Abstract
Misreporting characterized by the reporting of implausible energy intakes may undermine the valid estimation of diet-disease relations, but the methods to best identify and account for misreporting are unknown. The present study compared how alternate approaches affected associations between selected dietary factors and body mass index (BMI) by using data from the European Prospective Investigation Into Cancer and Nutrition-Spain. A total of 24,332 women and 15,061 men 29–65 years of age recruited from 1992 to 1996 for whom measured height and weight and validated diet history data were available were included. Misreporters were identified on the basis of disparities between reported energy intakes and estimated requirements calculated using the original Goldberg method and 2 alternatives: one that substituted basal metabolic rate equations that are more valid at higher BMIs and another that used doubly labeled water-predicted total energy expenditure equations. Compared with results obtained using the original method, underreporting was considerably lower and overreporting higher with alternative methods, which were highly concordant. Accounting for misreporters with all methods yielded diet-BMI relations that were more consistent with expectations; alternative methods often strengthened associations. For example, among women, multivariable-adjusted differences in BMI for the highest versus lowest vegetable intake tertile (β = 0.37 (standard error, 0.07)) were neutral after adjusting with the original method (β = 0.01 (standard error, 07)) and negative using the predicted total energy expenditure method with stringent cutoffs (β = −0.15 (standard error, 0.07)). Alternative methods may yield more valid associations between diet and obesity-related outcomes.
Keywords: body mass index, energy intake, fruit, obesity, vegetables
Underreporting of dietary intakes or low energy reporting is a major challenge to research on relations between diet and health. Although the reporting of implausibly low energy intakes is also related to factors such as age, sex, and psychosocial characteristics, numerous studies have found it to be particularly prevalent among obese subjects and to be characterized by a tendency to report relatively low intakes of foods high in fat and simple carbohydrates that may be perceived as socially undesirable (1–6). Thus, underreporting is especially problematic for studies in which investigators explore associations between diet and obesity or obesity-related disorders. Furthermore, underreporting has been observed to be widespread and to persist across methods of dietary assessment (1, 7–9). Although generally estimated to be less prevalent, overreporting is a problem as well, and it is also related to individual characteristics (10, 11).
Methods for identifying underreporters and overreporters have been proposed, although recent reviews on various dietary factors and obesity have suggested that relatively few studies account for the presence of subjects with implausible intakes beyond excluding subjects with extreme energy intakes (1, 12–14). Ideally, implausible reporters would be identified by comparing reported energy intakes (rEIs) with objective estimates of energy intake. However, such methods are often not feasible in large-scale studies, as they are relatively costly (1, 15). Other methods suggested for identifying implausible reporters (16–18) are more indirect and based on the extent of the disparity between rEIs and predicted energy requirements. Several studies have found that excluding underreporters through the use of such methods affects the magnitude and/or direction of diet-obesity relations, strengthening associations with factors such as fat, sugar, and fiber consumption (1, 2, 19, 20). Alternative indirect methods have been proposed, such as predicting energy needs on the basis of either estimated basal metabolic rates (BMRs) (18, 21) or doubly labeled water prediction equations, at times recommending more stringent cutoffs for classifying reported intakes as implausible (17, 22). No studies to date have directly compared these different methods.
Using diet history data from the Spanish cohort of the European Prospective Investigation Into Cancer and Nutrition (EPIC-Spain), we compared how these different methods affected the estimated prevalence of and characteristics associated with implausible reporting. We also examined how alternative methods of accounting for implausible reporting affected relations between body mass index (BMI, measured as weight in kilograms divided by height in meters squared) and intakes of energy, fat, and selected food groups hypothesized a priori to be susceptible to biased reporting.
MATERIALS AND METHODS
The EPIC-Spain cohort, part of the multicountry EPIC study, included men and women aged 29–69 years at enrollment in 1992–1996, recruited from the general population and among blood donors in 5 regions: Asturias, Granada, Murcia, Navarra, and Gipuzkoa. Details on the study design have been published previously (23, 24). Informed consent was obtained from all participants, and the ethics committee of the Spanish Carlos III Health Institute approved the study. The analysis sample excluded subjects ≥65 years of age (n = 453), those for whom height or weight data were missing (n = 113) or implausible (n = 7 subjects whose self-reported weight at a 3-year follow-up represented losses >90 kg), underweight subjects (BMI <18) (n = 18), and those who reported following weight-loss diets and who were perhaps not in energy balance (n = 1,456). Exclusions reduced the original sample of 25,808 women and 15,632 men to 24,332 women and 15,061 men.
Data collection and variable definition
Trained interviewers collected detailed data on habitual diet in the past year by using a validated, computerized diet history instrument with >600 items (23, 24). Briefly, a structured interview was used to ask subjects to report, for each meal or food intake occasion, frequency of consumption, usual portion size, and preparation methods of all foods consumed at least twice per month (lower intake frequencies were allowed for liver). For this analysis, in addition to energy and dietary fat, we selected 3 food groups hypothesized a priori to be susceptible to misreporting: vegetables, fruits, and pastries/cakes (hereafter referred to as pastries). Food groups were analyzed in grams per megajoule of energy; sex-specific tertiles were used because some relations were nonlinear. Energy and percentage of calories from fat were analyzed continuously after confirming the linearity of relations using quartiles.
Interviewers used standardized methods to measure height and weight; BMI was used as a measure of fatness, with standard cutoffs for overweight (≥25–30) and obesity (>30) (25). Data on sociodemographic characteristics, health history, and health behaviors were collected with interviewer-administered questionnaires. A validated index of physical activity was developed from questions about exercise, cycling, and occupational activity (26). The index was modified to classify subjects as “very active” if they reported strenuous manual labor or >7 hours of sports/exercise per week, with at least 3 hours reported to be vigorous. Food and Agriculture Organization physical activity level values were assigned to this index as follows: inactive = 1.35, moderately inactive = 1.55, moderately active = 1.75, active = 1.85, and very active = 2.2 (16, 27).
Classification of implausible reporters
Detailed descriptions of each method are provided in Web Appendix 1 (available at http://aje.oxfordjournals.org/) and summarized below.
Goldberg method.
BMRs were estimated using the recommended Schofield equations (27), and the ratios between BMRs and reported energy intakes (rEI:BMR) were calculated, providing an estimate of energy available for activity after meeting the needs for basic metabolism (i.e., weight maintenance). The plausibility of rEIs was determined by comparing this ratio with physical activity levels: Implausible reporters had rEI:BMR values that differed from physical activity levels by more than ±2 standard deviations when standard deviations were calculated as prescribed by Black (16), using estimates of variance in rEIs, BMR, and activity. More stringent cutoffs of ±1.5 standard deviations were also applied, because previous researchers using the predicted total energy expenditure (pTEE) method described below suggested that this cutoff yielded a sample in which associations between rEIs and estimated requirements were consistent with theoretical relations (22).
