Abstract
Traditional imaging modalities such as computed tomography, although perfectly adept at identifying and quantifying advanced calcification, cannot detect the early stages of this disorder and offer limited insight into the mechanisms of mineral dysregulation. This review presents optical molecular imaging as a promising tool that simultaneously detects pathobiological processes associated with inflammation and early stages of calcification in vivo at the (sub)cellular levels.
Research into treatment of cardiovascular calcification is lacking, as shown by clinical trials that have failed to demonstrate the reduction of calcific aortic stenosis. Hence the need to elucidate the pathways that contribute to cardiovascular calcification and to develop new therapeutic strategies to prevent or reverse calcification has driven investigations into the use of molecular imaging. This review discusses studies that have used molecular imaging methods to advance knowledge of cardiovascular calcification, focusing in particular on the inflammation-dependent mechanisms of arterial and aortic valve calcification.
Keywords: Aortic valve, atherosclerosis, inflammation, calcification, molecular imaging
Cardiovascular Calcification – An Unresolved Medical Problem
Cardiovascular calcification, a disease of dysregulated mineral metabolism, is by no means a new dilemma. Indeed some reports date its existence as far back as the Ice Age.1 Hypercholesterolemia, metabolic syndrome, end-stage renal disease, diabetes mellitus and increased age accelerate atherosclerosis and cardiovascular calcification. Ectopic mineralization mainly affects the aorta, coronary arteries, peripheral arteries, and aortic valves, with fully-formed bone observed in atherosclerotic plaques and stenotic aortic valves.2 Once believed to be a passive degenerative disease, cardiovascular calcification is now recognized as an active process, with evidence suggesting that it follows a mechanism similar to that of bone formation. Age and lifestyle are still major factors however, thus the rising average age of the population is accompanied by a growing burden of this disorder, translating into a large cost for society.3-6
Arterial Calcification
Cardiovascular calcification, typically measured and quantified in patients using imaging modalities such as computed tomography (CT), serves as a marker for atherosclerotic coronary artery disease and is associated with increased cardiovascular events.7 Coronary artery calcification scoring produced via the use of CT has been shown to predict future coronary heart disease events.8, 9 Arterial microcalcifications located in the thin (< 65 μm) fibrous cap overlying the necrotic core of atherosclerotic plaques may cause microfractures, which can lead to acute thrombosis and even fatal myocardial infarction.10-15 However, although evidence suggests that microcalcifications in thin fibrous caps increase the risk of plaque rupture, calcification remains a neglected pathology, and effective anti-calcification therapies are not available.
Aortic Valve Calcification
Massing evidence suggests that valvular calcification possesses characteristics of arterial calcification.16 Clinicopathological studies of human stenotic aortic valves have identified lesions similar to those in atherosclerotic plaques,17, 18 and atherosclerotic-like lesions have been noted in the aortic valve leaflets of rabbit and mouse models of atherosclerosis.19-23 Aortic valve stenosis and coronary atherosclerosis also share epidemiologic risk factors, further fueling recognition of their similarity.16, 24, 25 Calcific aortic valve disease can range from mild valve thickening to severe calcification with impaired leaflet motion or aortic valve stenosis, the most common form of heart valve disease.26 Thus calcification is a strong predictor of disease progression in patients with initially asymptomatic aortic stenosis.27 Approximately 85,000 patients in the United States and 275,000 worldwide annually undergo valve replacement due to aortic valve stenosis28, 29 – invasive and costly surgical intervention is the only effective treatment.23, 30
Current Treatment Strategies
Current research is aimed at revealing the mechanisms involved in cardiovascular calcification in order to target specific pathways pharmacologically. Various therapeutic agents have been investigated — including statins, which have decreased osteoblastic differentiation and cell mineralization in vitro31, 32 and prevented progression of macrophage burden and osteogenesis in vivo.33, 34 However thus far, statin therapy has not proved beneficial in clinical trials.3, 35 As no therapies are currently available to prevent or treat calcific disease progression, the Working Group on Calcific Aortic Stenosis of the National Heart, Lung, and Blood Institute (NHLBI) recently highlighted the importance of developing new imaging and therapeutic strategies to diagnose, prevent, and potentially reverse or delay the onset of the calcification process.36 This review aims to establish underlying mechanisms of cardiovascular calcification as elucidated by in vivo molecular imaging, and demonstrate the prognostic value of optical near-infrared fluorescence (NIRF) imaging for the detection of specific changes associated with arterial calcification and aortic valve disease.
The Evolving Molecular Imaging Approach
Conventional Imaging Modalities for Detection of Calcification
The detection and quantification of advanced calcification in coronary arteries and aortic valves can be readily achieved by conventional diagnostic imaging techniques, including CT, intravascular ultrasonography (IVUS), transthoracic echocardiography and MRI.4, 37 At present, IVUS is an excellent tool for detecting advanced calcification however calcium volume is hard to quantify using these method due to acoustic shadowing.4 It is clinically advantageous for diagnostic imaging techniques to be as noninvasive as possible to alleviate patient discomfort. Therefore noninvasive techniques such as CT and ultrasound are emerging as diagnostic contenders, particularly because they are more sensitive than other imaging modalities and can quantify calcium content. However, most imaging modalities have low spatial resolution and lack suitable molecular imaging agents, and thus cannot detect the earliest stages of calcification on cellular and molecular levels.
Optical Molecular Imaging
While conventional imaging modalities proficiently visualize anatomic structures and macro changes, high-resolution optical imaging, particularly intravital fluorescence microscopy enables observation of processes on molecular and (sub)cellular levels. Novel molecular imaging technologies utilize targeted and activatable imaging agents for the in vivo detection of pro-inflammatory, pro-osteogenic, and proteolytic activity.23, 38, 39 Chen et al. demonstrated the potential use of proteases as biomarkers for vulnerable plaques when probed with beacons40; we have since harnessed this technique to explore the pro-inflammatory mechanisms of cardiovascular calcification.23, 34, 41, 42 Imaging agents utilize specific molecular or cellular processes to generate image contrast using high-resolution imaging technology. Advances in nanotechnology have yielded targeted imaging agents by chemically attaching an affinity ligand, such as an antibody or small molecule, to a fluorochrome or magnetic compound (e.g., bisphosphonate-conjugated fluorescent agent for the detection of hydroxyapatite23, 34, 43-45, or cross-linked iron oxide fluorescent nanoparticles for the detection of macrophages23, 34, 38). Studies have also produced activatable imaging agents – chemically engineered substrates that interact with their targets (e.g., enzymes) and undergo a physicochemical change, resulting in signal amplification (e.g., protease-activatable imaging agents for the detection of matrix metalloproteinase or cathepsin activity).23, 39, 41, 46
Near-infrared fluorescence (NIRF; excitation in 650 to 900 nm wavelength) molecular imaging represents a useful platform for optical molecular imaging in vivo.47, 48 Indeed near-infrared (NIR) light has the potential to penetrate tissues in the magnitude of centimeters rather than micrometers,49 which makes NIRF imaging highly attractive, as it allows for greater depth sensing of a larger area of inflamed tissue or calcific lesion. In addition, fluorescence imaging in the NIR bandwidth offers reduced tissue autofluorescence.48 Other imaging detection systems can also be used, depending on the resolution required. One such platform is fluorescence-mediated tomography (FMT), which can detect femtomole quantities of fluorochromes in whole animals with millimeter resolution.39, 40
Fluorescence molecular imaging is able to utilize two or more spectrally distinct imaging agents to visualize different biological processes simultaneously.23, 34, 41, 42, 50 It can also be integrated with more conventional imaging techniques (e.g., magnetic resonance imaging (MRI) and CT) when using multimodality or multi-functional probes. A recently developed tri-modality iron oxide–based magnetic nanoparticle could be used for simultaneous NIRF, MRI, and positron emission tomography (PET) imaging of macrophages.51 Therefore it may enable the attainment of both high imaging sensitivity (from NIRF) and high spatial resolution (from MRI).52
Clinical Translation
For the successful treatment of calcification, we need to visualize the pathways involved in the earlier stages; studies that apply optical molecular imaging to the calcifying vasculo/valvulopathy or bone remodeling are therefore desired. We anticipate that clinical molecular imaging approaches could provide new biological insights into human arterial osteogenesis far before the development of advanced calcification detected by current methods. To accomplish this goal clinical multimodal molecular imaging approaches will likely be required to detect and monitor the dynamic changes in inflammation/macrophages and osteogenesis/calcification in calcified aortic valves and atherosclerotic plaques in cardiovascular calcification.
