Abstract
Angiotensin II is implicated in cardiovascular diseases, which is associated with a role in increasing vascular inflammation. The present study investigated how angiotensin II modulates vascular inflammatory signaling and expression of inducible nitric oxide synthase (iNOS) and vascular cell adhesion molecule (VCAM)-1. In cultured rat aortic vascular smooth muscle cells (VSMCs), angiotensin II suppressed interleukin-1β-induced prolonged phosphorylation of extracellular signal-regulated kinase (ERK) and ribosomal S6 kinase (RSK)-1, and nuclear translocation of nuclear factor (NF)-κB, leading to decreased iNOS but enhanced VCAM-1 expression, associated with an up-regulation of mitogen-activated protein kinase phosphatase-1 expression. Knock-down of RSK1 selectively down regulated interleukin-1β-induced iNOS expression without influencing VCAM-1 expression. In-vivo experiments showed that interleukin-1β, iNOS, and VCAM-1 expression were detectable in the aortic arches of both wild-type and apolipoprotein E-deficient (ApoE−/− ) mice. VCAM-1 and iNOS expression were higher in ApoE−/− than in wild type mouse aortic arches. Angiotensin II infusion (3.2 mg/kg/day, for 6 days, via subcutaneous osmotic pump) in ApoE−/− mice enhanced endothelial and adventitial VCAM-1 and iNOS expression, but reduced medial smooth muscle iNOS expression associated with reduced phosphorylation of ERK and RSK-1. These results indicate that angiotensin II can differentially modulate inflammatory gene expression in aortic smooth muscle cells through influencing ERK-NF-κB crosstalk, which may contribute to angiotensin II-induced inflammatory disorders related to cardiovascular diseases.
Keywords: adhesion molecules, angiotensin, nitric oxide, inflammation, vascular smooth muscle
Introduction
Angiotensin II (Ang II), a vasoconstrictor that maintains vascular homeostasis, has a role in promoting cardiovascular diseases such as fibrosis and atherogenesis in certain circumstances such as hypertension, hyperlipidemia, and diabetes.[1; 2; 3] The pathogenic effect of Ang II has been linked to its role in regulating inflammatory gene expression.[4; 5] However, how Ang II modulates vascular inflammatory response remains vague.
Activation of nuclear factor (NF)-κB is essential to initiate and process inflammation. NF-κB activation is tightly regulated by cellular inhibitory proteins such as IκBα and IκBβ. IκBα and IκBβ display different degradation kinetics and mediate transient and persistent NF-κB activation, respectively in response to stimulation.[6; 7; 8; 9] Evidence suggests that the temporal control of NF-κB activation may differentially regulate the transcription of distinct sets of inflammatory genes.[9; 10; 11; 12] However, the mechanisms that regulate the balance of transient and persistent NF-κB activation remain less understood, and their pathophysiological relevance to cardiovascular pathophysiology has not been established.
We have previously found that in cultured rat vascular smooth muscle cells (VSMCs), Ang II alone does not induce NF-κB activation and NF-κB-dependent gene expression, but it differentially regulates IL-1β-induced expression of inflammatory genes such as vascular cell adhesion molecule (VCAM)-1 and inducible nitric oxide synthase (iNOS) through temporal modulation of extracellular signal-regulated kinase (ERK) signaling [12] that subsequently influences the persistent but not transient NF-κB activation.[11; 12; 13] Here, we demonstrate that Ang II attenuates IL-1β-induced persistent activation of RSK1, an ERK downstream kinase that functionally leads to attenuated degradation of IκB and differential regulation of iNOS and VCAM-1 expression in cultured rat VSMCs and in apolipoprotein E-deficient (ApoE−/− ) mouse aorta. We hypothesize that the modulation of cytokine-induced inflammatory gene expression pattern by Ang II may contribute to its role in accelerating cardiovascular pathogenesis such as fibrosis and atherosclerosis.
Material and Methods
Materials
DMEM/F12 medium and FBS were purchased from Invitrogen. Recombinant murine IL-1β was from PeproTech. Ang II was from Sigma. PD98059, U0126, SB203580, and SP600125 were from Calbiochem.
