Hypothesis
In subjects with previous preeclampsia, differences in cardiovascular and/or blood biochemical parameters are present in the non-pregnant state and that a simultaneous assessment of multiple derived indices better differentiates between women with or without prior preeclampsia.
We examined 18 prior preeclamptic and 50 prior uncomplicated pregnancies, ~16 months post partum. Cardiovascular assessment included: (1) systemic hemodynamics and mechanics (Doppler echocardiography, tonometry, oscillometric sphygmomanometry) (2) endothelial function (plethysmography) (3) left ventricular properties (echocardiography), and (4) blood biochemical analyses.
Compared to women with prior uncomplicated pregnancies, prior preeclamptics had higher mean (80±1 vs. 86±3 mmHg, P=0.04) and diastolic (64±1 vs. 68±2 mmHg; P=0.04) pressures and total vascular resistance (1562±37 vs. 1784±114 dyne•s/cm5; P=0.03). Systolic blood pressure, arterial compliance, and left ventricular properties were not different. While heart-to-femoral pulse wave velocity was not different, heart-to-brachial pulse wave velocity tended to be faster in prior preeclamptics (374±8 vs. 404±20 cm/s; P=0.06). Stress-induced increase in forearm blood flow was less in prior preeclamptics (245±21% vs. 136±22%; P=0.01), indicating impaired endothelial function. No significant differences were observed in markers of endothelial activation, dyslipidemia, or oxidative stress; prior preeclamptics tended to have higher glucose level (58.7±1.9 vs. 95±5.2 mg/dl; P=0.06). Logistic regression analysis indicated that a simultaneous evaluation of multiple derived indices better discriminated between the two groups. The differences in the prior preeclamptic group are in directions known to be associated with greater cardiovascular disease risk later in life.
Keywords: preeclampsia, cardiac function, blood pressure, plethysmography, vascular resistance, compliance, endothelial function
Introduction
Preeclampsia is a pregnancy disorder, characterized by new onset elevation in blood pressure and proteinuria that occurs in 5–8% of pregnancies.1 Both maternal and fetal complications occur with this condition, including high rates of preterm delivery and intra-uterine growth restriction.2 Most of the pathological conditions associated with preeclampsia seem to resolve after delivery. However there is growing evidence that there are differences during the post partum period between subjects with prior preeclampsia and prior uncomplicated pregnancy.
Women with a history of preeclampsia are more likely to develop cardiovascular disease later in life.3–10 Some studies have looked at general hemodynamic parameters (e.g. blood pressure, cardiac output, heart rate), while others have measured biochemical markers of dysfunction (e.g. endothelial dysfunction, dyslipidemia, oxidative stress, and glucose homeostasis). The objective of this study was to provide a comprehensive cardiovascular and biochemical characterization of women with a history of preeclampsia versus women with an uncomplicated first pregnancy. Specifically, systemic arterial hemodynamic and global and regional mechanical properties, left ventricular structure, endothelial function, and several biochemical markers were examined. We hypothesized that, in subjects with a history of preeclampsia, there are differences in many of these parameters during the non-pregnant state and that a simultaneous assessment of multiple derived indices will better differentiate between the two groups of women.
Methods
Study Subjects
The study protocol was approved by the University of Pittsburgh Institutional Review Board, and all subjects provided written informed consent to participate. This cross-sectional study recruited study subjects from a list of women at the Magee-Womens Hospital central repository which created a list of names and addresses of women who had delivered a baby in the past 36 months. Over 6400 women were sent study fliers and given a phone number to call if interested in participating. Of these, more than 760 women were screened over the phone for eligibility. To participate in the study, subjects must meet the following inclusion criteria (1) have delivered a singleton pregnancy 6–36 months prior to test date, (2) be premenopausal, non-smoking and (3) currently not breastfeeding. Potential subjects were excluded for underlying medical conditions, specifically, pre-existing cardiac disease, diabetes mellitus, renal disease, systemic lupus, or a known disorder of lipid metabolism. Of the large pool of women screened over the phone, 71 women were invited to participate and scheduled study visits.
Once enrolled, two additional criteria had to be met prior to obtaining cardiovascular measurements. Subjects were excluded if they were currently pregnant or had >1+ protein detected on urine analysis. Two women were excluded for high protein; another woman was excluded due to pregnancy. Sixty-eight healthy women (18 with prior preeclampsia and 50 prior uncomplicated pregnancies) between the ages of 18 and 40 years were enrolled in the study. Subjects were evaluated in the Clinical Research Center of Magee-Womens Hospital in a quiet, temperature controlled room.
