Abstract
During development, sympathetic neurons and chromaffin cells originate from bipotential sympathoadrenal (SA) progenitors arising from neural crests (NC) in the trunk regions. Recently, we showed that AP-2β, a member of the AP2 family, plays a critical role in the development of sympathetic neurons and locus coeruleus and their norepinephrine (NE) neurotransmitter phenotype. In the present study, we investigated the potential role of AP-2β in the development of NC-derived neuroendocrine chromaffin cells of the adrenal medulla and the epinephrine (EPI) phenotype determination. In support of its role in chromaffin cell development, AP-2β is prominently expressed in both embryonic and adult adrenal medulla. In adrenal chromaffin cells of the AP-2β−/− mouse, the expression levels of catecholamine biosynthesizing enzymes, dopamine β-hydroxylase (DBH) and phenylethanolamine-N-methyl-transferase (PNMT), as well as the SA-specific transcription factor, Phox2b, are significantly reduced compared to wild type. In addition, ultrastructural analysis demonstrated that formation of large secretory vesicles, a hallmark of differentiated chromaffin cells, is defective in AP-2β−/− mice. Furthermore, the level of EPI content is largely diminished (>80%) in the adrenal gland of AP-2β−/− mice. Chromatin immunoprecipitation (ChIP) assays of rat adrenal gland showed that AP-2β binds to the upstream promoter of the PNMT gene in vivo; strongly suggesting that it is a direct target gene. Overall, our data suggest that AP-2β plays critical roles in the epinephrine phenotype and maturation of adrenal chromaffin cells.
Keywords: AP-2β, epinephrine, chromaffin cells, phenylethanolamine-N-methyl-transferase, large secretory vesicles, Chromatin immunoprecipitation (ChIP) assays
Introduction
Understanding the regulatory mechanisms underlying cell fate specification of a vast number of different neural lineage cell types from pluripotent neural stem cells is a central question in vertebrate development. NCs, which arise from the interface between the neural plate and the surface epidermis, represent a useful paradigm to understand the molecular networks involved in cell fate specification because they give rise to diverse cell types, including the neurons and glia of the peripheral nervous system, bones, and cartilage (Adams and Bronner-Fraser, 2009; Huber, 2006; Le Douarin et al., 2004; Sauka-Spengler and Bronner-Fraser, 2006). Through the regulatory network of extracellular signals and intrinsic transcription factors, NCs of trunk regions develop to be NE-producing sympathetic neurons, EPI-producing chromaffin cells in the adrenal gland, and melanocytes. Sympathetic neurons and chromaffin cells are thought to originate from bipotential SA progenitors (Anderson, 1993; Anderson and Axel, 1986; Huber et al., 2005; Unsicker et al., 2005). NCs migrate near the dorsal aorta and become SA progenitors in response to bone morphogenetic proteins (BMP). These SA progenitors further migrate to target sites and become NE neurons of the sympathetic ganglia (SG) and neuroendocrine chromaffin cells in the adrenal gland. Another theory suggests that sympathetic neurons and chromaffin cells have distinct origins. In this scenario, NCs develop into neural and chromaffin precursors, instead of bipotential SA progenitors, and further differentiate into specific cell types at the target sites (Ernsberger et al., 2005; Gut et al., 2005; Huber, 2006).
Fundamental mechanistic insights can be obtained by elucidating transcription factors that play critical roles in development and cell fate specification during the development of SG and adrenal chromaffin cells. Proteins of the AP2 family share unique structures consisting of an N-terminus transactivation domain, a C-terminal DNA binding, and a dimerization domain. They form hetero or homodimer, and bind to the palindromic recognition sequence 5′-GCCNNNGGC-3′ or its related GC-rich sequences (Eckert et al., 2005; Hilger-Eversheim et al., 2000). Since AP-2α was first isolated (Mitchell et al., 1987), four other homologous genes, AP-2β, AP-2γ, AP-2δ, and AP-2ε, have been reported as members of the AP2 family (Chazaud et al., 1996; Feng and Williams, 2003; Moser et al., 1995; Oulad-Abdelghani et al., 1996; Wang et al., 2004; Zhao et al., 2001). Though AP2 proteins are coexpressed in some developing organs, they show distinct spatiotemporal expression during early mouse embryogenesis. Also, gene inactivation studies suggest that the proteins have quite distinct roles in development (Eckert et al., 2005). AP-2α knockout mice display defects in neural tube, body wall, and craniofacial development (Schorle et al., 1996; Zhang et al., 1996). AP-2β knockout mice suffer from kidney malfunction (Moser et al., 1997), while AP-2γ knockout mice illustrate that it plays a role in the development of extraembryonic lineages (Auman et al., 2002; Werling and Schorle, 2002). Thus, despite sequence homologies and overlapping expression patterns, AP2 family members have non-redundant roles during embryonic development (Eckert et al., 2005).
We recently showed that of the three members of the AP2 family (AP-2α, β, and γ) that are expressed in the nervous system, AP-2β specifically regulates development of sympathetic neurons that are derived from SA progenitors (Hong et al., 2008). In this report, we addressed the role of AP-2β in the development of chromaffin cells. Expression levels of EPI and its biosynthetic enzymes, as well as SA determining transcription factors, were reduced in AP-2β knockout mice. We found abnormal ultrastructures in chromaffin cells of AP-2β knockout mice. ChIP assays showed that AP-2β directly binds to the upstream promoter of the PNMT gene, which converts NE to EPI, in rat adrenal glands. Taken together, we propose that AP-2β is critical for maturation of adrenal chromaffin cells.
