Abstract
OBJECTIVES
Proton pump inhibitors (PPIs) are frequently prescribed to patients with Barrett’ s esophagus (BE), but in a subset, they can induce significant hypergastrinemia. Elevated levels of gastrin have been associated with tumorigenic effects in a number of gastrointestinal cancers. We decided to investigate the association between serum gastrin levels and dysplasia in BE.
METHODS
We performed a cross-sectional study and enrolled patients with BE without dysplasia, low-grade dysplasia (LGD), high-grade dysplasia (HGD), or adenocarcinoma (AC), as well as gastroesophageal reflux disease controls, all chronically taking PPIs. Fasting serum gastrin was measured, and data were collected on patient characteristics, medication use, and the highest degree of BE neoplasia.
RESULTS
A total of 95 patients were enrolled. The mean age was 64.7 (±10.0) years, and 70.5 % were male. The median serum gastrin level was 40 pM. There was no significant difference in gastrin levels with increased degrees of BE neoplasia (overall P = 0.68). In multivariable analysis, the highest quartile of gastrin was associated with significantly increased odds of advanced neoplasia (HGD or AC) (odds ratio (OR): 5.46, 95 % confidence interval (CI): 1.20–24.8).
CONCLUSIONS
In BE patients taking PPIs, an elevated serum gastrin is associated with a history of HGD or AC. Prospective studies are needed to determine whether patients with nondysplastic BE and elevated serum gastrin are at increased risk for neoplastic progression.
INTRODUCTION
Barrett’ s esophagus (BE), the well-recognized precursor lesion for esophageal adenocarcinoma (EAC), is relatively common in Western countries with an estimated prevalence between 1 and 5% (1–3). In addition, the incidence of EAC has been steadily rising over the past three decades, likely, at least in part, because of an increase in gastroesophageal reflux disease (GERD) in the setting of the increasing prevalence of obesity (4,5). Concurrently, there has been a rapid increase in the use of proton pump inhibitors (PPIs) and other potent antisecretory agents, which have been used to treat both GERD and its complications (6). Given the strong association between acid reflux and BE, as well as the established association between EAC and a history of acid reflux (7,8), it has been common practice to treat BE patients with PPIs in the hope of reducing the risk of neoplastic progression.
Although the role of PPIs in relieving GERD symptoms and healing erosive esophagitis has been well established, the role of PPIs in preventing neoplastic progression in BE patients has been less clear. By inhibiting acid secretion and increasing intragastric pH, PPIs at therapeutic doses can cause a physiological secondary hypergastrinemia in some patients (9). Gastrin is a well-known growth factor for a number of gastrointestinal cell types that express the cholecystokinin-2/gastrin receptor (CCK2R). In recent years, several studies have suggested that BE cells have increased expression of CCK2R, and that gastrin is able to stimulate proliferation, antiapoptosis, and loss of cell–cell adhesion in BE cells (10–13). The expression of cyclooxygenase-2 (COX-2), which is known to be associated with esophageal and other gastrointestinal malignancies, can also be induced by gastrin in esophageal cells (14,15). There is epidemiological evidence to suggest that hypergastrinemia is associated with an increased risk of other gastrointestinal cancers, such as colorectal neoplasia (16,17).
The gastrin response to the use of PPIs is quite variable among patients, and thus any risk associated with the hypergastrinemic response to PPIs would not be evenly distributed. Most PPIs are metabolized in the liver by the cytochrome p450 2C19 enzyme, and polymorphisms in this gene may be partly responsible for the observed variations in serum gastrin levels among patients taking PPIs (18). Given the widespread use of PPIs in BE patients, as well as the potential carcinogenic effects of hypergastrinemia, we decided to investigate for a potential association between serum gastrin levels and dysplasia in a cross-section of BE patients, all of whom were taking PPIs.
METHODS
Study subjects
Subjects were recruited from the clinical practices of two of the investigators (C.J.L. and J.A.A.) at the Columbia University Medical Center. Patients with established BE, who were already scheduled to undergo upper endoscopy, were approached regarding participation in the study. Inclusion criteria included endoscopically suspected BE, confirmed by the presence of intestinal metaplasia on esophageal biopsies, PPI use on a daily basis at the initial diagnosis of BE, and age ≥18 years. PPI dosage and frequency were confirmed to have remained unchanged during the 2 weeks before study enrollment. Four quadrant random biopsies were taken every 1–2 cm along the length of BE for all cases. Patients were excluded from the study if they were not taking PPIs at the time of their diagnosis of high-grade dysplasia (HGD) or AC. Exclusion criteria also included any history of gastric or esophageal surgery or inability to provide informed consent.
