Abstract
Young shoots of Rubus species have been used for healing of wounds, infected insect bites and pimples in folk medicine for ages. In order to evaluate the wound healing activity of Rubus sanctus, four different extracts were prepared from the whole aerial parts of the plant by using n-hexane, chloroform, ethyl acetate and methanol, respectively. Incision wound healing model by using tensiometer on rats and excision model on mice were employed to assess the activity. Remarkable wound healing activity was observed with the ointment formulation of the methanol extract at 1% concentration on the mentioned models. The results of histopathological examination also supported the outcome of both incision and excision wound models. The wound healing effect was comparatively evaluated with a reference ointment Madecassol. The experimental data confirmed the ethnobotanical usage of R. sanctus.
1. Introduction
Rubus species (Rubus sp.) (Rosaceae) have been traditionally used for therapeutic purposes. For instance, extracts of leaves and roots of this genus have been used for the treatment of diabetes mellitus, rheumatism, sore throat, hemorrhoid, diarrhea and similar enteric disorders [1–5]. Particularly, decoction prepared from the roots of Rubus sanctus Schreber is used as herbal tea to alleviate pain and to heal rheumatism [6]. Moreover, dried and crushed young shoots of Rubus sp. have been applied onto wounds, infected insect bites and pimples [7].
Several biological activity tests have been carried out so far under laboratory conditions on Rubus species, which focus on antimicrobial [8, 9], radical scavenging [10], anticonvulsant muscle relaxant [11] and antinflammatory and antinociceptive activities [12, 13]. According to phytochemical analysis the plant extract characterized by their capability of synthesizing and accumulating ellagitannins containing a sanguisorboyl group [14]. They have also been found to metabolise several phenolic carboxylic acids, such as ellagic acid, and phenyl propanoids, particularly caffeic acid [15]. In a previous study, the aerial parts of R. sanctus, R. hirtus Walds. et Kit and their hybrid were evaluated for their anti-inflammatory activity using carrageenan-induced hind paw edema on mice and polar fractions, n-butanol and remaining aqueous fractions obtained by solvent extraction, were shown to possess significant activity [12]. In a different study on Rubus sp., ethanolic extracts of R. sanctus (root and aerial part) and R. hirtus (aerial parts) showed potent antinociceptive activity, while that of aqueous extracts had weak [13]. However, it should be noted that in the same study both plants' extracts had tendency to induce gastric damage. Ongoing studies revealed the novel anti-inflammatory triterpenoids, tormentic acid and euscaphic acid, which were isolated from the fresh leaves of R. sieboldii Blume [16].
Furthermore, phytochemical studies exposed chemical content of the aerial parts of some Rubus sp., which contain flavonoids (quercetin, kaempferol, caffeic acid and chlorogenic acid), phenolic acids, tannins, amino acids, sugars, pectins, carboxylic acids, anthocyanins, catechins, vitamin C and saturated or unsaturated fatty acids [17–20].
A survey of the published literatures revealed that wound healing property of R. sanctus has not been subjected to in vivo investigation by using incision and excision models.
The goals of the pharmacology of wound healing are to evaluate the influence of various measures in wound management programs on healing and to screen drugs that encourage healing. Several candidates have so far been used and were declared to affect healing in various ways. Nevertheless, thorough research in wound healing has not yielded, economic and efficient pro-healing agent that could preclude the long hospitalization of patients following surgery and wound imposition. The present investigations were planned to study the wound healing activity of R. sanctus Shreber. We undertook the present activity screening study in order to evaluate traditional use of this plant in terms of scientific point. The n-hexane, chloroform, ethyl acetate and methanol extracts prepared from the aerial parts of R. sanctus were tested in rats and mice for wound healing activity via incision by using tensiometer and excision wound models.
2. Methods
2.1. Plant Material
Rubus sanctus Schreber aerial parts were collected from Kıbrısköy village, Ankara, Turkey during June to July, 2007. The plant was authenticated by Serdar Arslan from Gazi University, Department of Biology, Faculty of Science and Art, Ankara, and a voucher specimen (GUE 2604) was deposited in the Herbarium of Faculty of Pharmacy, Gazi University, Ankara, Turkey.