Cell Culture
VSMCs and fibroblasts were isolated from rat thoracic aorta and cultured as described previously. [11; 14] Cells were used between passages 4 and 7. Cells at confluence were washed and then maintained in DMEM/F12 with 0.1% FBS for 24 hours, and then incubated with or without IL-1β, Ang II, inhibitors, or vehicle for designated times as indicated in the results. For knock-down of RSK1, cells were transfected with siRNA-Rsk1 or control siRNA (obtained from Qiagen) for 72 hours using the method as described previously.[15]
Immunoprecipitation and Western Blot Analysis
Immunoprecipitation and Western blot analysis were performed using the methods as described previously.[15]
Animals
All experiments with animals were carried out with the approval of the Institutional Animal Care and Use Committee of Boston University Medical Center. ApoE−/− mice (female, 10 weeks of age, obtained from Jackson Laboratory) were infused for 6 days with either saline or Ang II (3.2 mg/kg/day) via subcutaneous osmotic pumps (Alzet, model 1007D). C57BL/6 mice (female, 10 weeks of age, obtained from Jackson Laboratory) were used as wild-type normal controls. Systolic blood pressure (SBP) was measured by the tail cuff method [16] before and on day 5 of treatments. On day 6 of treatments, the mice were sacrificed under anesthetization with isoflurane, and the aortas were either perfusion-fixed with 10% buffered formalin acetate, processed, and embedded in paraffin for histology and immunohistochemistry or processed for RNA extraction.
Reverse Transcription (RT)-PCR
Mouse aortic medial layers were dissected from aortic arches by gentle removal of endothelium with a cotton swab and adventitia with tweezers, which was performed in ice-cold PBS under a dissecting microscope. Total RNA was extracted by using Trizol reagent. After cDNA synthesis by reverse transcription, PCR was performed using the following schedule: denaturation, annealing, and extension at 94, 57, and 72ºC for 40, 30, and 60 seconds, respectively, for 35 cycles for IL-1β, iNOS, and VCAM-1, and 26 cycles for GAPDH, respectively. PCR primers were: IL-1β forward 5′-CTTCAAATCTCACAGCAGCACATC-3′ and reverse 5′-CCAGCAGGTTATCATCATCATCC-3′, iNOS forward 5′-GCCCAACAATACAAGATGACC-3′ and reverse 5′-GTTCCGAGCGTCAAAGACC-3′, VCAM-1 forward 5′-TCCAGACATTTACCCAGTTTACAG-3′ and reverse 5′-TCATTCCTTACCACCCCATTG-3′, and GAPDH forward 5′-GCCATCAACGACCCCTTCAT-3′ and reverse 5′-CGCCTGCTTCACCACCTTCT-3′. PCR products were electrophoresed on 1.2% agarose gels containing ethidium bromide and visualized by UV-induced fluorescence. RT-PCR for cultured rat VSMCs was performed as described previously.[12] The primers for rat MKP-1 were forward 5′-AGCACCCCTCTCTACGACCAG-3′ and reverse 5′-AAACACCCTTCCTCCAGCATC-3′.
Histology and Immunohistochemistry
Masson’s trichrome staining [17] was performed in 5-μm thick sections of descending aorta. Immunohistochemistry was performed in the sections of ascending aorta with the method described previously [17]. The primary antibodies used were anti-iNOS (polyclonal, 1:2,000, BioMol Research) or anti-VCAM-1 (polyclonal, 4 μg/mL, Santa Cruz Biotechnology) in PBS containing 1% BSA. Non-immune rabbit IgG was used for negative control. For immunofluorescent staining, following incubation overnight at 4ºC with anti-p-ERK (Thr202/Tyr204) or anti-p-RSK1 (Thr359/Ser363) (2 μg/mL, Cell Signaling Technology), the sections were washed, incubated for 1 hour with FITC-conjugated secondary antibodies (1:100 dilution, Jackson Lab.), washed with PBS, and finally mounted with aqueous mounting medium. Double staining of NF-κB p65 and p-RSK1 in rat VSMCs cultured on chamber slides was performed using the method as described previously.[15] The images observed under a fluorescence microscope were recorded on a linked computer using Openlab software (version 2.2.5, Improvision).
Statistical Analysis
Data are expressed as means±SD. Student’s t test and 1-way ANOVA were performed for comparison between 2 groups and among multiple groups, respectively, and P<0.05 was considered significant.
