Abstract
Background: This study was designed to determine the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of brivanib in patients with advanced/metastatic solid tumors.
Patients and methods: Ninety patients enrolled in this two-part, phase I open-label study of oral brivanib alaninate. The primary objectives of this study were (in part A) dose-limiting toxicity, maximum tolerated dose (MTD) and the lowest biologically active dose level and (in part B) the optimal dose/dose range. The secondary objectives of this study were preliminary evidence of antitumor activity, PK and PD.
Results: Across part A (open-label dose escalation and MTD) and part B (open-label dose optimization), 68 patients received brivanib alaninate. Brivanib demonstrated a manageable toxicity profile at doses of 180–800 mg. Most toxic effects were mild. Systemic exposure of the active moiety brivanib increased linearly ≤1000 mg/day. The MTD was 800 mg/day. Forty-four patients were treated at the MTD: 20 with 800 mg continuously, 11 with 800 mg intermittently and 13 with 400 mg b.i.d. doses. Partial responses were confirmed in two patients receiving brivanib ≥600 mg. Dynamic contrast-enhanced magnetic resonance imaging demonstrated statistically significant decreases in parameters reflecting tumor vascularity and permeability after multiple doses in the 800-mg continuous q.d. and 400-mg b.i.d. dose cohorts.
Conclusion: In patients with advanced/metastatic cancer, brivanib demonstrates promising antiangiogenic and antitumor activity and manageable toxicity at doses ≤800 mg orally q.d., the recommended phase II study dose.
Keywords: antiangiogenesis, brivanib, fibroblast growth factor, vascular endothelial growth factor
introduction
Angiogenesis plays a key role in growth and metastasis of many tumors [1] and is generally mediated by a variety of growth factors, including vascular endothelial growth factor (VEGF)-A and fibroblast growth factor (FGF)-1 (aFGF) and FGF-2 (bFGF) [1]. Several VEGF-targeted agents, administered either as single agents or in combination with chemotherapy, provide survival benefits in patients with advanced-stage malignancies, including colorectal cancer (CRC), hepatocellular carcinoma (HCC), renal cell carcinoma (RCC) and non-small-cell lung cancer [2–6]. However, currently available VEGF-targeted agents fail to produce enduring clinical responses in most patients as a result of innate or evasive resistance [7]. Recent evidence suggests that up-regulation of alternate proangiogenic signals, such as the FGF signaling pathway, plays a role in the development of resistance, as in the case of HCC [7–9]. Thus, dual targeting of FGF and VEGF signaling pathways may provide another approach to avoiding and overcoming resistance to VEGF inhibition.
Brivanib alaninate, an ester prodrug of the active moiety brivanib, is an oral dual inhibitor of FGFR and VEGFR signaling pathways. Brivanib has shown strong antiangiogenic and antitumor effects across a range of tumor cell types, including colon, breast, liver and lung cancer [10–12].
Here, we report findings from a phase I study designed to evaluate the safety profile, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of brivanib in patients with metastatic solid tumors that were refractory to standard therapies or for whom no standard therapy existed or was inappropriate.
patients and methods
study design
This was a two-part, phase I open-label study of brivanib alaninate administered orally (p.o.) to patients with advanced/metastatic solid tumors. The study was conducted at nine sites: three in the United States, two in the UK, two in Canada, one in Australia and one in Italy. Part A was an open-label dose-escalation study in which brivanib alaninate was administered p.o. on a once-daily schedule at a starting dose of 180 mg. At least three patients, and a maximum of six, were treated at each dose level. If a dose-limiting toxicity (DLT; supplemental Appendix A, available at Annals of Oncology online) was observed in the first three patients in a given dosing cohort, an additional three patients were enrolled to that dose level before further dose escalation was considered. Dose escalation proceeded when at least three patients completed a given cycle (28 days) and continued until at least one third of patients at a particular dose level had a DLT. Part B was an open-label study with four cohorts in which patients were treated with different regimens of brivanib alaninate: (i) 320 mg q.d. [continuous dosing schedule at the lower end of the biological response curve based on dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters; 320-mg cohort]; (ii) 800 mg q.d. continuous [continuous dosing at maximum tolerated dose (MTD) defined in part A; 800-mg continuous cohort]; (iii) 800 mg q.d. intermittent [intermittent dosing (5 days on, 2 days off); 800-mg intermittent cohort] and (iv) 400 mg twice-daily (b.i.d.) continuous dosing (400-mg b.i.d. cohort). The lower dose of 320 mg for one expansion cohort was chosen based on preliminary analysis of DCE-MRI data of the dose-escalation cohorts, showing that the 320 mg dose was the lowest dose level with DCE-MRI changes.