Results
The transcription factor AP-2β is expressed in the adrenal gland
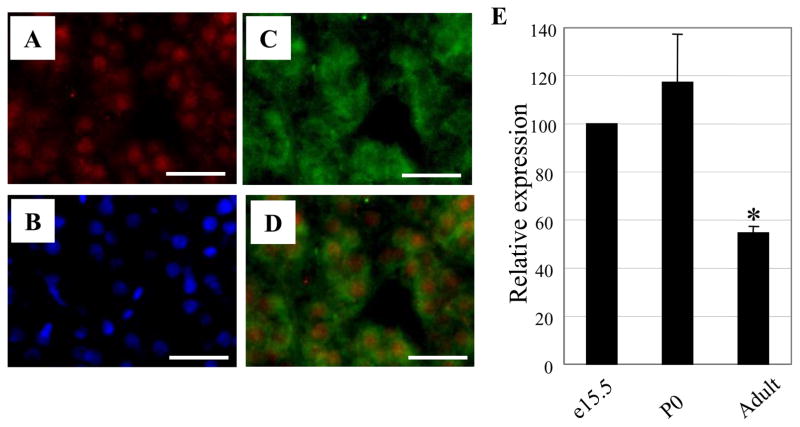
Our recent study showed that AP-2β is predominantly expressed in the SG of developing mouse embryos and is critical for the development and NE neurotransmitter phenotype determination of the SG (Hong et al., 2008). We hypothesized that AP-2β is also involved in the development of adrenal chromaffin cells. To address this, we first analyzed its in vivo expression pattern with AP-2β specific antibodies that were characterized in a previous report (Hong et al., 2008). Expression of AP-2β was detected in the adrenal gland of adult as well as at e15.5 and neonate mice (data not shown). Expression of AP-2β was more prominently detected in the adrenal gland of rats (Fig. 1A). Merging the immunostaining of tyrosine hydroxylase (TH) and AP-2β showed that their expression almost completely overlapped (Fig. 1D). Furthermore, reverse transcription quantitative PCR (RT-qPCR) analyses of mouse adrenal glands confirmed AP-2β expression and showed higher expression of AP-2β in e15.5 and P0 compared to adult mice (Fig. 1E). Together, these expression patterns support the potential role of AP-2β in the development and/or phenotype determination of adrenal glands.
Fig. 1.
AP-2β is expressed in the adrenal gland. (A-D) Co-expression of AP-2β and TH in the adrenal gland. Gene expression from adult rat’s adrenal medulla was analyzed. The same section was stained with rabbit anti-AP-2β (A) and sheep anti-TH specific (C) antibodies. Nuclei were stained with DAPI (B). Merged image of AP-2β and TH indicates that these genes are co-expressed in the adrenal medulla (D). Scale bar; 40 μm. (E) AP-2β mRNA expression analysis. Total RNAs from adrenal glands of e15.5, P0, and adult (eight weeks old) wild type mice were isolated, and RT-qPCR was performed. AP-2β expression was normalized according to the expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The level of gene expression of AP-2β of P0 and adult mice was compared to that of e15.5, which was set at 100%. Data are from three independent experiments. (*P<0.05; Student’s t-test)
The gross morphology and the number of adrenal chromaffin cells appear normal in AP-2β knockout mice
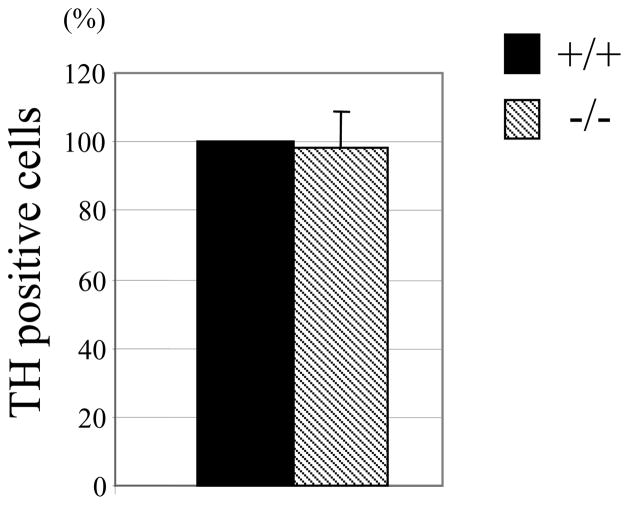
In AP-2β−/− embryos, the number of NE neurons in the SG was reduced to 40% of wild type (Hong et al., 2008). To address whether the number of chromaffin cells is similarly affected in AP-2β−/− mice, we stained adrenal medulla with TH specific antibodies. We could not detect significant differences in the size of adrenal medulla and the number of TH positive cells between wild type and AP-2β−/− mice at P0 (Fig. 2). In addition, the intensity of TH staining was comparable, suggesting that the level of TH gene expression is not detectably affected in chromaffin cells of AP-2β−/− mice (also see Fig. 3 and 4). Furthermore, we failed to detect any significant changes in apoptotic cell death in the adrenal medulla of AP-2β−/− mice at P0 (data not shown). Therefore, we conclude that the gross development of adrenal chromaffin cells is not detectably affected in AP-2β−/− mice.
Fig. 2.
The size of adrenal medulla is unchanged in AP-2β−/− mice. Adrenal glands at P0 were sectioned (10 μm thickness). Every fifth section was collected and stained with TH specific antibodies. Nuclei were stained with Hoechst dye. After merging TH and Hoechst stained sections, TH positive cells were counted. The number of TH positive cells in AP-2β homozygous mutant mice was compared to that of wild types, which was set at 100%. Data are from three littermates.