2.2. Preparation of Plant Extracts
The plant material was shade dried and powdered. Each 50 g of powdered aerial parts was submitted to successive solvent extractions separately with n-hexane, chloroform, ethyl acetate and methanol at room temperature for 24 h (3 × 500 ml each solvent). After filtration, the extracts were evaporated by using a rotary evaporator (Buchi, Switzerland) at 40°C to dryness in vacuo. Yields of each extracts were 8.3% for n-hexane, 17.6% for chloroform, 9.2% for ethyl acetate and 47.5% for methanol.
2.3. Wound Healing Activity Tests
2.3.1. Animals
Male, Sprague-Dawley rats (160–180 g) and Swiss albino mice (20–25 g) were purchased from the animal breeding laboratories of Refik Saydam Central Institute of Health (Ankara, Turkey).
The animals were left for 3 days at room conditions for acclimatization. They were maintained on standard pellet diet and water ad libitum throughout the experiment. A minimum of six animals were used in each group, otherwise described in procedure. The study was permitted by the Institutional Animal Ethics Committee (Gazi University Ethical Council Project Number: G.U.ET-08.037) and was performed according to the international rules considering the animal experiments and biodiversity right.
2.3.2. Preparation of Test Samples for Bioassay
Incision and excision wound models were used to evaluate the wound healing activity. For the in vivo wound models, test samples were prepared in an ointment base (vehicle) consisting of glycol stearat, 1,2 propylene glycol, liquid paraffin (3 : 6 : 1) in 1% concentration. Each test ointment (0.5 g) was applied topically on the wounded site immediately after wound was created by a surgical blade.
The vehicle group of animals was treated with the ointment base only, whereas the reference drug group of animals were treated with 0.5 g of Madecassol (Bayer, 00001199). Madecassol contains 1% extract of Centalla asiatica.
2.3.3. Linear Incision Wound Model
All the animals were anaesthetized with 0.15 cm3 Ketalar and the back hair of the rats were shaved by using a shaving machine. Five-centimeter long, two linear-paravertebral incisions were made with a sterile surgical blade through the full thickness of the skin at the distance of 1.5 cm from the midline of each side of the vertebral column [21]. The wounds were closed with three surgical interrupted sutures of 1 cm apart. All the sutures used in the experiments were non-absorbable braided non-capillary and siliconized. The animals were randomly distributed into four major groups; the extracts, the reference drug the vehicle and the negative control. Six rats (160–180 g) were kept in each group. The negative control group of animals was not treated with any material, whereas the extracts, the reference drug (Madecassol) and the vehicle were applied topically once in a day throughout 9 days. All the sutures were removed on the 9th post wound day. On Day 10 all the animals were killed under anesthesia. One linear-paravertebral incised skin was measured using tensiometer (Zwick/Roell Z0.5, Germany) for its tensile strength in Newtons, the other incised skin was sent for histopathological examination [22, 23].
2.3.4. Excision Wound Model
This model was employed to have information about wound contraction and wound closure time on extract applied mice compared to controls. Initially, all the animals were anaesthetized by 0.01 cm3 Ketalar. The back hairs of the animals were depilated by shaving. A circular wound was created on the dorsal interscapular region of each animal by excising the skin with a 5 mm biopsy punch; wounds were left open [24]. The extracts, the reference drug (Madecassol Bayer) and the vehicle were applied topically once a day on to each group (six mice) of animals, which were randomly distributed, till the wound was completely healed. The progressive changes in wound area were monitored by a camera (Fuji, S20 Pro, Japan) every other day. Later on, wound area was evaluated by using AutoCAD program. Wound contraction was calculated as percentage of the reduction in wounded area. A specimen sample of tissue was isolated from the healed skin of each group of mice for the histopathological examination [25].
2.4. Histopathology
The cross-sectional full-thickness skin specimens from each group were collected at the end of the experiment to evaluate for the histopathological alterations. Samples were fixed in 10% buffered formalin, processed and blocked with paraffin and then sectioned into 5 μm and stained with hematoxylin & eosin (HE), Van Gieson's (VG) and toluidine blue (TB) stains [26]. Sections were analyzed and scored as mild (+), moderate (++) and severe (+++) for epidermal or dermal re-modeling. Re-epithelization or ulcus in epidermis; fibroblast proliferation, mononuclear and/or polymorphonuclear cells, neovascularization and collagen depositions in dermis were analyzed to scor the epidermal or dermal re-modeling. Van Gieson's stained sections were checked for collagen deposition and toluidine blue stained sections checked for metachromatic staining of mast cells. At the end of the examination, all the wound healing processes were combined and staged for wound healing phases as inflammation, proliferation and re-modeling in all groups.