The last visit for each patient was defined as the follow-up visit and occurred 30 days after the patient was discontinued from the study. All patients gave informed consent to participate in the study, which was approved by local ethics committees and conducted in accordance with the Declaration of Helsinki and locally applicable guidelines on good clinical practice.
patient eligibility
In part A, patients with a histological or cytological diagnosis of a solid tumor (nonhematologic malignancy) were enrolled. Patients with measurable or nonmeasurable disease were eligible. However, patients planning to undergo DCE-MRI were required to have at least one lesion ≥2 cm in diameter that was suitable for DCE-MRI imaging. In Part B, patients with a histological or cytological diagnosis of a tumor type that is likely to benefit from antiangiogenic therapy—CRC, HCC or clear-cell RCC—with at least one lesion ≥2 cm in diameter that was suitable for DCE-MRI imaging were enrolled. Patient inclusion and exclusion criteria are described in supplemental Appendix B, available at Annals of Oncology online.
objectives
The primary objectives were to determine the DLTs and MTD of the active moiety brivanib on a continuous and an intermittent dose schedule in part A and to determine the lowest biologically active dose for further evaluation in part B. In part B, primary objectives were to determine the optimal dose or dose range and schedule for phase II trials from measurement of brivanib’s effects on DCE-MRI parameters, namely area under the plasma concentration–time curve for the first 60 s postcontrast agent injection (IAUC60) and transfer constant (Ktrans). Secondary objectives were to (i) assess any preliminary evidence of antitumor activity observed with brivanib; (ii) characterize the PK and PD profile of brivanib alaninate and brivanib following the first oral dose and on days 8 and 26 and (iii) assess brivanib’s effects on corrected QT (QTc) interval at baseline and on days 1, 8 and 26.
assessments
safety
Safety evaluations were based on medical review of adverse event (AE) reports and results of vital sign measurements, echocardiograms, electrocardiograms, physical examinations and clinical laboratory tests. AEs and other symptoms were graded according to National Cancer Institute–Common Terminology Criteria for Adverse Events (v.3.0, June, 2003).
pharmacokinetics
Serial plasma PK samples were drawn relative to the time of dose on days 1, 8 and 26 of cycle 1 only. For those subjects receiving brivanib alaninate b.i.d., an additional 12-h plasma PK sample was obtained at days 1, 8 and 26. Brivanib, the active moiety, was measured in plasma by a validated liquid chromatography/tandem mass spectrometry method as described in supplemental Appendix C, available at Annals of Oncology online.
efficacy
Tumor response was assessed at baseline, every 8 weeks and at the end of treatment using the modified World Health Organization criteria for tumor response. Tumor response was defined as best overall response with outcome of complete response (CR) or partial response (PR). Disease control was defined as a best overall response of CR, PR or stable disease. Prolonged disease control was defined as best overall response of CR, PR or stable disease lasting for at least 120 days.
PD biomarkers
DCE-MRI studies to assess changes in tumor perfusion characteristics were carried out at baseline and on days 2, 8 and 26. Further details are described in supplemental Appendix D, available at Annals of Oncology online.
statistical methods
All patients who received study medication were included in the safety dataset. All patients who received study mediation for at least one treatment cycle (28 days) were included in the efficacy dataset. In part B, a sample size of 12 patients in each cohort was calculated to provide 88% power to detect a 20% difference from baseline at each assessment time with respect to Ktrans and 98% power to detect a 20% difference from baseline at each assessment time with respect to AUC60. Therefore, an accrual target of 15 patients per cohort was planned to provide 12 patients to be assessable for DCE-MRI. Further details on statistical analyses are listed in supplemental Appendix E, available at Annals of Oncology online.
results
patient disposition and demographics
Ninety patients were enrolled, 68 of whom received at least one dose of brivanib; the 22 patients who did not receive study treatment were registered and began study-specific testing, but subsequently failed screening (Table 1). In part A, 18 patients were treated with brivanib alaninate in escalating doses; in part B, 50 patients received brivanib alaninate (see Table 1 for numbers in each cohort). Baseline demographics are also shown in Table 1. The majority of patients were white (93%), with a median age of 61 years, and 56% were male (Table 1). Sixty-five percent of patients had CRC and 10% had RCC. All 68 patients had received prior chemotherapy, including prior systemic anticancer agents, with the majority (96%) receiving three or more prior chemotherapy regimens. Per the exclusion criteria, no patient had prior VEGF-targeted therapy.