Revised Goldberg method.
Because the Schofield equations have been found to lead to overestimation of BMRs among obese and sedentary subjects (28, 29), we also classified implausible reporters by using alternative BMR equations, which have been shown to correspond well with measured values in both obese and nonobese subjects using indirect calorimetry (30). The calculations were otherwise identical to the Goldberg method.
pTEE method.
pTEE was estimated using the Dietary Reference Intakes prediction equations, derived by using large pooled data sets compiled from doubly labeled water studies, which were found to correlate well with measured TEE in independent samples (7, 31). Implausible reporters were identified on the basis of the ratio of reported intakes to estimated requirements (rEI:pTEE). As with the Goldberg method, standard deviations were calculated using published estimates of variation in energy balance components, as prescribed previously (7, 22). As the mean value for 1 standard-deviation was 15.1%, rEIs beyond approximately ±30.0% and ±23.0% of pTEEs were used to identify implausible reporters, corresponding to 2.0- and 1.5-standard-deviation cutoffs, respectively.
Web Appendix 2 shows the mean rEI:BMR and rEI:pTEE ratios for subjects classified as under-, over-, and plausible reporters who were identified using 2-standard-deviation cutoffs for each method. Means resembled expected values among plausible reporters: Values on the order of 1.55 were expected for rEI:BMR in a moderately inactive population (16), and on average rEIs should correspond to pTEEs among plausible reporters. Values for both ratios were substantially lower in underreporters and higher in overreporters.
Data analysis
Analyses were conducted separately for men and women. The concordance of under- and overreporting estimated using the different methods was assessed with kappa statistics. For each method, differences in subject characteristics and dietary intakes across reporting groups were evaluated using analysis of variance or chi-square tests. We used linear regression to estimate the effect of accounting for implausible reporting on multivariate associations between BMI and energy and fat (model 1) and between BMI and intakes of vegetables, fruits, and pastries (model 2). Results were adjusted for age, physical activity, education, center, height, smoking status, season, alcohol intakes, parity, diabetes, the other dietary variables shown in the model, and use of special diets related to hypertension, cholesterol, or diabetes. Excluding rather than adjusting for subjects on these diets did not meaningfully change the findings (data not shown). There were significant interactions (P < 0.05) between several food groups and smoking: Interaction terms were included in analyses of women, and models in men were stratified by smoking status, as there were multiple interactions with several food groups. To evaluate different strategies of accounting for implausible reporting, a baseline multivariate model was compared with results obtained after 1) restricting the sample to plausible reporters identified using each method, and 2) using dummy variables to adjust for under- and overreporting. Correlations between BMI and dummy variable indicators of underreporting (r = 0.13–0.25) and overreporting (r = −0.06–0.21) were low-to-moderate. Variance inflation factors indicated the absence of collinearity problems. In supplementary models, the effects of excluding only underreporters were examined, as were effects of excluding subjects with extreme energy intakes that fell outside the recommended cutoffs (<500 and <800 kcal or >3,500 and >4,000 kcal in women and men, respectively) (32) without taking energy requirements into account.
RESULTS
Characteristics of implausible reporters: 2.0-standard-deviation cutoffs
The estimated prevalences of underreporting in women and men determined using the Goldberg method (21.7% and 14.7%, respectively) were higher than estimates determined using the revised Goldberg method (14.4% and 9.1%, respectively) and the pTEE method (12.0% and 7.2%, respectively; chi-square P < 0.05 for all prevalence differences) (Table 1). Conversely, overreporting estimates determined by using the Goldberg method (4.8% and 5.9% in women and men, respectively) were lower than those seen when using the revised Goldberg method (9.2% and 10.5%, respectively) and the pTEE method (17.9% and 21.2%, respectively). Concordance of underreporting was highest for the revised Goldberg and pTEE methods (kappa = 0.83), intermediate for the Goldberg and revised Goldberg methods (kappa = 0.75), and lowest for the Goldberg and pTEE methods (kappa = 0.64). Similarly, for overreporting, the concordance of the revised Goldberg and pTEE (kappa = 0.62) and the Goldberg and revised Goldberg (kappa = 0.67) methods was higher than that for the Goldberg and pTEE (kappa = 0.38) methods.
Table 1.
Characteristics and Dietary Intakes of Underreporters, Plausible Reporters, and Overreporters Classified by Using Alternative Methods With 2.0-Standard-Deviation Cutoffsa in the Spanish Cohort of the European Investigation Into Cancer and Nutrition, 1992–1996