Preeclampsia during the first pregnancy was confirmed by the presence of gestational hypertension, proteinuria, and hyperuricemia beginning after the 20th week of pregnancy, with resolution of gestational hypertension and proteinuria post partum. We include hyperuricemia in our classification of preeclampsia as it identifies women with a greater frequency of unfavorable pregnancy outcomes, including preterm birth and small for gestational age infants.11 Gestational hypertension was defined as a new onset increased blood pressure to an absolute blood pressure ≥140 mmHg systolic and/or ≥90 mmHg diastolic after 20 weeks of gestation. Proteinuria was defined as ≥ 300 mg per 24-hour urine collection, ≥ 2+ protein on voided urine sample, ≥ 1+ protein on catheterized urine specimen, or a protein-creatinine ratio of ≥ 0.3. Hyperuricemia was defined as plasma uric acid concentration ≥ 1 SD above reference values at the gestational age the sample was obtained (e.g. term, > 5.5 mg/dl).12 Diagnosis of preeclampsia was determined retrospectively based on medical chart review by a jury of research and clinical investigators.
Experimental Measurements
At the study visit, the subjects’ demographic information (e.g., height, weight, age, prior pregnancy information) was recorded and urine and fasting blood samples were collected. The following experimental measurements were obtained with the woman supine: (1) vascular segment lengths were measured along the body surface, using the sternal notch as a landmark (heart-to-carotid, heart-to-femoral, and heart-to-brachial), (2) brachial artery blood pressure and heart rate by oscillometric sphygmomanometry (Critikon Dinamap, GE Healthcare, Wakesha, WI USA), (3) pressure waveforms at various vascular sites (carotid, femoral, and brachial) by applanation tonometry (Sphygmocor, AtCor Medical, Itasca, IL USA), (4) 2D, M-mode, and Doppler echocardiographic assessment of the left ventricle (GE Vivid 7, GE Healthcare, Wakesha, WI USA), and (5) endothelial function by venous occlusion plethysmography (D. E. Hokanson, Bellevue, WA USA). Biochemical analyses of blood included markers of endothelial dysfunction (cellular fibronectin, E-selectin), dyslipidemia (triglycerides, apo B, free fatty acids, total cholesterol, HDL, glycerol), oxidative stress (malondialdehyde), and glucose homeostasis (insulin, glucose). Additional methodological details (including a specific protocol for obtaining blood pressure measurements), can be found in the Online Supplementary material.
Calculated Variables
Systemic hemodynamic variables (various mean pressures and flows) and steady (total vascular resistance, TVR) and pulsatile (global arterial compliance) components of systemic arterial load were calculated from measured data (carotid pressure waveform, brachial artery cuff blood pressures, aortic blood flow). Pulse wave velocity (PWV) was used to characterize regional vascular stiffness. Echocardiography-based assessment of the left ventricle included measurements of systolic and diastolic chamber diameters and wall thicknesses and the calculation of muscle mass. Endothelial function was quantified using forearm blood flow (FBF) measurements at baseline and under mental stress.
Statistical Analysis
All statistical analyses were performed using the SPSS software (IBM Corporation, Somers, NY USA). Data are presented as mean ± standard error of the mean (SEM). Univariate (simple) logistic models were used to assess differences in individual variables, with P<0.10 denoting marginal significance and P<0.05 statistical significance. Multiple logistic regression was performed via both a forward selection algorithm and inclusion of all terms selected a priori to discriminate between the two groups on the basis of multiple variables. A forward selection approach was implemented to include only variables which were independently associated with outcome after adjusting for other variables (beginning with the most significant on a univariate level) and to avoid co-linearity. The receiver operating characteristic (ROC) curve was constructed to judge the performance of the various logistic models.
Results
Demographic data are presented in Table 1. There were no significant differences between the two groups in terms of age, body size, and time since delivery. Although subjects in the prior uncomplicated pregnancy group tended to be taller (P<0.01), there were no statistically significant differences in the calculated values of body mass index (BMI) or body surface area (BSA).
Table 1.
Demographic Findings
| Variable | Prior Uncomplicated Pregnancy n=50 | Prior Preeclamptic Pregnancy n=18 | P Value |
|---|---|---|---|
| Age (yrs) | 29.9 ± 0.6 | 28.1 ± 1.3 | 0.176 |
| Race | 46 caucasian 4 african american |
15 caucasian 3 african american |
|
| Height (cm) | 167 ± 1 | 161 ± 2 | 0.004* |
| Weight (kg) | 73.3 ± 2.3 | 68.7 ± 4.8 | 0.331 |
| BMI (kg•m−2) | 26.3 ± 0.8 | 26.4 ± 1.7 | 0.959 |
| BSA (m2) | 2.52 ± 0.06 | 2.35 ± 0.12 | 0.407 |
| Post Partum (mo) | 16.5 ± 0.6 | 16.5 ± 1.1 | 0.964 |
BMI indicates body mass index, BSA, body surface area. Data are mean ± SEM.
