Abstract
Background
Whether individuals with peripheral artery disease (PAD) identified by screening ankle-brachial index (ABI) benefit from preventative therapies to reduce cardiovascular risk is unknown. We aimed to determine the number of US adults with PAD not receiving preventative therapies and whether treatment is associated with reduced mortality in PAD subjects without known cardiovascular disease (CVD).
Methods and Results
We analyzed data from the National Health and Nutrition Examination Survey (NHANES) 1999-2004 with mortality follow-up through December 31, 2006. PAD was defined as ABI ≤ 0.90. Of 7458 eligible participants ≥ 40 years, weighted PAD prevalence was 5.9% ± SE 0.3%, corresponding to approximately 7.1 million US adults with PAD. Statin use was reported in only 30.5% ± 2.5%, angiotensin converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) use in 24.9% ± 1.9%, and aspirin use in 35.8% ± 2.9%, corresponding to 5.0 million adults with PAD not taking statins, 5.4 million not taking ACEI/ARBs, and 4.5 million not receiving aspirin. Adjusting for age, gender, and race/ethnicity, PAD was associated with all-cause mortality (HR 2.4 [1.9-2.9], p<0.0001). Even after excluding individuals with known CVD, subjects with PAD had higher mortality rates (16.1% ± 2.1%) than subjects without PAD or CVD (4.1% ± 0.3%) with adjusted HR 1.9 [1.3-2.8], p=0.001. Among PAD subjects without CVD, use of multiple preventative therapies was associated with 65% lower all-cause mortality (HR 0.35 [0.20-0.86], p=0.02).
Conclusions
Millions of US adults with PAD are not receiving secondary prevention therapies. Treatment with multiple therapies is associated with reduced all-cause mortality.
Keywords: Peripheral vascular disease, Prevention, Cardiovascular disease, Mortality
Background
Cardiovascular disease remains a major cause of morbidity and mortality in the United States. Individuals with peripheral artery disease (PAD), a manifestation of systemic atherosclerosis, are known to be at significantly increased risk of adverse cardiovascular events regardless of symptoms.1, 2 Despite multiple treatments known to decrease cardiovascular risk, several studies have shown that PAD remains under-recognized and under-treated.3, 4 However, the number of individuals in the US with PAD who are not receiving preventative therapies that may reduce the risk of myocardial infarction, stroke, or death remains unknown.
Current guidelines for the management of patients with PAD recommend lipid-lowering therapy with a statin to achieve a goal LDL < 100 mg/dL (or < 70 mg/dL in high risk patients),5, 6 anti-hypertensive therapy to achieve a systolic blood pressure below 140 mm Hg, recognizing that ACE inhibitors may have a unique role,7 and anti-platelet therapy.8 Most trials of secondary prevention have included PAD patients with previously recognized and symptomatic disease (such as intermittent claudication or prior peripheral revascularization), but whether these guidelines can be extended to patients with PAD identified by population screening, the majority of whom are likely to be asymptomatic, has not been well studied.
The ankle-brachial index (ABI) is a simple non-invasive test that can identify high-risk adults with PAD.9-11 However, it is not known whether secondary prevention therapies can reduce mortality in patients identified solely by population-based ABI screening, especially in those without other manifestations of atherosclerotic vascular disease in whom these preventative therapies would already be indicated. Furthermore, recent studies have called into question certain preventative therapies, such as aspirin therapy in particular, in patients with PAD.12-14 This lack of evidence for screening-guided use of treatments for patients with unrecognized PAD has precluded the US Preventative Services Task Force from recommending screening for PAD using the ABI.15
We used the National Health and Nutrition Examination Survey (NHANES) to estimate the absolute number of US adults at high risk based on a low screening ABI who are not receiving preventative therapies recommended by established guidelines.1, 2 Furthermore, we aimed to determine whether treatment with multiple risk factor modifying therapies was associated with reduced all-cause morality in adults identified with PAD who are otherwise free of established cardiovascular disease (CVD).
Methods
NHANES is an ongoing series of surveys that have been conducted by the National Center for Health Statistics (NCHS) since the early 1960s to assess the heath and nutritional status of the civilian US population using a complex, stratified, multi-stage survey design. NHANES has been reviewed and approved by the Institutional Review Board at the NCHS.
Ankle-brachial index
During the survey years 1999-2004, ABI measurements were obtained as part of the NHANES lower extremity examination in adults ≥ 40 years. With subjects in the supine position, systolic blood pressure was measured in the right arm and in the posterior tibial arteries at both ankles using an 8-MHz Doppler probe. We calculated the ABI for each leg by dividing the ankle pressure by the arm pressure. A diagnosis of PAD was assigned if either leg had an ABI ≤ 0.90.