Data were collected on patient age, gender, race and ethnicity, and medication use, including PPIs, as well as aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs). The duration of PPI use in months was recorded. The highest degree of neoplasia confirmed with biopsy at any point in time (past or present) was also recorded. Subjects were classified on the basis of their highest degree of neoplasia, as BE and no dysplasia, low-grade dysplasia (LGD), HGD, or AC (both invasive and intramucosal). No patients with a history of HGD “spontaneously” regressed to lesser degrees of dysplasia. Histological interpretation was performed by at least one pathologist experienced in BE, and the pathologists were unaware of gastrin results. Advanced neoplasia was defined a priori as a history of either HGD or AC.
This study was approved by the Columbia University Institutional Review Board.
Selection of controls
GERD controls were chosen by enrolling consecutive patients who presented for upper endoscopy with symptoms of acid reflux as the clinical indication and who had been on PPIs for at least 2 weeks before endoscopy. If potential control subjects had a previous history of BE or were found to have either endoscopically suspected BE or columnar mucosa (with or without intestinal metaplasia) on any esophageal biopsies, they were excluded from the study. Eligible control subjects were selected from patients already scheduled to undergo upper endoscopy, with consent of their primary gastroenterologist.
Sample collection
On the day of endoscopy, 10 ml of blood was drawn by sterile peripheral venipuncture. All patients fasted for at least 8 hours in preparation for upper endoscopy. During the endoscopy, two random biopsies were taken from the gastric antrum to evaluate for the presence of Helicobacter pylori. If a subject was found to be H. pylori positive, this status was recorded and appropriate eradication therapy was prescribed.
Sample processing and radioimmunoassay
Whole blood of subjects was centrifuged at 2,700 rpm (1,600g) for 10 min to separate out serum, which was then stored at −80°C until shipment to one of the coinvestigators (AV). Serum gastrin concentrations were determined by radioimmunoassay, as described earlier by Nemeth et al. (19). Quantitative serum gastrin concentrations were reported in units of pM. The individuals performing the quantitative gastrin assays were blinded to the histological classification of samples.
Statistical analysis
Categorical variables were evaluated using Fisher’s exact tests. When normally distributed, continuous variables were evaluated using two-sided Student’s t-tests. If continuous variables were not normally distributed, Wilcoxon rank-sum tests (for two groups) or Kruskal–Wallis tests (for three or more groups) were used. Gastrin was analyzed as a continuous variable and as a categorical variable (quartiles). Among BE patients, secondary analyses were performed comparing those with and without a history of advanced neoplasia.
Multivariable regression modeling was performed to assess for associations between serum gastrin quartiles and degree of neoplasia in patients with BE, adjusted for potential confounders. The initial model contained all potential confounders. Covariates were then removed sequentially on the basis of the highest P-value that was also >0.15. The final model contained gastrin quartiles, sex, and aspirin/NSAID use.
Statistical significance was defined as P < 0.05 or a 95% confidence interval (CI) that did not cross 1.00. All analyses were performed using STATA 9.2 (STATACorp, College Station, TX).
RESULTS
A total of 95 patients, 82 with BE and 13 GERD controls, were enrolled. The mean age was 64.7 (±10.0) years and 70.5 % were male (Table 1). All patients were on chronic PPI therapy at the time of blood collection, with 31 patients (33%) receiving a once-daily dose, and the remaining 64 patients (67%) receiving at least a twice-daily dose at the time of study enrollment. Two patients (2.1%) had H. pylori detected on gastric antral biopsies, and 39 patients (41.1%) were taking regular aspirin or NSAIDs. There was no difference between BE patients and GERD controls with regard to mean age (65.5 vs. 64.6, P = 0.78), but a higher proportion of BE patients was male (76 % vs. 38%, P = 0.02).
Table 1.
Patient demographics, medication use, and histology
| n | % | |
|---|---|---|
| Total subjects | 95 | 100 |
| Mean age, years (s.d.) | 64.7 | (10.0) |
| Race, white | 90 | 94.7 |
| Sex, male | 67 | 70.5 |
| PPI dosing frequency | ||
| Daily | 31 | 32.6 |
| Twice daily or more | 64 | 67.4 |
| Mean PPI duration, months (s.d.) | 74.1 | (63.5) |
| H. pylori positive | 2 | 2.1 |
| Regular aspirin/NSAID use | 39 | 41.1 |
| Histology | ||
| GERD (no BE) | 13 | 13.7 |
| BE | 16 | 16.8 |
| LGD | 16 | 16.8 |
| HGD | 38 | 40.0 |
| AC | 12 | 12.7 |
AC, adenocarcinoma; BE, Barrett’s esophagus; GERD, gastroesophageal reflux disease; HGD, high-grade dysplasia; LGD, low-grade dysplasia; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitor.