Results
Ang II Attenuates IL-1β-Induced Persistent Activation of NF-κB through down regulating prolonged RSK1 activation
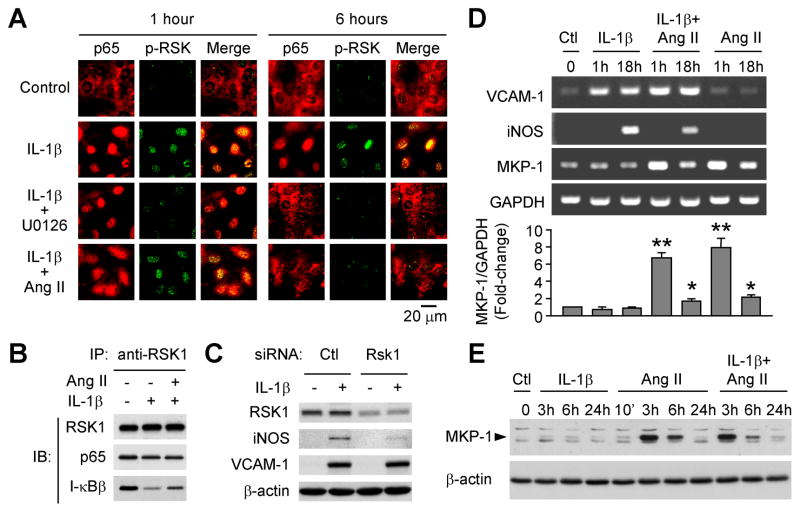
We have previously found that in VSMCs, RSK1 physically interacts with the IκBβ/NF-κB complex.[15] Treatment with IL-1β reduces IκBβ in the complex, which is prevented by inhibition of ERK activation.[15] To determine whether Ang II may affect IL-1β-induced NF-κB activation through influencing RSK1 phosphorylation, we treated rat VSMCs with IL-1β in the absence or presence of Ang II, with mitogen-activated protein kinase kinase (MEK) inhibitor U0126 treatment serving as a control for ERK pathway inhibition. As shown by immunofluorescent double staining (Figure 1A), in control cells, weak signals for phospho-RSK1 were observed in the nuclei and NF-κB p65 was predominantly stained in the cytoplasm. Upon IL-1β stimulation, nuclear accumulation of phospho-RSK1 was seen at both 1 hour and 6 hours, accompanied by nuclear translocation of p65. Although IL-1β plus Ang II induced nuclear accumulation of both phospho-RSK1 and p65 in the cells treated for 1 hour, Ang II attenuated RSK1 phosphorylation and p65 nuclear translocation after the cells treated for 6 hours. U0126 inhibited IL-1β-induced RSK1 phosphorylation during the 6 hours treatment. U0126 did not influence IL-1β-induced p65 translocation at 1 hour after treatment but reduced the translocation at the 6 hour time point. These results indicate that associated with Ang II-induced down-regulation of prolonged activation of RSK1, Ang II selectively down-regulates IL-1β-induced persistent activation of NF-κB. Furthermore, Ang II attenuated IL-1β-induced decrease in IκBβ in the IκBβ/NF-κB complex (Figure 1B), suggesting that Ang II attenuates IL-1β-induced persistent activation of NF-κB through suppressing ERK/RSK1-mediated IκBβ degradation.
Figure 1.
Ang II attenuates IL-1β-induced prolonged activation of RSK1 and NF-κB associated with up-regulation of MKP-1 expression. Rat VSMCs were treated with IL-1β (3 ng/mL), or Ang II (10−7 mol/L), or both for the designated times. A, Immunofluorescent double staining shows that Ang II inhibits IL-1β-induced p-RSK1 and NF-κB nuclear translocation at 6 hours. U0126 (20 mmol/L) was added 1 hour before IL-1β. B, Ang II attenuates IL-1β-induced dissociation of IκBβ from RSK1/IκBβ/p65 complex. Rat VSMCs were treated for 6 hours with IL-1β or IL-1β plus Ang II. C, Knock-down of RSK1 reduces iNOS, but not VCAM-1, expression induced by IL-1β (24 hours). D, Ang II differentially regulates IL-1β-induced VCAM-1 and iNOS transcription associated with increased MKP-1 mRNA levels. *P<0.05, **P<0.01, vs. control (Ctl, set as 1-fold), n=3. E, Western blot analysis of MKP-1 protein levels. β-actin serves as an equal loading reference.
We have previously reported that Ang II inhibits IL-1β-induced iNOS expression but enhances IL-1β-induced VCAM-1 expression.[12] To determine whether RSK1 could be functionally involved in the differential regulation of IL-1β-induced gene expression, rat VSMCs were transfected with either control siRNA or RSK1 siRNA and then treated with or without IL-1β. Western blot shows that knockdown of RSK1 reduced IL-1β-induced iNOS expression without obvious effect on VCAM-1 expression (Figure 1C), indicating that in the ERK signaling cascade, RSK1 is a key component contributing to the differential regulation of the NF-κB responsive genes.
Ang II Induces MKP-1 Expression
We further examined whether IL-1β or Ang II, or both may change the expression of MKP-1 that is known to participate in regulating the duration and magnitude of ERK activation.[18] As shown in Figure 1D and 1E, treatment of VSMCs with IL-1β alone did not affect MKP-1 expression. However, both mRNA and protein levels of MKP-1 were increased after the cells were treated with either Ang II alone or Ang II plus IL-1β, with a dramatic increase detected at 1 hour for mRNA and 3 hours for protein, respectively. The Ang II-induced MKP-1 increase sustained for several hours, and was not significantly influenced by the presence of IL-1β. This suggests that Ang II may down regulate IL-1β-induced prolonged ERK activation through inducing MKP-1 expression.