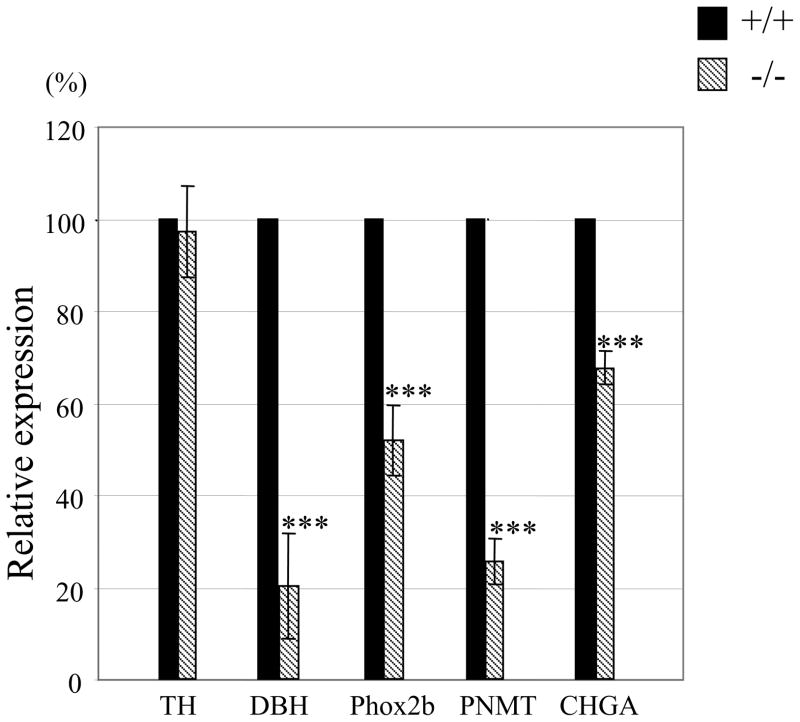
Fig. 3.
mRNA level of SA specific gene is reduced in the adrenal gland of AP-2β−/− mice. Total RNAs were isolated from adrenal glands of P0 mice, and RT-qPCR was performed. Each gene expression was normalized according to the expression level of GAPDH. The level of gene expression of AP-2β homozygous mutant mice was compared to that of wild types, which was set at 100%. Data from four independent experiments. (***P<0.001; Student’s t-test)
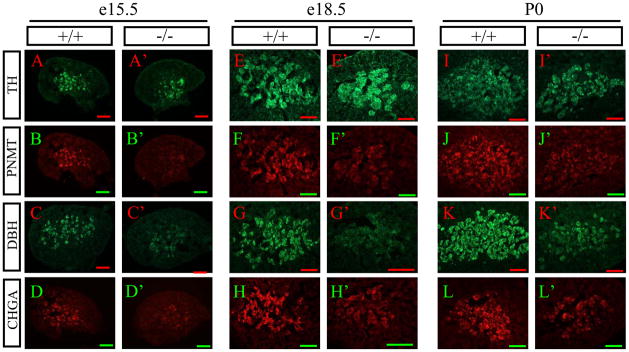
Fig. 4.
Expression of SA specific genes is reduced in adrenal medulla of AP-2β−/− mice. (A–L′) Cryosectioned adrenal gland of e15.5 (A–D′), e18.5 (E–H′), and P0 (I–L′) wild type (A–L) and AP-2β homozygous mutant mice (A′–L′) was analyzed. TH (A, A′, E, E′, I, I′), PNMT (B, B′, F, F′, J, J′), DBH (C, C′, G, G′, K, K′), and CHGA (D, D′, H, H′, L, L′) are stained with their specific antibodies, and visualized using fluorescence conjugated secondary antibodies. The same section was used to detect TH and PNMT. Scale bar; 200 μm.
Expression of SA specific genes is significantly compromised in adrenal chromaffin cells of AP-2β knockout mice
We next tested if expression of SA-specific marker genes such as TH, DBH, and PNMT is affected in adrenal chromaffin cells of AP-2β−/− mice. Consistent with the initial TH immunostaining analysis described above, RT-qPCR analysis of mRNAs from adrenal glands showed that the level of TH expression was almost identical in neonate AP-2β−/− mice (Fig. 3). In sharp contrast, in AP-2β−/− mice the level of DBH mRNA expression was dramatically reduced to 20% of wild type (Fig. 3). We next addressed if the absence of AP-2β affected the RNA levels of EPI-synthesizing PNMT gene. Interestingly, in AP-2β−/− mice, the mRNA expression level of PNMT was also greatly diminished to <30% of wild type (Fig. 3). In addition, we found that mRNA expression of the transcription factor Phox2b, which is required for the development of chromaffin cells (Huber et al., 2005), was diminished by approximately 50% in chromaffin cells of AP-2β−/− mice (Fig. 3), whereas Phox2a expression appeared to be intact (data not shown). We also found that mRNA expression of chromogranin A (CHGA), a major soluble protein in large dense-core secretory granules of chromaffin cells (Taupenot et al., 2003), was significantly reduced in AP-2β−/− mice (Fig. 3). Thus, our results show that AP-2β does not control all genes critical for chromaffin cell development but rather regulates a subset of genes critical for phenotypic specification.
We next investigated whether these marker gene expressions are attenuated in chromaffin cells of the AP-2β−/− mice at protein level by immunohistochemistry (IHC) analysis. In line with our mRNA expression analysis (Fig. 3), we found that expression of DBH (Fig. 4C′, G′, K′) and PNMT protein (Fig. 4B′, F′, J′) was greatly diminished in the AP-2β−/− mice, while TH expression was not detectably affected (Fig. 4A′, E′, I′). In addition, the expression level of Phox2b, which is required for the development of chromaffin cells (Huber et al., 2005), was significantly reduced in AP-2β−/− mice during development (data not shown), which is consistent with mRNA expression analysis (Fig. 3). Furthermore, we found that the expression level of CHGA was significantly reduced in the adrenal gland of AP-2β−/− mice (Fig. 4D′, H′, L′).