2.5. Statistical Analysis of the Data
The data on percentage wound healing was statistically analyzed using one-way analysis of variance (ANOVA). The values of P ≤ .001 were considered statistically significant. Histopathologic data were considered to be nonparametric; therefore, no statistical tests were performed.
3. Results
In this study, an investigation on the in vivo wound healing activity of a medicinal plant, R. sanctus was carried out to verify the claimed traditional uses of the plant. To assess the wound healing activity of the aerial parts, extracts were prepared with different solvents; n-hexane, chloroform, ethyl acetate and methanol, respectively, from the aerial parts of R. sanctus. Incision by using tensiometer and excision wound models were employed for this activity assessment
3.1. Excision Wound Model
The measurements of the progression of wound healing induced by the extracts, reference drug, negative and vehicle groups are shown in Figure 1. In this excision wound model, the methanolic extract treated groups of animals showed 56.5% contraction on the wounds at Day 6. The same extract demonstrated 80.6% contraction on the day 12, which was close to contraction value of the reference drug Madecassol (100%). However, the other extracts presented no significant results.
Figure 1.
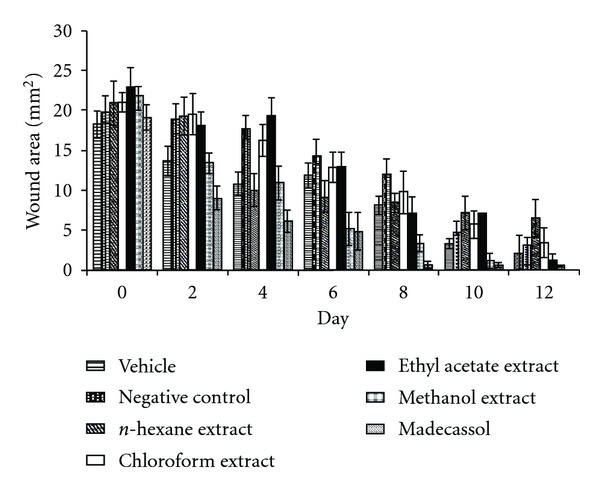
Effects of the extracts from R. sanctus on excision wound model.
3.2. Incision Wound Model
The results of the measurements of tensile strength (in Newtons) are shown in Figure 2. Tensile strength of the animals treated with the methanolic extract demonstrated the highest value (38.9%) at day 10. Topical application of the methanolic extract on the incision wound model demonstrated a remarkable improvement in wound tensile strength compared to other groups.
Figure 2.
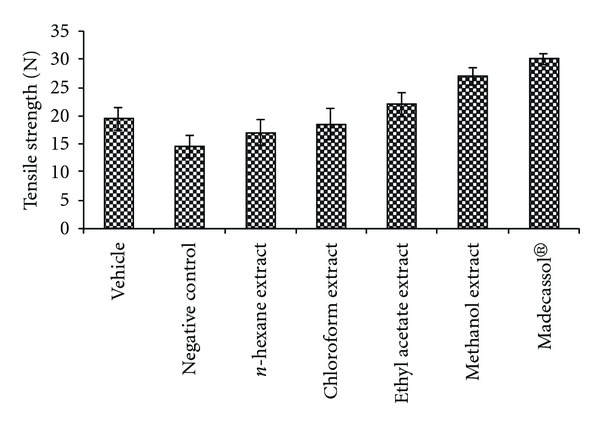
Effects of the extracts from R. sanctus on linear incision wound model. N: Newton.
3.3. Histopathological Examination
Following histopathological examination, evaluated, scored and staged results were combined, summarized and presented in Table 1. for demonstrating of wound healing process, representative figures (Figure 3), which stained with HE, VG and TB, were also added.
Table 1.