Ang II Differentially Modulates IL-1β-induced VCAM-1 and iNOS Expression in Aortic Smooth Muscle Cells but not in Adventitial Fibroblasts
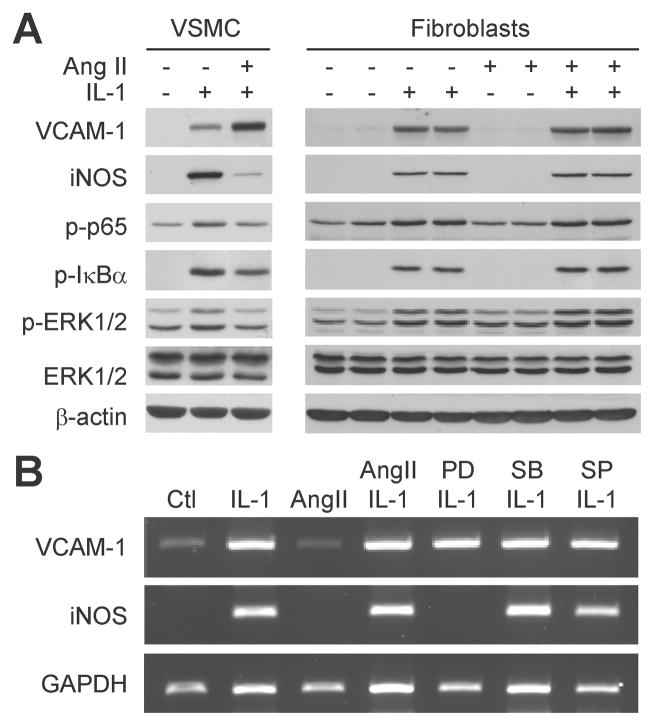
We further tested in cultured VSMCs and adventitial fibroblasts whether the effect of Ang II on IL-1β-induced ERK activation and gene expression could be cell type-dependent. In VSMCs, Ang II up-regulated VCAM-1 expression, but down-regulated iNOS expression, associated with down-regulation of IL-1β-induced phosphorylation of ERK, NF-κB p65, and IκBα (Figure 2A, left panels). However, in adventitial fibroblasts, Ang II had no effect on IL-1β-induced VCAM-1 and iNOS expression, as well as ERK, p65, and IκBα phosphorylation (Figure 2A, right panels). In addition, RT-PCR showed that IL-1β-induced VCAM-1 and iNOS mRNA increases in the fibroblasts were not influenced by Ang II (Figure 2B). However, IL-1β-induced iNOS, but not VCAM-1, transcription was inhibited by the presence of PD98059 (MEK1 inhibitor) in adventitial fibroblasts, whereas SB203580 (p38MAPK inhibitor) or SP600125 (JNK inhibitor) showed no effect. These results demonstrate that Ang II regulates iNOS and VCAM-1 expression in response to IL-1β in a cell type-specific manner that depends on its modulation of ERK and NF-κB signaling cascades.
Figure 2.
Ang II differentially modulates IL-1β-induced VCAM-1 and iNOS expression in aortic VSMCs but not in adventitial fibroblasts. A, Western blot shows that Ang II (10−7 mol/L) specifically targets VSMCs, but not fibroblasts, for down regulating the phosphorylation of ERK, IκBα, p65, and differential regulating the expression of VCAM-1 and iNOS induced by IL-1β (3 ng/mL, 24 hours). Right panels show samples from duplicate culture plates for each treatment. B, RT-PCR shows that Ang II has no effect on IL-1β-induced VCAM-1 and iNOS mRNA transcription in fibroblasts at 16 hours after the treatment, whereas inhibitor (added 1 hour before IL-1β and Ang II) targeting inhibition of ERK activation (PD98059, 20 μmol/L), but not p38MAPK (SB203580, 10 μmol/L) or JNK (SP600125, 1 μmol/L), inhibits iNOS but not VCAM-1 expression.