Studies to improve molecular imaging methods, and increase their chances for clinical use, are ongoing. Clinical molecular imaging has already made significant headway into visualizing targets in larger vessels.53 There is substantial evidence supporting the use of (18)F-fluorodeoxyglucose positron emisson tomography (FDG-PET) imaging for the evaluation of coronary artery disease.54 Furthermore a novel intravascular NIRF catheter has been developed, which has been demonstrated to detect inflammation-associated protease activity in vessels the size of human coronary arteries in real time with an activatable-NIRF agent.55 In addition more recently a new two-dimensional NIRF imaging catheter system based on rotational fiber design has been developed that will allow seamless integration of molecular imaging into the cardiac catheterization laboratory.56 These advanced molecular imaging techniques do not only offer the potential to be sensitive diagnostic tools, but they also enable in vivo study of the mechanisms of atherosclerosis and cardiovascular calcification. For example the availability of a clinical intravascular NIRF catheter could accelerate the detection of high-risk plaques.53 Due to the significant technological developments made in the field of molecular imaging over the past 2 decades, it is now deemed a clinically feasible diagnostic tool.52
Imaging Identifies Underlying Molecular Mechanisms Involved in Early Aortic Valve Calcification
The mechanistic pathways involved in the development of calcific aortic valve disease remain largely unknown. Therefore the use of molecular imaging is thought advantageous to detect early molecular and functional abnormalities in aortic valves. Our recent research tested the hypothesis that molecular imaging can detect early changes in aortic valve disease, with positive outcomes.23 We used a panel of distinct NIRF imaging agents to map endothelial cells, macrophages, proteolytic activity, and osteogenesis in the aortic valves of hypercholesterolemic apolipoprotein E–deficient (apoE-/-) mice.
Imaging of Valvular Endothelial Cell Activation
MRI and NIRF microscopy clearly demonstrated ex vivo that most of the VCAM-1-targeted agent57 was distributed in the aortic valve leaflets near the attachment of the aortic root — a region known as the commissures — which was corroborated by immunohistochemical evidence using immunoreactive VCAM-1.23 Increased expression of VCAM-1, ICAM-1, and E-selectin has been noted in surgically removed diseased heart valves,58, 59 illustrating that injury to endothelial cells causes increased expression of adhesion molecules. The leaflets of the heart valves open and close at least 3 × 109 times over a single lifetime, therefore they have to endure a certain amount of ‘wear and tear’ due to the repetitive forces exerted upon them. During the cardiac cycle, the aortic valve leaflets are subjected to continual bending, shearing, and tensile and compressive stresses.60 As the flexion area of the aortic valve leaflets near the commissures encounter the greatest amount of mechanical stress within the leaflet,61 these areas might induce endothelial cell activation/injury, and thus the subsequent expression of adhesion molecules.
Imaging of Macrophages and Proteolytic Activity
Elevated plasma lipids and other atherogenic factors may also induce valve endothelial cell activation. This results in an amplification cascade of events, including monocyte recruitment and subsequent macrophage accumulation within the extracellular matrix of the valve, as visualized by macrophage-targeted NIRF-conjugated iron nanoparticles. The use of NIRF protease-activatable probes provided direct evidence that valvular interstitial cells (in their activated form as myofibroblasts) and macrophages elaborate excessive levels of matrix metalloproteinases (e.g., collagenase-1/MMP-1, collagenase-3/MMP-13, gelatinase-A/MMP-2, and gelatinase-B/MMP-9) and cysteine endoproteases (cathepsins K and S), which corroborates with other studies.41, 62-67 These proteolytic enzymes degrade collagen and elastin in the extracellular matrix, leading to vascular and valvular remodeling and subsequent structural changes. Activation of MMP-9 by osteopontin may play a role in aortic valve calcification68 by initiating elastin degradation that could be a nidus for hydroxyapatite crystal formation.69 Proteolytic activity not only affects the extracellular matrix, but also other substrates (e.g., IL-1β precursor, tissue factor pathway inhibitor), which may in turn enhance valve inflammation.70, 71 Molecular imaging has thus enabled us to analyze proteolytic activity, which could provide diagnostic information on inflammation and matrix degradation and thereby predict risk of subclinical aortic valve stenosis.
Imaging of Osteogenic Activity
The need to closely follow and evaluate changes once lesions are identified in patients with aortic valve thickening, which could eventually lead to aortic sclerosis, is evident due to the high morbidity and mortality rates reported.72 The early stages of mineralization were observed using a bisphosphonate-conjugated imaging agent that binds to nanomolar concentrations of calcium hydroxyapatite complexes elaborated by valvular interstitial cells (e.g., myofibroblasts). Like all bisphosphonates, this non-cleavable pyrophosphate analog avidly binds calcium, thus accumulating at sites of active biomineralization and osteogenesis44, 50 as detected by alkaline phosphatase activity. This calcium imaging agent can be excited at NIRF wave lengths that are spectrally distinctive from other imaging agents (e.g., macrophage-targeted or cathepsin-activatable agents), thus enabling simultaneous correlation of osteogenic activity with other biological processes. In our study immunohistochemical analysis of osteogenic markers was used to validate data produced via NIRF. Valvular interstitial myofibroblast-like cells were demonstrated to express osteopontin, osteocalcin, and osteogenic transcription factors such as Runx2 and Osterix, indicating the active regulation of mineralization prior to the development of end-stage calcification. Additionally, large populations of myofibroblast-like cells contained a cleaved form of Notch1,23 which has been suggested to direct osteoblast differentiation.73 Valve lesions have been identified in this study to possess features similar to atherosclerotic plaques, including endothelial cell activation, inflammation/macrophages, proteolytic activity, and osteogenesis — supporting the hypothesis that aortic stenosis and atherosclerosis share a similar pathogenesis.
Inflammation-Dependent Mechanisms of Calcific Aortic Valve Disease
Although the cause of mineral dysregulation in early aortic valve disease requires further investigation, molecular imaging has enabled simultaneous visualization of the roles of various cells and enzymes in the early stages of mineralization in vivo, supporting the concept of inflammation-dependent development of calcific aortic valve disease. In summary, atherogenic factors and mechanical forces may activate valve endothelial cells and initiate recruitment of inflammatory monocytes/macrophages, which when activated produce a cocktail of pro-osteogenic cytokines, growth factors, and proteolytic enzymes. Extracellular matrix remodeling and thickening/stiffening of the leaflets due to proteolytic activity may result in valvular dysfunction and alterations of mechanical stresses across the valve leaflet. The resulting change in flow patterns may further induce inflammation and the activation of fibroblasts into myofibroblasts, and subsequently into osteoblast-like cells through augmentation of the Runx2 pathway. The end result would be the deposition of calcium primarily in the regions of high mechanical stress and eventual immobilization of the aortic leaflets due to increased stiffening (Figure 1).