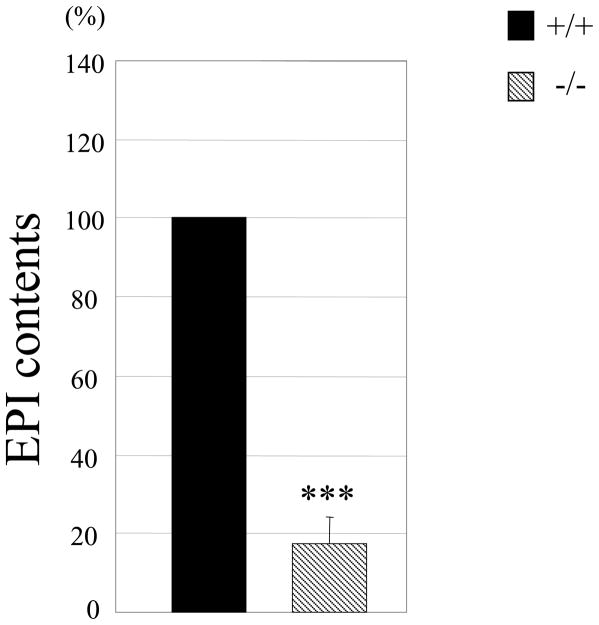
Based on the altered gene expression, we speculated that the major neurotransmitter of chromaffin cells, EPI, may be affected in the adrenal gland of AP-2β−/− mice. Indeed, we found that the content of EPI was diminished by more than 80% in the adrenal gland of neonate AP-2β−/− mice (Fig. 5). Taken together, our data revealed that, although the gross structure and TH-positive cell number appeared normal, SA-specific marker gene expression and the level of EPI were significantly defective in the adrenal gland of AP-2β−/− mice.
Fig. 5.
EPI content in adrenal gland is reduced in AP-2β−/− mice. The amount of EPI from adrenal glands of neonate mice (P0) was measured by HPLC. Normalized EPI contents to the total proteins in AP-2β homozygous mutant mice were compared with those from their wild type littermates. EPI contents in wild type are set as 100%. The data are from nine littermates. (***P<0.001; Student’s t-test)
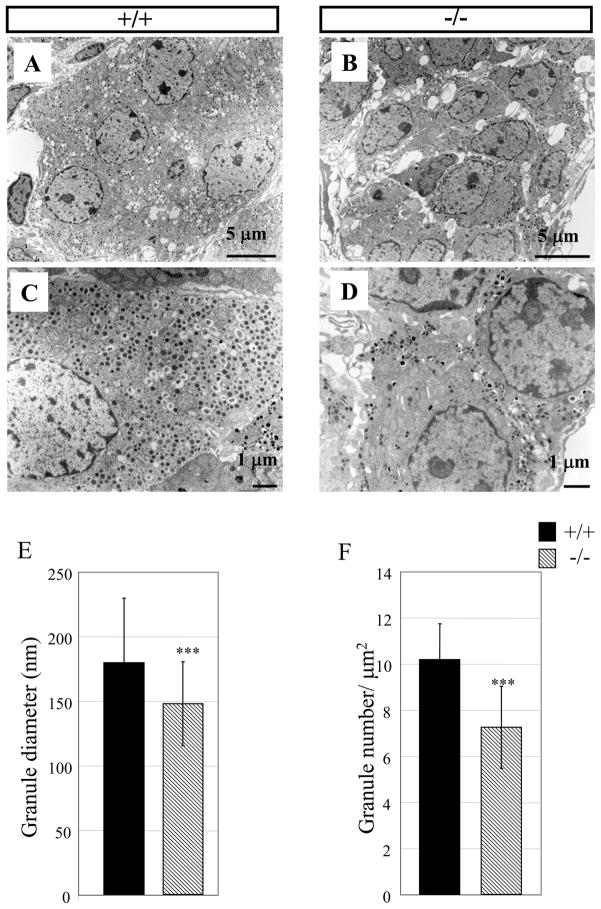
The number of secretory granule is reduced in AP-2β knockout mice
Chromaffin cells usually contain large chromaffin granules, which is a feature distinct from sympathetic neurons (Coupland and Tomlinson, 1989). Next, we sought to find if there was any defect in chromaffin cells of AP-2β−/− mice at the ultrastructural level. To accomplish this goal, we compared the cellular structure of chromaffin cells of wild type and AP-2β−/− neonates (P0) using electron microscopy. As shown in Fig. 6A & C, this analysis demonstrated the typical chromaffin cell structures in which many large dense core secretory granules (core diameter > 100 nm) are distributed throughout the cytoplasm in wild type mice. On the contrary, large secretory granules were found in the periphery of cells and their number was greatly reduced in adrenal chromaffin cells of AP-2β−/− mice (Fig. 6B & D). Higher numbers of secretory granules were found in wild type (10.2 per μm2) compared to AP-2β−/− mice (7.3 per μm2) (Fig. 6F). The diameter of the secretory granules in wild type (179 nm) was larger than that of AP-2β−/− mice (147 nm) (Fig. 6E). Adrenal chromaffin cells were less attached to each other in AP-2β−/− mice (Fig. 6B), indicating the abnormal structural and subcellular organization of chromaffin cells.
Fig. 6.