Definitions of variables of interest
Age, gender, race/ethnicity, smoking status, and history of atherosclerotic cardiovascular disease (CVD) were based on self-report as previously reported.16, 17 Diagnosis of CVD was based on an affirmative response to the question, “Has a doctor or other health professional ever told you that you had [coronary heart disease, angina (also called angina pectoris), heart attack (also called myocardial infarction), stroke]?” A diagnosis of hypertension was assigned if SBP ≥ 140 mmHg and/or diastolic blood pressure ≥ 90, based on prior physician diagnosis, or if subjects self-reported taking a prescription medication for hypertension. Hyperlipidemia was considered present if subjects reported a physician diagnosis of elevated cholesterol or had a total cholesterol level ≥ 240 mg/dL (6.21 mmol/L). Low density lipoprotein (LDL) levels were available in a subset of participants (n=3224) who had fasting blood samples drawn. Subjects were considered to have diabetes if they reported a physician diagnosis, were taking prescription medications for diabetes (either insulin or oral agents), or had blood non-fasting glucose values ≥200 mg/dL (11.1 mmol/L) or fasting glucose values ≥ 126 mg/dL (7 mmol/L). The MDRD (Modification of Diet in Renal Disease) study equation was used to estimate glomerular filtration rate (eGFR), and eGFR <60 mL/min/m2 indicated chronic kidney disease (CKD).18 Socioeconomic status (SES) was categorized based on the poverty-income ratio (PIR), a ratio of self-reported income relative to the poverty threshold, with PIR less than 1.0 indicating income below poverty level. Other self-reported socioeconomic variables included highest education level attained and health insurance status.
Definition of preventative treatments
Medication use was ascertained by self-report, and NHANES interviewers confirmed medication use by direct visualization of all prescription medication containers when available. Medication dose was not available. We specifically chose to evaluate guideline-recommended treatments: 1) anti-platelet therapy (including aspirin, clopidogrel, dipyridamole, ticlopidine, or combinations), 2) any statin therapy, and 3) any angiotensin converting enzyme inhibitor or angiotensin receptor blocker (ACEI/ARB).1 We did not include treatments (such as smoking cessation aids or diabetes medications) that would be indicated irrespective of a diagnosis of PAD.
Primary outcome
Mortality status was determined based on a probabilistic record match with the National Death Index (NDI) using demographic identifiers.19, 20 The primary outcome was all-cause mortality. For participants in NHANES 1999-2004, mortality follow-up data was available through December 31, 2006.
Statistical Methods
Analyses were performed with SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) callable SUDAAN with use of appropriate sample weights, stratum, and PSU variables to account for NHANES' complex sample design. Data are reported as weighted mean and standard error (SE) or weighted percentile and SE. Comparisons of categorical variables were achieved using χ2 test. Population estimates were determined by multiplying the weighted prevalence estimates by the average population total of US adults ≥ 40 years of age provided in the Current Population Survey by the US Census Bureau for the years 1999-2004.
The primary mortality analysis estimated the association between mortality and the number of secondary prevention treatments used (0, 1, or multiple (≥2)) in PAD subjects without established CVD using univariate and multivariate Cox proportional hazards models. Multivariable analyses included demographics (age, gender, race/ethnicity), atherosclerotic risk factors (diabetes, hypertension, hyperlipidemia, smoking, and chronic kidney disease), and socioeconomic factors (health insurance, education level, and income).
Relative risks are reported as hazard ratios (HR) with corresponding 95% confidence intervals (CI). Time to event was calculated as the number of days from initial NHANES study visit to date of death. Subjects were censored if no death occurred by the end of the follow up period, December 31, 2006. Time-varying covariates were included in the models to test the proportional hazards assumption, and no covariates were found to violate the proportional hazards assumption. A two-sided p-value of < 0.05 was considered to be statistically significant for all analyses.