Ang II Infusion Differentially Regulates VCAM-1 and iNOS Expression in ApoE−/− Mouse Aorta
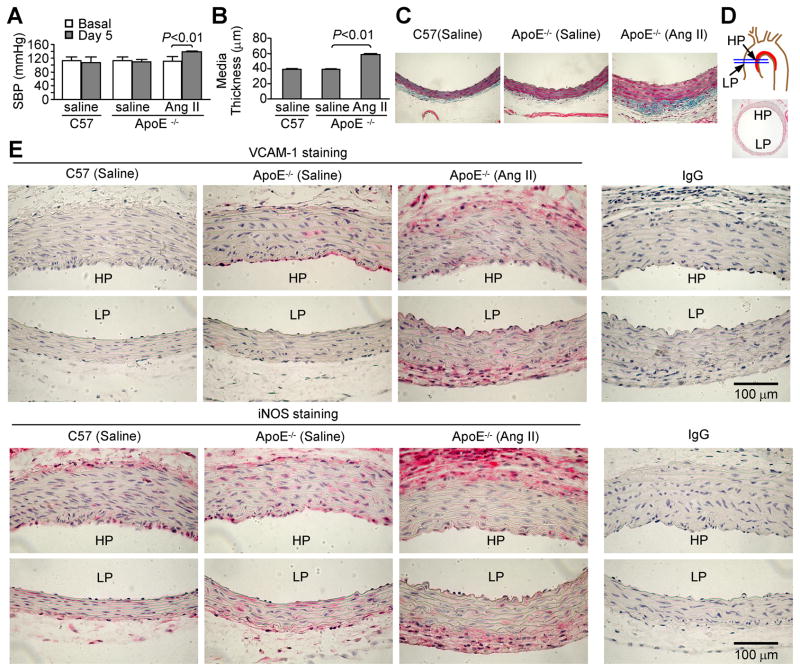
We examined the in vivo role of Ang II in modulating the expression of two well-known NF-κB-inducible inflammatory gene products, VCAM-1 and iNOS, in ApoE−/− mice. Ang II infusion enhanced SBP (Figure 3A), and caused increased aortic media thickness (Figure 3B and 3C), smooth muscle hypertrophy, and increased adventitial extracellular matrix deposition (Figure 3C, stained in blue). As shown in Figure 3E immunohistochemical staining on the sections of mouse ascending aorta (HP and LP indicate the “high-prone” and “low-prone” atherogenic regions, respectively, as shown in Figure 3D), VCAM-1 was not evident in C57BL/6 mouse aorta, but was clearly detectable in ApoE−/− mouse aorta, particularly in the endothelium of the HP region. VCAM-1 was increased in Ang II-infused ApoE−/− mouse aorta, not only in the endothelium but also throughout the smooth muscle and adventitial layers. Positive iNOS immunoreactivity was scattered in the ascending aortas from either ApoE−/− or C57BL/6 mice infused with saline, but in the media it was more obvious in ApoE−/− than in C57BL/6 mice. Interestingly, in the aorta of ApoE−/− mice infused with Ang II, the expression of iNOS was increased in the adventitia but attenuated in the medial smooth muscle.
Figure 3.
Ang II infusion change systolic blood pressure (SBP), aortic morphology and inflammatory gene expression in ApoE−/− mice. A and B, Ang II enhances SBP and media thickness of the descending aorta. C57BL/6J mice infused with saline were used as wild-type normal controls. n=5 for each group. C, Representative of trichrome staining on the descending aorta shows increased media thickness and adventitial extracellular matrix deposition (in blue) caused by Ang II-infusion. D, The section cutting sites (blue lines), and the high-prone (HP) and low-prone (LP) atherogenic regions in an aortic arch are shown. E, Immunohistochemical staining of VCAM-1 and iNOS on mouse ascending aorta. IgG was used as non-immune isotype controls. Each image is a representative of 5 for each treatment. Note that Ang II differentially regulates VCAM-1 and iNOS expression in mouse aortic medial layers.
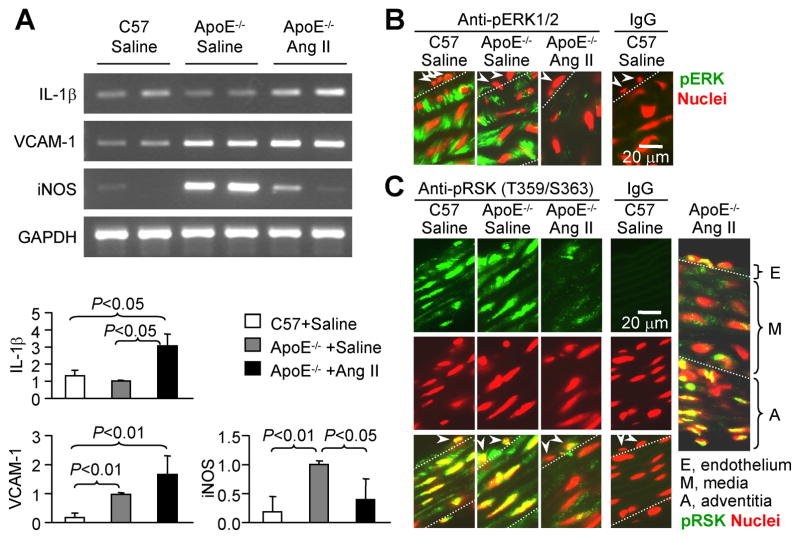
To confirm the unique feature of Ang II in regulating the medial inflammatory response, the medial layers were carefully isolated from pooled aortic arches and RNA was extracted and used for RT-PCR. As shown in Figure 4A, IL-1β mRNA was detected in the aortic arch smooth muscle layers, with similar levels in saline-infused C57BL/6 mice and ApoE−/− mice, but a significantly increased level in Ang II-infused ApoE−/− mice. Both VCAM-1 and iNOS mRNA levels were significantly higher in saline-infused ApoE−/− than in wild type mice. Ang II infusion further enhanced VCAM-1, but lowered iNOS mRNA levels. These results are consistent with those observed by immunohistochemistry, and provide clear in vivo evidence that Ang II differentially modulates the expression of VCAM-1 and iNOS in aortic arch smooth muscle cells.