Ultrastructural analysis of adrenal medulla of AP-2β−/− mice. (A-D) Electron microscopy was performed in the adrenal gland of wild type (A, C) and AP-2β homozygous mutant mice (B, D) at P0. Chromaffin granules are found in the periphery of cells and their number is reduced in AP-2β homozygous mutants (compare C, D). Scale bar; 5 μm (A, B), 1 μm (C, D). (E, F) Secretory granules were analyzed in the adrenal gland of wild type and AP-2β homozygous mutant mice. (E) Diameter of granules was measured using the ImageJ program and presented as nm. Data are from 200 granules. (F) The number of granules was counted in the cytosol of adrenal medullar cells and presented as granule number per surface area (μm2). Data are from 10 random fields of three wild type and AP-2β homozygous mutant mice. (***P<0.001; Student’s t-test)
The transcription factor AP-2β directly binds to the rat PNMT promoter
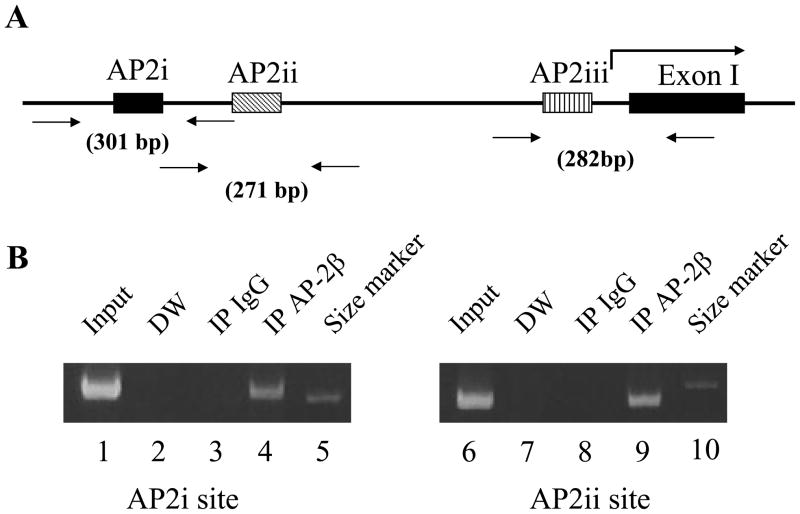
We next sought to address whether AP-2β directly activates transcription of the PNMT gene promoter in vivo. Previous co-transfection study showed that AP-2α is able to interact with the rat PNMT promoter and to activate its promoter function in vitro (Ebert et al., 1998). AP-2α and AP-2β show similar transactivation and DNA binding activities in vitro (data not shown). In order to determine whether AP-2β directly binds to the PNMT gene promoter in vivo, we performed ChIP assays. Rats’ adrenal glands were homogenized and cross-linked with 1% formaldehyde, followed by sonication to generate 200–500 bp DNA fragments. Following immunoprecipitation, specifically bound DNA fragments were purified and PCR was performed using specific primer sets (Fig. 7A). ChIP assays using AP-2β specific antibody yielded the expected products including the AP2i (Fig. 7B, lane 4) and AP2ii (Fig. 7B, lane 9) sites from the rat PNMT gene promoter. ChIP assays using normal rabbit IgG did not detect clear bands (Fig. 7B, lanes 3, 8). We were not able to detect the binding of AP-2β to the AP2iii site by ChIP assay, most likely due to its very low binding affinity to AP2 (Ebert et al., 1998). The results strongly suggest that the PNMT gene is a direct target of AP-2β.
Fig. 7.
ChIP analysis showing in vivo binding of AP-2β to the AP2 binding sites of the rat PNMT promoter. (A) Schematic drawing of the three AP2 binding sites (i, ii, iii) in the rat PNMT promoter as described (Ebert et al., 1998). Primer sets to detect the AP2 binding sites and the size of expected PCR products are shown. The bent arrow represents the PNMT transcription start site. The first PNMT exon is shown (Exon I). (B) The protein-DNA complexes were immunoprecipitated using antibodies against AP-2β (lane 4, 9). Normal rabbit IgG was used as a negative control (lane 3, 8). PCR was performed without template and used for PCR control (lane 2, 7). Input DNA (lane 1, 6) and 300 bp DNA size marker (lane 5, 10) are shown. PCR products were analyzed by electrophoresis on 7% polyacrylamide gel.
Discussion
Although sympathetic NE neurons and chromaffin cells are generally thought to originate from common SA progenitors and share many characteristics such as catecholamine synthesizing enzymes, TH and DBH, they also have distinct features. For example, while typical neuronal markers are induced in sympathetic neurons, their expression is suppressed or downregulated in chromaffin cells. Thus, chromaffin cells do not exhibit typical neuronal properties or morphology. Chromaffin cells have large secretory granules, which are not found in sympathetic neurons that have extended neurites (Coupland and Tomlinson, 1989; Langley and Grant, 1999). In addition, the major neurotransmitters of SG and chromaffin cells are NE and EPI, respectively. Expression of PNMT converting NE to EPI is an essential and unique phenotype of chromaffin cells. Several signaling molecules (e.g., BMPs), and transcription factors (e.g., Mash1, Phox2a/2b, GATA-2/3, and dHand) have been identified to be critical for the specification of sympathetic neurons (Brunet and Pattyn, 2002; Goridis and Rohrer, 2002; Howard, 2005; Lan and Breslin, 2009; Lim et al., 2000; Morikawa et al., 2007; Morikawa et al., 2009; Wildner et al., 2008). Among these, Mash1, Phox2b, and GATA-2/3 are also important for chromaffin cell differentiation. Also, a zinc finger transcription factor Insm1 has recently been shown to be critical for the development of the SG and adrenal chromaffin cells (Wildner et al., 2008).