| Goldberg Method |
Revised Goldberg Method |
Predicted Total Energy Expenditure Method |
|||||||
| Underreporters | Plausible Reporters | Overreporters | Underreporters | Plausible Reporters | Overreporters | Underreporters | Plausible Reporters | Overreporters | |
| Women | |||||||||
| No. | 5,280 | 17,891 | 1,161 | 3,505 | 18,593 | 2,234 | 2,926 | 17,043 | 4,363 |
| % of sample | 21.7 | 73.5 | 4.8 | 14.4 | 76.4 | 9.2 | 12.0 | 70.0 | 17.9 |
| Age, years*b | 49.4 (8.2) | 48.0 (8.2) | 46.1 (8.1) | 48.8 (8.4) | 48.3 (8.2) | 47.2 (8.0) | 48.9 (8.4) | 38.4 (8.3) | 47.3 (8.0) |
| Body mass index, kg/m2* | 30.0 (5.1) | 27.6 (4.4) | 26.0 (4.2) | 30.5 (5.3) | 27.8 (4.5) | 26.2 (4.0) | 30.8 (5.3) | 28.0 (4.5) | 26.4 (4.0) |
| Reported dietary intake | |||||||||
| Reported energy intake, MJ* | .58 (0.98) | 8.84 (1.72) | 14.28 (2.28) | 5.28 (0.96) | 8.42 (1.68) | 13.06 (2.25) | 5.03 (0.84) | 8.01 (1.38) | 12.2 (1.98) |
| % of energy as fat* | 35.2 (6.7) | 36.9 (5.9) | 38.5 (6.7) | 35.0 (6.8) | 36.7 (6.0) | 38.3 (6.3) | 34.8 (6.9) | 36.5 (6.0) | 38.1 (6.0) |
| Vegetable intake, g/MJ* | 40.7 (26.5) | 28.5 (16.5) | 20.4 (10.8) | 42.7 (28.4) | 29.6 (17.4) | 21.5 (11.5) | 43.6 (29.4) | 30.6 (18.0) | 22.9 (12.1) |
| Fruit intake, g/MJ* | 2.4 (36.2) | 37.7 (26.7) | 24.4 (19.9) | 53.9 (37.4) | 39.3 (27.8) | 26.9 (21.0) | 55.3 (38.6) | 40.6 (28.4) | 28.9 (21.3) |
| Pastry and cake intake, g/MJ* | 2.92 (4.15) | 4.72 (4.99) | 6.63 (5.77) | 2.73 (4.00) | 4.49 (4.89) | 6.50 (5.72) | 2.57 (3.94) | 4.29 (4.78) | 8.20 (5.55) |
| Other characteristics | |||||||||
| University education, %* | 7.2 | 10.1 | 13.0 | 7.3 | 9.9 | 11.4 | 6.9 | 9.8 | 10.9 |
| Smoker, %* | 16.2 | 19.1 | 25.8 | 17.1 | 18.7 | 21.9 | 16.8 | 18.7 | 20.6 |
| Men | |||||||||
| No. | 2,206 | 11,964 | 891 | 1,373 | 12,113 | 1,575 | 1,088 | 10.788 | 3.185 |
| % of sample | 14.7 | 79.4 | 5.9 | 9.1 | 80.4 | 10.5 | 7.2 | 71.6 | 21.2 |
| Age, years* | 0.1 (6.7) | 50.7 (7.1) | 52.2 (7.9) | 50.1 (7.2) | 50.6 (7.1) | 51.2 (7.1) | 50.3 (7.4) | 50.7 (7.1) | 50.8 (6.9) |
| Body mass index, kg/m2* | 29.7 (3.6) | 28.2 (3.2) | 27.6 (3.4) | 29.8 (3.6) | 28.3 (3.3) | 27.7 (3.4) | 30.1 (3.6) | 28.5 (3.3) | 27.6 (3.3) |
| Reported dietary intake | |||||||||
| Relative energy intake, MJ* | 8.01 (1.57) | 11.90 (2.47) | 17.53 (3.54) | 7.51 (1.50) | 11.48 (2.36) | 16.66 (3.17) | 6.90 (1.14) | 10.90 (1.90) | 15.86 (2.66) |
| % of energy as fat* | 34.7 (6.4) | 35.1 (6.0) | 35.3 (6.4) | 34.7 (6.6) | 35.0 (6.0) | 35.4 (6.3) | 34.7 (6.9) | 35.0 (6.0) | 35.0 (6.1) |
| Vegetable intake, g/MJ* | 33.2 (22.4) | 23.5 (14.1) | 18.1 (10.8) | 35.3 (24.2) | 24.2 (14.7) | 18.5 (10.7) | 37.4 (26.0) | 25.0 (15.0) | 18.9 (10.8) |
| Fruit intake, g/MJ* | 38.6 (29.4) | 27.5 (21.4) | 19.4 (17.7) | 41.4 (31.5) | 28.4 (21.9) | 19.7 (17.2) | 43.3 (32.9) | 29.4 (22.3) | 21.1 (17.7) |
| Pastry and cake intake, g/MJ* | 1.94 (3.01) | 2.63 (3.48) | 2.92 (4.07) | 1.86 (2.99) | 2.57 (3.45) | 2.91 (3.85) | 1.75 (2.90) | 2.43 (3.42) | 2.84 (3.71) |
| Other characteristics | |||||||||
| University education, %* | 17.2 | 14.8 | 9.0 | 17.9 | 15.1 | 10.4 | 19.4 | 15.9 | 9.5 |
| Smoker, %* | 29.1 | 30.8 | 37.6 | 29.2 | 30.2 | 37.8 | 28.8 | 29.7 | 35.8 |
P < 0.05.
See text and Web Appendix 1 for details on alternative methods used for identifying likely underreporters, plausible reporters, and overreporters.
Chi-square test or analysis of variance for differences by reporting group using each method.
Regardless of the method used, underreporters had higher mean BMIs and overreporters had lower mean BMIs than did plausible reporters, with especially marked differences among women (analysis of variance P < 0.05) (Table 1). Thus, the estimated prevalence of underreporting found with each method was higher among obese women than among overweight and normal-weight women (underreporting prevalences of 32.6%, 20.4%, and 12.2% with the Goldberg method; 23.3%, 12.9%, and 7.2% with the revised Goldberg method; and 20.4%, 10.9%, and 4.8% with the pTEE method, respectively). Patterns were similar among men (not shown).
Diet-BMI relations accounting for implausible reporters: 2.0-standard-deviation cutoffs
Across all methods, underreporters reported lower overall intakes of energy and of energy from fat than did plausible reporters (analysis of variance P < 0.05) (Table 1). Underreporters also reported lower intakes of pastries and higher intakes of fruits and vegetables as a proportion of energy intakes (g/MJ), whereas the opposite was true for overreporters (analysis of variance P < 0.05). Consequently, there were strong effects of accounting for implausible reporters. Compared with the baseline model, in women (Table 2), either excluding or adjusting for implausible reporters identified using any method resulted in positive rather than negative multivariate-adjusted associations between BMI and energy and pastry intakes, null or negative rather than positive associations between BMI and vegetable intakes, and negative rather than null associations with fruit intakes (among nonsmokers). As shown, however, accounting for implausible reporters had little added effect on associations with percentage of energy from fat.
Table 2.