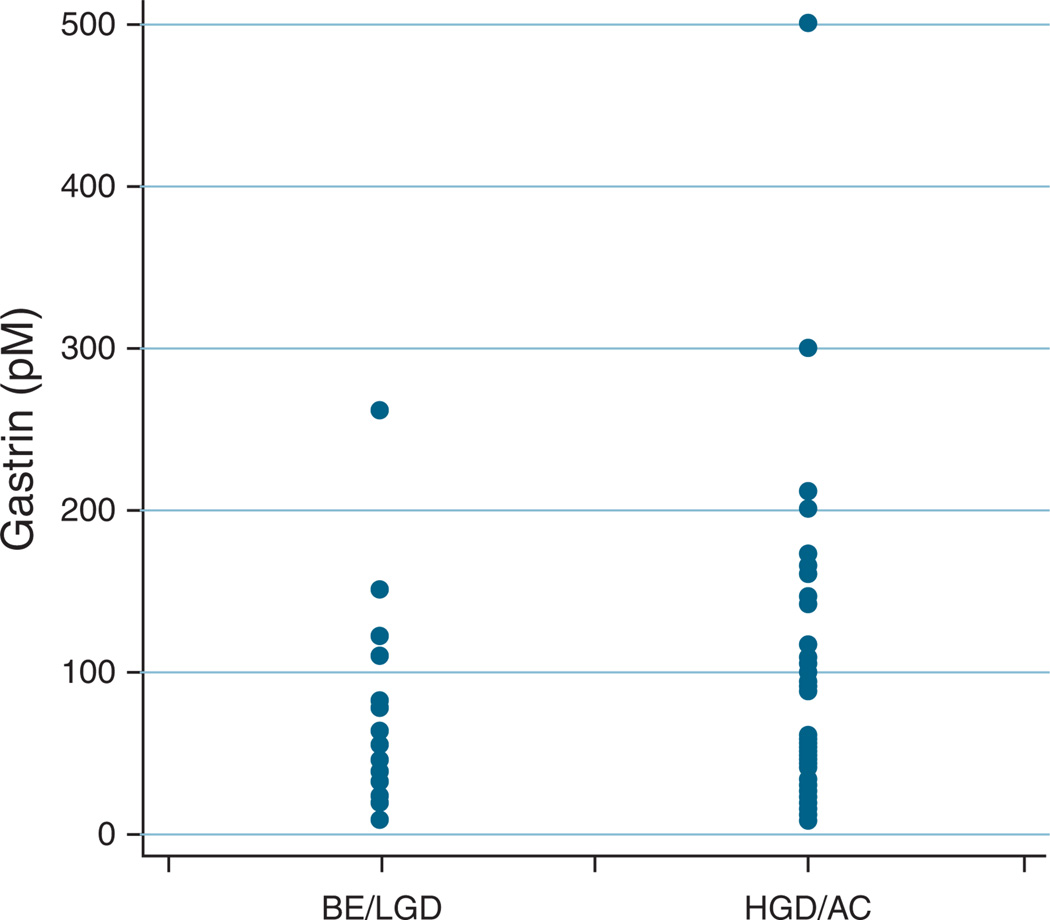
Results of serum gastrin analyses are shown in Table 2 and Figure 1. The median gastrin level was 40 pM (interquartile range: 24–87). There was no significant difference in gastrin levels between patients with GERD and with BE without dysplasia (33.6 vs. 45.2, respectively; P = 0.78). Serum gastrin levels did not trend upward with increasing degrees of neoplasia (BE 45.2 pM, LGD 32.1 pM, HGD 49 pM, AC 35 pM; overall P-value = 0.68). There was no significant difference in gastrin levels between BE subjects with and without advanced neoplasia (35.7 vs. 46.5 pM, respectively; P = 0.25). Twenty patients (21.1%) had a threefold elevation in serum gastrin compared with the median for GERD controls (≥101 pM). Among these, 2 (10%) patients had GERD, 4 (20%) had BE with no dysplasia or LGD, and 14 (70%) had HGD or EAC.
Table 2.