Table 1.
Patient disposition in each brivanib dose cohort and baseline demographics and characteristics
| Brivanib dose cohort | Patients, n |
||
| Part A | Part B | Total | |
| 180 mg | 3 | 0 | 3 |
| 320 mg | 3 | 11 | 14 |
| 600 mg | 3 | 0 | 3 |
| 800 mg continuous | 5 | 15 | 20 |
| 800 mg intermittent | 0 | 11 | 11 |
| 400 mg b.i.d. | 0 | 13 | 13 |
| 1000 mg | 4 | 0 | 4 |
| Demographic | N = 68 | ||
| Age, years | |||
| Median (range) | 61 (29–85) | ||
| Age category, n (%), years | |||
| <65 | 46 (68) | ||
| ≥65 | 22 (32) | ||
| Gender, n (%) | |||
| Male | 38 (56) | ||
| Female | 30 (44) | ||
| Race, n (%) | |||
| White | 63 (93) | ||
| Black | 1 (1) | ||
| Asian | 4 (6) | ||
| ECOG, n (%) | |||
| 0 | 33 (49) | ||
| 1 | 35 (51) | ||
| Tumor type, n (%) | |||
| Colorectal | 44 (65) | ||
| Kidney | 7 (10) | ||
| Hepatocellular carcinoma | 2 (3) | ||
| Other | 15 (22) | ||
| Prior therapy, n (%) | |||
| Prior surgery | 67 (99) | ||
| Prior hormonal, immunologic or biologic therapy | 19 (28) | ||
| Prior radiotherapy | 23 (34) | ||
| Prior chemotherapya | 68 (100) | ||
| No. of regimens | |||
| 1 | 0 (0) | ||
| 2 | 3 (4) | ||
| ≥3 | 65 (96) | ||
Included prior systemic anticancer agents.
ECOG, Eastern Cooperative Oncology Group.
safety
Brivanib alaninate, at doses of 180, 320, 600 and 800 mg demonstrated a manageable safety profile in patients with metastatic or advanced solid tumors across all doses. DLTs were observed in the 1000-mg dose group; hence, the MTD was determined to be 800 mg p.o. q.d. At the end of the study, 44 patients had been treated with brivanib alaninate 800 mg/day: 20 patients with 800 mg continuous, 11 patients with 800 mg intermittent and 13 patients with 400 mg b.i.d. Safety analysis in the expansion cohort at MTD confirmed a manageable safety profile.
In patients treated at the MTD (n = 44), the most frequent serious toxic effects recorded were nausea, pyrexia, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevations, and thrombocytopenia each in 4.5% of patients. Most AEs were mild, with no major differences in tolerability between dose schedules. Frequently occurring treatment-related toxic effects (>15% with each dosing schedule) at the MTD (800 mg continuous, 800 mg intermittent and 400 mg b.i.d.) included nausea, diarrhea, fatigue, dizziness, hypertension, headache and anorexia. The most frequently occurring treatment-related toxic effects in brivanib-treated patients are shown in Tables 2 and 3. No grade five AEs were reported and no clinically significant QTc prolongation was observed across the range of brivanib doses investigated. VEGF inhibition class effects observed with brivanib included hypertension (33.8%), proteinuria (14.7%), hemorrhage (11.8%), thrombosis-related events (4.4%) and reversible posterior leukoencephalopathy (1.5%); there were no reports of bowel perforation or fistulas.
Table 2.