Associationsa Between Body Mass Index and Dietary Factors With Various Adjustments for the Plausibility of Reported Energy Intakes in Women With 2.0-Standard-Deviation Cutoffsb in the Spanish Cohort of the European Investigation Into Cancer and Nutrition, 1992–1996
| Baseline | Goldberg Method |
Revised Goldberg Method |
Predicted Total Energy Expenditure Method |
||||
| Restricted | Adjusted | Restricted | Adjusted | Restricted | Adjusted | ||
| Model 1c: n | 24,025 | 17,654 | 24,025 | 18,350 | 24,025 | 16,826 | 24,025 |
| R2 | 0.237 | 0.252 | 0.286 | 0.269 | 0.312 | 0.272 | 0.319 |
| Energy, MJ | −0.10 (0.01)* | 0.31 (0.02)* | 0.39 (0.02)* | 0.42 (0.02)* | 0.52 (0.02)* | 0.63 (0.02)* | 0.65 (0.02)* |
| % of fat | 0.18 (0.02)* | 0.18 (0.03)* | 0.17 (0.02)* | 0.18 (0.02)* | 0.18 (0.02) | 0.18 (0.03)* | 0.19 (0.02)* |
| Model 2c: R2 | 0.236 | 0.237 | 0.268 | 0.246 | 0.282 | 0.237 | 0.280 |
| Vegetables (tertile 2) | 0.15 (0.07)* | 0.10 (0.07) | 0.05 (0.06) | 0.09 (0.07) | 0.06 (0.06) | 0.05 (0.08) | 0.06 (0.06) |
| Vegetables (tertile 3) | 0.37 (0.07)* | 0.07 (0.08) | 0.06 (0.07) | 0.01 (0.08) | 0.05 (0.07) | 0.03 (0.08) | 0.05 (0.07) |
| Fruit (tertile 2): nonsmokers | −0.13 (0.07)* | −0.17 (0.08)* | 0.18 (0.07)* | −0.24 (0.08)* | −0.18 (0.07)* | 0.26 (0.09)* | −0.21 (0.07)* |
| Fruit (tertile 3): nonsmokers | 0.00 (0.08) | −0.25 (0.08)* | −0.31 (0.07)* | 0.32 (0.08)* | 0.33 (0.07)* | 0.36 (0.09)* | −0.36 (0.07)* |
| Fruit (tertile 2): smoking interactiond | 0.34 (0.16)* | 0.35 (0.17)* | 0.36 (0.15)* | 0.45 (0.17)* | 0.38 (0.15)* | 0.46 (0.18)* | 0.39 (0.15)* |
| Fruit (tertile 3): smoking interactiond | 0.28 (0.18) | 0.43 (0.20)* | 0.35 (0.17)* | 0.48 (0.20)* | 0.35 (0.17)* | 0.44 (0.21)* | 0.35 (0.17)* |
| Cakes and pastries (tertile 2) | −0.16 (0.07)* | 0.15 (0.08)* | 0.16 (0.08)* | 0.18 (0.08)* | 0.19 (0.07)* | 0.22 (0.08)* | 0.22 (0.07)* |
| Cakes and pastries (tertile 3) | −0.16 (0.07)* | 0.23 (0.07)* | 0.21 (0.06)* | 0.27 (0.07)* | 0.28 (0.06)* | 0.33 (0.07)* | 0.32 (0.06)* |
P < 0.05.
Associations are expressed as β coefficient (standard error) except where noted.
See text and Web Appendix 1 for details on alternative methods used for identifying likely underreporters, plausible reporters, and overreporters.
Both models included adjustment for age, study center, height, activity level, educational level, smoking status, season, alcohol intake, parity, diabetes, and use of special diets. In each model, results were additionally adjusted for all dietary variables shown.
Coefficient (standard error) for smoking × fruit intake tertile interaction term. The body mass index-fruit intake association in smokers was obtained by summing the 2 β coefficients. Coefficients for the relation between body mass index and current smoking in women ranged from −1.26 (standard error, 0.10) to −1.39 (standard error, 0.10) in different models.
Although all 3 methods had consistent effects on estimates, the magnitude of these diet-BMI associations was generally stronger when we used the pTEE and revised Goldberg methods than when we used the standard Goldberg method. In men, models were stratified by smoking status, as there were significant interactions between current smoking and several food groups (Table 3). Accounting for implausible reporting had effects similar to those seen in analyses of women: Positive associations with energy and pastry intakes and negative associations with fruit intake (among nonsmokers) were seen after accounting for implausible reporters, whereas positive associations with vegetable intakes were strongly attenuated.
Table 3.
Associationsa Between Body Mass Index and Dietary Factors With Various Adjustments for the Plausibility of Reported Energy Intakes in Men With 2.0-Standard-Deviation Cutoffs in the Spanish Cohort of the European Investigation Into Cancer and Nutrition, 1992–1996
| Baseline | Goldberg Method |
Revised Goldberg Method |
Predicted Total Energy Expenditure Method |
||||
| Restricted | Adjusted | Restricted | Adjusted | Restricted | Adjusted | ||
| Model 1a: n | 14,890 | 11,826 | 14,890 | 11,974 | 14,890 | 10,671 | 14,890 |
| R2 | 0.085 | 0.120 | 0.151 | 0.115 | 0.142 | 0.131 | 0.173 |
| Energy, MJ | −0.06 (0.01)* | 0.17 (0.01)* | 0.22 (0.01)* | 0.16 (0.01)* | 0.21 (0.01)* | 0.33 (0.02)* | 0.36 (0.01)* |
| % fat | 0.29 (0.02)* | 0.24 (0.03)* | 0.27 (0.02)* | 0.25 (0.03)* | 0.28 (0.02)* | 0.23 (0.03)* | 0.27 (0.02)* |
| Model 2a—nonsmokersb: n | 10,285 | 8,185 | 10,285 | 8,352 | 10,285 | 7,449 | 10,285 |
| R2 | 0.094 | 0.122 | 0.138 | 0.118 | 0.130 | 0.114 | 0.137 |
| Vegetables (tertile 2) | 0.09 (0.08) | 0.04 (0.08) | 0.02 (0.08) | 0.06 (0.08) | 0.03 (0.08) | 0.00 (0.09) | 0.01 (0.08) |
| Vegetables (tertile 3) | 0.47 (0.08)* | 0.21 (0.09)* | 0.18 (0.08)* | 0.23 (0.09)* | 0.22 (0.08)* | 0.15 (0.09) | 0.16 (0.08)* |
| Fruit (tertile 2) | −0.03 (0.08) | 0.01 (0.08) | −0.00 (0.08) | 0.02 (0.09) | 0.01 (0.08) | 0.06 (0.09) | 0.02 (0.08) |
| Fruit (tertile 3) | −0.01 (0.08) | −0.11 (0.09) | −0.13 (0.08)* | −0.06 (0.06) | −0.12 (0.08) | −0.11 (0.09) | −0.15 (0.08)* |
| Cakes and pastries (tertile 2) | −0.09 (0.13) | 0.17 (0.13) | 0.08 (0.12) | 0.13 (0.13) | 0.07 (0.12) | 0.23 (0.15) | 0.12 (0.12) |
| Cakes and pastries (tertile 3) | –0.11 (0.07) | 0.07 (0.07) | 0.08 (0.07) | 0.03 (0.08) | 0.05 (0.07) | 0.06 (0.08) | 0.09 (0.07) |
| Model 2b—smokers: n | 4,605 | 3,641 | 4,605 | 3,622 | 4,605 | 3,171 | 4,605 |
| R2 | 0.052 | 0.061 | 0.095 | 0.060 | 0.089 | 0.058 | 0.104 |
| Vegetables (tertile 2) | 0.40 (0.12)* | 0.43 (0.13)* | 0.34 (0.12)* | 0.43 (0.13)* | 0.35 (0.12)* | 0.36 (0.14)* | 0.30 (0.12)* |
| Vegetables (tertile 3) | 0.66 (0.13)* | 0.52 (0.15)* | 0.41 (0.13)* | 0.54 (0.15)* | 0.44 (0.13)* | 0.34 (0.15)* | 0.33 (0.13)* |
| Fruit (tertile 2) | 0.12 (0.12) | 0.13 (0.13) | 0.15 (0.12) | 0.15 (0.15) | 0.13 (0.12) | 0.12 (0.14) | 0.11 (0.12) |
| Fruit (tertile 3) | 0.36 (0.14)* | 0.05 (0.15) | 0.19 (0.13) | 0.13 (0.15) | 0.19 (0.13) | 0.07 (0.15) | 0.14 (0.13) |
| Cakes and pastries (tertile 2) | 0.17 (0.21) | 0.23 (0.22) | 0.32 (0.21) | 0.23 (0.23) | 0.31 (0.21) | 0.15 (0.24) | 0.34 (0.21)* |
| Cakes and pastries (tertile 3) | 0.03 (0.11) | 0.21 (0.12)* | 0.24 (0.11)* | 0.13 (0.12) | 0.22 (0.11)* | 0.16 (0.13) | 0.28 (0.11)* |
P < 0.05.