Median and mean serum gastrin levels (pM) among study subjects with GERD and Barrett’s esophagus, with or without dysplasia
| Median | Mean | P value a | |
|---|---|---|---|
| All subjects | 40 | 69.2 | |
| Age, years (by group) | 0.05 | ||
| < 60 | 32.8 | 44.8 | |
| 60 – 69 | 44.5 | 62.9 | |
| ≥70 | 67.4 | 100.0 | |
| Sex | 0.49 | ||
| Female | 46.5 | 80.1 | |
| Male | 40 | 64.7 | |
| PPI frequency | 0.11 | ||
| Daily | 32 | 61.9 | |
| Twice daily or more | 49 | 72.8 | |
| Aspirin/NSAID use | 0.23 | ||
| No | 39.5 | 64.1 | |
| Yes | 50 | 76.7 | |
| Histology | 0.82 | ||
| GERD | 33.6 | 70.6 | |
| BE, no dysplasia | 45.2 | 59.7 | |
| LGD | 32.1 | 49.8 | |
| HGD | 49 | 76.0 | |
| AC | 35 | 85.2 |
AC, adenocarcinoma; BE, Barrett’s esophagus; GERD, gastroesophageal reflux disease; HGD, high-grade dysplasia; LGD, low-grade dysplasia; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitor.
Using Wilcoxon or Kruskal–Wallis tests.
Figure 1.
Serum gastrin levels in Barrett’s esophagus (BE) patients with and without advanced neoplasia (high-grade dysplasia (HGD) or adenocarcinoma (AC)). LGD, low-grade dysplasia.
Older age was associated with a significantly increased serum gastrin (P = 0.05) (Table 2). There was no significant difference in serum gastrin by patient sex, frequency of PPI use, or aspirin/NSAID use.
When analyzed as a continuous variable, serum gastrin was not associated with a history of advanced neoplasia (unadjusted odds ratio (OR) 1.01, P = 0.19; adjusted OR 1.00, P = 0.31). When categorized by quartile, 80% of subjects in the highest gastrin quartile had a history of advanced neoplasia (Table 3).
Table 3.
Proportion of patients with and without a history of advanced neoplasia (HGD/AC) in each quartile of serum gastrin
| Gastrin quartile | Highest degree of neoplasia | |
|---|---|---|
| No dysplasia/LGD | HGD/adenocarcinoma | |
| 1st | 10 (48%) | 11 (52%) |
| 2nd | 8 (40%) | 12 (60%) |
| 3rd | 10 (48%) | 11 (52%) |
| 4th | 4 (20%) | 16 (80%) |
AC, adenocarcinoma; HGD, high-grade dysplasia; LGD, low-grade dysplasia.
Overall P value = 0.22.
In multivariable analysis, the highest gastrin quartile was associated with significantly increased odds of a history of advanced neoplasia (OR: 5.46, 95% CI: 1.20–24.8) (Table 4). There was a nonsignificant trend across quartiles for association with a history of advanced neoplasia (P for trend = 0.06). Male sex was associated with a nonsignificantly increased odds of advanced neoplasia (OR: 2.76, 95% CI: 0.86–8.82), and aspirin/NSAID use was associated with a decreased but non-significant odds of advanced neoplasia (OR: 0.47, 95% CI: 0.17–1.33). Patient age and duration of PPI use were not associated with a history of advanced neoplasia in multivariable analyses and were removed from the final model.
Table 4.
Multivariable logistic regression of risk factors associated with a history of advanced neoplasia (HGD or AC) in patients with Barrett’s esophagus (n = 82)
| Odds ratio | 95% CI | |
|---|---|---|
| Gastrin quartilea | ||
| 1st (lowest) | 1.00 | Referent |
| 2nd | 1.63 | 0.43 – 6.28 |
| 3rd | 1.21 | 0.34 – 4.29 |
| 4th (highest) | 5.46 | 1.20 – 24.8 |
| Sex | ||
| Female | 1.00 | Referent |
| Male | 2.76 | 0.86 – 8.82 |
| Aspirin/NSAID use | ||
| No | 1.00 | Referent |
| Yes | 0.47 | 0.17 – 1.33 |
AC, adenocarcinoma; CI, confidence interval; HGD, high-grade dysplasia; NSAID, nonsteroidal anti-inflammatory drug.
P for trend = 0.06.
As older age was found to be associated with higher gastrin levels, and age is an established risk factor for HGD and AC, we once again added age to the model that included gastrin quartile, sex, and aspirin/NSAID use. The highest quartile of serum gastrin remained significantly associated with a history of advanced neoplasia (OR: 4.69, 95% CI: 1.00–22.0). Age was associated with a nonsignificantly increased odds of advanced neoplasia (per year, OR: 1.03, 95% CI: 0.99–1.09).