P<0.05, prior preeclamptic versus prior uncomplicated pregnancy by univariate logistic regression.
Data regarding systemic arterial hemodynamics and mechanical properties are presented in Table 2. Compared to the prior uncomplicated pregnancy group, women with prior preeclampsia had elevated MAP mostly due to a rise in diastolic blood pressure (both of which were significant at P=0.04). Systolic blood pressure was also higher in the prior preeclamptic group but this was not significant (P=0.07). There were no significant differences in heart rate and cardiac output between the two groups. Total vascular resistance (TVR) was significantly elevated (P=0.03) in the prior preeclamptic group; however two measures of global arterial compliance (stroke volume to pulse pressure and area method) were not significantly different. Regional vascular stiffness, as quantified by PWV, was not different for large vessels (no significant difference in heart-to-femoral or heart-to-carotid PWV). Heart-to-brachial PWV tended to be higher (P=0.06) in women with a history of preeclampsia, which suggests that smaller vessels may be stiffer in this group.
Table 2.
Systemic Arterial Hemodynamic and Mechanical Properties
| Variable | Prior Uncomplicated Pregnancy | Prior Preeclamptic Pregnancy | P Value |
|---|---|---|---|
| HR (bpm) | 65 ± 1 | 68 ± 3 | 0.292 |
| Systolic BP (mmHg) | 99 ± 2 | 107 ± 4 | 0.069 |
| Diastolic BP (mmHg) | 64 ± 1 | 68 ± 2 | 0.037* |
| MAP (mmHg) | 80 ± 1 | 86 ± 3 | 0.038* |
| PP (mmHg) | 36 ± 1 | 39 ±3 | 0.240 |
| CO (L/min) | 4.2 ± 0.1 | 4.1 ± 0.2 | 0.491 |
| SV (ml) | 66 ± 2 | 62 ± 4 | 0.251 |
| TVR (dyne•s/cm5) | 1562 ± 37 | 1784 ± 114 | 0.027* |
| ACG (ml/mmHg) (Area method) | 1.7 ± 0.1 | 1.5 ± 0.2 | 0.410 |
| ACG (ml/mmHg) (SV-to-PP ratio) | 2.0 ± 0.1 | 1.8 ± 0.2 | 0.258 |
| Heart-to-Carotid PWV (cm/s) | 326 ± 14 | 343 ± 31 | 0.561 |
| Heart-to-Femoral PWV (cm/s) | 254 ± 7 | 239 ± 8 | 0.255 |
| Heart-to-Brachial PWV (cm/s) | 374 ± 8 | 405 ± 20 | 0.061 |
HR indicates heart rate; BP, blood pressure; MAP, mean arterial pressure; CO, cardiac output; SV, stroke volume; PP, pulse pressure; ACG, global arterial compliance; TVR, total vascular resistance; and PWV, pulse wave velocity. Data are mean ± SEM.
P<0.05, prior preeclamptic versus prior uncomplicated pregnancy by univariate logistic regression.
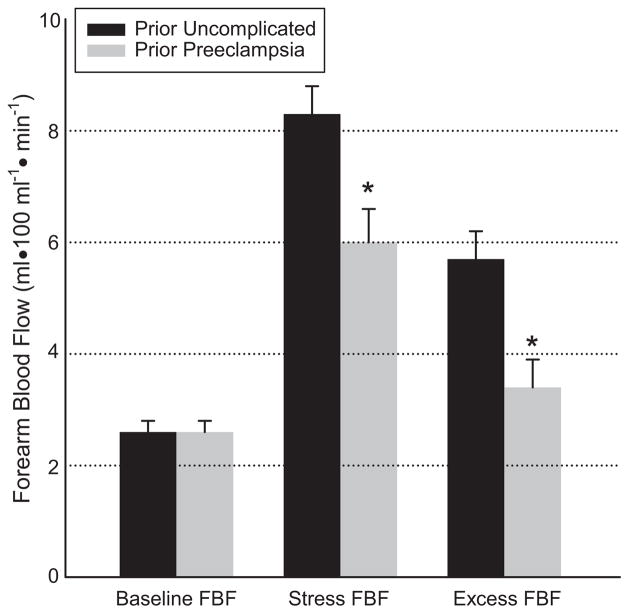
Results for endothelial function testing by venous occlusion plethysmography are presented in Figure 1. Baseline FBF was comparable between the two groups. The mental stress test led to a similar increase in heart rate and blood pressure in the two groups, with blood pressure higher in the prior preeclamptic group (data not shown). Stress-induced blood flow is characterized by three measurements: (1) stress FBF, (2) excess FBF (i.e., difference between stress FBF and baseline FBF), and (3) percent increase of FBF (i.e., excess FBF divided by baseline FBF). Stress and excess FBF were significantly lower in the prior preeclamptic group compared to the uncomplicated pregnancy group (stress FBF 6.0±0.6 vs. 8.3±0.5 ml•100 ml−1•min−1; P=0.02 and excess FBF 3.4±0.5 vs. 5.7±0.5 ml•100 ml−1•min−1; P=0.01). Percent increase in FBF was significantly attenuated in the prior preeclamptic group (245%±21% vs. 136%±22% P=0.01). These data suggest impaired endothelial function in the prior preeclamptic group.