We recently found that AP-2β could be another potential transcription factor that may critically regulate the development of both the SG and adrenal chromaffin cells. In AP-2β−/− mice we observed that the SG becomes gradually hypomorphic during embryonic development (Hong et al., 2008). In the present study, we determined that AP-2β is also important for chromaffin cell development. Notably, our results indicate that the specific role and mechanism of AP-2β underlying SG and chromaffin cell development are significantly distinct. For instance, in contrast to hypomorphism of SG in AP-2β−/− mice, apparent development of chromaffin cells of the adrenal gland and TH gene expression appeared to be generally normal, suggesting that sympathetic neurons and chromaffin cells are differentially affected by AP-2β. However, the expression of DBH and PNMT was greatly reduced in chromaffin cells of AP-2β−/− mice. Most significantly, this study revealed that the level of EPI, the major neurotransmitter produced by chromaffin cells, was diminished by more than 80% in AP-2β−/− mice compared to wild type mice. It is important to note that in AP-2β−/− mice chromaffin cells’ developmental defects were not limited to the neurotransmitter phenotype. Ultrastructural analysis revealed that formation of large secretory vesicles, which is a hallmark of differentiated chromaffin cells, was significantly defective. This observation is corroborated by the result that the expression of CHGA, known to be critical for the genesis of large secretory vesicles (Huh et al., 2003; Kim et al., 2001b), is significantly diminished. Furthermore, in these mice the cellular organization of chromaffin cells appears to be abnormal at the ultrastructural level. AP-2β−/− mice die during neonatal days 1–2 and this lethality has been attributed to kidney failure and a small fraction (~10%) of newborn AP-2β−/− mice survive beyond P2, but none of them survived past P20 (Hong et al., 2008; Moser et al., 1997). Thus follow-up of the development of the adrenal gland during the postnatal period is not feasible.
Based on initial studies of AP-2α (Mitchell et al., 1991; Williams et al., 1988) it was generally assumed that AP-2α may be important for differentiation of neural crest-derived cells such as SA cells. In support of this notion, mutant zebra fish deficient in AP-2α (tfap2a) showed defect in NE neuron development in both the locus coeruleus and sympathetic neurons (Holzschuh et al., 2003). Several laboratories have shown that AP-2α is able to bind to the TH, DBH, and PNMT gene promoters and activate their transcriptional activities using in vitro assays (Ebert et al., 1998; Greco et al., 1995; Kim et al., 2001a). In addition, AP-2α was shown to be able to interact with the rat PNMT promoter and activate its activity in vitro (Ebert et al., 1998). In contrast, our data indicate that AP-2β is prominently expressed in SA lineage cells and participates in the neurotransmitter phenotype specification by regulating the expression of genes involved in neurotransmitter biosynthesis. In support of this conclusion, our ChIP assays show that AP-2β directly binds to the PNMT gene promoter in vivo in adrenal chromaffin cells, strongly suggesting that it is a direct downstream target of AP-2β. Thus, it is likely that AP-2α may have a different role during the development of neural crest lineage cell types. Conditional knockout (Brewer et al., 2004) and gain-of-function studies (Hong et al., 2008) indicated that AP-2α may be critical for melanocyte differentiation. However, we cannot completely exclude a role for AP-2α in chromaffin cell development because expression of AP-2α is detected in adrenal medulla by immunohistochemical method (Ebert et al., 1998) and by RT-PCR, although its signal is significantly weaker than AP-2β (data not shown). At present, it is not completely known how AP-2β regulates downstream genes. For example, our in vitro data indicate that AP-2β binds to rat TH, rat PNMT, and human DBH promoters (Kim et al., 2001a). In contrast, the lack of an AP-2 binding site in the 2kb upstream promoter of the rat DBH gene, which is present in the human DBH promoter, suggests that the promoter structure and regulation mechanisms of human and rat DBH genes by AP-2β may be significantly different. Taken together, this study shows that AP-2β critically regulates the neurotransmitter phenotype of adrenal chromaffin cells by controlling expression of epinephrine-synthesizing DBH and PNMT genes and also controls their maturation. The development of NE-producing sympathetic neurons, EPI-producing chromaffin cells is controversial; common precursor cells versus distinct origins (Huber, 2006). It is important to address these two possibilities but our study does not completely prove which one is true. Instead, our AP-2β knockout mice analyses focused more on its role in the regulation of expression of specific genes critical for the neurotransmitter phenotype and maturation of chromaffin cells.
Together with our previous study showing regulation of SG development, our data suggest that AP-2β plays a critical role in the development of sympathoadrenergic lineage cells. Notably, however, our results indicate that AP-2β regulates SG and chromaffin cell development in a distinct manner and that it differentially controls only a subset of genes critical for phenotypic specification and maturation, emphasizing the importance of the cellular context and functional interaction with other transcription factors. Further investigation is warranted to delineate its molecular and mechanistic insights and to establish the AP-2β’s relation to other transcription factors in the transcriptional regulatory cascade during the development of the SA system (Adams and Bronner-Fraser, 2009; Huber, 2006; Le Douarin et al., 2004; Sauka-Spengler and Bronner-Fraser, 2006).
Experimental methods
Animals
Overnight-mated female AP-2β heterozygous mice (Moser et al., 1997) were tested for the presence of a vaginal plug, which was recorded as gestation day 0.5 (e0.5). Embryos were genotyped by PCR with reverse primer (5′-TTCTCTGAACTTCGCCCACAGTG-3′) and forward primer (5′-TTCTTGGGAGGAATGTCAGTCAAC-3′) or (5′-TGGATGTGGAATGTGTGCGAGG-3′) to detect the wild type or knockout loci of AP-2β, respectively.