Figure 4.
Ang II differentially regulates VCAM-1 and iNOS expression associated with down-regulates constitutive activation of ERK and RSK1 in ApoE−/− mouse aortic media. A, RT-PCR detection of mRNA levels. Total RNA were extracted from the aortic arch medial layers. Each lane represents a pooled RNA preparation from 2 aortic arches. Data shown in bar-graph are mRNA levels normalized by GAPDH and set the ratio of ApoE−/− +saline as 1 fold (n=3). B and C, Immunofluorescent staining of pERK1/2 and pRSK1, respectively, on mouse ascending aorta. Green (FITC): anti-pERK1/2 (B) or anti-pRSK1 (C); Red (propidium iodide): nuclear counter staining. Arrowheads indicate endothelial cells. Each image is a representative of 3 for each treatment. IgG, non-immune isotype control shows negative staining.
Ang II Infusion Down-Regulates Constitutive Activation of ERK and RSK1 in Aortic Arch Smooth Muscle
To examine whether the in vivo role of Ang II in modulating inflammatory gene expression is associated with altered ERK signaling activity, we examined the phosphorylation of ERK and its downstream kinase RSK1 in mouse ascending aorta by immunofluorescent staining. Phospho-ERK was intensely detected in the aortic media from either wild-type or ApoE−/− mice infused with saline, but strikingly less stained in those from ApoE−/− mice infused with Ang II (Figure 4B). Furthermore, consistent with the changes observed in phospho-ERK, phospho-RSK1 (Thr359/Ser363) was clearly detected in the ascending aorta from either wild-type or ApoE−/− mice infused with saline, but obviously less stained in those from ApoE−/− mice infused with Ang II (Figure 4C). The phospho-ERK was predominantly located in the cytoplasm of medial smooth muscle cells, whereas phospho-RSK1 was predominantly in the nuclei. Ang II infusion selectively suppressed RSK1 phosphorylation in the media, but not in the endothelium and adventitia (Figure 4C, far right panel), which is consistent with the observation that Ang II specifically down-regulated iNOS expression in the aortic medial smooth muscle.
Discussion
Our study demonstrate that both VCAM-1 and iNOS expression are increased in the atherosclerosis-prone aortic arch in ApoE−/− mice in comparison to C57BL/6 mice (the wild type normal control), and that Ang II infusion of the ApoE−/− mice differentially modulates VCAM-1 and iNOS expression specifically in the aortic medial smooth muscle by down-regulating ERK signaling.
Inflammation is essential for tissue response to injury and initiating healing process. Following vascular injury, the inflammatory response could be either protective or pathogenic, depending on how stimuli program the NF-κB-initiated gene expression. We observed that IL-1β was similarly expressed in the aortic arches of both ApoE−/− and wild-type mice, but VCAM-1 and iNOS expression was increased in ApoE−/− mice compared with those in wild-type mice. This may suggest the involvement of other synergistic factors such as interferon-γ and TNFα that can enhance IL-1β-induced VCAM-1 and iNOS expression [11] and are increased in ApoE−/− mice.[19] The constitutive expression of IL-1β in the aortic arches of either wild-type or ApoE− /− mice is consistent with the inflammation described by others in this atherosclerosis-prone region of the aorta, even if in normal mice.[19; 20] This could be related to turbulent blood flow and low fluid shear stress that may constitutively cause endothelial dysfunction and vascular remodeling.[21]
The present study provides the first in vivo evidence that down-regulation of iNOS expression in the medial smooth muscle by Ang II is related to Ang II-induced desensitization of ERK signaling, as shown by decreased phosphorylation of both ERK and RSK1. Cell culture studies suggest that this desensitization of ERK signaling is likely resulted from Ang II-induced rapid up-regulation of MKP-1 expression. We previously reported that activation of p38MAPK is required for Ang II down regulating persistent activation of ERK and NF-κB.[12] It is possible that Ang II induces MKP-1 expression, a negative feedback regulatory mechanism,[18] through its early and transient activation of p38MAPK, or both p38MAPK and ERK.[12; 18] The fact that knock-down of RSK1 attenuates IL-1β-induced iNOS expression without affecting VCAM-1 expression in VSMCs suggests that RSK1 could selectively regulate the expression of certain genes such as iNOS that require persistent NF-κB activation. In adventitial fibroblasts, because IL-1β-induced iNOS expression remains clearly attenuated by MEK-1 inhibitor PD98059, the fact that Ang II shows no effect on the prolonged ERK activation in the fibroblasts can explain its lack of effect on IL-1β-induced iNOS expression in this cell type.