Figure 1.
Endothelial function by venous occlusion plethysmography. FBF indicates forearm blood flow. Data are mean ± SEM. *P<0.05, prior preeclampsia versus prior uncomplicated pregnancy by univariate logistic regression.
No differences in left ventricular properties were observed; these data are presented in the Online Supplement (Table S1, supplementary material).
The values of the biochemical markers are presented in Table 3. No differences were detected in the two markers for endothelial dysfunction (cellular fibronectin or E-selectin), dyslipidemia (triglycerides, apo B, free fatty acids, total cholesterol, HDL, and glycerol), or oxidative stress (malondialdehyde). Circulating concentrations of insulin were similar between the two groups, but glucose levels tended to be elevated in prior preeclamptics (P=0.06).
Table 3.
Biochemical Markers
| Variable | Prior Uncomplicated Pregnancy | Prior Preeclamptic Pregnancy | P Value |
|---|---|---|---|
| Cellular Fibronectin (μg/ml) | 21 ± 2 | 24 ± 3 | 0.337 |
| E-selectin (ng/ml) | 29 ± 2 | 34 ± 4 | 0.241 |
| Triglycerides (mg/dl) | 84 ± 6 | 73 ± 10 | 0.363 |
| apo B (mg/dl) | 79 ± 3 | 80 ± 5 | 0.824 |
| Free Fatty Acids (mEq/L) | 0.29 ± 0.03 | 0.36 ± 0.04 | 0.201 |
| Total Cholesterol (mg/dl) | 180 ± 5 | 176 ± 12 | 0.726 |
| High Density Lipoprotein (mg/dl) | 52 ± 2 | 54 ± 3 | 0.449 |
| Glycerol (mg/dl) | 7.6 ± 0.6 | 5.9 ± 0.8 | 0.144 |
| Insulin (μU/ml) | 5.9 ± 0.5 | 6.5 ± 1.2 | 0.636 |
| Glucose (mg/dl) | 86 ± 2 | 95 ± 5 | 0.056 |
| HOMA Index | 1.28 ± 0.13 | 1.65 ± 0.47 | 0.312 |
| Malondialdehyde (μM) | 0.57 ± 0.03 | 0.55 ± 0.05 | 0.700 |
HOMA indicates homeostatic model assessment. Data are mean ± SEM.
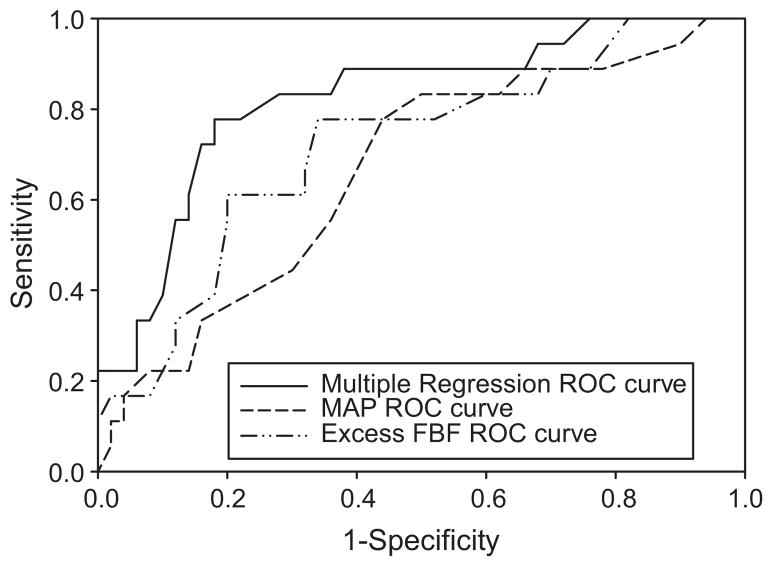
Odds ratios and P values for logistic regression modeling are shown in Table 4. The specific variables listed in Table 4 were selected based upon physiological relevance; in cases where multiple variables represented the same or similar construct (e.g. BMI and BSA) we considered both physiological and statistical significance. Multiple logistic regression models were run with and without a forward selection approach. The purpose of this step was to build a model that could discriminate between the two groups of women. The three variables selected from the forward selection process were then specified as predictors of preeclampsia (BSA, MAP, and excess FBF). Although not significant on the univariate level, BSA got included in the final multiple logistic regression model based on having the highest level of significance after adjusting for other already-included covariates in the forward selection process. An ROC curve (shown in Figure 2) was also generated to display the sensitivity and specificity across a range of cut-off values. Using a predicted proportion of 0.40 as the cut-off value, the model is able to discriminate 81% of the women correctly; with a sensitivity of 72% and a specificity of 84%. Also included on this graph are individual ROC curves for MAP and excess FBF, which reflect less optimal discrimination.