Immunohistochemistry (IHC)
Adrenal glands were isolated from e15.5, e18.5, and neonate mice and fixed in 4% paraformaldehyde in PBS at 4°C overnight. Adrenal glands were isolated from adult rats or mice perfused with 4% paraformaldehyde. Adrenal glands were treated with 30% sucrose overnight at 4°C prior to embedding in OCT compound and stored at −70°C. Adrenal glands were sectioned with 10 μm thickness and IHC was performed. The following primary antibodies were used: mouse anti- TH, 1: 200 (Chemicon, Temecula, CA); rabbit anti-PNMT, 1: 200 (Chemicon, Temecula, CA); rabbit anti-DBH, 1:500 (gift of Dr. Eipper); rabbit anti-chromogranin A, 1:500. For immunofluorescence microscopy, Alexa Fluor 488 or 594 (Molecular Probe, Carlsbad, CA) conjugated secondary antibodies were used to detect specific primary antibodies.
EPI measurement
Adrenal glands from newborn mice were frozen and stored at −70°C. Samples were homogenized in the presence of 0.2 M perchloric acid and 0.1 mM EDTA. Samples were disrupted by ultrasonication and centrifuged at 13,000 rpm for 15 min at 4°C. Supernatants were filtered through a 0.45 μM syringe filter and then used to measure the amount of CA by HPLC (high performance liquid chromatography). Pellets were resuspended in phosphate buffer (10 mM potassium phosphate containing 0.2% Triton X-100) and the amount of protein was determined.
Reverse transcription quantitative PCR (RT-qPCR) analysis
Total RNA from adrenal gland was prepared by treatment with Trizol Reagent (Sigma St. Louis, MO). Five μg of total RNA were mixed with oligo (dT)12–18 and superscript II (Invitrogen, Carlsbad, CA) to generate cDNA. RT-qPCR analyses were performed in duplicate using SYBR green I on a DNA engine Opticon™ (MJ Research, Waltham, MA). Primer sets are shown in Table 1.
Electron microscopy
Adrenal glands of wild-type and homozygous AP-2β knockout mice were fixed in sodium cacodylate buffer containing 2% glutaraldehyde, 2% paraformaldehyde, and 3.5% sucrose for 2 hr at 4°C. After three washes in sodium cacodylate buffer, tissues were postfixed with 1% osmium tetroxide on ice for 2 hr and washed three times with sodium cacodylate buffer. The tissues were dehydrated in an ethanol and propylene oxide series then embedded in Epon 812. Ultrathin sections were collected on collodion-coated cupper grids. The grids were stained with uranyl acetate (7 min) and lead citrate (2 min), and were viewed with a JEM 1011 (JEOL, Tokyo, Japan) electron microscope. The diameter of granules was measured using the ImageJ program. Granules’ density is presented as granule number per cytoplasm area (μm2) as described (Huh et al., 2003).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed according to the manufacturer’s protocol (Upstate, Lake Placid, NY). Briefly, ten rat adrenal glands were homogenized and cross-linked with 1% formaldehyde for 20 min and harvested in the presence of protease inhibitor (EDTA-free Complete, Roche, IN). These cells were then lysed and sonicated to generate 200–500 bp DNA fragments. One tenth of the lysates was used for input control. The remaining lysates were halved, and each half was treated with 2 μg of anti-AP-2β antibody or normal rabbit IgG as negative control (Santa Cruz Biotechnology, Santa Cruz, CA) and incubated overnight at 4°C. Complexes were captured by addition of salmon sperm DNA/Protein A agarose slurry and the precipitates were extensively washed and eluted with 1% SDS, 0.1M NaHCO3. The cross-linked protein-DNA complexes were treated with NaCl to reverse the crosslinks and the DNA was recovered by phenol extraction. PCR was performed to detect specifically bound DNA using GoTaq polymerase (Promega, Madison, WI). The following primers were used to detect AP2i, ii, iii: 5′-AGGATTGTTGACTGAGGGGTGG-3′ and 5′-GCATTTGATGGCAGCAGAGATAC-3′, 5′-GCATTAGACCCCACTCACCTGTATC-3′ and 5′-GCATCCCATCCCTTCTCTTTCC-3′, 5′-ACTGAGATGGATGGGGTGACAG-3′ and 5′-TAGTTGTTGCGGAGGTAGGCAC-3′, respectively (Fig. 7A).
Acknowledgments
We would like to thank Betty Eipper for the DBH antibody and Matthew Geringer for his critical review of the manuscript. This work was supported by NIH grants (MH48866 and MH087903).