Wound healing processes and healing phases of the vehicle, negative control, R. sanctus extracts and Madecassol administered animalsa.
| Groups | Wound healing processes | Healing phases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | U | RE | FP | CD | MNC | PMN | NV | MC | I | P | R | |
| Vehicle | +/+++ | ++ | −/+ | ++ | ++ | + | + | +/++ | + | +++ | ++ | − |
| Negative control | −/++ | + | −/++ | +/++ | +/++ | −/+ | + | +/++ | + | +++ | ++ | −/+ |
| Hexane extract | + | − | ++ | +/++ | ++ | −/+ | − | + | + | + | +/++ | ++ |
| Chloroform extract | + | − | ++ | +/++ | ++ | + | − | + | + | + | +/++ | ++ |
| Ethyl acetate extract | + | − | ++ | ++ | ++ | + | − | + | +++ | + | +/++ | ++ |
| Methanol extract | + | − | +++ | + | ++ | −/+ | − | −/+ | + | −/+ | −/+ | +++ |
| Madecassol | +/++ | − | ++ | ++ | ++ | − | − | ++ | +/++ | +/++ | ++ | + |
S: Scab; U: Ulcus; RE: re-epithelization; FP: fibroblast proliferation; CD: collagen depositions; MNC: mononuclear cells; PMN: polymorphonuclear cells; NV: neovascularization; MC: Mast cells; I: inflammation phase; P: proliferation phase; R: re-modeling phase.
aHE, VG and TB stained sections were scored as mild (+), moderate (++) and severe (+++) for epidermal and/or dermal re-modeling.
Figure 3.
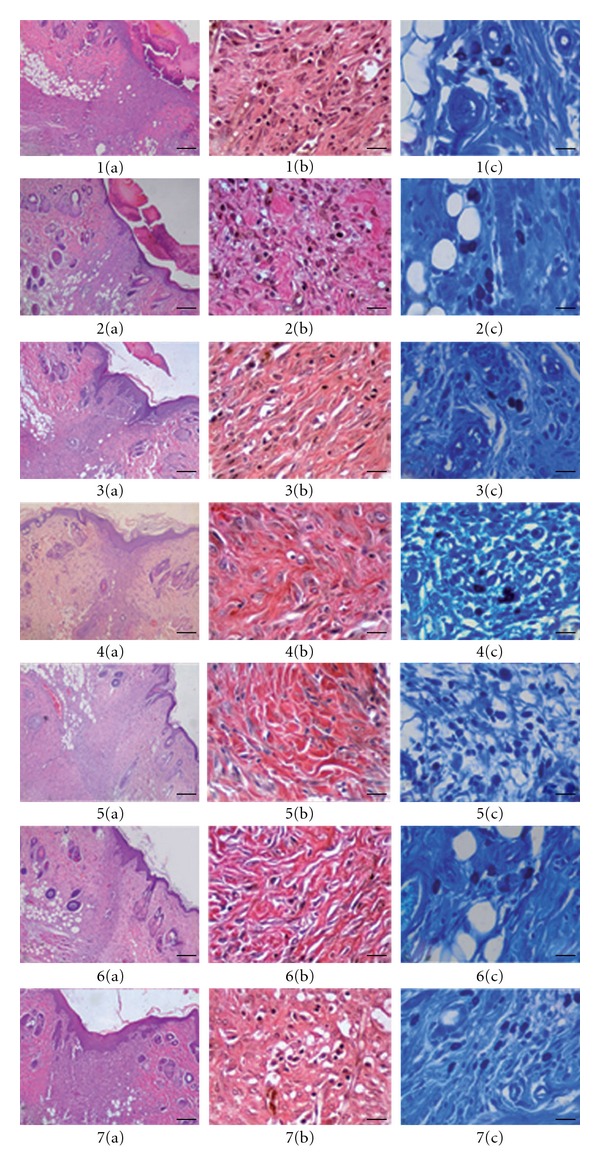
Histopathological view of wound healing and epidermal/dermal re-modeling in the vehicle, negative control, R. sanctus extracts and Madecassol administered animals. Skin sections show the hematoxylin & eosin (HE) stained epidermis and dermis in (a) and the dermis stained with Van Gieson's (VG) and toluidine blue (TB) in (b) and (c) respectively. The original magnification was 40× and the scale bars represent 25 μm for figures in (a), and the original magnification was 400× and the scale bars represent 100 μm for both (b) and (c). Data are representative of six animal per group. (i) Vehicle group, 10 days old wound tissue treated with only vehicle, (ii) negative c ontrol group (untreated), 10 days old wound tissue (iii) n-hexane extract group, 10 days old wound tissue treated with n-hexane extract, (iv) chloroform extract group, 10 days old wound tissue treated with chloroform extract, (v) ethyl acetate extract group, 10 days old wound tissue treated with ethyl acetate extract, (vi) methanol extract group, 10 days old wound tissue treated with methanol extract and (vi) Madecassol group, 10 day old wound tissue treated with Madecassol.