Figure 1. Molecular imaging correlated inflammatory activity, defined as macrophage accumulation, with osteogenesis in the aortic valves and aortas of apoE-/- mice.
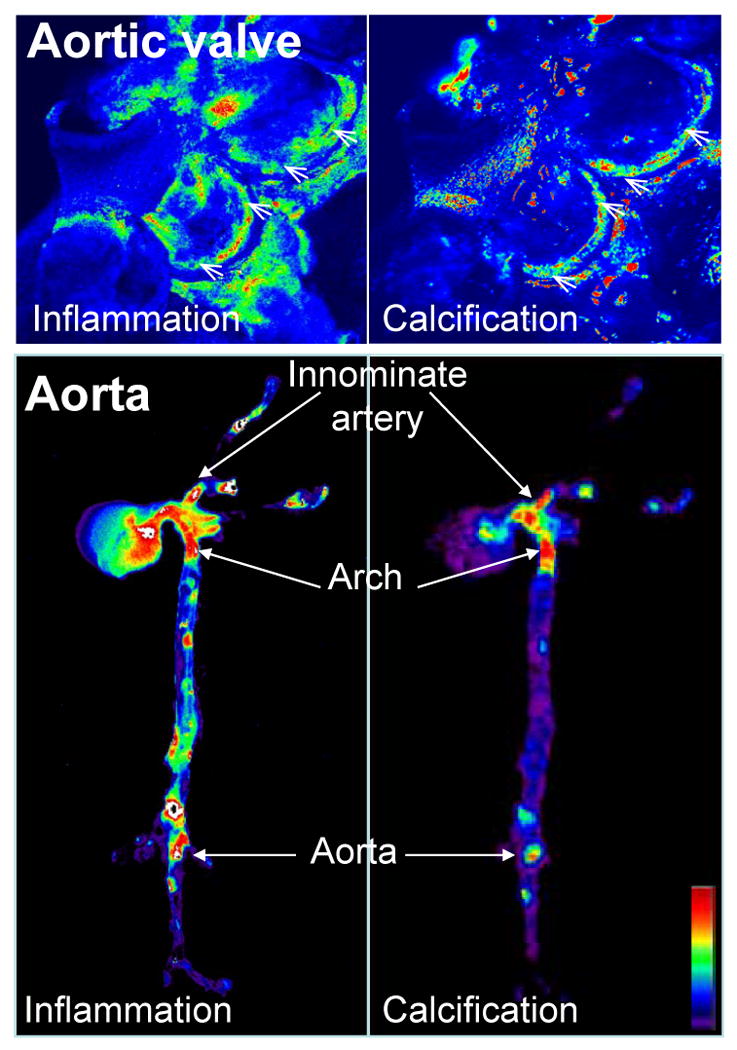
Mice were injected with magneto-fluorescent nanoparticles to visualize macrophage accumulation (left panels), and a spectrally distinct bisphosphonate-imaging agent that binds to nanomolar concentrations of hydroxyapatite to detect osteogenic activity (right panels). In the aortic valve (top) and in the aorta (bottom) molecular imaging detected that inflammatory and osteogenic activities colocalized in the areas of highest mechanical stresses at the aortic valve attachment (arrowheads) and at the atherosclerosis prone areas such as innominate artery, aortic arch, and abdominal aorta (arrows). Images were processed using Osirix software. High signal intensities are shown in red-yellow-green. (Adapted with permission from Aikawa E. et al., Circulation 2009 and Aikawa E. et al., Circulation 2007).
Monitoring Changes in Osteogenic Activity during Atherosclerotic Plaque Progression and after Anti-inflammatory Treatment
Monitoring Calcification and Inflammation in Living Animals
The limited knowledge regarding cardiovascular calcification has been blamed on the inability to spatially and temporally resolve and quantify the dynamic pro-osteogenic molecular mechanisms in vivo.74 These limitations can be overcome using innovative molecular imaging tools to visualize and quantify components of inflammation, along with osteogenic activity associated with early-stage atherosclerosis.34, 42 Though in vitro studies have suggested the potential role of inflammation in calcification,75-79 few in vivo reports pursued this idea. We therefore employed molecular imaging to test the hypothesis in vivo that atherosclerotic plaque inflammation, determined as macrophage infiltration, triggers osteogenic activity, and that further reduction of inflammation may decrease arterial calcification. Nanoparticle technology was once again utilized to image macrophages and the calcium imaging agent used to image the osteogenic differentiation of smooth muscle cells and areas of active mineralization processes in the arteries34, 42 — demonstrating the value of these imaging methods in analyzing calcific processes within both aortic valves and arteries. In this study, apoE-/- mice were fed an atherogenic, high-cholesterol diet supplemented with atorvastatin. For the first time intravital microscopy was performed sequentially on the carotid arteries of untreated mice and statin-treated cohort of mice at 20 weeks and 30 weeks of age. Macrophage number was found to increase in association with advanced osteogenic signal by the later time period, however this progression of macrophage burden and osteogenesis was prevented by anti-inflammatory statin therapy, which further supported our hypothesis that inflammation may trigger calcification.
Arterial Calcification as an Inflammatory Disease
A series of groundbreaking in vitro studies by Demer's group demonstrated that macrophage-derived cytokines (e.g., IL-1β, IL-6, IL-8, TNF-α, IGF-1, and TGF-β) induce osteogenic differentiation and mineralization of vascular smooth muscle cells.75-78 The results of these studies produced the theory that pro-inflammatory cytokines promote atherosclerosis-associated calcification by regulating the differentiation of calcifying vascular smooth muscle cells. Our in vivo molecular imaging studies corroborated previous reports and further linked macrophages with osteogenesis. Fluorescence reflectance imaging ex vivo elegantly visualized the real-time association of inflammation and early calcification.34 Similar to macrophage and calcification signals noted in regions of high flexure and increased mechanical forces in the aortic valve, macrophage burden and osteogenic activity colocalized predominantly in proatherogenic regions of high mechanical stress, including the lesser curvature of the aortic arch, the aortic root, the innominate artery, the carotid bifurcation, and the aortic root (Figure 1). This evidence further supports the importance of macrophages in calcification.
The advancement of in vivo molecular imaging techniques has enabled us to further elaborate on the inflammation-dependent calcification paradigm (Figure 2), which can be split into three distinct phases: initiation, propagation, and end-stage calcification. We suggest that in the initiation phase, macrophage infiltration and inflammation precede calcification, and activated pro-inflammatory pathways induce osteogenic transformation of vascular wall cells. This phase can be characterized by the expression of pro-osteogenic cytokines (e.g., IL-1β, IL-6, IL-8, TNF-α, IGF-1, and TGF-β) by macrophages and other inflammatory cells.5, 80 In the propagation phase, vascular smooth muscle cells undergo osteogenic differentiation, characterized by the expression of transcription factors normally associated with differentiated osteoblasts and chondrocytes (e.g., Runx2, Osterix, and Sox 9)23, 34, 81 and mineralization-regulating proteins (e.g., alkaline phosphatase, osteopontin, osteocalcin, osteonectin, collagen I and II, and bone morphogenic proteins).82 In addition, in the absence of inhibitors, matrix vesicles released from living cells, and apoptotic bodies from dying macrophages and smooth muscle cells may provide a nidus for calcification.83, 84 Indeed these events are associated with the generation of microcalcification. In our imaging study, these early stages of mineralization could also be seen using electron microscopy — matrix vesicles (30–300 nm) and apoptotic bodies (300–1000 nm) contained structures compatible with nanocrystals of hydroxyapatite.34, 85 Additionally a recent study has demonstrated that apoptosis can be monitored in real-time using fluorescently-tagged Annexin A5 imaging agent.86 If inflammation continues, as the plaque advances it will induce further formation of microcalcifications, fibrous cap thinning, and eventually plaque rupture. Using molecular imaging at this stage, we showed that inflammation and microcalcification evolved within close proximity and overlapped at border regions, and suggested that plaque ruptures may occur in these adjacent areas.34
Figure 2. The inflammation-dependent mechanism of calcification visualized by molecular imaging.
