). Additionally, behavioural hypersensitivity to challenge with a nitric oxide donor was prevented by treatment with CGRP8-37, a CGRP receptor antagonist and the hypersensitivity to environmental stress was prevented by NXN-323. Treatment with triptans produced increased labelling for CGRP in dural afferents as well as for neuronal NOS and the most prominent increase in neuronal NOS labelling in multiple classes of dural afferents associated with the most prominent increase observed in profiles co-labelled for CGRP. Thus, possible increases in endogenous nitric oxide signalling may likely be sufficient to promote central sensitization as well as the release of CGRP in response to stimuli such as environmental stress. However, direct measurement of enhanced CGRP release may be difficult to test directly because of the relatively small numbers of neuronal profiles co-expressing neuronal NOS and this peptide. These observations are consistent with clinical literature linking CGRP and nitric oxide activity in the pathophysiology of migraine and medication overuse headache. Migraineurs show elevated blood levels of both CGRP and of nitric oxide production (Sarchielli et al., 2000). Donors of nitric oxide administered to human volunteers with migraine provoked a migraine attack along with elevated blood levels of CGRP, suggesting a possible causal relationship (Juhasz et al., 2003). Finally, CGRP infused in human volunteers increased forearm blood flow, and this effect was attenuated by N-methylarginine (de Hoon et al., 2003). Additionally, the selective inducible NOS inhibitor GW274150 was found to be ineffective against acute migraine headache pain in clinical studies (Van der Schueren et al., 2009). Such findings are consistent with the possibility that enhanced nitric oxide production, possibly as a consequence of increased neuronal NOS expression, facilitated the development of allodynia induced by CGRP release in response to endogenous or exogenous triggers of headache.
Persistent expression of neuronal NOS in trigeminal dural afferents may therefore provide a mechanism that could contribute to latent sensitization state preclinically as well as to increased responsiveness to migraine triggers and medication overuse headache. This interpretation is also consistent with observations suggesting the presence of adaptive processes in response to other analgesic medications. Stress-induced allodynia was used to unmask latent sensitization after prolonged exposure to opioids (Rivat et al., 2007) and numerous changes in expression of neural mediators in dural afferents have been observed following opiate administration (De Felice and Porreca, 2009). It should be pointed out that an environmental stress was employed in this study. Although environmental stress in the form of exposure to bright light has reliably induced enhanced responsiveness to sensory stimulation of cutaneous tissues in sensitized animals (Rivat et al., 2007), the possibility exists that this stimulus may differ mechanistically from the psychosocial stressors commonly associated with precipitation of migraine clinically (Barton-Donovan and Blanchard, 2005; White and Farrell, 2006; Hashizume et al., 2008).
Recent studies have also demonstrated that administration of paracetamol for 30 days led to an increase in cortical spreading depression frequency and cortical spreading depression-evoked Fos expression in the cerebral cortex (Supornsilpchai et al., 2009) suggesting an increase in neuronal excitability. Paracetamol treatment also facilitated trigeminal nocioception as shown by increases in cortical spreading depression-evoked Fos expression in trigeminal nucleus caudalis. These observations were suggested to result from up-regulation of the 5-HT2A receptor in the cortex (Supornsilpchai et al., 2009) raising the possibility of central changes that may additionally contribute to hyperexcitability following analgesic treatment. This possibility is supported for opioid-induced hyperalgesia as animals treated with opiates demonstrate a loss of diffuse noxious inhibitory control (Okada-Ogawa et al., 2009). Whether such central changes may occur with triptan treatment remains to be established. An important difference, however, between triptan-induced latent sensitization and other states of hyperexcitability seen with opiates and with paracetamol is the degree of brain penetration associated with these classes of molecules. Unlike these other analgesics, triptans do not readily penetrate the central nervous system and our studies show that cutaneous allodynia elicited in rats with triptan-induced latent sensitization is reversed by intravenous CGRP8-37 (De Felice et al., 2010) a large peptide that is unlikely to achieve significant central nervous system penetration. These observations suggest that the peripheral adaptations seen in the present study are likely to be of greatest significance to triptan-induced medication overuse headache and suggest the possibility that peripheral mechanisms may be significant in enhanced sensitivity to migraine triggers.