We repeated the analyses, restricted to those BE patients who had been taking PPIs for at least 12 months (n = 74). The association between a history of advanced neoplasia and the highest quartile of serum gastrin was qualitatively similar (adjusted OR: 4.47, 95% CI: 0.92–21.7).
DISCUSSION
The results of this study show a significant association between elevated serum gastrin and a history of advanced neoplasia (HGD or EAC) in patients with BE, with an OR of 5.46 for these advanced lesions. Although these findings do not imply causation between hypergastrinemia and EAC, the data add to the existing literature with regard to a potential role for gastrin in BE-associated neoplastic progression. The association observed with the highest quartile of serum gastrin suggests a possible “threshold” effect for gastrin.
In contrast to the findings regarding advanced neoplasia, we did not find any differences in gastrin levels between GERD controls and nondysplastic BE patients, suggesting that gastrin may not have a major role in the development of BE. This is consistent with earlier work, which also did not find a difference in serum gastrin levels between patients with nonerosive reflux disease, reflux esophagitis, and BE (20). We observed higher gastrin levels in older subjects, possibly because of a progressive decrease in parietal cell mass with age.
Earlier reports have suggested an association between hypergastrinemia and a number of solid malignancies, particularly colorectal cancer (10). In a nested case–control study from a large cohort of patients in northern California, there was no overall difference in baseline median serum gastrin levels between cases of colorectal cancer and matched controls (17). However, an elevated serum gastrin level (defined as >90 pg/ml) was associated with a significantly increased risk of colorectal cancer (adjusted OR: 3.9, 95% CI: 1.5–9.7), consistent with our findings in esophageal neoplasia. In a more recent case–control study of patients with colorectal adenomas, elevated serum gastrin was seen more frequently in cases compared with controls (29.5% vs. 11.5%, P = 0.006) and was found to be an independent risk factor for adenomas (adjusted OR: 3.2, 95% CI: 1.4–7.5) (16). In contrast, studies have failed to find an increased risk of colorectal cancer associated with PPI use, including two nested case–control studies from the United Kingdom and Denmark (21,22). However, these studies were not designed to evaluate the subset of patients with PPI-induced secondary hypergastrinemia.
Similarly, earlier studies have been inconclusive regarding the impact of PPI use on EAC risk. In the study by Lagergren et al. on the risk of EAC in GERD patients, treatment with a PPI was associated with an increased risk for developing EAC even after adjusting for severity of symptoms (OR: 2.9) (8). In contrast, several more recent epidemiological studies suggest that PPI use in patients with BE may be associated with lower rates of neoplastic progression (23,24). In a study by El-Serag et al. of veterans with BE, PPI use (compared with histamine-2 antagonists and no acid suppression) was associated with a lower risk of progression to dysplasia (HR: 0.25, 95% CI: 0.13–0.47) (24). However, a case–control study from England showed an increased risk of esophageal AC in patients who received acid suppressive therapy (H2-antagonists or PPIs) for GERD symptoms or BE (OR: 5.42, 95% CI: 3.13–9.39) (25). Overall, the evidence is inconclusive as to whether acid suppressive therapy prevents progression to EAC, and none of these earlier studies included serum gastrin levels in analyses. Indeed, gastrin levels in patients on PPIs are extremely variable, as shown in this study, and our data suggesting that only 20% of patients have a greater than threefold elevation are consistent with earlier studies (26). Thus, future studies investigating the effects of PPI-induced hypergastrinemia will likely need to focus on this subset of patients with significantly elevated gastrin levels.
The rationale for treating Barrett’s patients with PPIs has been based on the notion that continued acid exposure is one of the factors that can contribute to esophageal proliferation and thus neoplastic progression. Indeed, patients with more severe acid reflux tend to be at higher risk for developing HGD and EAC than those with less acid reflux (27). Acid suppression has been shown to reduce the recurrence of other complications of severe GERD, including erosive esophagitis and esophageal strictures (28,29). In addition, several studies have suggested that acid exposure can lead to increased proliferation and activation of MAPK and COX-2 expression in EAC cell lines (30,31)
However, in studies with nondysplastic BE cells in culture, acid exposure does not seem to induce cellular proliferation (32,33), or in some cases to have antiproliferative effects (34,35). In addition, although ex vivo studies using endoscopic biopsy specimens have suggested that pulsed acid exposure (as compared with continuous acid exposure) resulted in increased cellular proliferation (36), in vivo studies of proliferative markers in BE tissue in patients on PPI therapy have been inconsistent in their findings (37–39).