Selected grade 1/2 treatment-related adverse events commonly reported with vascular endothelial growth factor inhibitors and laboratory test change by dose (all treated subjects)
| 180 mg | 320 mg | 600 mg | 800 mg C | 800 mg I | 1000 mg | 400 mg b.i.d. | |
| AEa related to study drug | n = 3 | n = 14 | n = 3 | n = 20 | n = 11 | n = 4 | n = 13 |
| Nausea | 1 (33) | 6 (43) | 2 (67) | 7 (35) | 3 (27) | 3 (75) | 5 (38.5) |
| Diarrhea | 2 (67) | 2 (14) | 0 (0) | 8 (40) | 3 (27) | 0 (0) | 2 (15) |
| Fatigue | 1 (33) | 4 (29) | 1 (33) | 4 (20) | 9 (82) | 1 (25) | 6 (46) |
| Dizziness | 0 (0) | 5 (36) | 2 (67) | 4 (20) | 5 (46) | 1 (25) | 3 (23) |
| Hypertension | 0 (0) | 2 (14) | 2 (67) | 4 (20) | 5 (46) | 2 (50) | 2 (15) |
| Vomiting | 0 (0) | 6 (43) | 0 (0) | 6 (30) | 1 (9) | 0 (0) | 6 (46) |
| Constipation | 1 (33) | 2 (14) | 0 (0) | 2 (10) | 0 (0) | 0 (0) | 0 (0) |
| Abdominal pain | 0 (0) | 1 (7) | 0 (0) | 1 (5) | 1 (9) | 0 (0) | 0 (0) |
| Dyspepsia | 0 (0) | 1 (7) | 1 (33) | 0 (0) | 1 (9) | 0 (0) | 0 (0) |
| Stomatitis | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (9) | 1 (25) | 0 (0) |
| Anorexia | 2 (67) | 4 (29) | 3 (100) | 6 (30) | 3 (27) | 3 (75) | 7 (54) |
| Proteinuria | 0 (0) | 2 (14) | 0 (0) | 2 (10) | 1 (9) | 0 (0) | 1 (8) |
| Laboratory testsb | n = 3 | n = 14 | n = 3 | n = 20 | n = 11 | n = 4 | n = 10–12 |
| AST increased | 0 (0) | 0 (0) | 1 (33) | 2 (10) | 0 (0) | 2 (50) | 1 (8) |
| ALT increased | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bilirubin increased | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Hyponatremia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
National Cancer Institute–Common Terminology Criteria for AEs, v 3.
Grade increase from baseline.
AE, adverse event; C, continuous; I, intermittent; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Table 3.
Selected grade 3/4 treatment-related adverse events commonly reported with vascular endothelial growth factor inhibitors and laboratory test change by dose (all treated patients)
| 180 mg | 320 mg | 600 mg | 800 mg C | 800 mg I | 1000 mg | 400 mg b.i.d. | |
| n = 3 | n = 14 | n = 3 | n = 20 | n = 11 | n = 4 | n = 13 | |
| AEa related to study drug | |||||||
| Fatigue | 0 (0) | 3 (21) | 0 (0) | 3 (15) | 0 (0) | 1 (25) | 2 (15) |
| Hypertension | 0 (0) | 0 (0) | 0 (0) | 3 (15) | 0 (0) | 1 (25) | 1 (8) |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 2 (10) | 1 (9) | 0 (0) | 0 (0) |
| Dizziness | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (8) |
| Nausea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) |
| Vomiting | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) |
| Laboratory testsb | |||||||
| ALT increased | 0 (0) | 0 (0) | 1 (33) | 3 (15) | 0 (0) | 2 (50) | 1 (8) |
| AST increased | 0 (0) | 1 (7) | 1 (33) | 1 (5) | 1 (9) | 0 (0) | 0 (0) |
| Bilirubin increased | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hyponatremia | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) |
National Cancer Institute–Common Terminology Criteria for AEs, v 3.
Grade increase from baseline.
AE, adverse event; C, continuous; I, intermittent; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
According to prespecified definitions (see supplemental Appendix A, available at Annals of Oncology online), DLTs were observed in two of four patients treated in the brivanib alaninate 1000-mg/day dose cohort. One patient experienced grade 3 altered mental status considered possibly related to brivanib, and grade 3 dehydration considered not related to brivanib, on day 13. The second patient had grade 3 fatigue related to treatment on day 17. As DLTs occurred in <33% of patients treated at 800 mg daily, this dose was defined as the MTD.
Toxic effects led to brivanib discontinuation in 12 (17.6%) patients; due to study drug toxicity in eight patients (12%) and non-drug-related AEs in four patients (6%). Reasons for brivanib discontinuation in these patients included grade 2/3 ALT/AST increased, grade 3 dizziness, grade 3 peripheral neuropathy, grade 3 reversible posterior leukoencephalopathy syndrome, grade 2 nausea, grade 1 vomiting, grade 2 chills, grade 3 fatigue, grade 2 hemorrhagic cystitis, grade 3 renal failure, grade 2 thrombocytopenia, grade 3 dehydration, grade 3 hypertension and grade 4 elevated bilirubin. Other reasons for brivanib discontinuation included patient’s request in three patients (4%), physician’s decision in two patients (3%) and withdrawn consent in one patient (1%). Two patients discontinued study therapy due to grade 2/3 elevated liver function tests and two patients discontinued due to grade 1 vomiting. Of the eight deaths reported during the study, all were due to disease progression; three patients died within 30 days of receiving the last dose of brivanib alaninate.