In summary, the present investigation shows that prolonged exposure to sumatriptan produces persistent neural adaptations and enhanced excitability that is neuronal NOS and CGRP dependent. CGRP receptor antagonists have been demonstrated clinically to be effective in abortive treatment of migraine though it is not yet known if these medications would lead to enhanced excitability as might be relevant for medication overuse headache. Additionally, non-specific inhibition of NOS, but not inducible NOS, has been demonstrated to be clinically effective in abortive treatment of migraine headache. For these reasons, adaptive changes following administration of analgesic agents may ultimately reflect increased pronocioceptive adaptations in dural afferents including neuronal NOS and subsequent activity of CGRP. Highly selective neuronal NOS inhibitors are currently in clinical development and may offer opportunities for both abortive and prophylactic treatment of migraine without the likelihood of development of medication overuse headache.
Funding
Support was provided in part by Grant No. R01DA012656 from NIDA and by NeurAxon Inc., Toronto, ON, Canada.
Acknowledgements
J.S.A., S.R. and S.M. are employed by NeurAxon.
Glossary
Abbreviations
- CGRP
calcitonin gene related peptide
- NOS
nitric oxide synthase
References
- Afridi SK, Kaube H, Goadsby PJ. Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain. 2004;110:675–80. doi: 10.1016/j.pain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Akerman S, Williamson DJ, Kaube H, Goadsby PJ. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br J Pharmacol. 2002;137:62–8. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulmani U, Maassenvandenbrink A, Villalon CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–30. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Ayzenberg I, Obermann M, Nyhuis P, Gastpar M, Limmroth V, Diener HC, et al. Central sensitization of the trigeminal and somatic nociceptive systems in medication overuse headache mainly involves cerebral supraspinal structures. Cephalalgia. 2006;26:1106–14. doi: 10.1111/j.1468-2982.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- Bahra A, Walsh M, Menon S, Goadsby PJ. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43:179–90. doi: 10.1046/j.1526-4610.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Barton-Donovan K, Blanchard EB. Psychosocial aspects of chronic daily headache. J Headache Pain. 2005;6:30–9. doi: 10.1007/s10194-005-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: current concepts and synthesis. Curr Pain Headache Rep. 2003;7:371–6. doi: 10.1007/s11916-003-0036-y. [DOI] [PubMed] [Google Scholar]
- Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23:2057–66. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525–33. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821–8. doi: 10.1212/01.wnl.0000335946.53860.1d. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Excessive opioid use and the development of chronic migraine. Pain. 2009;142:179–82. doi: 10.1016/j.pain.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026–34. [PubMed] [Google Scholar]
- Boes CJ, Black DF, Dodick DW. Pathophysiology and management of transformed migraine and medication overuse headache. Semin Neurol. 2006;26:232–41. doi: 10.1055/s-2006-939924. [DOI] [PubMed] [Google Scholar]
- Bolay H, Moskowitz MA. The neurobiology of migraine and transformation of headache therapy. In: Waxman S, editor. From neuroscience to neurology: neuroscience, molecular medicine, and the therapeutic transformation of neurology. San Diego: Elsevier; 2004. pp. 107–23. [Google Scholar]
- Brain SD, Hughes SR, Cambridge H, O’Driscoll G. The contribution of calcitonin gene-related peptide (CGRP) to neurogenic vasodilator responses. Agents Actions. 1993;38 doi: 10.1007/BF01991124. (Spec No):C19–21. [DOI] [PubMed] [Google Scholar]
- Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–10. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–9. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol. 2005;161:658–60. doi: 10.1016/s0035-3787(05)85109-4. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- Centonze V, Bassi A, Causarano V, Dalfino L, Cassiano MA, Centonze A, et al. Sumatriptan overuse in episodic cluster headache: lack of adverse events, rebound syndromes, drug dependence and tachyphylaxis. Funct Neurol. 2000;15:167–70. [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neuro Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19:660–7; discussion 26. doi: 10.1046/j.1468-2982.1999.019007660.x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Yashpal K. Intracellular messengers contributing to persistent nociception and hyperalgesia induced by L-glutamate and substance P in the rat formalin pain model. Eur J Neurosci. 1994;6:1328–34. doi: 10.1111/j.1460-9568.1994.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Goadsby PJ. Functional neuroimaging of primary headache disorders. Curr Neurol Neurosci Rep. 2004;4:105–10. doi: 10.1007/s11910-004-0023-7. [DOI] [PubMed] [Google Scholar]