Gastrin seems to exert trophic effects in BE as a result of binding to the cholecystokinin-2 receptor (CCK2R), also known as the gastrin receptor (11–13). Using real-time PCR, Haigh et al. (11) showed that BE tissue had a 2 orders of magnitude higher expression of CCK2R than did normal esophageal squamous mucosa, and [(125)I]-G17 bound to epithelia within glandular regions of Barrett’s mucosa. In addition, treatment with gastrin-17 resulted in an increase in cellular proliferation. The presence of an increased CCK2R expression in Barrett’s mucosa was confirmed in a separate in vitro study by Harris et al. (12). Administration of exogenous gastrin resulted in the activation of protein kinase B/Akt (PKB/Akt), a potent inhibitor of apoptosis. Interestingly, the investigators also found increased endogenous gastrin production in BE. These studies support the notion that gastrin may promote carcinogenesis in BE, and that this may be because of both endocrine and autocrine effects of gastrin.
Gastrin could potentially promote neoplastic progression in BE through its effects on COX-2 expression. COX-2 is expressed at progressively increased levels from BE to dysplasia to AC (15,40–42). Gastrin has been shown to induce COX-2 expression through CCK2R in Barrett’s neoplasia (14). Inhibition of COX-2 in vitro and in vivo decreases the expression of markers of cellular proliferation and induces apoptosis in both BE and EAC (43–45), and also results in decreased development of EAC in a rat model (46). In gastric cancer, gastrin seems to increase COX-2 mRNA stability and binding (47).
This study does have certain limitations. Although it is notable that elevated serum gastrin was associated with a history of advanced neoplasia in BE, the findings should not be interpreted as evidence of causation. The CI for this association was relatively wide, and the study sample size was not large. The design was cross-sectional in nature, and our study should therefore be seen as hypothesis generating. There are several questions that need to be answered to assess whether elevated levels of circulating gastrin can promote tumorigenesis. For example, it is unknown whether serum gastrin correlates with CCK2R expression in Barrett’s tissue. A single gastrin level was measured, and it is unclear whether this can serve as a proxy for chronic gastrin “exposure.” Our study was not designed to determine the duration of gastrin exposure. A large prospective trial of nondysplastic BE patients with baseline and serial follow-up serum gastrin measurements would be required to determine whether hypergastrinemia is an independent risk factor for neoplastic progression in BE.
Data on cumulative PPI exposure would have strengthened the study findings. We found that a retrospective assessment of this variable on the basis of patient recall and medical records was too unreliable to be included in this study. H. pylori status was determined by antral biopsies; patients on chronic PPI therapy can have proximal migration of H. pylori, and additional biopsies in the body may identify more subjects with H. pylori positivity. Obesity, also a risk factor for acid reflux, BE, and EAC, and smoking history were not recorded in this study.
Despite the fact that this study was cross-sectional, we excluded patients not on PPIs before their diagnosis of advanced neoplasia. In this manner, we attempted to study only BE patients who were on PPIs as they progressed to high-grade dysplasia or AC, although the cumulative PPI dose before the initial diagnosis of HGD or AC was not known for all cases. In our analyses, we accounted for some important potential confounders in the association with neoplastic progression, including patient sex and age, and aspirin or NSAID use.
In this study, we showed that elevated serum gastrin is associated with a history of HGD and AC in patients with BE. There is indirect evidence to support the notion that gastrin may have an important role in esophageal tumorigenesis. Further studies are currently underway to determine whether hypergastrinemia in BE patients also correlates with increased tissue expression of CCK2R and COX-2. Given the widespread use of PPIs for acid suppression in BE patients, it will also be important to determine whether there may be a subset of BE patients with PPI-induced secondary hypergastrinemia who may actually be at increased risk for neoplastic progression.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Guidelines recommend proton pump inhibitor (PPI) therapy for all patients with Barrett’s esophagus.
-
✓
Acid suppression with PPIs can result in secondary hypergastrinemia.
WHAT IS NEW HERE
-
✓
High serum gastrin is associated with a history of advanced neoplasia in Barrett’s esophagus.
ACKNOWLEDGMENTS
This research was supported in part by the National Institutes of Health (Dr T.C. Wang, R01 DK52778; Dr Abrams, K07 CA132892) and by the Medical Research Council (Dr Varro).
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Julian A. Abrams, MD, MS.
Specific author contributions: Responsible for study design, data analysis, data interpretation, and article preparation and review: Julian A. Abrams; participated in the study design, data collection, and article preparation: Judy S. Wang; responsible for performing study assays and contributed to data interpretation and article review: Andrea Varro; responsible for performing study assays: Nantaporn Lertkowit; assisted with data collection: Michael L. Fingerhood; assisted with biostatistical interpretation as well as article preparation and review: Wei Yann Tsai; responsible for study design and article review: Charles J. Lightdale; responsible for study design, data interpretation, and article preparation and review: Timothy C. Wang. All authors actively participated in the study and contributed to the final version of the article.