Phases in wound healing processes (inflammation, proliferation and remodeling) were observed and recorded successfully within the experimental groups (Table 1). The vehicle and the negative control groups demonstrated delayed wound healing processes compared to the other groups. In comparison with the vehicle and negative control groups, faster re-modeling were noticed in extracts treated groups. The best re-modeling, particularly, re-epithelization were detected with the methanol extract group. On the other hand, faster keratinization characterized with minor intraepithelial cornification was seen in n-hexane, chloroform and ethyl acetate extract groups. Weak foreign body reaction, superfluous process in wound healing, characterized with a few foreign body giant cells, which generally localized in peripheral sides of some hair follicles were detected in all groups except for the reference drug Madecassol group.
4. Discussion
Wound healing process begins with the restoration of a damaged tissue as closely as possible to its natural state and wound contraction is the course of shrinkage in wounded area. The healing primarily depends on the repairing ability of the tissue in addition to type and degree of damage and general health status of the tissue. The granulation tissue of the wound is primarily composed of edema, fibroblast, collagen and new blood vessels. The mesenchymal cells of the wound area adjust themselves into fibroblast then begin migrating into the wound gap together with the fibrin strands. The collagen is the main constituent of extra cellular tissue, which is responsible for support and strength. Free hydroxyproline and its peptides are released with collapse of collagen. Thus, measurement of the hydroxyproline could be used as an indicator for collagen turnover. Furthermore, increase in dry tissue also indicates the presence of elevated protein content. The phytochemical analysis of aerial parts of R. sanctus revealed the presence of flavonoids and phenolic acid derivatives [14–16, 19]. Phenolic compounds, which include tannins and flavonoids, serve as floral pigments, structural components, feeding attractants and deterrents, of plants [27, 28]. Flavonoids have therapeutic uses due to their anti-inflammatory, anti-fungal, antioxidant and wound healing properties [29–32]. Moreover, flavonoids and their derivatives are known to decrease lipid peroxidation by improving vascularity and by preventing or slowing down the progress of cell necrosis. Hence, any drug that inhibits lipid peroxidation is supposed to increase the viability of collagen fibrils by increasing the circulation and the strength of collagen fibres, by encouraging the DNA synthesis and preventing the cell damage [33, 34]. Flavonoids [35] are also known to endorse the wound-healing process primarily due to their antimicrobial and astringent properties, which appears to be responsible for wound contraction and elevated rate of epithelization. Corresponding types of wound-healing effect were reported on medicinal plants [36, 37]. Therefore, wound-healing potential of R. sanctus may be attributed to the phytoconstituents present in the aerial parts, which may be either due to their individual or additive effect that speeds up the process most probably the proliferation phase of wound healing (Figure 4).
Figure 4.
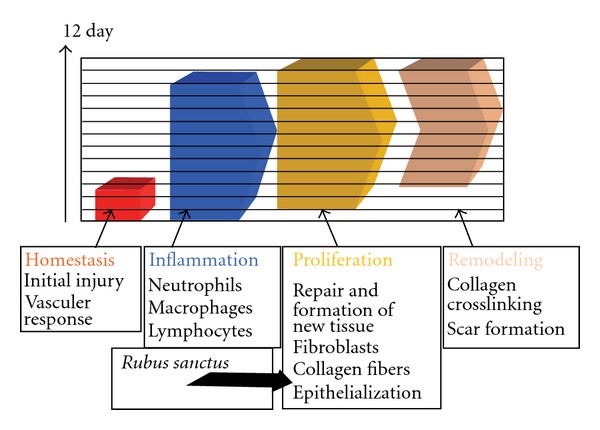
Hypothetical diagram of the wound healing mechanism of methanolic extract of R. sanctus
5. Conclusion
In conclusion, the present study demonstrated that the aerial parts of R. sanctus promote wound healing activity in animal as a preclinical study. The methanolic extract showed remarkable wound healing activity and it may be suggested for treating various types' wounds in animal and human beings. Further studies with purified constituents compared to the crude extracts might be needed to comprehend the complete mechanism of wound healing activity of R. sanctus.
Funding
Scientific Research Project Foundation of Gazi University, Ankara, Turkey (Project code no: 02/2007-04).