Table 4.
Logistic Regression Modeling
| Variable | Logistic Regression Univariate | Multiple Regression Forward Selection | Multiple Regression Without Selection | |||
|---|---|---|---|---|---|---|
| OR | P value | OR | P value | OR | P value | |
| TVR (dyne•s/cm5) | 1.002 | 0.027 | ---- | ---- | 1.002 | 0.252 |
| Age (yrs) | 0.925 | 0.176 | ---- | ---- | 0.844 | 0.083 |
| BSA (m2) | 0.177 | 0.407 | 0.139 | 0.029 | 0.190 | 0.181 |
| Diastolic BP (mmHg) | 1.094 | 0.037 | ---- | ---- | 0.595 | 0.258 |
| MAP (mmHg) | 1.065 | 0.038 | 1.152 | 0.005 | 1.890 | 0.138 |
| PP (mmHg) | 1.029 | 0.240 | ---- | ---- | 0.845 | 0.297 |
| ACG (ml/mmHg) | 1.000 | 0.410 | ---- | ---- | 1.002 | 0.191 |
| H-B PWV (cm/s) | 1.008 | 0.061 | ---- | ---- | 1.015 | 0.128 |
| CFn (μg/ml) | 1.022 | 0.337 | ---- | ---- | 0.984 | 0.657 |
| Glycerol (mg/dl) | 0.884 | 0.144 | ---- | ---- | 0.790 | 0.079 |
| Glucose (mg/dl) | 1.036 | 0.056 | ---- | ---- | 1.061 | 0.058 |
| Excess FBF (ml•100ml−1•min−1) | 0.695 | 0.007 | 0.576 | 0.004 | 0.617 | 0.077 |
OR indicates odds ratio; TVR, total vascular resistance; BSA, body surface area; BP, blood pressure; MAP, mean arterial pressure; PP, pulse pressure; ACG, global arterial compliance (area method); H-B PWV, heart-to-brachial pulse wave velocity; CFn, cellular fibronectin; and FBF, forearm blood flow.
Figure 2.
Receiver operating characteristic (ROC) curves for various logistic regression analyses. The multiple logistic regression equation used to construct the ROC curve (solid line) was logit(y) = −5.56 + (−0.551•excess FBF) + (0.141•MAP) + (−1.972•BSA), with the area under the curve of 0.82. Univariate logistic regression equation used to construct the ROC curve for excess FBF (dash-to-dot line) was logit(y) = 0.573 + (−0.364•excess FBF); with the area under the curve of 0.71. Univariate logistic regression equation used to construct the ROC curve for MAP (dash line) was logit(y) = −6.269 + (0.063•MAP), with the area under the curve of 0.66.
Discussion
The present study provides a comprehensive functional and biochemical assessment of the cardiovascular status of women with prior preeclamptic and uncomplicated pregnancies. At ~16 months post partum, we found differences between these two groups, with prior preeclamptics having higher MAP and diastolic blood pressure, higher TVR, tendency towards higher peripheral (small vessel) vascular stiffness, endothelial dysfunction, and marginal insulin resistance. A simultaneous consideration of multiple measures resulted in better discrimination between the two groups.
Arterial Hemodynamic and Mechanical Properties
During pregnancy, blood pressure decreases slightly, increasing towards nonpregnant values as pregnancy progresses.13 A hallmark of preeclampsia is new onset elevation of blood pressure during the gestational period, which is expected to fall after delivery. We observed higher blood pressures in prior preeclamptics during the post partum period, consistent with other studies.14–17 Higher blood pressure in prior preeclamptics, although still well within the normal range, may precede development of hypertension later in life.4–9
TVR rapidly decreases during the first trimester of uncomplicated (normal) pregnancy and this decrease is maintained until the end of pregnancy.13 A common feature of preeclamptic pregnancy is the significantly higher TVR during late gestation (third trimester) as compared to that in uncomplicated pregnancy.18 Our data indicate that TVR is also higher in women with a history of preeclampsia during the post partum state, consistent with other published data.5,14 If TVR remains elevated over time, the risk for developing heart disease also increases.5,14
In uncomplicated pregnancy, vasodilation (i.e., reduced TVR) is accompanied by an increase in global arterial compliance (i.e., reduced vascular stiffness).13 This increased global arterial compliance is adaptive from multiple perspectives:13,19 it accommodates greater intravascular volume without increasing MAP, helps maintain the efficiency of LV-to-arterial system mechanical energy transfer, and helps preserve coronary perfusion pressure by reducing aortic diastolic pressure decay. Global arterial compliance is significantly lower during late gestation in preeclamptic subjects, even after adjusting for higher blood pressures.18 The current data show no difference in global arterial compliance between women with prior preeclampsia and uncomplicated pregnancies, indicating that unlike TVR, the gestational differences in global compliance do not persist into the post partum period.