Financial support: None.
Potential competing interests: None.
REFERENCES
- 1.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 2.Conio M, Cameron AJ, Romero Y, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted County, Minnesota. Gut. 2001;48:304–309. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron AJ, Zinsmeister AR, Ballard DJ, et al. Prevalence of columnar-lined (Barrett’s) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990;99:918–922. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag H. Role of obesity in GORD-related disorders. Gut. 2008;57:281–284. doi: 10.1136/gut.2007.127878. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Nocon M, Labenz J, Jaspersen D, et al. Long-term treatment of patients with gastro-oesophageal reflux disease in routine care - results from the ProGERD study. Aliment Pharmacol Ther. 2007;25:715–722. doi: 10.1111/j.1365-2036.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287:1972–1981. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 8.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 9.Jansen JB, Klinkenberg-Knol EC, Meuwissen SG, et al. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology. 1990;99:621–628. doi: 10.1016/0016-5085(90)90946-x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238:15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Haigh CR, Attwood SE, Thompson DG, et al. Gastrin induces proliferation in Barrett’s metaplasia through activation of the CCK2 receptor. Gastroenterology. 2003;124:615–625. doi: 10.1053/gast.2003.50091. [DOI] [PubMed] [Google Scholar]
- 12.Harris JC, Clarke PA, Awan A, et al. An antiapoptotic role for gastrin and the gastrin/CCK-2 receptor in Barrett’s esophagus. Cancer Res. 2004;64:1915–1919. doi: 10.1158/0008-5472.can-03-2713. [DOI] [PubMed] [Google Scholar]
- 13.Moore TC, Jepeal LI, Boylan MO, et al. Gastrin stimulates receptor-mediated proliferation of human esophageal adenocarcinoma cells. Regul Pept. 2004;120:195–203. doi: 10.1016/j.regpep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Abdalla SI, Lao-Sirieix P, Novelli MR, et al. Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res. 2004;10:4784–4792. doi: 10.1158/1078-0432.CCR-04-0015. [DOI] [PubMed] [Google Scholar]
- 15.Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol. 2001;96:990–996. doi: 10.1111/j.1572-0241.2001.03599.x. [DOI] [PubMed] [Google Scholar]
- 16.Georgopoulos SD, Polymeros D, Triantafyllou K, et al. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion. 2006;74:42–46. doi: 10.1159/000096593. [DOI] [PubMed] [Google Scholar]
- 17.Thorburn CM, Friedman GD, Dickinson CJ, et al. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–280. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- 18.Furuta T, Shirai N, Sugimoto M, et al. Pharmacogenomics of proton pump inhibitors. Pharmacogenomics. 2004;5:181–202. doi: 10.1517/phgs.5.2.181.27483. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth J, Varro A, Bridson J, et al. Increased tissue concentrations of the gastrin precursor in patients treated with omeprazole. Eur J Clin Invest. 1992;22:638–644. doi: 10.1111/j.1365-2362.1992.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 20.Monkemuller K, Neumann H, Nocon M, et al. Serum gastrin and pepsinogens do not correlate with the different grades of severity of gastro-oesophageal reflux disease: a matched case-control study. Aliment Pharmacol Ther. 2008;28:491–496. doi: 10.1111/j.1365-2036.2008.03769.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang YX, Hennessy S, Propert K, et al. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133:748–754. doi: 10.1053/j.gastro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Robertson DJ, Larsson H, Friis S, et al. Proton pump inhibitor use and risk of colorectal cancer: a population-based, case-control study. Gastroenterology. 2007;133:755–760. doi: 10.1053/j.gastro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Hillman LC, Chiragakis L, Shadbolt B, et al. Effect of proton pump inhibitors on markers of risk for high-grade dysplasia and oesophageal cancer in Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:321–326. doi: 10.1111/j.1365-2036.2007.03579.x. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2004;99:1877–1883. doi: 10.1111/j.1572-0241.2004.30228.x. [DOI] [PubMed] [Google Scholar]