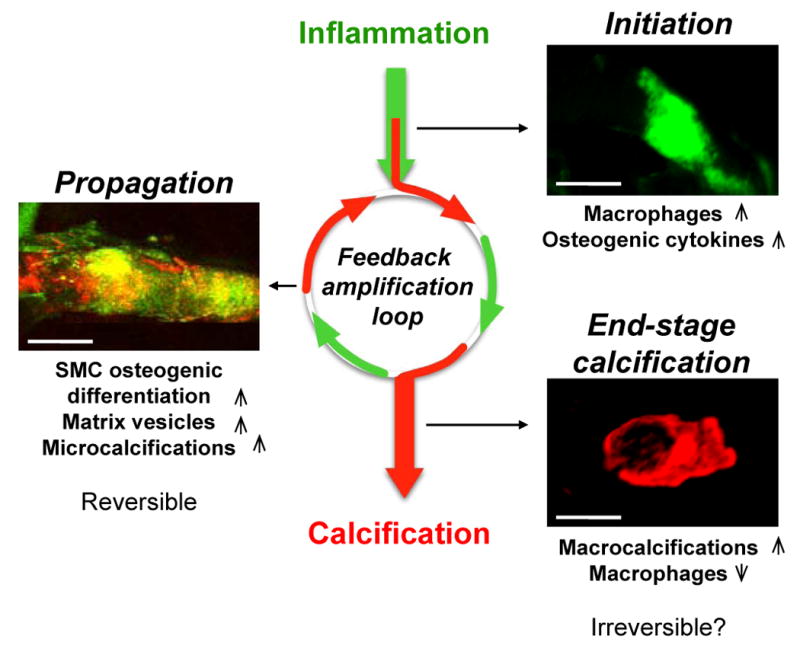
Sequential intravital fluorescence microscopy was used to observe changes in inflammation and osteogenesis in atherosclerotic arteries. Two spectrally distinct molecular imaging agents were administered through intravenous injection into apoE−/− mice and the common carotid artery was visualized: magneto-fluorescent nanoparticle to target macrophages (green), and a bisphosphonate-imaging agent to detect osteogenic activity/microcalcifications (red). Three different stages could be identified depending on atheroma progression in 20, 30 and 72 week-old apoE-/- mice feed high-fed diet. In the initiation phase – associated with increased macrophages and pro-osteogenic cytokines – only inflammation was observed (green). In the propagation phase – associated with osteogenic activity and generation of microcalcification – inflammation (green) and calcification (red) overlapped (yellow), suggesting that these two processes develop in parallel. Continued inflammation, in parallel with advancing plaque induces further formation of microcalcifications that provoke additional pro-inflammatory responses from macrophages suggesting that feedback amplification loop of calcification and inflammation drives disease progression. Reducing inflammation through anti-inflammatory therapy at this stage may retard osteogenesis and subsequent calcification. In the end-stage phase – associated with increased mineralization and decreased macrophages – macrocalcifications (red) were observed with limited inflammation. Reversing advanced calcification at this late stage is deemed difficult.
The deposition of hydroxyapatite progresses quickly,34 and microcalcifications evoke additional pro-inflammatory responses from macrophages, demonstrating that a positive feedback loop of calcification and inflammation drives disease progression.87, 88 Moreover, the onset of microcalcifications is an added complication, as they may cause plaque rupture as a result of debonding, leading to acute clinical events as predicted by Vengrenyuk and colleagues.13, 15, 89 It is hypothesized however, that reducing inflammation through anti-inflammatory therapy at the early stages could retard subsequent osteogenesis and stabilize the plaque until further inflammatory events are initiated.34, 42, 50, 84 As reversing or halting the mineralization process in later stages of calcific disorders may be more difficult, the final of the three phases — end-stage calcification — is classically viewed as irreversible. This final phase is associated with advanced tissue mineralization and reduced inflammation, and can be readily detected by conventional imaging approaches (e.g., CT).90
Detecting Elastolysis-triggered Calcification in Chronic Renal Disease
Cardiovascular disease is the most common cause of death in individuals with chronic renal disease (CRD).91-93 In addition to the classic risk factors mentioned earlier, patients with CRD have hyperphosphatemia, an independent risk factor for cardiovascular death.94, 95 While cardiovascular disease in the general population is associated with older age, lesions of cardiovascular calcification have been reported in dialysis patients of a much younger age,95, 96 confirming that cardiovascular calcification is not simply a degenerative disorder. This issue, in our mind, also heightens the necessity for research into this area. We have already highlighted the implication of proteolytic enzymes expressed by activated macrophages in atherosclerotic plaque progression and aortic valve disease.62, 97-100 Although the contribution of cathepsins to the pathogenesis of atherosclerosis and aortic stenosis has been previously established, our study using optical molecular imaging produced direct in vivo evidence for the role of cathepsin S, one of the most potent elastases, in accelerating arterial and valvular calcification in mice with atherosclerosis and CRD induced by 5/6 nephrectomy – a 2-step CRD-promoting procedure that is known to aggravate atherosclerosis and intimal calcification in apoE-/- mice.41,101
Role of Elastin Degradation in Calcification
Elastolytic cathepsin S is abundantly expressed by macrophages and smooth muscle cells in atheroma.102 Previous studies have suggested that the structure of the elastic fibers allows them to withstand proteolytic degradation.103, 104 But during the progression of atherosclerosis, an imbalance in the regulation of cathepsins may enable proteases such as cathepsin S to degrade elastin.69, 105 Elastolysis induced by inflammation in the atherosclerotic plaques may promote the release of biologically active, soluble elastin-derived peptides that trigger the osteogenic differentiation of smooth muscle cells.41, 106, 107 These elastin-derived peptides act as biologically active molecules known as matrikines or elastokines, which can regulate cell processes such as migration and proliferation, as well as the release of bone-regulating proteins from myofibroblasts or smooth muscle cells, resulting in calcific lesions.106-110
Imaging Elastolytic Activity
Our study utilized a new protease-activatable imaging agent that was specific for cathepsin S.41, 111 Molecular imaging detected increased cathepsin S and osteogenic activities in CRD mice, whereas calcification was decreased in atherosclerotic plaques and aortic valves in mice lacking cathepsin S activity. These results were corroborated using optical projection tomography and quantitative histology, and provided the first direct in vivo evidence for the role of cathepsin S in calcification.