pharmacokinetics
Systemic exposure (AUCTAU) of the active moiety of brivanib alaninate increased in a linear manner with brivanib alaninate with doses of up to 1000 mg/day (see supplemental Appendix F, available at Annals of Oncology online). On day 26, the geometric mean AUCTAU following administration of brivanib alaninate 800 mg continuous was 62 813 ng·h/ml [coefficient of variation (CV), 22%], geometric mean Cmax was 6610 ng/ml (CV, 39%), median Tmax was 2 h (range 0.77–6.0) and mean T1/2 was 12.3 h (standard deviation 7.05). At 800 mg continuous, the median accumulation index of brivanib in plasma was small (1.62-fold; CV, 53%).
PD activity
Forty-nine patients had double baseline DCE-MRI scans before treatment. Post-treatment scans were carried out in 52 patients on day 2 (postdose), 51 patients on day 8 (predose) and 47 patients on day 26 (predose). Reduction in DCE-MRI parameters was dependent on dose and schedule. A statistically significant reduction in Ktrans and IAUC60 occurred with brivanib alaninate for the 800 mg continuous dose starting on day 2 and for the 800 mg continuous and 400 mg b.i.d. doses on both day 8 and day 26. However, DCE-MRI parameter changes were not significant for the 320- and the 800-mg intermittent dose cohorts (with the exception of day 2; Table 4). Overall, 14 patients (27% of subjects on day 8 and 30% of subjects on day 26) had an MRI response in IAUC60 (reductions >50%, the IAUC60 repeatability coefficient threshold); 18 (35%) and 14 (30%) subjects had an MRI response for Ktrans (reductions >52%, the Ktrans repeatability coefficient threshold) on days 8 and 26, respectively. Figure 1A and B, respectively, show each patient’s day 26 percent change from baseline in MRI IAUC60 and Ktrans.
Table 4.
Percent changes from baseline in IAUC60 and Ktrans on days 2, 8 and 26
| DCE-MRI parameter | Study day | Percent change from baseline point estimate (95% CI) |
|||
| 320 mg | 800 mg C | 800 mg I | 400 mg b.i.d. | ||
| IAUC60, % (95% CI) | 2 | −4 (–23 to +19) | −28a (−46 to −8)a | −36a (−56 to −7) | −20a (−50 to +28) |
| 8 | −4 (−28 to +19) | −37a (−51 to −19) | −27 (−50 to +7) | −64a (−78 to −42) | |
| 26 | −3 (−22 to +21) | −47a (−60 to −30) | −29 (−53 to +5) | −57a (−74 to −30) | |
| Ktrans (95% CI) | 2 | −4 (−24 to +21) | −31a (−49 to −9)a | −44a (−63 to −16) | −31 (−58 to +15) |
| 8 | −6 (−25 to +17) | −45a (−59 to −26) | −31 (−54 to +5) | −64a (−78 to −39) | |
| 26 | −6 (−26 to +18) | −51a (−65 to −32) | −34 (−58 to +2) | −59a (−76 to −31) | |
Statistically significant differences from baseline (P < 0.017).
CI, confidence interval; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; C, continuous; I, intermittent; IAUC60, area under the plasma concentration–time curve for the first 60 s postcontrast agent injection; Ktrans, transfer constant.
Figure 1.