References
- 1.Jouad H, Maghrani M, Eddouks M. Hypoglycaemic effect of Rubus fructicosis L. and Globularia alypum L. in normal and streptozotocin-induced diabetic rats. Journal of Ethnopharmacology. 2002;81(3):351–356. doi: 10.1016/s0378-8741(02)00118-6. [DOI] [PubMed] [Google Scholar]
- 2.Marquina MA, Corao GM, Araujo L, Buitrago D, Sosa M. Hyaluronidase inhibitory activity from the polyphenols in the fruit of blackberry (Rubus fruticosus B.) Fitoterapia. 2002;73(7-8):727–729. doi: 10.1016/s0367-326x(02)00222-8. [DOI] [PubMed] [Google Scholar]
- 3.Panizzi L, Caponi C, Catalano S, Cioni PL, Morelli I. In vitro antimicrobial activity of extracts and isolated constituents of Rubus ulmifolius . Journal of Ethnopharmacology. 2002;79(2):165–168. doi: 10.1016/s0378-8741(01)00363-4. [DOI] [PubMed] [Google Scholar]
- 4.Patel AV, Rojas-Vera J, Dacke CG. Therapeutic constituents and actions of Rubus species. Current Medicinal Chemistry. 2004;11(11):1501–1512. doi: 10.2174/0929867043365143. [DOI] [PubMed] [Google Scholar]
- 5.Guarrera PM. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium) Fitoterapia. 2005;76(1):1–25. doi: 10.1016/j.fitote.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Honda G, Yeşilada E, Tabata M, et al. Traditional medicine in Turkey VI. Folk medicine in West Anatolia: Afyon, Kutahya, Denizli, Mugla, Aydin provinces. Journal of Ethnopharmacology. 1996;53(2):75–87. doi: 10.1016/S0378-8741(96)01426-2. [DOI] [PubMed] [Google Scholar]
- 7.Uncini Manganelli RE, Tomei PE. Ethnopharmacobotanical studies of the Tuscan Archipelago . Journal of Ethnopharmacology. 1999;65(3):181–202. doi: 10.1016/s0378-8741(98)00177-9. [DOI] [PubMed] [Google Scholar]
- 8.Richards RME, Durham DG, Liu X. Antibacterial activity of compounds from Rubus pinfaensis . Planta Medica. 1994;60(5):471–473. doi: 10.1055/s-2006-959536. [DOI] [PubMed] [Google Scholar]
- 9.Rauha J-P, Remes S, Heinonen M, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology. 2000;56(1):3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 10.Costantino L, Albasini A, Rastelli G, Benvenuti S. Activity of polyphenolic crude extracts as scavengers of superoxide radicals and inhibitors of xanthine oxidase. Planta Medica. 1992;58(4):342–344. doi: 10.1055/s-2006-961481. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira E, Vassilieff VS. Hypnotic, anticonvulsant and muscle relaxant effects of Rubus brasiliensis. Involvement of GABA(A)-system. Journal of Ethnopharmacology. 2000;70(3):275–280. doi: 10.1016/s0378-8741(99)00205-6. [DOI] [PubMed] [Google Scholar]
- 12.Akcos Y, Yeşilada E, Ezer N. Anti-inflammatory activity of some Turkish Rubus species. Hacettepe University Journal of the Faculty of Pharmacy. 1998;18:33–8. [Google Scholar]
- 13.Erdemoglu N, Küpeli E, Yeşilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. Journal of Ethnopharmacology. 2003;89(1):123–129. doi: 10.1016/s0378-8741(03)00282-4. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Morita A, Nonaka G-I, Lin T-C, Nishioka I, Ho F-C. Tannins and related compounds. CIII. Isolation and characterization of new monomeric, dimeric and trimeric ellagitannins, calamansanin and calamanins A, B and C, from Terminalia calamansanai (Blanco) Rolfe. Chemical and Pharmaceutical Bulletin. 1991;39(1):60–63. [Google Scholar]
- 15.Häkkinen S, Neinonen M, Kärenlampi S, Mykkänen H, Ruuskanen J, Törrönen R. Screening of selected flavonoids and phenol acids in 19 berries. Food Research International. 1999;32:345–353. [Google Scholar]
- 16.Murakami C, Ishijima K, Hirota M, Sakaguchi K, Yoshida H, Mizushina Y. Novel anti-inflammatory compounds from Rubus sieboldii, triterpenoids, are inhibitors of mammalian DNA polymerases. Biochimica et Biophysica Acta. 2002;1596(2):193–200. doi: 10.1016/s0167-4838(02)00227-3. [DOI] [PubMed] [Google Scholar]
- 17.Tzouwara-Karayanni SM, Philianos SM. Chemical constituents of Rubus ulmifolius Schott. Quarterly Journal of Crude Drug Research. 1981;19:127–130. [Google Scholar]