We have previously shown that proteolytic activity, in the form of cathepsins (cathepsin S and cathepsin K) and MMPs (MMP-2 and MMP-9), plays a role in valvular diseases.62 Therefore, as mature aortic valves also have an elastin-rich structure 100 and the ability to develop lesions similar to those of atherosclerotic plaques,23, 34 we proposed that similar mechanisms of cathepsin S-associated elastin degradation contribute to the development of calcific aortic valve disease. In the early stages of aortic valve and artery calcification, macrophage-derived elastolytic enzymes participate in the degradation of elastin matrix. This remodeling of the matrix leads to proliferation of vascular smooth muscle cells or valvular myofibroblasts, causing lesion formation and growth. The elastolysis-triggered release of biologically active peptides attracts more macrophages, which in turn produce more proteolytic enzymes, promoting further expansion of the lesion. These biologically active peptides promote osteogenic differentiation of the cells. Patients with CRD have the additional complication of hyperphosphatemia, a mineral imbalance leading to phosphate-induced release of matrix vesicles and apoptosis — which, in turn, accelerates calcification of vascular smooth muscle cells or valvular myofibroblasts.41, 84, 112
Inverse Correlation of Arterial and Aortic Valve Calcification with Osteoporotic Bone Remodeling: A Role for Inflammation
Clinical studies have suggested associations between cardiovascular calcification, atherosclerosis, CRD, and osteoporosis.113,114 Although this link initially was thought to be age-related, epidemiological evidence has demonstrated an age-independent correlation between bone mineral density (BMD) and cardiovascular events.115-117 These studies noted a reduction in BMD along with arterial calcification in humans; this was corroborated by mouse studies that further demonstrated that atherosclerosis susceptibility corresponds with reduced bone mineralization.118, 119 Limited studies suggest a mechanism behind this seemingly paradoxical event — a recent literature review, for example, discussed the possibility that osteoporosis and cardiovascular calcification are tissue-specific responses to chronic inflammation.2 However the precise nature of the reciprocal regulation of arterial calcification, calcific aortic valve disease, and bone osteogenesis remains unknown.
Arterial, Valvular and Bone Mineralization have Shared Pro-inflammatory Mechanisms
In our recent study42 the relationship between cardiovascular calcification (arterial and valvular) and long bone remodeling (cortical and trabecular) was simultaneously quantified for the first time in mice with hyperlipidemia and with CRD using optical molecular imaging and high-resolution 3D micro-CT.41 We hypothesized that cardiovascular calcification progresses with inflammation and inversely correlates with BMD. The study sought to provide mechanistic evidence on the role of inflammation in calcification and osteoporosis. Our results on the opposing effects of inflammation in cardiovascular organs (soft tissues) and in bone agree with previous reports. This study provided new insight into the relationship between osteoporosis and cardiovascular calcification, and suggested shared inflammatory mechanisms of ectopic calcification and bone osteolysis. Directly comparing macrophage burden and progression of osteogenic changes via NIRF imaging in each region of the same animals in vivo and ex vivo, enabled us to discover that bone osteogenic activity and BMD decrease as atherosclerosis and calcific aortic valve disease develop, and that the degree of cardiovascular calcification correlates directly with loss of BMD. Molecular imaging identified strong inflammatory activity in arteries, aortic valves, and long bones of mice with atherosclerosis and CRD, demonstrating that inflammation at these three locations is related probably via systemic or circulating inflammatory cues.
Osteoporosis was associated epidemiologically with atherosclerosis and hyperlipidemia many decades ago.120 More recently, a clinical study reported that stenotic coronary narrowing was more prevalent among women with low BMD,121 while preclinical studies have suggested that hyperlipidemia promotes cardiovascular calcification and reduces BMD via increased bone resorption.118 Other evidence suggests that osteoporosis may contribute to cardiovascular calcification by releasing biochemical factors, such as increased amounts of circulating phosphate and calcium and decreased amounts of parathyroid hormone, which promote osteogenesis and mineralization of the arterial wall and aortic valve.122, 123 This evidence agrees with results from studies showing that agents that block bone resorption in animals also block vascular calcification.124 Biphosphonates, used in the management of osteoporosis, have been associated with decreased cardiovascular calcification in elderly subjects and increased prevalence of valvular, aortic and coronary arterial calcification in younger women with subclinical cardiovascular disease.125 The reduction of cardiovascular calcification in elderly women may be due to decreased cholesterol levels and pro-inflammatory cytokines, or alternatively due to declining bone resorption and subsequent decrease of circulating calcium phosphate. Additional studies are needed to elucidate the beneficial effect of biphosphonates on bone metabolism in elderly patients; however further evaluation of unfavorable effects in younger women is warranted.
The use of molecular imaging in our imaging study has strongly suggested that systemic and local inflammation, seemingly paradoxically, drives both cardiovascular calcification and bone loss. In simplified terms, inflammation causes differential tissue responses that result in “hardening” of soft tissue and “softening” of hard tissue,126 but it is unclear whether the pathways are similar. It has been suggested that inflammation in cardiovascular and bone regions may act through the NF-κB-RANKL pathway, but whether this mechanism is utilized simultaneously for cardiovascular calcification and osteoporosis is uncertain. Future studies could use molecular imaging to elucidate these signaling pathways, as there are still many questions to answer.
Conclusions
Both in vitro and clinical studies have suggested that a sequence of active osteogenic processes contributes to cardiovascular calcification, and that osteogenic activity is initiated by inflammation. State-of-the-art multimodality molecular imaging has provided the opportunity to effectively visualize in vivo these different biological processes simultaneously, by using two spectrally distinct imaging agents, and thus substantiate this theory. The studies cited in this review have led us to the hypothesis that calcification of the artery and the aortic valve are mechanistically similar, and thus the same sophisticated imaging modalities can examine both processes (Figure 3). A key finding of our studies was that molecular imaging techniques can visualize atherosclerotic plaques and aortic valve lesions in the early stages,23, 34, 41, 50 which are undetectable by conventional imaging modalities. However this review does not aim to lessen the value of studies using histopathology and conventional imaging modalities, but rather support the development of innovative imaging techniques to enable further exploration of the pathogenesis of cardiovascular calcification.
Figure 3. Imaging shared molecular mechanisms underlying arterial and aortic valve calcification.
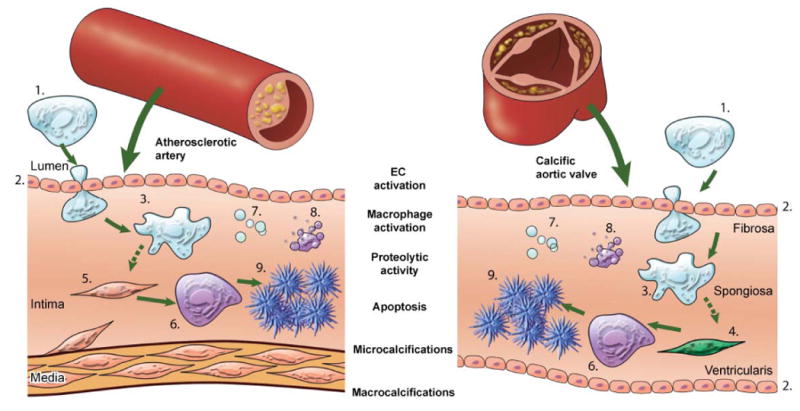
The studies cited in this review showed that calcification is an active process composed of a sequence of events, initiated by inflammation and resulting in mineralization. Pro-inflammatory monocytes (1) are recruited to a site via activated endothelial cells (EC) (2). The activation of EC causes increased expression of adhesion molecules, such as VCAM-1, which can be visualized by NIRF imaging using a VCAM-1-targeted agent. Subsequent macrophage (3) accumulation follows, which can be analyzed using NIRF macrophage-targeted nanoparticles. The release of proteolytic enzymes — including matrix metalloproteases and cathepsins — by macrophages, which stimulates the differentiation of myofibroblasts (4) and smooth muscle cells (5) into osteoblasts, can be visualized by molecular imaging using activatable imaging agents. Osteogenic activity in the form of osteoblast (6) formation and microcalcifications associated with generation of calcified matrix vesicles (7) can be identified by bisphosphonate-conjugated imaging agent, while apoptotic bodies (8) can be detected by fluorescently-tagged Annexin A5 imaging probe. Macrocalcifications (9) can be readily detected by molecular imaging and conventional imaging techniques. This schematic visualizes the theory that calcification follows similar processes in both the artery and the aortic valve, and summarizes how NIRF imaging can visualize dynamic sequences of calcification process.