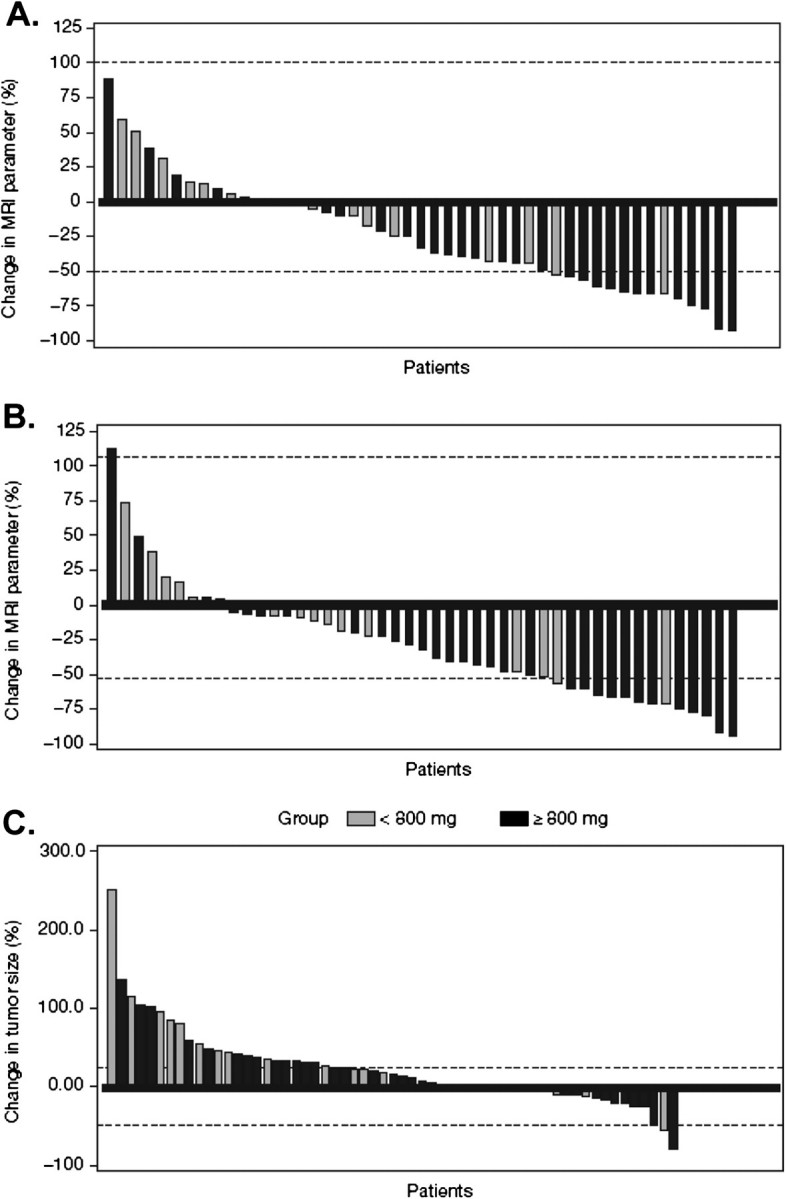
(A) Day 26 percent change from baseline in dynamic contrast-enhanced magnetic resonance imaging (MRI) area under the plasma concentration–time curve for the first 60 s postcontrast agent injection. Horizontal lines indicate 95% repeatability coefficients of −50% to 99%. (B) Ktrans. Horizontal lines indicate 95% repeatability coefficients of and –52% to 106%. (C) Change in tumor size on study with brivanib treatment in the total study population (n = 68). Panels A through C are grouped by patient in ascending order.
antitumor activity: tumor measurements
Preliminary evidence of brivanib antitumor activity was observed in this study; PRs were confirmed in two patients treated with brivanib ≥600 mg, one with RCC in the 600-mg dose cohort and one with ampulla of Vater carcinoma in the 1000-mg dose cohort. Based on the investigator’s assessment in response-assessable patients, 24 patients (35%) had stable disease and 32 (47%) had progressive disease (Table 5). A waterfall plot of the maximum reduction in tumor size in 20 patients (29%) treated with a dose <800 mg and 48 (71%) treated with a dose of 800 mg or more on study is shown in Figure 1C.
Table 5.
Best overall response based on modified World Health Organization assessment and length of time on study
| Brivanib dose (mg) (assessable patientsa) | All doses, n (%) | 180 (3) | 320 (14) | 600 (3) | 800 C (20) | 800 I (11) | 400 b.i.d. (13) | 1000 (4) |
| Response, n | ||||||||
| Partial response, n | 2 (3) | 1b | 1c | |||||
| Stable disease | 24 (35) | 1 | 4 | 1 | 8 | 5 | 4 | 1 |
| Progressive disease | 32 (47) | 2 | 9 | 1 | 8 | 5 | 6 | 1 |
| Not assessable | 10 (15) | 1 | 4 | 1 | 3 | 1 | ||
At least one post-treatment scan.
Renal cell carcinoma.
Ampulla of Vater.
C, continuous; I, intermittent.
discussion
In the phase I portion of this study, single-agent brivanib alaninate had a half-life of 12 h and demonstrated a manageable toxicity profile at doses of 180–800 mg in patients with advanced/metastatic cancer. The MTD was determined to be 800 mg/day and was the recommended dose for phase II study based on the following: PRs were seen at 600 mg/day and 1000 mg/day doses of brivanib; the 1000 mg/day dose was intolerable and prolonged disease control was also observed at 800 mg/day in these heavily pretreated patients. Significant changes in DCE-MRI parameters were observed with 800 mg/day brivanib in the 400-mg p.o. b.i.d. and 800-mg continuous dosing cohorts. Similar effects were not observed in the 800-mg intermittent cohort, a finding that lends weight to the hypothesis that continued inhibition of angiogenic processes is important for maximum therapeutic effect. Based on observed toxic effects, splitting the daily dose did not confer a significant safety advantage over once-daily dosing.