Associations are expressed as β coefficient (standard error) except where noted.
Both models included adjustment for age, study center, height, activity level, educational level, smoking status, season, alcohol intake, parity, diabetes, and use of special diets; nonsmoker models adjusted for past smoking. In each model, results were additionally adjusted for all dietary variables shown.
Diet-BMI relations accounting for implausible reporters: 1.5-standard-deviation cutoffs
With the more stringent 1.5-standard-deviation cutoffs, underreporting estimates based on the Goldberg, revised Goldberg, and pTEE methods increased to 31.2%, 21.6%, and 19.4% in women and 22.7%, 14.9%, and 13.0% in men, respectively. Estimates for overreporting also increased substantially, to 8.8%, 15.4%, and 24.1% in women and 11.1%, 17.9%, and 28.4% in men, respectively. Applying these cutoffs to the revised Goldberg and pTEE methods led to underreporter classification that was highly concordant with the Goldberg method using 2.0-standard-deviation cutoffs (kappa = 0.90 and kappa = 0.83 for agreement with the revised Goldberg and pTEE methods, respectively).
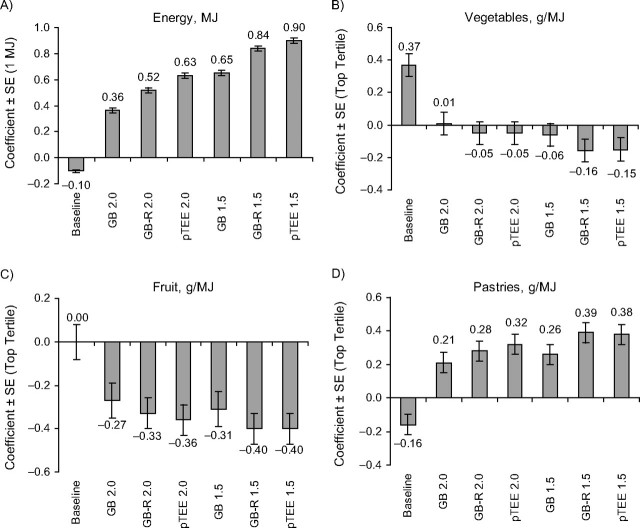
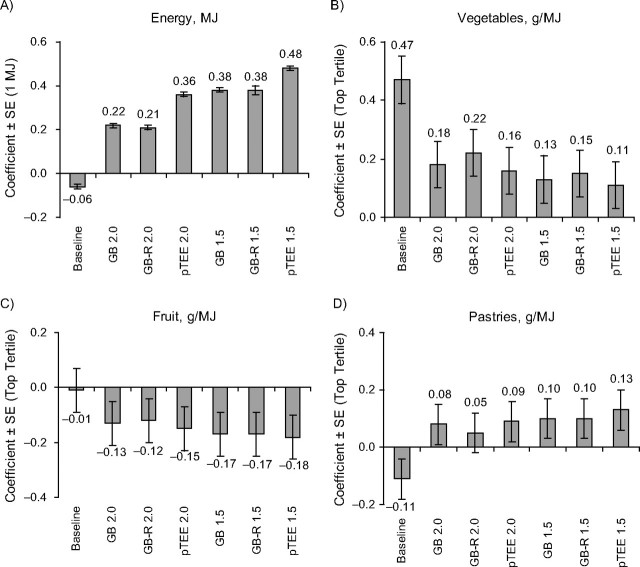
As shown in Figure 1, among women, adjusting for implausible reporters by using more stringent cutoffs generally increased the magnitude of BMI-diet associations. Again, the revised Goldberg and pTEE methods generally yielded somewhat stronger associations than did the Goldberg method. For example, coefficients for the highest vegetable intake tertile among women were negative and significant when we used the revised Goldberg and pTEE methods but remained neutral when we used the Goldberg method. Similar patterns were observed among men (Figure 2).
Figure 1.
Associations between dietary intakes and body mass index among women, determined using alternative adjustments for estimated under- and overreporting in the Spanish cohort of the European Investigation Into Cancer and Nutrition, 1992–1996. Coefficients from multivariable linear regression models were adjusted for age, center, height, activity, educational level, smoking status, season, alcohol intakes, parity, diabetes, and use of special diets. We adjusted for energy and fat simultaneously, and we adjusted food group models (intakes in g/MJ) simultaneously for all food groups. Fruit intakes are shown in nonsmokers (81% of women), as there was an interaction with smoking status. Coefficients for energy were significant in all models. For fruit, coefficient P < 0.05 for all except the baseline model. For vegetables, coefficient P < 0.05 in the following models: baseline, revised Goldberg method, 1.5-standard-deviation cutoffs (GB-R 1.5), and predicted total energy expenditure (pTEE) method, 1.5-standard-deviation cutoffs (pTEE 1.5). For pastries, the coefficient P < 0.05 in all models. GB 1.5, Goldberg method, 1.5-standard-deviation cutoffs; GB 2.0, Goldberg method, 2.0-standard-deviation cutoffs; GB-R 2.0, revised Goldberg, 2.0-standard-deviation cutoffs; pTEE 2.0, pTEE method, 2.0-standard-deviation cutoffs. Bars, standard error (SE).
Figure 2.
Associations between dietary intakes and body mass index among men, determined using alternative adjustments for estimated under- and overreporting, in the Spanish cohort of the European Investigation Into Cancer and Nutrition, 1992–1996. Coefficients from multivariable linear regression models adjusted for age, center, height, activity, educational level, smoking status, season, alcohol intakes, parity, diabetes, and use of special diets. We adjusted for energy and fat simultaneously, and food group models (intakes in g/MJ) adjusted simultaneously for all food groups. Food group intakes are shown in nonsmokers (69% of men), as there was an interaction with smoking status. Coefficients for energy were significant in all models. For fruit, coefficient P < 0.05 in the following models: Goldberg method, 1.5-standard-deviation cutoffs (GB 1.5), revised Goldberg method, 1.5-standard-deviation cutoffs (GB-R 1.5), and predicted total energy expenditure (pTEE) method, 1.5-standard-deviation cutoffs (pTEE 1.5); coefficient P < 0.10 for pTEE method, 2.0-standard-deviation cutoffs (pTEE 2.0). For vegetables, coefficient P < 0.05 in the following models: baseline, Goldberg, 2.0-standard-deviation cutoffs (GB 2.0), revised Goldberg method, 2.0-standard-deviation cutoffs (GB-R 2.0) and pTEE 2.0; coefficient P < 0.10 for GB-R 1.5. For pastries, coefficient P < 0.10 only for the model adjusting for misreporters identified using pTEE 1.5. Bars, standard error (SE).