The cell type-specific regulation of smooth muscle inflammation may influence the important role that these cells play in inflammatory and proliferative vascular diseases. It is well documented that iNOS has a critical role in inhibiting smooth muscle cell proliferation in the response-to-injury program of the arterial tissues.[22; 23; 24] However, it is also well documented that iNOS-deficient ApoE− /− mice on Western diet have attenuated atherosclerotic lesions,[25; 26; 27] which suggests a pro-atherogenic role of iNOS expression. It is possible that the consequence of iNOS expression may differ with its expression in different cells (endothelial cells, smooth muscle cells, fibroblasts, and macrophages), and also probably in different microenvironment. Particularly in the endothelium and adventitia, increased superoxide generation induced by diet-induced dyslipidemia and/or Ang II can reduce NO availability by converting NO to cell toxic products such as peroxynitrite that promotes the oxidative modification of low density lipoproteins [28] and other proteins as evidenced, for example, by tyrosine nitration.[16; 29] In this regard, either decrease in NO availability or increase in peroxynitrite production could contribute to the acceleration of neointimal hyperplasia, vascular remodeling, and atherogenesis.
In conclusion, attenuation of ERK-RSK1 signaling activity in VSMCs by Ang II represents an important and novel mechanism that selectively regulates the expression of certain NF-κB-dependent genes and may change the inflammatory consequence in response to vascular injury. The differential regulation of NF-κB-dependent genes by modulation of MAPK signaling pathways implicates a new avenue targeting vascular inflammation and cardiovascular disease.
Highlights.
We examine how angiotensin II modulates ERK-NF-κB crosstalk and gene expression.
Angiotensin II suppresses IL-1β-induced prolonged ERK and NF-κB activation.
ERK-RSK1 signaling is required for IL-1β-induced prolonged NF-κB activation.
Angiotensin II modulates NF-κB responsive genes via regulating ERK-NF-κB crosstalk.
ERK-NF-κB crosstalk is a novel mechanism regulating inflammatory gene expression.
Acknowledgments
This work was supported in part by an American Heart Association award 09GRNT2110017 and National Institute of Health grants HL083358.
Abbreviations
- Ang II
angiotensin II
- ApoE
apolipoprotein E
- ERK
extracellular signal-regulated kinase
- IκB
inhibitor of NF-κB
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- MEK
mitogen-activated protein kinase kinase
- MKP
mitogen-activated protein kinase phosphatase
- NF-κB
nuclear factor κB
- NO
nitric oxide
- RSK1
ribosomal S6 kinase-1
- SBP
systolic blood pressure
- siRNA
small interfering RNA
- TNF
tumor necrosis factor
- VCAM
vascular cell adhesion molecule
- VSMC
vascular smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Refrences
- 1.Ohishi M, Ueda M, Rakugi H, Naruko T, Kojima A, Okamura A, Higaki J, Ogihara T. Enhanced expression of angiotensin-converting enzyme is associated with progression of coronary atherosclerosis in humans. J Hypertens. 1997;15:1295–302. doi: 10.1097/00004872-199715110-00014. [DOI] [PubMed] [Google Scholar]
- 2.Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–54. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 3.Candido R, Allen TJ, Lassila M, Cao Z, Thallas V, Cooper ME, Jandeleit-Dahm KA. Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation. 2004;109:1536–42. doi: 10.1161/01.CIR.0000124061.78478.94. [DOI] [PubMed] [Google Scholar]
- 4.Graninger M, Reiter R, Drucker C, Minar E, Jilma B. Angiotensin receptor blockade decreases markers of vascular inflammation. J Cardiovasc Pharmacol. 2004;44:335–9. doi: 10.1097/01.fjc.0000137160.76616.cc. [DOI] [PubMed] [Google Scholar]