- 18.Rommel A, Wrolstad RE. Ellagic acid content of red raspberry juice as influenced by cultivar, processing, and environmental factors. Journal of Agricultural and Food Chemistry. 1993;41(11):1951–1960. [Google Scholar]
- 19.Gudej J, Tomczyk M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Archives of Pharmacal Research. 2004;27(11):1114–1119. doi: 10.1007/BF02975114. [DOI] [PubMed] [Google Scholar]
- 20.Tomczyk M, Gudej J. Polyphenolic compounds from Rubus saxatilis . Chemistry of Natural Compounds. 2005;41(3):349–351. [Google Scholar]
- 21.Ehrlich HP, Hunt TK. The effects of cortisone and anabolic steroids on the tensile strength of healing wounds. Annals of Surgery. 1969;170(2):203–206. doi: 10.1097/00000658-196908000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodhi S, Pawar RS, Jain AP, Singhai AK. Wound healing potential of Tephrosia purpurea (Linn.) Pers. in rats. Journal of Ethnopharmacology. 2006;108(2):204–210. doi: 10.1016/j.jep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. Phytotherapy Research. 2002;16(3):227–231. doi: 10.1002/ptr.827. [DOI] [PubMed] [Google Scholar]
- 24.Tramontina VA, Machado MA, Nogueira Filho GR, Kim SH, Vizzioli MR, Toledo S. Effect of bismuth subgallate (local hemostatic agent) on wound healing in rats. Histological and histometric findings. Brazilian Dental Journal. 2002;13(1):11–16. [PubMed] [Google Scholar]
- 25.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid-ul N. Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. Journal of Ethnopharmacology. 2006;107(2):161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Jagetia GC, Rajanikant GK. Curcumin treatment enhances the repair and regeneration of wounds in mice exposed to hemibody γ-irradiation. Plastic and Reconstructive Surgery. 2005;115(2):515–528. doi: 10.1097/01.prs.0000148372.75342.d9. [DOI] [PubMed] [Google Scholar]
- 27.Harborne JB. Introduction to Ecological Biochemistry. New York, NY, USA: Academic Press; 1982. [Google Scholar]
- 28.Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London, UK: Chapman & Hall; 1998. [Google Scholar]
- 29.Nayak BS, Sandiford S, Maxwell A. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evidence-Based Complementary and Alternative Medicine. 2009;6(3):351–356. doi: 10.1093/ecam/nem127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuanazzi JAS, Montanha JA. Farmacognosia: da Planta ao Medicamento. Porto Alegre/Florianópolis, Brazil: Editora UFRGS/UFSC; 2004. Flavonóides. (in Portuguese) [Google Scholar]
- 31.Santos SC, Mello JCP. Farmacognosia: da Planta ao Medicamento. Porto Alegre/Florianópolis, Brazil: Editora UFRGS/UFSC; 2004. Taninos. (in Portuguese) [Google Scholar]
- 32.Okuda T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry. 2005;66(17):2012–2031. doi: 10.1016/j.phytochem.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Getie M, Gebre-Mariam T, Rietz R, Neubert RHH. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae) Pharmazie. 2002;57(5):320–322. [PubMed] [Google Scholar]
- 34.Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evidence-Based Complementary and Alternative Medicine. 2008;5(1):95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuchiya H, Sato M, Miyazaki T, et al. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus . Journal of Ethnopharmacology. 1996;50(1):27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 36.Manjunatha BK, Vidya SM, Rashmi KV, Mankani KL, Shilpa HJ, Jagadeesh Singh SD. Evaluation of wound-healing potency of Vernonia arborea Hk. Indian Journal of Pharmacology. 2005;37(4):223–226. [Google Scholar]
- 37.Nayak BS, Vinutha B, Geetha B, Sudha B. Experimental evaluation of Pentas lanceolata flowers for wound healing activity in rats. Fitoterapia. 2005;76(7-8):671–675. doi: 10.1016/j.fitote.2005.08.007. [DOI] [PubMed] [Google Scholar]