PWV has been used to quantify regional vascular stiffness – the higher the PWV higher the stiffness and vice versa. A longitudinal study has reported that both central and peripheral PWVs drop between the first and second trimesters of uncomplicated pregnancy, returning back to control values one month after delivery.20 Cross-sectional studies indicate that both central and peripheral PWVs are elevated in the preeclamptic pregnancy as compared to the uncomplicated pregnancy.21–23 Regarding the post partum period, some studies have reported no differences in central PWV.16 However, a recent report indicates increased central PWV in the prior preeclamptic group.24 In our study population, we observed no difference in central PWV between the groups; however, heart-to-brachial PWV tended to be higher, suggesting peripheral vessels may continue to be stiffer during the post partum state in women with prior preeclampsia.
Arterial Endothelial Function
During pregnancy, endothelial function is improved such that forearm plethysmography-based indices are increased.25 Endothelial dysfunction appears to be a central component of the pathophysiology of preeclampsia26 and flow mediated dilation has also been shown to be reduced during early pregnancy in women who later develop preeclampsia.25 In our study, endothelial function was significantly lower post partum in women with a history of preeclampsia. This is consistent with previous reports17,27 and indicates that preeclampsia-associated endothelial dysfunction persists after pregnancy.
Biochemical Markers
Several biochemical markers were examined; all having important physiological significance in pregnancy and preeclampsia. Markers of endothelial dysfunction (e.g., cellular fibronectin and E-selectin) and oxidative stress (malondialdehyde and uric acid) are elevated during preeclamptic pregnancy.28–30 Lipid metabolism changes dramatically during pregnancy,31 with increases in circulating concentrations of triglycerides and cholesterols; and these are even greater during preeclamptic pregnancy.30,32 Free fatty acid levels are elevated prior to the onset of clinical findings of preeclampsia and contribute to endothelial dysfunction.33 In the current study, endothelial dysfunction, dyslipidemia, and oxidative stress markers were not different, consistent with other post partum studies.34–37 There are however, several studies demonstrating differences in these parameters. It is possible that the population of prior preeclamptics studied may not have been homogeneous.
Pregnancy is associated with minimal changes in glucose homeostasis during early-to-mid gestation, with maternal insulin resistance developing in the third trimester.38–39 Insulin resistance occurs in preeclamptic pregnancies much earlier in gestation.38–39 Most, but not all, studies have reported that insulin resistance is also present during the post partum period in women with a history of preeclampsia.2,4,15,17,34–35,37 Our results are somewhat difficult to interpret with regards to insulin resistance. The hallmark of insulin resistance is elevated insulin in relationship to glucose. This is usually assessed, as we did, in fasting samples. In our study only glucose seemed to be elevated, but this did not reach statistical significance (P=0.06). Although HOMA and insulin values were in the direction of insulin resistance, the differences with respect to previously uncomplicated pregnant women were small.
Left Ventricular Structure
During mid to late gestation in uncomplicated pregnancy, the left ventricular end-diastolic diameter, muscle mass, and outflow tract diameter are higher than values during the post partum period.13 Similar changes are observed in preeclamptic pregnancy.40 We did not observe any differences in the indices of left ventricular structure during the post partum state between the two groups (prior uncomplicated pregnancy and prior preeclamptics).
Utility of the Multivariate Characterization
A number of variables seem to be different for the prior preeclamptic group, with some reaching statistical significance based on the univariate analysis. We conducted the multiple logistic regression analysis to examine whether the two groups can be better discriminated when several variables are considered simultaneously. As discussed in the Results section, the logistic regression model containing BSA, excess FBF, and MAP provided the best discrimination (area under the ROC curve = 0.82). The discrimination based on a single variable was not as good, e.g.: the area under the ROC curve using excess FBF alone was 0.71 and based on MAP alone was 0.66 (Figure 2). Because of the relatively small number of observations (total observations = 68; 50 for prior uncomplicated pregnancies and 18 for prior preeclampsia), we did not test the predictive ability of the regression model on an independent dataset. In addition, it is likely that a larger sample size will yield a different, and larger, set of variables in the model. However, the current multiple regression model makes physical sense: the odds ratio of having had a prior preeclamptic pregnancy increases with BSA and MAP and decreases with excess FBF. Thus, we emphasize the point that the prior preeclamptic group is best discriminated from the prior uncomplicated pregnancy group during the post partum state when multiple variables are considered simultaneously; the definitive identification of these discriminatory variables and unbiased prediction in an independent test set should be investigated further.