Forty-four patients were treated with an 800 mg/day dose (MTD) of brivanib alaninate, none of whom reported a DLT. The most frequently reported drug-related toxic effects were nausea, diarrhea, fatigue, dizziness, hypertension and anorexia. Many of these toxic effects are common to the class of VEGFR-2 inhibitors [2, 4, 13–17]; of note, those reported here with brivanib at 800 mg/day were mainly mild at grade 1/2. Across all dose cohorts, the incidence of grade 3/4 hypertension was low at 7.4% compared with bevacizumab (5%–18%) but comparable to that observed with sorafenib and sunitinib (2%–8%) [4, 18–20]. Likewise, in this small sample size with brivanib alaninate treatment, there were no reported cases of grade 3/4 hand–foot syndrome, clinically significant oral toxic effects or bleeding, events which are commonly (up to 35%) reported following sunitinib or sorafenib treatment [4, 13, 16, 21–23]. The overall incidence of grade 3/4 AST and ALT elevation (13.2% and 11.8%, respectively) was higher than that observed with sunitinib and sorafenib in phase III studies in HCC and RCC patients; only 2% of patients had grade 3 AST/ALT elevations in a phase III study of sunitinib in RCC patients [16] and no reported grade 3/4 AST/ALT elevations have been reported with sorafenib [4, 13]. The reason for this is unclear, but it has been noted in a separate absorption, distribution, metabolism and excretion (ADME) study that brivanib was primarily metabolized by multiple liver enzymes, including CYP1A2, CYP3A4 and multiple sulfotransferases [24]. However, similar toxicity has been seen with the VEGF/PDGF/c-kit inhibitor pazopanib in a phase III study in which 20% of patients had grade 3/4 AST elevations [25].
Data from this study confirmed that brivanib has linear PK, with AUCTAU increasing in approximate proportion to brivanib alaninate doses. Brivanib PK parameters are comparable to those observed in an ADME study of brivanib in patients with advanced/metastatic solid tumors [24].
The DCE-MRI imaging technique was used to assess brivanib’s effects on tumor permeability and vascularity. This technique has been used with several VEGF inhibitors, although the relationship of DCE-MRI changes to clinical benefit still awaits validation [26–30]. AUC60 and Ktrans parameter values were very highly correlated within a subject; moreover, due to the smaller variability and fewer missing values for AUC60, AUC60 was seen as the primary parameter. Statistically significant decreases from baseline after multiple doses, in parameters that reflect tumor vascularity and permeability as measured by DCE-MRI, were seen at the 800-mg continuous q.d. dose and 400-mg b.i.d. dose cohorts; a small association between longer PFS and decrease in DCE-MRI as measured by AUC60 was observed, but samples were too small to make formal comparison. No significant decreases from baseline were seen in the 320- or 800-mg intermittent dose cohort. The inability of the 800-mg intermittent dose to significantly reduce DCE-MRI parameters at both day 8 and day 26 might be explained by a rebound in these parameters during periods when patients are off therapy. Moreover, this observation indicates that intermittent suppression of angiogenesis by brivanib may be less effective than continuous inhibition.
In addition to sample size being too small for a formal statistical analysis of the association between clinical outcomes and DCE-MRI parameter changes, other limitations of this study include that patients were not randomized to the different dose expansion cohorts and that is was a muticenter study (although site selection included a qualification process for DCE-MRI).
Although tumor evaluation was not a primary objective of this study, preliminary evidence of antitumor activity was observed, with PRs observed in one patient in the 600-mg dose cohort and in one patient in the 1000-mg dose cohort.
An ongoing phase II study of brivanib in HCC patients [31] has demonstrated antitumor activity and manageable tolerability with brivanib at 800 mg once daily as both first- and second-line treatment, including those with prior sorafenib failure. These results, together with its dual inhibition of FGFR and VEGFR signaling and the potential to overcome resistance seen with previous studies of VEGFR tyrosine kinase inhibitors, suggest that brivanib represents an agent of interest in the management of several malignancies. Determination of the role of this promising novel agent depends on ongoing trials as monotherapy and in combination with other treatment modalities such as transarterial chemoembolization. Molecular biomarker studies, to be reported separately, will also be important to identify those patients who may benefit most from brivanib therapy.