Clinical trials have failed to demonstrate the benefit of statin therapy in slowing the progression of valve calcification.21 This may be due to the late implementation of the statins – after aortic valve calcification has progressed to the irreversible stage. Adjusting atherogenic factors and/or using pharmacological therapies that target pro-inflammatory pathways may impede or even halt the progression of cardiovascular calcification, when implemented during the early stages of calcification. For example, anti-inflammatory therapies or the preservation of elastin integrity — via the inhibition of elastolytic cathepsins such as cathepsin S — might prevent cardiovascular calcification and its complications when introduced early. In addition, macrophage- or smooth muscle cell-targeted silencing of pro-inflammatory or pro-osteogenic factors with siRNA may retard the progression of calcification. Combining optical imaging agents with anti-calcification drugs (e.g., bisphosphonate) within the targeted construct may provide a unique platform for specific imaging and therapy for preclinical models and for future clinical translation. Moreover, therapeutic interventions for calcification-prone patients with CRD could target inflammation, matrix degradation, or mineral imbalance. Further studies are required to establish the relationship between cardiovascular calcification and osteoporosis, to identify factors to target for the reciprocal regulation of these processes.
Molecular imaging and particularly optical imaging is anticipated to have the most impact on preclinical research, including identification of novel targets and mechanisms and evaluation of imaging tools in preclinical models. It is apparent that various limitations and issues need to be addressed before the molecular imaging approach will be used clinically. Despite this several studies are producing favorable results in regards to the clinical translation of this evolving modality. Molecular imaging techniques, which utilize radionuclides could be available for clinical use within the next 1-3 years, as many of the imaging probes have already been routinely used clinically.52 The complementary use of targeted molecular imaging agents with MRI has demonstrated promising results in animal studies. In addition, molecular imaging agents are being considered for clinical trials.50, 53 Indeed the use of cysteine protease-activatable NIRF agent, is already believed to be clinically favourable55 the backbone of this imaging agent has been found to be safe in clinical trials127 and the NIR fluorochromes are similar to those of an agent used widely in retinal angiography.128 The development of promising clinically feasible technology (e.g. intravascular NIRF for coronary artery imaging55,56) is ongoing and each study leads us closer to the clinical translation of this imaging modality.
Acknowledgments
The authors would like to thank Dr. Farouc Jaffer, Massachusetts General Hospital, for the critical reading of the manuscript and Ms. Sara Karwacki for excellent editorial assistance.
Funding: This work was supported in part by grants from the American Heart Association (#0835460N), Foundation Leducq Transatlantic Network (#07CVD04), Donald W. Reynolds Foundation and Translational Program of Excellence in Nanotechnology (5-UO1-HL080731).
Abbreviations
- apoE
apoliprotein-E
- BMD
bone mineral density
- CRD
chronic renal disease
- CT
computed tomography
- FMT
fluorescence-mediated tomography
- IGF-1
insulin-like growth factor 1
- IL
interleukin
- IVUS
intravascular ultrasonography
- ICAM-1
intercellular adhesion molecule 1
- MMP
matrix metalloproteinases
- MRI
magnetic resonance imaging
- NF-kB
nuclear factor kappa B
- NHLBI
National Heart, Lung and Blood Institute
- NIR
near-infrared
- NIRF
near-infrared fluorescence
- PET
positron emission tomography
- RANK
receptor activator of nuclear factor kappa B
- RANKL
RANK ligand
- Runx2
runt-related transcription factor 2
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
Disclosures: The authors report no conflict of interest.
References
- 1.Murphy WA, Jr, Nedden Dz D, Gostner P, Knapp R, Recheis W, Seidler H. The iceman: Discovery and imaging. Radiology. 2003;226:614–629. doi: 10.1148/radiol.2263020338. [DOI] [PubMed] [Google Scholar]
- 2.Demer LL, Tintut Y. Mechanisms linking osteoporosis with cardiovascular calcification. Curr Osteoporos Rep. 2009;7:42–46. doi: 10.1007/s11914-009-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto CM. Calcific aortic stenosis--time to look more closely at the valve. N Engl J Med. 2008;359:1395–1398. doi: 10.1056/NEJMe0807001. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: Pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 5.Demer LL, Tintut Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towler DA. Vascular calcification: A perspective on an imminent disease epidemic. IBMS BoneKEy. 2008;5:41–58. [Google Scholar]
- 7.Doherty TM, Asotra K, Fitzpatrick LA, Qiao JH, Wilkin DJ, Detrano RC, Dunstan CR, Shah PK, Rajavashisth TB. Calcification in atherosclerosis: Bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci U S A. 2003;100:11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenker MP, Dorbala S, Hong EC, Rybicki FJ, Hachamovitch R, Kwong RY, Di Carli MF. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: A combined positron emission tomography/computed tomography study. Circulation. 2008;117:1693–1700. doi: 10.1161/CIRCULATIONAHA.107.717512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmermund A, Mohlenkamp S, Berenbein S, Pump H, Moebus S, Roggenbuck U, Stang A, Seibel R, Gronemeyer D, Jockel KH, Erbel R. Population-based assessment of subclinical coronary atherosclerosis using electron-beam computed tomography. Atherosclerosis. 2006;185:177–182. doi: 10.1016/j.atherosclerosis.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Abedin M, Tintut Y, Demer LL. Vascular calcification: Mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 11.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 13.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL. Mechanical stress analysis of a rigid inclusion in distensible material: A model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol. 2009;297:H802–810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vengrenyuk Y, Cardoso L, Weinbaum S. Micro-ct based analysis of a new paradigm for vulnerable plaque rupture: Cellular microcalcifications in fibrous caps. Mol Cell Biomech. 2008;5:37–47. [PubMed] [Google Scholar]
- 16.Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. A1396. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 18.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 19.Rajamannan NM. Calcific aortic stenosis: A disease ready for prime time. Circulation. 2006;114:2007–2009. doi: 10.1161/CIRCULATIONAHA.106.657759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–2665. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K, Sata M, Fukuda D, Suematsu Y, Motomura N, Takamoto S, Hirata Y, Nagai R. Age-associated aortic stenosis in apolipoprotein e-deficient mice. J Am Coll Cardiol. 2005;46:134–141. doi: 10.1016/j.jacc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 22.Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in c57bl/6j mice. J Am Coll Cardiol. 2006;47:850–855. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 23.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 24.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 25.Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–866. doi: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 26.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 27.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 28.Rabkin-Aikawa E, Mayer JE, Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol. 2005;94:141–179. doi: 10.1007/b100003. [DOI] [PubMed] [Google Scholar]
- 29.Badylak SF. Regenerative medicine approach to heart valve replacement. Circulation. 2005;111:2715–2716. doi: 10.1161/CIRCULATIONAHA.105.542837. [DOI] [PubMed] [Google Scholar]
- 30.Cannata-Andia JB, Rodriguez-Garcia M, Carrillo-Lopez N, Naves-Diaz M, Diaz-Lopez B. Vascular calcifications: Pathogenesis, management, and impact on clinical outcomes. J Am Soc Nephrol. 2006;17:S267–273. doi: 10.1681/ASN.2006080925. [DOI] [PubMed] [Google Scholar]
- 31.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 32.Monzack EL, Gu X, Masters KS. Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment. Arterioscler Thromb Vasc Biol. 2009;29:246–253. doi: 10.1161/ATVBAHA.108.179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the lrp5 receptor pathway. Circulation. 2005;112:I229–234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 35.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 36.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Heistad DD, Masters KS, Mathieu P, O'Brien K, Otto CM, Schoen FJ, Simmons G, Towler D, Yoganathan A. National heart, lung and blood institute (NHLBI) working group on calcific aotic stenosis. 2010 http://www.Nhlbi.Nih.Gov/meetings/workshops/cas.Htm.