Preeclampsia and Cardiovascular Risk Later in Life
Several studies have demonstrated that having preeclampsia is associated with an increased risk of cardiovascular disease later in life.3–4,8,10,36–37 Preeclamptic pregnancy is associated with characteristic cardiovascular and biochemical alterations: vasomotor dysfunction, hypertension, endothelial damage, inflammation, and metabolic disturbances (oxidative stress, dyslipidemia, and insulin resistance). These alterations are known predictors of cardiovascular risk later in life. We observed that some of these alterations continue during the post partum state in prior preeclamptic women. Whether these are the prior preeclamptic women at a higher risk of cardiovascular disease later in life compared to women with preeclamptic pregnancy without these differences post partum is not known.
Supplementary Material
Perspectives.
There are cardiovascular functional differences ~16 months post partum among women with prior preeclampsia compared to women with previous uncomplicated pregnancies. The observed differences in the prior preeclamptic group are in the direction associated with greater cardiovascular disease risk later in life. It is presently unclear whether these differences are the remnants of the changes due to the preeclamptic pregnancy or whether they were present before pregnancy.
Acknowledgments
Sources of Funding
All studies were conducted at the Clinical Research Center, Magee-Womens Hospital, Pittsburgh, PA. Primary support for this project was provided by P01 HD30367, a grant from the National Institutes of Health (NIH). Additional support was provided by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of NIH, and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
The authors would like to thank Dr. Patricia Agatisa (Research Coordinator, Department of Bioethics, Cleveland Clinic, Cleveland, OH) for assistance with analyzing and interpreting the plethysmography data.
Footnotes
DISCLOSURES
None.
References
- 1.Cunningham FG, Lindheimer MD. Hypertension in pregnancy. N Engl J Med. 1992;326:927–932. doi: 10.1056/NEJM199204023261405. [DOI] [PubMed] [Google Scholar]
- 2.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 3.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42:39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- 5.Spaanderman ME, Ekhart TH, van Eyck J, Cheriex EC, de Leeuw PW, Peeters LL. Latent hemodynamic abnormalities in symptom-free women with a history of preeclampsia. Am J Obstet Gynecol. 2000;182:101–107. doi: 10.1016/s0002-9378(00)70497-2. [DOI] [PubMed] [Google Scholar]
- 6.Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 7.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 10.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, Powers RW. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–1269. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 12.Hill LM, Furness C, Dunlop W. Diurnal variation of serum urate in pregnancy. Br Med J. 1977;2:1520. doi: 10.1136/bmj.2.6101.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poppas A, Shroff SG, Korcarz CE, Hibbard JU, Berger DS, Lindheimer MD, Lang RM. Serial assessment of the cardiovascular system in normal pregnancy. Role of arterial compliance and pulsatile arterial load. Circulation. 1997;95:2407–2415. doi: 10.1161/01.cir.95.10.2407. [DOI] [PubMed] [Google Scholar]
- 14.Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873–880. doi: 10.1161/HYPERTENSIONAHA.108.117358. [DOI] [PubMed] [Google Scholar]
- 15.Wolf M, Hubel CA, Lam C, Sampson M, Ecker JL, Ness RB, Rajakumar A, Daftary A, Shakir AS, Seely EW, Roberts JM, Sukhatme VP, Karumanchi SA, Thadhani R. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89:6239–6243. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- 16.Ronnback M, Lampinen K, Groop PH, Kaaja R. Pulse wave reflection in currently and previously preeclamptic women. Hypertens Pregnancy. 2005;24:171–180. doi: 10.1081/PRG-200059871. [DOI] [PubMed] [Google Scholar]
- 17.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 18.Hibbard JU, Korcarz CE, Nendaz GG, Lindheimer MD, Lang RM, Shroff SG. The arterial system in pre-eclampsia and chronic hypertension with superimposed pre-eclampsia. BJOG. 2005;112:897–903. doi: 10.1111/j.1471-0528.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 19.Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990;76:1061–1069. [PubMed] [Google Scholar]