Diet-BMI relations that accounted only for underreporting
Accounting only for underreporters yielded results that were substantially different than when both types of misreporters were considered for some dietary factors. For example, when only underreporters were excluded, energy-BMI associations among women were strongly attenuated compared with values shown in Table 2, with β coefficients of 0.12 (standard error (SE), 0.01), 0.10 (SE, 0.01), and 0.07 (SE, 0.01) using 2.0-standard-deviation cutoffs, and 0.18 (SE, 0.01), 0.16 (SE, 0.01), and 0.14 (SE, 0.01) using 1.5-standard-deviation cutoffs for the Goldberg, revised Goldberg, and pTEE methods, respectively (P < 0.01 for all methods). When we used the Goldberg method, the association between BMI and the highest vegetable intake tertile among women remained weakly positive rather than null after excluding only underreporters using 2.0-standard-deviation cutoffs (β coefficient = 0.13, SE, 0.08; P < 0.10). Using the pTEE method with the more stringent 1.5-standard-deviation cutoffs, excluding only underreporters rather than both types of implausible reporters yielded null versus negative associations (β coefficient = 0.05 (SE, 0.07), P > 0.10, vs. −0.15 (SE, 0.07), P < 0.01). Although attenuated, associations with other food groups, for which associations were more consistent across the different methods, were not meaningfully different when overreporters were not taken into account (data not shown). Results were similar when adjusting for rather than excluding underreporters, and patterns were similar among men (data not shown).
Excluding extreme energy intakes
When subjects with extreme energy intakes (1.1% of women and 2.2% of men) were excluded rather than using methods to identity implausible reporters based on estimated energy requirements, coefficients in all models were similar to baseline multivariate models (e.g., in women the energy-BMI coefficient was −0.12 (SE, 0.01), P < 0.01).
DISCUSSION
Dietary misreporting characterized by implausible energy intakes is often overlooked. In the absence of objective measures of energy intake, however, indirect methods are typically used to identify implausible reporters and to evaluate how misreporting may influence associations between dietary intakes and health outcomes (16). Recently, some researchers have proposed that pTEE equations may be better suited than previous methods to estimate energy requirements and identify implausible reporters (17, 22); others have suggested that the equations most frequently used to estimate BMR may be insufficiently valid among overweight and obese subjects (28–30). In the present study, we assessed how these alternative methods of estimating energy needs affected estimated prevalences of implausible reporting and influenced associations between dietary factors and obesity.
Levels of under- and overreporting obtained using the traditional Goldberg method—19% and 5%, respectively, in the sample as a whole—were comparable to those reported in the literature that used diet histories or food frequency questionnaires (1). In comparison, when we used the revised Goldberg and pTEE methods, which were highly concordant with each other, levels of underreporting were 7%–10% lower and levels of overreporting were 13%–15% higher. Nonetheless, regardless of the method used, underreporters had higher mean BMIs and overreporters had lower mean BMIs than did plausible reporters, as observed elsewhere (1, 2, 10). As in earlier studies (2, 19, 20, 22), likely underreporters identified with each method reported higher intakes of healthy foods, such as fruits and vegetables, and lower intakes of energy and less-healthy foods, such as pastries, than did plausible reporters. The opposite pattern was true for overreporters. After excluding implausible reporters using each approach, coefficients for several diet-BMI associations changed in magnitude or direction, becoming more consistent with hypotheses relating energy-dense foods to obesity (14, 33), again consistent with several earlier reports (2, 20, 22). For example, among women, initially negative associations between BMI and intakes of energy and pastries were reversed, whereas a neutral association with fruit became negative. In contrast, excluding subjects with extreme energy intakes by using recommended cutoffs (32) had no meaningful effect. Coefficients for percentage of energy from fat were not meaningfully affected by adjustment for misreporting. Although reasons for this finding are uncertain, Huang et al. (22) also found that associations between BMI and percentage of energy from fat were not influenced by excluding implausible reporters. Similarly, coefficients for the percentage of energy from saturated, polyunsaturated, and monounsaturated fat in the baseline multivariate model (β = −0.02 (SE, 0.01), 0.17 (SE, 0.01), and 0.04 (SE, 0.01), respectively) were consistent with those obtained excluding (β = −0.01 (SE, 0.01), 0.14 (SE, 0.01), and 0.03 (SE, 0.01), pTEE method 1.5-standard-deviation cutoffs) or adjusting for implausible reporters (not shown). In separate models, we briefly examined associations between BMI and the percentage of energy from carbohydrates. As for fat, misreporting adjustments had little effect (not shown).
Although the effects of accounting for misreporting were generally consistent across methods, the magnitude of associations observed after these adjustments was frequently stronger when using the revised Goldberg and pTEE methods to identify misreporters than when using the original Goldberg method. This was observed despite the lower prevalence of underreporting found when using these alternative methods. As observed previously, using more restrictive cutoffs to identify implausible reporters tended to strengthen associations (17, 22). Results also suggested that in some cases, overreporting might have been influential, as excluding or adjusting only for underreporters at times yielded associations that differed when also accounting for overreporters. Additionally, as in a previous study on a different population (2), we found that adjusting for rather than excluding implausible reporters yielded consistent results: Relations between dietary intakes and BMI that emerged after stratifying by reporting group were similar to those observed among plausible reporters (Web Figure 1). This suggested that adjustment—effectively summarizing across reporting groups—was a viable alternative to omitting a substantial proportion of subjects, which some researchers have suggested may lead to bias (34). Similarly, in another recent study, de Castro et al. (35) found that positive relations between variables such as energy density and energy intakes were preserved within reporting subgroups defined on the basis of the rEI:BMR ratio, despite the disparate levels of intake reported across these groups.