- Dahlof CG, Dodick D, Dowson AJ, Pascual J. How does almotriptan compare with other triptans? A review of data from placebo-controlled clinical trials. Headache. 2002;42:99–113. doi: 10.1046/j.1526-4610.2002.02025.x. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Zervas NT, Moskowitz MA. From spreading depression to the trigeminovascular system. Neurol Sci. 2006;27(Suppl. 2):S86–90. doi: 10.1007/s10072-006-0577-z. [DOI] [PubMed] [Google Scholar]
- De Felice M, Ossipov MH, Wang R, Lai J, Chichorro JG, Meng I, et al. Triptan-induced latent sensitiation: A possible basis for medication overuse headache. Annals of Neurology. 2010;67:325–37. doi: 10.1002/ana.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Porreca F. Opiate-induced persistent pronociceptive trigeminal neural adaptations: potential relevance to opiate-induced medication overuse headache. Cephalalgia. 2009;29:1277–84. doi: 10.1111/j.1468-2982.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon JN, Pickkers P, Smits P, Struijker-Boudier HA, Van Bortel LM. Calcitonin gene-related peptide: exploring its vasodilating mechanism of action in humans. Clin Pharmacol Ther. 2003;73:312–21. doi: 10.1016/s0009-9236(03)00007-9. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dahlof CGH. Headache associated with chronic use of substances. In: Olesen J, Tfelt-Hansen P, Welch K, editors. The Headaches. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 871–8. [Google Scholar]
- Diener HC, Limmroth V. Medication-overuse headache: a worldwide problem. Lancet Neurol. 2004;3:475–83. doi: 10.1016/S1474-4422(04)00824-5. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dodick D, Freitag F. Evidence-based understanding of medication-overuse headache: clinical implications. Headache. 2006;46(Suppl. 4):S202–11. doi: 10.1111/j.1526-4610.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- Dowson AJ, Dodick DW, Limmroth V. Medication overuse headache in patients with primary headache disorders: epidemiology, management and pathogenesis. CNS Drugs. 2005;19:483–97. doi: 10.2165/00023210-200519060-00002. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Elsas T, Suzuki N, Shimizu T, Lee TJ. Origin and co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc Res Tech. 2001;53:221–8. doi: 10.1002/jemt.1086. [DOI] [PubMed] [Google Scholar]
- Ekbom K, Krabbe A, Micieli G, Prusinski A, Cole JA, Pilgrim AJ, et al. Cluster headache attacks treated for up to three months with subcutaneous sumatriptan (6 mg). Sumatriptan Cluster Headache Long-term Study Group. Cephalalgia. 1995;15:230–6. doi: 10.1046/j.1468-2982.1995.015003230.x. [DOI] [PubMed] [Google Scholar]
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–58. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- Freire MA, Guimaraes JS, Leal WG, Pereira A. Pain modulation by nitric oxide in the spinal cord. Front Neurosci. 2009;3:175––81. doi: 10.3389/neuro.01.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiotto N, Sances G, Galli F, Tassorelli C, Guaschino E, Sandrini G, et al. Medication overuse headache and applicability of the ICHD-II diagnostic criteria: 1-year follow-up study (CARE I protocol) Cephalalgia. 2009;29:233–43. doi: 10.1111/j.1468-2982.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Is medication-overuse headache a distinct biological entity? Nat Clin Pract Neurol. 2006;2:401. doi: 10.1038/ncpneuro0236. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. The vascular theory of migraine–a great story wrecked by the facts. Brain. 2009;132:6–7. doi: 10.1093/brain/awn321. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Gordh T, Karlsten R, Kristensen J. Intervention with spinal NMDA, adenosine, and NO systems for pain modulation. Ann Med. 1995;27:229–34. doi: 10.3109/07853899509031964. [DOI] [PubMed] [Google Scholar]
- Guy N, Marques AR, Orliaguet T, Lanteri-Minet M, Dallel R, Clavelou P. Are there differences between cephalic and extracephalic cutaneous allodynia in migraine patients? Cephalalgia. 2009 doi: 10.1111/j.1468-2982.2009.02008.x. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Yamada U, Sato A, Hayashi K, Amano Y, Makino M, et al. Stress and psychological factors before a migraine attack: A time-based analysis. Biopsychosoc Med. 2008;2:14. doi: 10.1186/1751-0759-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009;37:886–91. doi: 10.1042/BST0370886. [DOI] [PubMed] [Google Scholar]
- Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001;909:112–20. doi: 10.1016/s0006-8993(01)02645-2. [DOI] [PubMed] [Google Scholar]
- Hughes SR, Brain SD. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol. 1994;111:425–30. doi: 10.1111/j.1476-5381.1994.tb14752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey PP, Goadsby PJ. The mode of action of sumatriptan is vascular? A debate. Cephalalgia. 1994;14:401–10. doi: 10.1046/j.1468-2982.1994.1406401.x. discussion 393. [DOI] [PubMed] [Google Scholar]
- Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38:17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Zsombok T, Modos EA, Olajos S, Jakab B, Nemeth J, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106:461–70. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Katsarava Z, Jensen R. Medication-overuse headache: where are we now? Curr Opin Neurol. 2007;20:326–30. doi: 10.1097/WCO.0b013e328136c21c. [DOI] [PubMed] [Google Scholar]
- Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31:430–9. doi: 10.1016/s0891-5849(01)00597-4. [DOI] [PubMed] [Google Scholar]
- Landy S, Rice K, Lobo B. Central sensitisation and cutaneous allodynia in migraine: implications for treatment. CNS Drugs. 2004;18:337–42. doi: 10.2165/00023210-200418060-00001. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Ashina M, Christiansen I, Ulrich V, Grover R, Donaldson J, et al. Nitric oxide synthase inhibition: a new principle in the treatment of migraine attacks. Cephalalgia. 1998;18:27–32. doi: 10.1046/j.1468-2982.1998.1801027.x. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang M, Yang C, Dun Y, Zhang Y, Hao Y. Nitroglycerin protects small intestine from ischemia-reperfusion injury via NO-cGMP pathway and upregulation of alpha-CGRP. J Gastrointest Surg. 2009;13:478–85. doi: 10.1007/s11605-008-0728-z. [DOI] [PubMed] [Google Scholar]
- Limmroth V, Katsarava Z, Fritsche G, Przywara S, Diener HC. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011–4. doi: 10.1212/wnl.59.7.1011. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–58. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu MS, Cohen AS, McGonigle DJ, Ward N, Frackowiak RS, Goadsby PJ. Posterior hypothalamic and brainstem activation in hemicrania continua. Headache. 2004;44:747–61. doi: 10.1111/j.1526-4610.2004.04141.x. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Zhu J, Lu J, Mayer DJ. The inhibition of nitric oxide-activated poly(ADP-ribose) synthetase attenuates transsynaptic alteration of spinal cord dorsal horn neurons and neuropathic pain in the rat. Pain. 1997;72:355–66. doi: 10.1016/s0304-3959(97)00063-8. [DOI] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–36. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–16. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Neeb L, Reuter U. Nitric oxide in migraine. CNS Neurol Disord Drug Targets. 2007;6:258–64. doi: 10.2174/187152707781387233. [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Zinck T, Hoffmann J, Schiemann K, Schuh-Hofer S, Rohde W, et al. CGRP release and c-fos expression within trigeminal nucleus caudalis of the rat following glyceryltrinitrate infusion. Cephalalgia. 2005;25:225–36. doi: 10.1111/j.1468-2982.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- Okada-Ogawa A, Porreca F, Meng ID. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J Neurosci. 2009;29:15828–35. doi: 10.1523/JNEUROSCI.3623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120:157–71. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport. 1993;4:1027–30. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- Olesen J, Bousser MG, Diener HC, Dodick D, First M, Goadsby PJ, et al. The International Classification of Headache Disorders, 2nd Edition. Cephalalgia. 2004;24:1–160. doi: 10.1111/j.1468-2982.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–90. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- Olesen J, Bousser MG, Diener HC, Dodick D, First M, Goadsby PJ, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742–6. doi: 10.1111/j.1468-2982.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- Paemeleire K, Bahra A, Evers S, Matharu MS, Goadsby PJ. Medication-overuse headache in patients with cluster headache. Neurology. 2006;67:109–13. doi: 10.1212/01.wnl.0000223332.35936.6e. [DOI] [PubMed] [Google Scholar]