- 20.Oyama-Kato M, Ohmichi M, Takahashi K, Suzuki S, Henmi N, Yokoyama Y, Kurachi H. Change in pulse wave velocity throughout normal pregnancy and its value in predicting pregnancy-induced hypertension: a longitudinal study. Am J Obstet Gynecol. 2006;195:464–469. doi: 10.1016/j.ajog.2006.01.104. [DOI] [PubMed] [Google Scholar]
- 21.Elvan-Taspinar A, Franx A, Bots ML, Bruinse HW, Koomans HA. Central Hemodynamics of hypertensive disorders in pregnancy. American Journal of Hypertension. 2004;17:941–946. doi: 10.1016/j.amjhyper.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Tihtonen KMH, Koobi T, Uotila JT. Arterial stiffness in preeclamptic and chronic hypertensive pregnancies. Eur J Obstet Gyn R B. 2006;128:180–186. doi: 10.1016/j.ejogrb.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Kaihura C, Savvidou MD, Anderson JM, McEniery CM, Nicolaides KH. Maternal arterial stiffness in pregnancies affected by preeclampsia. Am J Physiol Heart Circ Physiol. 2009;297:H759–764. doi: 10.1152/ajpheart.01106.2008. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Watanabe Y, Arima H, Kobayashi K, Ohno Y, Kanno Y. Short- and long-term prognosis of blood pressure and kidney disease in women with a past history of preeclampsia. Clin Exp Nephrol. 2008;12:102–109. doi: 10.1007/s10157-007-0018-1. [DOI] [PubMed] [Google Scholar]
- 25.Anim-Nyame N, Sooranna SR, Johnson MR, Gamble J, Steer PJ. A longitudinal study of resting peripheral blood flow in normal pregnancy and pregnancies complicated by chronic hypertension and pre-eclampsia. Cardiovasc Res. 2001;50:603–609. doi: 10.1016/s0008-6363(01)00236-x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 27.Agatisa PK, Ness RB, Roberts JM, Costantino JP, Kuller LH, McLaughlin MK. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;286:H1389–1393. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 28.Powers RW, Evans RW, Ness RB, Crombleholme WR, Roberts JM. Homocysteine and cellular fibronectin are increased in preeclampsia, not transient hypertension of pregnancy. Hypertens Pregnancy. 2001;20:69–77. doi: 10.1081/PRG-100104173. [DOI] [PubMed] [Google Scholar]
- 29.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 30.Lorentzen B, Henriksen T. Plasma lipids and vascular dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:33–39. doi: 10.1055/s-2007-1016250. [DOI] [PubMed] [Google Scholar]
- 31.Montelongo A, Lasuncion MA, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992;41:1651–1659. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- 32.Sattar N, Clark P, Greer IA, Shepherd J, Packard CJ. Lipoprotein (a) levels in normal pregnancy and in pregnancy complicated with pre-eclampsia. Atherosclerosis. 2000;148:407–411. doi: 10.1016/s0021-9150(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 33.Hubel CA, McLaughlin MK, Evans RW, Hauth BA, Sims CJ, Roberts JM. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am J Obstet Gynecol. 1996;174:975–982. doi: 10.1016/s0002-9378(96)70336-8. [DOI] [PubMed] [Google Scholar]
- 34.Pouta A, Hartikainen AL, Sovio U, Gissler M, Laitinen J, McCarthy MI, Ruokonen A, Elliott P, Jarvelin MR. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension. 2004;43:825–831. doi: 10.1161/01.HYP.0000120122.39231.88. [DOI] [PubMed] [Google Scholar]
- 35.Berends AL, de Groot CJ, Sijbrands EJ, Sie MP, Benneheij SH, Pal R, Heydanus R, Oostra BA, van Duijn CM, Steegers EA. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008;51:1034–1041. doi: 10.1161/HYPERTENSIONAHA.107.101873. [DOI] [PubMed] [Google Scholar]
- 36.He S, Silveira A, Hamsten A, Blomback M, Bremme K. Haemostatic, endothelial and lipoprotein parameters and blood pressure levels in women with a history of preeclampsia. Thromb Haemost. 1999;81:538–542. [PubMed] [Google Scholar]
- 37.Laivuori H, Tikkanen MJ, Ylikorkala O. Hyperinsulinemia 17 years after preeclamptic first pregnancy. J Clin Endocrinol Metab. 1996;81:2908–2911. doi: 10.1210/jcem.81.8.8768850. [DOI] [PubMed] [Google Scholar]
- 38.Kaaja R, Laivuori H, Laakso M, Tikkanen MJ, Ylikorkala O. Evidence of a state of increased insulin resistance in preeclampsia. Metabolism. 1999;48:892–896. doi: 10.1016/s0026-0495(99)90225-1. [DOI] [PubMed] [Google Scholar]
- 39.Parretti E, Lapolla A, Dalfra M, Pacini G, Mari A, Cioni R, Marzari C, Scarselli G, Mello G. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47:449–453. doi: 10.1161/01.HYP.0000205122.47333.7f. [DOI] [PubMed] [Google Scholar]
- 40.Lang RM, Pridjian G, Feldman T, Neumann A, Lindheimer M, Borow KM. Left ventricular mechanics in preeclampsia. Am Heart J. 1991;121:1768–1775. doi: 10.1016/0002-8703(91)90024-c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.