- 25.Garcia Rodriguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538–1544. doi: 10.1136/gut.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eissele R, Brunner G, Simon B, et al. Gastric mucosa during treatment with lansoprazole: Helicobacter pylori is a risk factor for argyrophil cell hyperplasia. Gastroenterology. 1997;112:707–717. doi: 10.1053/gast.1997.v112.pm9041231. [DOI] [PubMed] [Google Scholar]
- 27.Avidan B, Sonnenberg A, Schnell TG, et al. Hiatal hernia size, Barrett’s length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97:1930–1936. doi: 10.1111/j.1572-0241.2002.05902.x. [DOI] [PubMed] [Google Scholar]
- 28.Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106–1110. doi: 10.1056/NEJM199510263331703. [DOI] [PubMed] [Google Scholar]
- 29.Marks RD, Richter JE, Rizzo J, et al. Omeprazole vs. H2-receptor antagonists in treating patients with peptic stricture and esophagitis. Gastroenterology. 1994;106:907–915. doi: 10.1016/0016-5085(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 30.Souza RF, Shewmake K, Pearson S, et al. Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett’s adenocarcinoma cells. Am J Physiol. 2004;287:G743–G748. doi: 10.1152/ajpgi.00144.2004. [DOI] [PubMed] [Google Scholar]
- 31.Souza RF, Shewmake K, Terada LS, et al. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett’s esophagus. Gastroenterology. 2002;122:299–307. doi: 10.1053/gast.2002.30993. [DOI] [PubMed] [Google Scholar]
- 32.Hao Y, Sood S, Triadafilopoulos G, et al. Gene expression changes associated with Barrett’s esophagus and Barrett’s-associated adenocarcinoma cell lines after acid or bile salt exposure. BMC Gastroenterol. 2007;7:24. doi: 10.1186/1471-230X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan C, Alazawi W, Sirieix P, et al. In vitro acid exposure has a differential effect on apoptotic and proliferative pathways in a Barrett’s adenocarcinoma cell line. Am J Gastroenterol. 2004;99:218–224. doi: 10.1111/j.1572-0241.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 34.Feagins LA, Zhang HY, Hormi-Carver K, et al. Acid has antiproliferative effects in nonneoplastic Barrett’s epithelial cells. Am J Gastroenterol. 2007;102:10–20. doi: 10.1111/j.1572-0241.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang HY, Zhang X, Hormi-Carver K, et al. In non-neoplastic Barrett’s epithelial cells, acid exerts early antiproliferative effects through activation of the Chk2 pathway. Cancer Res. 2007;67:8580–8587. doi: 10.1158/0008-5472.CAN-07-2023. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett’s esophagus. An ex vivo proliferation and differentiation model. J Clin Invest. 1996;98:2120–2128. doi: 10.1172/JCI119018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology. 1999;117:327–335. doi: 10.1053/gast.1999.0029900327. [DOI] [PubMed] [Google Scholar]
- 38.Umansky M, Yasui W, Hallak A, et al. Proton pump inhibitors reduce cell cycle abnormalities in Barrett’s esophagus. Oncogene. 2001;20:7987–7991. doi: 10.1038/sj.onc.1204947. [DOI] [PubMed] [Google Scholar]
- 39.Lao-Sirieix P, Roy A, Worrall C, et al. Effect of acid suppression on molecular predictors for esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:288–293. doi: 10.1158/1055-9965.EPI-05-0528. [DOI] [PubMed] [Google Scholar]
- 40.Shirvani VN, Ouatu-Lascar R, Kaur BS, et al. Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 41.Wilson KT, Fu S, Ramanujam KS, et al. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 42.Mehta S, Boddy A, Johnson IT, et al. Systematic review: cyclo-oxygenase-2 in human oesophageal adenocarcinogenesis. Aliment Pharmacol Ther. 2006;24:1321–1331. doi: 10.1111/j.1365-2036.2006.03119.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaur BS, Khamnehei N, Iravani M, et al. Rofecoxib inhibits cyclooxygenase 2 expression and activity and reduces cell proliferation in Barrett’s esophagus. Gastroenterology. 2002;123:60–67. doi: 10.1053/gast.2002.34244. [DOI] [PubMed] [Google Scholar]
- 44.Souza RF, Shewmake K, Beer DG, et al. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]
- 45.Buttar NS, Wang KK, Anderson MA, et al. The effect of selective cyclooxygenase-2 inhibition in Barrett’s esophagus epithelium: an in vitro study. J Natl Cancer Inst. 2002;94:422–429. doi: 10.1093/jnci/94.6.422. [DOI] [PubMed] [Google Scholar]
- 46.Buttar NS, Wang KK, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–1112. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 47.Subramaniam D, Ramalingam S, May R, et al. Gastrin-mediated interleukin-8 and cyclooxygenase-2 gene expression: differential transcriptional and posttranscriptional mechanisms. Gastroenterology. 2008;134:1070–1082. doi: 10.1053/j.gastro.2008.01.040. [DOI] [PubMed] [Google Scholar]