The stronger associations observed using the revised Goldberg and pTEE methods versus the original Goldberg method might be due in part to improved classification of implausible reporters, as these methods could better estimate energy requirements. pTEE equations have high R2 values (7), and the revised BMR equations have been reported to yield better estimates across the range of BMIs (28, 29). It is noteworthy that these revised approaches, although based on independent equations for estimating energy needs, yielded highly concordant estimates of both under- and overreporting. It is important to keep in mind, however, that although the Goldberg method has been evaluated against doubly labeled water, the true validity of these alternative methods is uncertain. In previous studies, researchers have shown the Goldberg method with cutoffs of 2.0 standard deviations to be specific (97%–98%) and reasonably sensitive (72%–74%) for identifying underreporters (21). Reassuringly, when 1.5-standard-deviation cutoffs were applied, the numbers of underreporters identified with these updated methods were highly concordant (94%–96% agreement) with the Goldberg method. Thus, the major discrepancy was the substantially higher level of overrreporting identified using these methods. Indeed, the validation of the original Goldberg method suggested this method had limited ability to identify overreporters (21).
The substantial differences in the prevalence of implausible reporting across alternative methods highlight that in the absence of valid objective measures of habitual energy intakes, it is not possible to determine to what extent implausible rEIs reflect misreporting rather than true habitual intakes in subjects whose energy requirements may be poorly estimated (31). However, the findings that emerge after accounting for implausible reporters are consistent with the disparity in associations observed in several studies comparing how questionnaire data versus biomarker-based markers of intake relate to obesity or related health outcomes. For example, in one recent study, urinary sugars and plasma vitamin C, but not food frequency questionnaire-based estimates of intake, were found to be associated with obesity (36). In another population, estimates of vitamin C intake derived from plasma or food records, but not from food frequency questionnaires, were associated with ischemic heart disease (37). In yet another study, positive associations between energy intakes and obesity-related cancers, such as breast and colon cancer, emerged only after using biomarker-calibrated measures of intake, whereas associations with non-obesity-related cancers such as lung cancer and lymphoma remained neutral (38). The absence of objective measures of energy intake is an important limitation of this analysis. However, there are important strengths, including the large sample size with measured anthropometry, and the availability of a validated physical activity level measure to aid estimation of energy needs (21). Nonetheless, as household activities were not included, activity levels might have been assessed with some degree of error (39, 40).
Recent literature has suggested that imprecise or biased intake reporting, often more prevalent among obese subjects, may undermine the validity of research on diet and numerous health outcomes (11, 36, 38, 41, 42). In the absence of objective biomarkers, the updated methods used in this study, which attempted to address limitations identified with the original approach, appear to be a reasonable alternative, enabling researchers to examine the effects of accounting for likely overreporters as well as underreporters. Although its relevance may vary across populations and dietary assessment methods, additionally accounting for overreporting appeared to influence associations with some dietary factors, and this type of misreporting should be considered. Future studies to assess the sensitivity and specificity of these alternative methods against objective measures of energy intake are needed to better evaluate their ability to identify under- and overreporters compared with the Goldberg method.
Supplementary Material
Acknowledgments
Author affiliations: Center for Research in Environmental Epidemiology/Municipal Institute for Medical Research-Hospital del Mar, Barcelona, Spain (Michelle A. Mendez); Unit of Nutrition, Environment and Cancer/Cancer Epidemiology Research Programme, Catalan Institute of Oncology, Barcelona, Spain (Michelle A. Mendez, Genevieve Buckland, Carlos A González); Department of Nutrition and Carolina Population Center, University of North Carolina, Chapel Hill, North Carolina (Barry M. Popkin); Cardiovascular Risk and Nutrition Research Group/Municipal Institute for Medical Research-Hospital del Mar, Barcelona, Spain (Helmut Schroder); Public Health Division of Gipuzkoa and IIS Instituto Investigación Sanitaria BioDonostia, Basque Government, San Sebastian, Spain (Pilar Amiano); Navarre Public Health Institute, Pamplona, Spain (Aurelio Barricarte); Epidemiology Department, Health Council of Murcia, Murcia, Spain (José-María Huerta); Public Health and Health Planning Directorate of Asturias, Oviedo, Spain (José R. Quirós); Andalusian School of Public Health, Granada, Spain (María-José Sánchez); Consortium for Biomedical Research in Epidemiology and Public Health (CIBERESP), Barcelona, Spain (Michelle A. Mendez, Pilar Amiano, Aurelio Barricarte, José-María Huerta, José R. Quirós, María-José Sánchez); and Consortium for Biomedical Research in Obesity and Nutrition Physiopathology, Barcelona, Spain (Helmut Schroder).
Data are from the Spanish cohort of the European Prospective Investigation Into Cancer and Nutrition (EPIC), coordinated by the International Agency for Research on Cancer (agreement NTR/2000/01). The project was financed by the European Commission (agreement SPC.2002332) and participating regional governments, including the Health Research Fund of the Spanish Ministry of Health (exp. 96 0032). Centers from Barcelona, Granada, and Murcia received funding from the Epidemiology and Public Health Centers Network sponsored by the Carlos III Health Institute.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- BMR
basal metabolic rate
- EPIC
European Prospective Investigation Into Cancer and Nutrition
- pTEE
predicted total energy expenditure
- rEI
reported energy intake
- SE
standard error
References
- 1.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133(suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 2.Mendez MA, Wynter S, Wilks R, et al. Under- and overreporting of energy is related to obesity, lifestyle factors and food group intakes in Jamaican adults. Public Health Nutr. 2004;7(1):9–19. doi: 10.1079/phn2003508. [DOI] [PubMed] [Google Scholar]
- 3.Lissner L. Measuring food intake in studies of obesity. Public Health Nutr. 2002;5(6A):889–892. doi: 10.1079/phn2002388. [DOI] [PubMed] [Google Scholar]
- 4.Heitmann BL, Lissner L, Osler M. Do we eat less fat, or just report so? Int J Obes Relat Metab Disord. 2000;24(4):435–442. doi: 10.1038/sj.ijo.0801176. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari P, Slimani N, Ciampi A, et al. Evaluation of under- and overreporting of energy intake in the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5(6B):1329–1345. doi: 10.1079/PHN2002409. [DOI] [PubMed] [Google Scholar]