- 5.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–74. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Le Bail O, Schmidt-Ullrich R, Israel A. Promoter analysis of the gene encoding the I kappa B-alpha/MAD3 inhibitor of NF-kappa B: positive regulation by members of the rel/NF-kappa B family. EMBO J. 1993;12:5043–9. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:57–82. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Ghosh S. Differential phosphorylation of the signal-responsive domain of I kappa B alpha and I kappa B beta by I kappa B kinases. J Biol Chem. 2003;278:31980–7. doi: 10.1074/jbc.M304278200. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–5. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J. 2003;22:5530–9. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J Biol Chem. 2004;279:1323–9. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- 12.Jiang B, Xu S, Hou X, Pimentel DR, Cohen RA. Angiotensin II differentially regulates interleukin-1-beta-inducible NO synthase (iNOS) and vascular cell adhesion molecule-1 (VCAM-1) expression: role of p38 MAPK. J Biol Chem. 2004;279:20363–8. doi: 10.1074/jbc.M314172200. [DOI] [PubMed] [Google Scholar]
- 13.Jiang B, Brecher P, Cohen RA. Persistent activation of nuclear factor-kappaB by interleukin-1beta and subsequent inducible NO synthase expression requires extracellular signal-regulated kinase. Arterioscler Thromb Vasc Biol. 2001;21:1915–20. doi: 10.1161/hq1201.099424. [DOI] [PubMed] [Google Scholar]
- 14.Gu M, Brecher P. Nitric oxide-induced increase in p21(Sdi1/Cip1/Waf1) expression during the cell cycle in aortic adventitial fibroblasts. Arterioscler Thromb Vasc Biol. 2000;20:27–34. doi: 10.1161/01.atv.20.1.27. [DOI] [PubMed] [Google Scholar]
- 15.Xu S, Bayat H, Hou X, Jiang B. Ribosomal S6 kinase-1 modulates interleukin-1beta-induced persistent activation of NF-kappaB through phosphorylation of IkappaBbeta. Am J Physiol Cell Physiol. 2006;291:C1336–45. doi: 10.1152/ajpcell.00552.2005. [DOI] [PubMed] [Google Scholar]
- 16.Matsui R, Xu S, Maitland KA, Mastroianni R, Leopold JA, Handy DE, Loscalzo J, Cohen RA. Glucose-6-phosphate dehydrogenase deficiency decreases vascular superoxide and atherosclerotic lesions in apolipoprotein E(-/-) mice. Arterioscler Thromb Vasc Biol. 2006;26:910–6. doi: 10.1161/01.ATV.0000205850.49390.3b. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes. 2006;55:110–9. [PubMed] [Google Scholar]
- 18.Duff JL, Monia BP, Berk BC. Mitogen-activated protein (MAP) kinase is regulated by the MAP kinase phosphatase (MKP-1) in vascular smooth muscle cells. Effect of actinomycin D and antisense oligonucleotides. J Biol Chem. 1995;270:7161–6. doi: 10.1074/jbc.270.13.7161. [DOI] [PubMed] [Google Scholar]
- 19.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–62. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–83. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci U S A. 2001;98:6476–81. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Z, Hansson GK. Overexpression of inducible nitric oxide synthase by neointimal smooth muscle cells. Circ Res. 1998;82:21–9. doi: 10.1161/01.res.82.1.21. [DOI] [PubMed] [Google Scholar]
- 23.Koglin J, Glysing-Jensen T, Mudgett JS, Russell ME. Exacerbated transplant arteriosclerosis in inducible nitric oxide-deficient mice. Circulation. 1998;97:2059–65. doi: 10.1161/01.cir.97.20.2059. [DOI] [PubMed] [Google Scholar]
- 24.Cooney R, Hynes SO, Sharif F, Howard L, O’Brien T. Effect of gene delivery of NOS isoforms on intimal hyperplasia and endothelial regeneration after balloon injury. Gene Ther. 2007;14:396–404. doi: 10.1038/sj.gt.3302882. [DOI] [PubMed] [Google Scholar]
- 25.Detmers PA, Hernandez M, Mudgett J, Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock J, Weikel R, Bergstrom JD, Shevell DE, Hermanowski-Vosatka A, Sparrow CP, Chao YS, Rader DJ, Wright SD, Pure E. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J Immunol. 2000;165:3430–5. doi: 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation. 2001;103:3099–104. doi: 10.1161/01.cir.103.25.3099. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life Sci. 2006;79:525–31. doi: 10.1016/j.lfs.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Graham A, Hogg N, Kalyanaraman B, O’Leary V, Darley-Usmar V, Moncada S. Peroxynitrite modification of low-density lipoprotein leads to recognition by the macrophage scavenger receptor. FEBS Lett. 1993;330:181–5. doi: 10.1016/0014-5793(93)80269-z. [DOI] [PubMed] [Google Scholar]
- 29.Ginnan R, Guikema BJ, Halligan KE, Singer HA, Jourd’heuil D. Regulation of smooth muscle by inducible nitric oxide synthase and NADPH oxidase in vascular proliferative diseases. Free Radic Biol Med. 2008;44:1232–45. doi: 10.1016/j.freeradbiomed.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]