- 37.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: Part ii: Approaches by noninvasive computed tomographic/magnetic resonance imaging. J Am Coll Cardiol. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 38.Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5:85–92. [PubMed] [Google Scholar]
- 39.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: Visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105:2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 41.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin s deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: A role for inflammation. Eur Heart J. 2010;31:1975–1984. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol. 2001;19:1148–1154. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 44.Zaheer A, Murshed M, De Grand AM, Morgan TG, Karsenty G, Frangioni JV. Optical imaging of hydroxyapatite in the calcified vasculature of transgenic animals. Arterioscler Thromb Vasc Biol. 2006;26:1132–1136. doi: 10.1161/01.ATV.0000210016.89991.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozloff KM, Volakis LI, Marini JC, Caird MS. Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo. J Bone Miner Res. 2010;25:1748–1758. doi: 10.1002/jbmr.66. [DOI] [PubMed] [Google Scholar]
- 46.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin k activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 47.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: Insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1017–1024. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ntziachristos V, Ripoll J, Weissleder R. Would near-infrared fluorescence signals propagate through large human organs for clinical studies? Errata. Opt Lett. 2002;27:1652. doi: 10.1364/ol.27.001652. [DOI] [PubMed] [Google Scholar]
- 50.Chang K, Francis SA, Aikawa E, Figueiredo JL, Kohler RH, McCarthy JR, Weissleder R, Plutzky J, Jaffer FA. Pioglitazone suppresses inflammation in vivo in murine carotid atherosclerosis: Novel detection by dual-target fluorescence molecular imaging. Arterioscler Thromb Vasc Biol. 2010;30:1933–1939. doi: 10.1161/ATVBAHA.110.206342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle pet-ct imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen IY, Wu JC. Cardiovascular molecular imaging: Focus on clinical translation. Circulation. 2011;123:425–443. doi: 10.1161/CIRCULATIONAHA.109.916338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osborn EA, Jaffer FA. The year in molecular imaging. JACC Cardiovasc Imaging. 2010;3:1181–1195. doi: 10.1016/j.jcmg.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdelbaky A, Tawakol A. Noninvasive positron emission tomography imaging of coronary arterial inflammation. Curr Cardiovasc Imaging Rep. 2011;4:41–49. doi: 10.1007/s12410-010-9062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, Ntziachristos V, Libby P, Weissleder R. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Razansky RN, Rosenthal A, Mallas G, Razansky D, Jaffer FA, Ntziachristos V. Near-infrared fluorescence catheter system for two-dimensional intravascular imaging in vivo. Opt Express. 2010;18:11372–11381. doi: 10.1364/OE.18.011372. [DOI] [PubMed] [Google Scholar]
- 57.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik D, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies activated endothelium in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 58.Muller AM, Cronen C, Kupferwasser LI, Oelert H, Muller KM, Kirkpatrick CJ. Expression of endothelial cell adhesion molecules on heart valves: Up-regulation in degeneration as well as acute endocarditis. J Pathol. 2000;191:54–60. doi: 10.1002/(SICI)1096-9896(200005)191:1<54::AID-PATH568>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 59.Ghaisas NK, Foley JB, O'Briain DS, Crean P, Kelleher D, Walsh M. Adhesion molecules in nonrheumatic aortic valve disease: Endothelial expression, serum levels and effects of valve replacement. J Am Coll Cardiol. 2000;36:2257–2262. doi: 10.1016/s0735-1097(00)00998-0. [DOI] [PubMed] [Google Scholar]
- 60.Deck JD, Thubrikar MJ, Schneider PJ, Nolan SP. Structure, stress, and tissue repair in aortic valve leaflets. Cardiovasc Res. 1988;22:7–16. doi: 10.1093/cvr/22.1.7. [DOI] [PubMed] [Google Scholar]
- 61.Davies MJ, Treasure T, Parker DJ. Demographic characteristics of patients undergoing aortic valve replacement for stenosis: Relation to valve morphology. Heart. 1996;75:174–178. doi: 10.1136/hrt.75.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 63.Rabkin E, Hoerstrup SP, Aikawa M, Mayer JE, Jr, Schoen FJ. Evolution of cell phenotype and extracellular matrix in tissue-engineered heart valves during in-vitro maturation and in-vivo remodeling. J Heart Valve Dis. 2002;11:308–314. [PubMed] [Google Scholar]
- 64.Rabkin-Aikawa E, Aikawa M, Farber M, Kratz JR, Garcia-Cardena G, Kouchoukos NT, Mitchell MB, Jonas RA, Schoen FJ. Clinical pulmonary autograft valves: Pathologic evidence of adaptive remodeling in the aortic site. J Thorac Cardiovasc Surg. 2004;128:552–561. doi: 10.1016/j.jtcvs.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 65.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13:841–847. [PubMed] [Google Scholar]
- 66.Deguchi JO, Aikawa E, Libby P, Vachon JR, Inada M, Krane SM, Whittaker P, Aikawa M. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation. 2005;112:2708–2715. doi: 10.1161/CIRCULATIONAHA.105.562041. [DOI] [PubMed] [Google Scholar]
- 67.Helske S, Syvaranta S, Lindstedt KA, Lappalainen J, Oorni K, Mayranpaa MI, Lommi J, Turto H, Werkkala K, Kupari M, Kovanen PT. Increased expression of elastolytic cathepsins s, k, and v and their inhibitor cystatin c in stenotic aortic valves. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000228824.01604.63. [DOI] [PubMed] [Google Scholar]
- 68.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. An osteopontin-nadph oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–1489. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 69.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–1516. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 70.Belaaouaj AA, Li A, Wun TC, Welgus HG, Shapiro SD. Matrix metalloproteinases cleave tissue factor pathway inhibitor. Effects on coagulation. J Biol Chem. 2000;275:27123–27128. doi: 10.1074/jbc.M004218200. [DOI] [PubMed] [Google Scholar]
- 71.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 72.Cosmi JE, Kort S, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med. 2002;162:2345–2347. doi: 10.1001/archinte.162.20.2345. [DOI] [PubMed] [Google Scholar]
- 73.O'Brien KD. Pathogenesis of calcific aortic valve disease: A disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–1728. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 74.Towler DA. Imaging aortic matrix metabolism: Mirabile visu! Circulation. 2007;115:297–299. doi: 10.1161/CIRCULATIONAHA.106.675397. [DOI] [PubMed] [Google Scholar]
- 75.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. Tgf-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parhami F, Basseri B, Hwang J, Tintut Y, Demer LL. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91:570–576. doi: 10.1161/01.res.0000036607.05037.da. [DOI] [PubMed] [Google Scholar]
- 77.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the camp pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 78.Radcliff K, Tang TB, Lim J, Zhang Z, Abedin M, Demer LL, Tintut Y. Insulin-like growth factor-i regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ Res. 2005;96:398–400. doi: 10.1161/01.RES.0000157671.47477.71. [DOI] [PubMed] [Google Scholar]
- 79.Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-alpha and oncostatin m derived from macrophages. Circ Res. 2002;91:9–16. doi: 10.1161/01.res.0000026421.61398.f2. [DOI] [PubMed] [Google Scholar]
- 80.Demer LL, Tintut Y. Return to ectopia: Stem cells in the artery wall. Arterioscler Thromb Vasc Biol. 2005;25:1307–1308. doi: 10.1161/01.ATV.0000172633.74942.e6. [DOI] [PubMed] [Google Scholar]
- 81.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ Res. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Abbadi M, Giachelli CM. Mechanisms of vascular calcification. Adv Chronic Kidney Dis. 2007;14:54–66. doi: 10.1053/j.ackd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 84.Shanahan CM. Inflammation ushers in calcification: A cycle of damage and protection? Circulation. 2007;116:2782–2785. doi: 10.1161/CIRCULATIONAHA.107.749655. [DOI] [PubMed] [Google Scholar]