References
- Adams MS, Bronner-Fraser M. Review: The Role of Neural Crest Cells in the Endocrine System. Endocr Pathol. 2009;20:92–100. doi: 10.1007/s12022-009-9070-6. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986;47:1079–1090. doi: 10.1016/0092-8674(86)90823-8. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T. Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002;129:2733–2747. doi: 10.1242/dev.129.11.2733. [DOI] [PubMed] [Google Scholar]
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev Biol. 2004;267:135–152. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes - from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Oulad-Abdelghani M, Bouillet P, Decimo D, Chambon P, Dolle P. AP-2.2, a novel gene related to AP-2, is expressed in the forebrain, limbs and face during mouse embryogenesis. Mech Dev. 1996;54:83–94. doi: 10.1016/0925-4773(95)00463-7. [DOI] [PubMed] [Google Scholar]
- Coupland RE, Tomlinson A. The development and maturation of adrenal medullary chromaffin cells of the rat in vivo: a descriptive and quantitative study. Int J Dev Neurosci. 1989;7:419–438. doi: 10.1016/0736-5748(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Ebert SN, Ficklin MB, Her S, Siddall BJ, Bell RA, Ganguly K, Morita K, Wong DL. Glucocorticoid-dependent action of neural crest factor AP-2: stimulation of phenylethanolamine N-methyltransferase gene expression. J Neurochem. 1998;70:2286–2295. doi: 10.1046/j.1471-4159.1998.70062286.x. [DOI] [PubMed] [Google Scholar]
- Eckert D, Buhl S, Weber S, Jager R, Schorle H. The AP-2 family of transcription factors. Genome Biol. 2005;6:246. doi: 10.1186/gb-2005-6-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsberger U, Esposito L, Partimo S, Huber K, Franke A, Bixby JL, Kalcheim C, Unsicker K. Expression of neuronal markers suggests heterogeneity of chick sympathoadrenal cells prior to invasion of the adrenal anlagen. Cell Tissue Res. 2005;319:1–13. doi: 10.1007/s00441-004-0996-1. [DOI] [PubMed] [Google Scholar]
- Feng W, Williams T. Cloning and characterization of the mouse AP-2 epsilon gene: a novel family member expressed in the developing olfactory bulb. Mol Cell Neurosci. 2003;24:460–475. doi: 10.1016/s1044-7431(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Greco D, Zellmer E, Zhang Z, Lewis E. Transcription factor AP-2 regulates expression of the dopamine beta-hydroxylase gene. J Neurochem. 1995;65:510–516. doi: 10.1046/j.1471-4159.1995.65020510.x. [DOI] [PubMed] [Google Scholar]
- Gut P, Huber K, Lohr J, Bruhl B, Oberle S, Treier M, Ernsberger U, Kalcheim C, Unsicker K. Lack of an adrenal cortex in Sf1 mutant mice is compatible with the generation and differentiation of chromaffin cells. Development. 2005;132:4611–4619. doi: 10.1242/dev.02052. [DOI] [PubMed] [Google Scholar]
- Hilger-Eversheim K, Moser M, Schorle H, Buettner R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene. 2000;260:1–12. doi: 10.1016/s0378-1119(00)00454-6. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Barrallo-Gimeno A, Ettl AK, Durr K, Knapik EW, Driever W. Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development. 2003;130:5741–5754. doi: 10.1242/dev.00816. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Huh YH, Choi HJ, Ding Y, Kang UJ, Kim KS. Regulation of the noradrenaline neurotransmitter phenotype by the transcription factor AP-2b. J Biol Chem. 2008;283:16860–16867. doi: 10.1074/jbc.M709106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol. 2006;298:335–343. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Huber K, Karch N, Ernsberger U, Goridis C, Unsicker K. The role of Phox2B in chromaffin cell development. Dev Biol. 2005;279:501–508. doi: 10.1016/j.ydbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Huh YH, Jeon SH, Yoo SH. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- Kim HS, Hong SJ, LeDoux MS, Kim KS. Regulation of the tyrosine hydroxylase and dopamine beta-hydroxylase genes by the transcription factor AP-2. J Neurochem. 2001a;76:280–294. doi: 10.1046/j.1471-4159.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001b;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- Lan MS, Breslin MB. Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. FASEB J. 2009;23:2024–2033. doi: 10.1096/fj.08-125971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Grant NJ. Molecular markers of sympathoadrenal cells. Cell Tissue Res. 1999;298:185–206. doi: 10.1007/pl00008810. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet. 2000;25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Timmons PM, Hebert JM, Rigby PW, Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991;5:105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Wang C, Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987;50:847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development. 2009;136:3575–3584. doi: 10.1242/dev.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Imhof A, Pscherer A, Bauer R, Amselgruber W, Sinowatz F, Hofstadter F, Schule R, Buettner R. Cloning and characterization of a second AP-2 transcription factor: AP-2 beta. Development. 1995;121:2779–2788. doi: 10.1242/dev.121.9.2779. [DOI] [PubMed] [Google Scholar]
- Moser M, Pscherer A, Roth C, Becker J, Mucher G, Zerres K, Dixkens C, Weis J, Guay-Woodford L, Buettner R, Fassler R. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev. 1997;11:1938–1948. doi: 10.1101/gad.11.15.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Chazaud C, Dolle P, Chambon P. AP-2.2: a novel AP-2-related transcription factor induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Exp Cell Res. 1996;225:338–347. doi: 10.1006/excr.1996.0184. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Development and evolution of the migratory neural crest: a gene regulatory perspective. Curr Opin Genet Dev. 2006;16:360–366. doi: 10.1016/j.gde.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Huber K, Schutz G, Kalcheim C. The chromaffin cell and its development. Neurochem Res. 2005;30:921–925. doi: 10.1007/s11064-005-6966-5. [DOI] [PubMed] [Google Scholar]
- Wang HV, Vaupel K, Buettner R, Bosserhoff AK, Moser M. Identification and embryonic expression of a new AP-2 transcription factor, AP-2 epsilon. Dev Dyn. 2004;231:128–135. doi: 10.1002/dvdy.20119. [DOI] [PubMed] [Google Scholar]
- Werling U, Schorle H. Transcription factor gene AP-2 gamma essential for early murine development. Mol Cell Biol. 2002;22:3149–3156. doi: 10.1128/MCB.22.9.3149-3156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008;135:473–481. doi: 10.1242/dev.011783. [DOI] [PubMed] [Google Scholar]
- Williams T, Admon A, Luscher B, Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988;2:1557–1569. doi: 10.1101/gad.2.12a.1557. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–241. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- Zhao F, Satoda M, Licht JD, Hayashizaki Y, Gelb BD. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J Biol Chem. 2001;276:40755–40760. doi: 10.1074/jbc.M106284200. [DOI] [PubMed] [Google Scholar]