- Pardutz A, Krizbai I, Multon S, Vecsei L, Schoenen J. Systemic nitroglycerin increases nNOS levels in rat trigeminal nucleus caudalis. Neuroreport. 2000;11:3071–5. doi: 10.1097/00001756-200009280-00008. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–52. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat C, Laboureyras E, Laulin JP, Le RC, Richebe P, Simonnet G. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217–28. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–18. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Alberti A, Russo S, Codini M, Panico R, Floridi A, et al. Nitric oxide pathway, Ca2+, and serotonin content in platelets from patients suffering from chronic daily headache. Cephalalgia. 1999;19:810–6. doi: 10.1046/j.1468-2982.1999.1909810.x. [DOI] [PubMed] [Google Scholar]
- Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation–a 3T magnetic resonance angiography study. Brain. 2008;131:2192–200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132:16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754–62. doi: 10.1212/wnl.55.6.754. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Olesen J, Bousser MG, Diener HC, Dodick D, First M, et al. The International Classification of Headache Disorders, 2nd Edition (ICHD-II)–revision of criteria for 8.2 Medication-overuse headache. Cephalalgia. 2005;25:460–5. doi: 10.1111/j.1468-2982.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Lectin and neuropeptide labeling of separate populations of dorsal root ganglion neurons and associated “nociceptor” thin axons in rat testis and cornea whole-mount preparations. Somatosens Res. 1988;5:259–67. doi: 10.3109/07367228809144630. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Supornsilpchai W, le Grand SM, Srikiatkhachorn A. Involvement of pro-nociceptive 5-HT(2A) receptor in the pathogenesis of medication-overuse headache. Headache. 2009;50:185–97. doi: 10.1111/j.1526-4610.2009.01591.x. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Nagatani Y, Saitoh K, Takasu K, Ono H. Pharmacological assessments of nitric oxide synthase isoforms and downstream diversity of NO signaling in the maintenance of thermal and mechanical hypersensitivity after peripheral nerve injury in mice. Neuropharmacology. 2009;56:702–8. doi: 10.1016/j.neuropharm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995a;682:167–81. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Joseph SA. NADPH-diaphorase activity and Fos expression in brain nuclei following nitroglycerin administration. Brain Res. 1995b;695:37–44. doi: 10.1016/0006-8993(95)00732-6. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P. Triptans vs other drugs for acute migraine. Are there differences in efficacy? A comment. Headache. 2008;48:601–5. doi: 10.1111/j.1526-4610.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Kruuse C, Iversen H, Olesen J. A nitric oxide donor (nitroglycerine) triggers genuine migraine attacks. Eur J Neurol. 1994;1:73–80. doi: 10.1111/j.1468-1331.1994.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Olesen J. A pivotal role of nitric oxide in migraine pain. Ann NY Acad Sci. 1997;835:363–72. doi: 10.1111/j.1749-6632.1997.tb48642.x. [DOI] [PubMed] [Google Scholar]
- Van der Schueren BJ, Lunnon MW, Laurijssens BE, Guillard F, Palmer J, Van Hecken A, et al. Does the unfavorable pharmacokinetic and pharmacodynamic profile of the iNOS inhibitor GW273629 lead to inefficacy in acute migraine? J Clin Pharmacol. 2009;49:281–90. doi: 10.1177/0091270008329548. [DOI] [PubMed] [Google Scholar]
- Vetter G, Geisslinger G, Tegeder I. Release of glutamate, nitric oxide and prostaglandin E2 and metabolic activity in the spinal cord of rats following peripheral nociceptive stimulation. Pain. 2001;92:213–8. doi: 10.1016/s0304-3959(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson SM, Becker WJ, Heine JA. Opiate use to control bowel motility may induce chronic daily headache in patients with migraine. Headache. 2001;41:303–9. doi: 10.1046/j.1526-4610.2001.111006303.x. [DOI] [PubMed] [Google Scholar]
- White KS, Farrell AD. Anxiety and psychosocial stress as predictors of headache and abdominal pain in urban early adolescents. J Pediatr Psychol. 2006;31:582–96. doi: 10.1093/jpepsy/jsj050. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Fos expression is induced by increased nitric oxide release in rat spinal cord dorsal horn. Neuroscience. 2000;96:351–7. doi: 10.1016/s0306-4522(99)00534-5. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Yao D, Sessle BJ. Nitroglycerin facilitates calcitonin gene-related peptide-induced behavior. Neuroreport. 2008;19:1307–11. doi: 10.1097/WNR.0b013e32830b0f9d. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–30. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]