- 85.Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of apoe-deficient mice: Potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 86.Corsten MF, Bennaghmouch A. Optical characterization of arterial apoptosis. Methods Mol Biol. 2011;680:117–129. doi: 10.1007/978-1-60761-901-7_8. [DOI] [PubMed] [Google Scholar]
- 87.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, McCarthy GM, Landis RC, Haskard DO. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase c and map kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–1256. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 88.Bostrom K. Proinflammatory vascular calcification. Circ Res. 2005;96:1219–1220. doi: 10.1161/01.RES.0000172407.20974.e5. [DOI] [PubMed] [Google Scholar]
- 89.Vengrenyuk Y, Kaplan TJ, Cardoso L, Randolph GJ, Weinbaum S. Computational stress analysis of atherosclerotic plaques in apoe knockout mice. Ann Biomed Eng. 2010;38:738–747. doi: 10.1007/s10439-009-9897-5. [DOI] [PubMed] [Google Scholar]
- 90.Rudd JH, Fayad ZA. Imaging atherosclerotic plaque inflammation. Nat Clin Pract Cardiovasc Med. 2008;5 2:S11–17. doi: 10.1038/ncpcardio1160. [DOI] [PubMed] [Google Scholar]
- 91.Amann K, Tyralla K, Gross ML, Eifert T, Adamczak M, Ritz E. Special characteristics of atherosclerosis in chronic renal failure. Clin Nephrol. 2003;60 1:S13–21. [PubMed] [Google Scholar]
- 92.Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D, Leip EP, O'Donnell CJ, D'Agostino RB, Sr, Benjamin EJ. Cross-sectional association of kidney function with valvular and annular calcification: The framingham heart study. J Am Soc Nephrol. 2006;17:521–527. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- 93.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 94.Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176–181. doi: 10.1007/s00774-005-0668-6. [DOI] [PubMed] [Google Scholar]
- 95.Goodman WG. Vascular calcification in chronic renal failure. Lancet. 2001;358:1115–1116. doi: 10.1016/S0140-6736(01)06299-7. [DOI] [PubMed] [Google Scholar]
- 96.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 97.Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: A potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- 98.Aikawa M, Libby P. Atherosclerotic plaque inflammation: The final frontier? Can J Cardiol. 2004;20:631–634. [PubMed] [Google Scholar]
- 99.Aikawa M, Libby P. The vulnerable atherosclerotic plaque: Pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004;13:125–138. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- 100.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: Implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 101.Massy ZA, Ivanovski O, Nguyen-Khoa T, Angulo J, Szumilak D, Mothu N, Phan O, Daudon M, Lacour B, Drueke TB, Muntzel MS. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein e knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 102.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin s reduces atherosclerosis in ldl receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 104.Mecham RP. Methods in elastic tissue biology: Elastin isolation and purification. Methods. 2008;45:32–41. doi: 10.1016/j.ymeth.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brassart B, Fuchs P, Huet E, Alix AJ, Wallach J, Tamburro AM, Delacoux F, Haye B, Emonard H, Hornebeck W, Debelle L. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J Biol Chem. 2001;276:5222–5227. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 106.Simionescu A, Simionescu DT, Vyavahare NR. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-beta1: Role of myofibroblasts in vascular calcification. Am J Pathol. 2007;171:116–123. doi: 10.2353/ajpath.2007.060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 108.Jacob MP, Fulop T, Jr, Foris G, Robert L. Effect of elastin peptides on ion fluxes in mononuclear cells, fibroblasts, and smooth muscle cells. Proc Natl Acad Sci U S A. 1987;84:995–999. doi: 10.1073/pnas.84.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J. The 67-kd elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest. 1993;91:1198–1205. doi: 10.1172/JCI116280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duca L, Floquet N, Alix AJ, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 111.Galande AK, Hilderbrand SA, Weissleder R, Tung CH. Enzyme-targeted fluorescent imaging probes on a multiple antigenic peptide core. J Med Chem. 2006;49:4715–4720. doi: 10.1021/jm051001a. [DOI] [PubMed] [Google Scholar]
- 112.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in esrd. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 113.Ouchi Y, Akishita M, de Souza AC, Nakamura T, Orimo H. Age-related loss of bone mass and aortic/aortic valve calcification--reevaluation of recommended dietary allowance of calcium in the elderly. Ann N Y Acad Sci. 1993;676:297–307. doi: 10.1111/j.1749-6632.1993.tb38743.x. [DOI] [PubMed] [Google Scholar]
- 114.Banks LM, Lees B, MacSweeney JE, Stevenson JC. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: Links between osteoporosis and cardiovascular disease? Eur J Clin Invest. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 115.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric bmd and vascular calcification in middle-aged women: The study of women's health across the nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 116.Farhat GN, Strotmeyer ES, Newman AB, Sutton-Tyrrell K, Bauer DC, Harris T, Johnson KC, Taaffe DR, Cauley JA. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: The health, aging, and body composition study. Calcif Tissue Int. 2006;79:102–111. doi: 10.1007/s00223-006-0052-0. [DOI] [PubMed] [Google Scholar]
- 117.Frost ML, Grella R, Millasseau SC, Jiang BY, Hampson G, Fogelman I, Chowienczyk PJ. Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif Tissue Int. 2008;83:112–120. doi: 10.1007/s00223-008-9153-2. [DOI] [PubMed] [Google Scholar]
- 118.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 119.Hirasawa H, Tanaka S, Sakai A, Tsutsui M, Shimokawa H, Miyata H, Moriwaki S, Niida S, Ito M, Nakamura T. Apoe gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res. 2007;22:1020–1030. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 120.Boukhris R, Becker KL. Calcification of the aorta and osteoporosis. A roentgenographic study. JAMA. 1972;219:1307–1311. [PubMed] [Google Scholar]
- 121.Tekin GO, Kekilli E, Yagmur J, Uckan A, Yagmur C, Aksoy Y, Turhan H, Yetkin E. Evaluation of cardiovascular risk factors and bone mineral density in post menopausal women undergoing coronary angiography. Int J Cardiol. 2008;131:66–69. doi: 10.1016/j.ijcard.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Jono S, Nishizawa Y, Shioi A, Morii H. 1,25-dihydroxyvitamin d3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302–1306. doi: 10.1161/01.cir.98.13.1302. [DOI] [PubMed] [Google Scholar]
- 123.Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006;70:1577–1583. doi: 10.1038/sj.ki.5001841. [DOI] [PubMed] [Google Scholar]
- 124.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin d. Arterioscler Thromb Vasc Biol. 2001;21:1610–1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 125.Elmariah S, Delaney JA, O'Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, Kronmal RA, Halperin JL. Bisphosphonate use and prevalence of valvular and vascular calcification in women mesa (the multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2010;56:1752–1759. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Callahan RJ, Bogdanov A, Jr, Fischman AJ, Brady TJ, Weissleder R. Preclinical evaluation and phase i clinical trial of a 99mtc-labeled synthetic polymer used in blood pool imaging. AJR Am J Roentgenol. 1998;171:137–143. doi: 10.2214/ajr.171.1.9648777. [DOI] [PubMed] [Google Scholar]
- 128.Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, Krupsky S, Orlock DA, Puliafito CA. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101:529–533. doi: 10.1016/s0161-6420(94)31303-0. [DOI] [PubMed] [Google Scholar]