In conclusion, results from this study indicate that brivanib demonstrates a tolerable safety profile and preliminary antitumor activity in patients with advanced malignancies. Given the role of FGF in VEGF resistance, brivanib as a dual inhibitor of FGFR and VEGFR has the potential to provide clinical benefits to patients across several tumor types.
funding
This work was supported by research funding from Bristol-Myers Squibb (Study No. CA182002). This trial is registered at www.clinicaltrials.gov as NCT00207051.
disclosure
FDB, GCJ, JK, GAM, MBS, CJS, GW, GR and DJJ have no conflicts of interest to disclose. LSR and GG have received researching funding from Bristol-Myers Squibb. DMF, LV, SG, GK, SS, DSAN, OM hold stock in Bristol-Myers Squibb and are employees of the company.
Supplementary Material
Acknowledgments
The authors acknowledge the contributions of the Study 002 investigators and Suso Platero, Mark Ayers, Nga Kit Eliza Fung, Jianing Zeng and Amit Roy in the study analyses. Medical writing support was provided by Mark English, PhD, and U. Lena Prisco, PhD, of PAREXEL.
References
- 1.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 3.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6(7):395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Poon RT, Ng IO, Lau C, et al. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182(3):298–304. doi: 10.1016/s0002-9610(01)00708-5. [DOI] [PubMed] [Google Scholar]
- 10.Bhide RS, Cai ZW, Zhang YZ, et al. Discovery and preclinical studies of (R)-1-(4-(4-Fluoro-2-methyl-1 H-indol-5-yloxy)-5-methylpyrrolo[2,1- f][1,2,4]triazin-6-yloxy)propan-2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem. 2006;49(7):2143–2146. doi: 10.1021/jm051106d. [DOI] [PubMed] [Google Scholar]
- 11.Huynh H, Ngo VC, Fargnoli J, et al. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 12.Bhide RS, Lombardo LJ, Hunt JT, et al. The antiangiogenic activity in xenograft models of brivanib alaninate, a dual inhibitor of VEGFR-2 and FGFR-1 kinases. Mol Cancer Ther. 2010;9:369–378. doi: 10.1158/1535-7163.MCT-09-0472. [DOI] [PubMed] [Google Scholar]
- 13.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morabito A, De Maio E, Di Maio M, et al. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11(7):753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 17.Launay-Vacher V, Deray G. Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs. 2009;20(1):81–82. doi: 10.1097/CAD.0b013e3283161012. [DOI] [PubMed] [Google Scholar]
- 18.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 20.Avastin [Prescribing Information] South San Francisco, CA: Genentech; 2009. [Google Scholar]
- 21.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(26):4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 22.Worns MA, Weinmann A, Pfingst K, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43:489–495. doi: 10.1097/MCG.0b013e31818ddfc6. [DOI] [PubMed] [Google Scholar]
- 23.Okita K, Imanaka N, Chida N, et al. Phase III study of sorafenib in patients in Japan and Korea with advanced hepatocellular carcinoma (HCC) treated after transarterial chemoembolization (TACE) In ASCO Gastrointestinal Cancers Symposium (Abstr LBA128). Orlando, FL 2010. [Google Scholar]
- 24.Gong J, Gan J, Comezoglu SN, et al. Metabolism and disposition of [14C]brivanib alaninate after oral administration to rats, monkeys and humans. In 16th North American International Society for the Study of Xenobiotics Regional Meeting (Abstr 284). Baltimore, MD 2009. [Google Scholar]
- 25.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 26.Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21(21):3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 27.Flaherty KT, Rosen MA, Heitjan DF, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther. 2008;7(4):496–501. doi: 10.4161/cbt.7.4.5624. [DOI] [PubMed] [Google Scholar]
- 28.Thomas AL, Morgan B, Horsfield MA, et al. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23(18):4162–4171. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell A, Padhani A, Hayes C, et al. A Phase I study of the angiogenesis inhibitor SU5416 (semaxanib) in solid tumours, incorporating dynamic contrast MR pharmacodynamic end points. Br J Cancer. 2005;93(8):876–883. doi: 10.1038/sj.bjc.6602797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24(5):769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 31.Raoul J-L, Finn R, Kang YK, et al. An open-label phase II study of first- and second-line treatment with brivanib in patients with hepatocellular carcinoma (HCC) [abstract] J Clin Oncol. 2009;27:15s. (Abstr 4577) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