- 6.Tooze JA, Subar AF, Thompson FE, et al. Psychosocial predictors of energy underreporting in a large doubly labeled water study. Am J Clin Nutr. 2004;79(5):795–804. doi: 10.1093/ajcn/79.5.795. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: The National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 8.Schatzkin A, Kipnis V, Carroll RJ, et al. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol. 2003;32(6):1054–1062. doi: 10.1093/ije/dyg264. [DOI] [PubMed] [Google Scholar]
- 9.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- 10.Black AE, Cole TJ. Biased over- or under-reporting is characteristic of individuals whether over time or by different assessment methods. J Am Diet Assoc. 2001;101(1):70–80. doi: 10.1016/S0002-8223(01)00018-9. [DOI] [PubMed] [Google Scholar]
- 11.Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab. 2001;281(5):E891–E899. doi: 10.1152/ajpendo.2001.281.5.E891. [DOI] [PubMed] [Google Scholar]
- 12.Newby PK. Plant foods and plant-based diets: protective against childhood obesity? Am J Clin Nutr. 2009;89(5):1572S–1587S. doi: 10.3945/ajcn.2009.26736G. [DOI] [PubMed] [Google Scholar]
- 13.Buckland G, Bach A, Serra-Majem L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev. 2008;9(6):582–593. doi: 10.1111/j.1467-789X.2008.00503.x. [DOI] [PubMed] [Google Scholar]
- 14.Tohill BC, Seymour J, Serdula M, et al. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62(10):365–374. doi: 10.1111/j.1753-4887.2004.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 15.Samuel-Hodge CD, Fernandez LM, Henríquez-Roldán CF, et al. A comparison of self-reported energy intake with total energy expenditure estimated by accelerometer and basal metabolic rate in African-American women with type 2 diabetes. Diabetes Care. 2004;27(3):663–669. doi: 10.2337/diacare.27.3.663. [DOI] [PubMed] [Google Scholar]
- 16.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 2000;24(9):1119–1130. doi: 10.1038/sj.ijo.0801376. [DOI] [PubMed] [Google Scholar]
- 17.McCrory MA, McCrory MA, Hajduk CL, et al. Procedures for screening out inaccurate reports of dietary energy intake. Public Health Nutr. 2002;5(6A):873–882. doi: 10.1079/PHN2002387. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg GR, Black AE, Jebb SA, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45(12):569–581. [PubMed] [Google Scholar]
- 19.Macdiarmid JI, Vail A, Cade JE, et al. The sugar-fat relationship revisited: differences in consumption between men and women of varying BMI. Int J Obes Relat Metab Disord. 1998;22(11):1053–1061. doi: 10.1038/sj.ijo.0800724. [DOI] [PubMed] [Google Scholar]
- 20.Howarth NC, Huang TT, Roberts SB, et al. Dietary fiber and fat are associated with excess weight in young and middle-aged US adults. J Am Diet Assoc. 2005;105(9):1365–1372. doi: 10.1016/j.jada.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Black AE. The sensitivity and specificity of the Goldberg cut-off for EI: BMR for identifying diet reports of poor validity. Eur J Clin Nutr. 2000;54(5):395–404. doi: 10.1038/sj.ejcn.1600971. [DOI] [PubMed] [Google Scholar]
- 22.Huang TT, Roberts SB, Howarth NC, et al. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes Res. 2005;13(7):1205–1217. doi: 10.1038/oby.2005.143. [DOI] [PubMed] [Google Scholar]
- 23.González CA, Pera G, Quirós JR, et al. Types of fat intake and body mass index in a Mediterranean country. Public Health Nutr. 2000;3(3):329–336. doi: 10.1017/s1368980000000379. [DOI] [PubMed] [Google Scholar]
- 24.EPIC Group of Spain. Relative validity and reproducibility of a diet history questionnaire in Spain. I. Foods. Int J Epidemiol. 1997;26(suppl 1):S91–S99. doi: 10.1093/ije/26.suppl_1.s91. [DOI] [PubMed] [Google Scholar]
- 25.Haftenberger M, Lahmann PH, Panico S, et al. Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5(6B):1147–1162. doi: 10.1079/PHN2002396. [DOI] [PubMed] [Google Scholar]
- 26.Wareham NJ, Jakes RW, Rennie KL, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6(4):407–413. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 27.Food and Agriculture Organization of the United Nations. Human Energy Requirements: Report of a Joint FAO/WHO/ONU Expert Consultation. Rome, Italy: Food and Agriculture Organization of the United Nations; 2001. [Google Scholar]
- 28.Horgan GW, Stubbs J. Predicting basal metabolic rate in the obese is difficult. Eur J Clin Nutr. 2003;57(2):335–340. doi: 10.1038/sj.ejcn.1601542. [DOI] [PubMed] [Google Scholar]
- 29.Alfonzo-González G, Doucet E, Alméras N, et al. Estimation of daily energy needs with the FAO/WHO/UNU 1985 procedures in adults: comparison to whole-body indirect calorimetry measurements. Eur J Clin Nutr. 2004;58(8):1125–1131. doi: 10.1038/sj.ejcn.1601940. [DOI] [PubMed] [Google Scholar]
- 30.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105(5):775–789. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Tooze JA, Schoeller DA, Subar AF, et al. Total daily energy expenditure among middle-aged men and women: the OPEN Study. Am J Clin Nutr. 2007;86(2):382–387. doi: 10.1093/ajcn/86.2.382. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 33.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105(5 suppl 1):S98–S103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Poslusna K, Ruprich J, de Vries JH, et al. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(suppl 2):S73–S85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- 35.de Castro JM. Varying levels of food energy self-reporting are associated with between-group, but not within-subject, differences in food intake. J Nutr. 2006;136(5):1382–1388. doi: 10.1093/jn/136.5.1382. [DOI] [PubMed] [Google Scholar]
- 36.Bingham S, Luben R, Welch A, et al. Epidemiologic assessment of sugars consumption using biomarkers: comparisons of obese and nonobese individuals in the European Prospective Investigation of Cancer Norfolk. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1651–1654. doi: 10.1158/1055-9965.EPI-06-1050. [DOI] [PubMed] [Google Scholar]
- 37.Bingham S, Luben R, Welch A, et al. Associations between dietary methods and biomarkers, and between fruits and vegetables and risk of ischaemic heart disease, in the EPIC Norfolk Cohort Study. Int J Epidemiol. 2008;37(5):978–987. doi: 10.1093/ije/dyn111. [DOI] [PubMed] [Google Scholar]
- 38.Prentice RL, Shaw PA, Bingham SA, et al. Biomarker-calibrated energy and protein consumption and increased cancer risk among postmenopausal women. Am J Epidemiol. 2009;169(8):977–989. doi: 10.1093/aje/kwp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cust AE, Smith BJ, Chau J, et al. Validity and repeatability of the EPIC physical activity questionnaire: a validation study using accelerometers as an objective measure. Int J Behav Nutr Phys Act. 2008;5:33. doi: 10.1186/1479-5868-5-33. (doi:10.1186/1479-5868-5-33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamatakis E, Hillsdon M, Primatesta P. Domestic physical activity in relationship to multiple CVD risk factors. Am J Prev Med. 2007;32(4):320–327. doi: 10.1016/j.amepre.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Prentice RL. Dietary assessment and the reliability of nutritional epidemiology research reports. J Natl Cancer Inst. 2010;102(9):583–585. doi: 10.1093/jnci/djq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bingham SA, Luben R, Welch A, et al. Are imprecise methods obscuring a relation between fat and breast cancer? Lancet. 2003;362(9379):212–214. doi: 10.1016/S0140-6736(03)13913-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.