Results
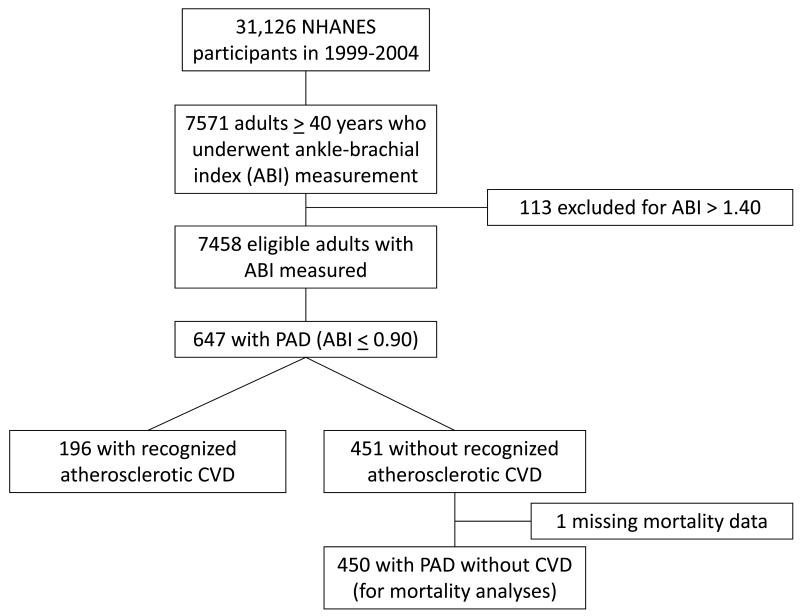
During the years 1999-2004, 7571 adults ≥ 40 years underwent measurement of ABI. Of those, we excluded 113 based on an ABI ≥ 1.40, indicating vascular calcification artifact. Among the remaining 7458 eligible subjects (figure 1), PAD (ABI ≤ 0.90) was identified in 647 individuals for a weighted prevalence of 5.9% ± 0.3%. Of these subjects, 196 had an established diagnosis of CVD (coronary heart disease, myocardial infarction, angina, stroke), leaving 451 individuals with PAD but without recognized CVD. Final mortality status was missing for one individual. Baseline characteristics of all PAD subjects are shown in table 1.
Figure 1. Flow diagram of derivation of sample population.
Table 1. Baseline characteristics of all PAD subjects (n=647).
| Age, years (mean, SE) | 67.8 (0.7) |
| Gender, male (%, SE) | 41.6 (2.5) |
| Race/ethnicity (% non-Hispanic white) | 78.1 (2.1) |
| Smoking status (% current or former) | 65.9 (2.0) |
| Diabetes (%) | 24.4 (2.6) |
| Hypertension (%) | 73.8 (2.4) |
| Hyperlipidemia (%) | 58.6 (2.3) |
| Chronic kidney disease (% with eGFR <60) | 34.2 (2.5) |
| History of cardiovascular disease (including coronary and cerebrovascular disease) (%) | 30.4 (2.4) |
| Ankle-brachial index (mean, SE) | 0.77 (0.007) |
Secondary Prevention in Individuals with PAD with and without known CVD
Among all 647 subjects with PAD, 69.5% ± 2.5% were not taking a statin, 75.1% ± 1.9% were not taking an ACE inhibitor or ARB, 64.2% ± 2.9% were not taking aspirin, and 61% ± 3.2% were taking no anti-platelet therapy (including aspirin, clopidogrel, dipyridamole, ticlopidine, or combination treatments) (table 2). There were 68.4% ± 3.8% of PAD subjects with an LDL cholesterol level above the recommend goal of 100 mg/dL, and the vast majority had an LDL cholesterol level above the more aggressive target of 70 mg/dL (94.7% ± 1.4%). Although 90.5% ± 2.2% of PAD subjects with hypertension reported currently taking an anti-hypertensive medication, 45.7% ± 2.3% of all PAD subjects still had a systolic blood pressure above 140 mmHg and 63.9% ± 2.4% had systolic blood pressures above 130 mmHg.
Table 2.
| Table 2A: Prevalence and population estimates of PAD in the US | ||||||
|---|---|---|---|---|---|---|
| All subjects | Subjects with recognized CVD | Subjects without CVD | ||||
| Weighted percent, % (SE) | Population estimate, N (SE) | Weighted percent, % (SE) | Population estimate, N (SE) | Weighted percent, % (SE) | Population estimate, N (SE) | |
| Total US population estimate* | 121,373,175 | 11.7% (0.6) | 14,200,662 (740,376) | 88.3% (0.6) | 107,172,513 (728,239) | |
| PAD prevalence % (SE) | 5.9% (0.3) | 7,185,171 (384,267) | 15.4% (1.6) | 2,186,902 (227,211) | 4.7% (0.3) | 5,004,956 (265,788) |
| Table 2B: Population estimates of non-use of recommended therapies in PAD | ||||||
|---|---|---|---|---|---|---|
| All PAD subjects (n=647) |
PAD subjects with recognized CVD (n=196) |
PAD subjects without CVD (n=451) |
||||
| Statin non-use % (SE) | 69.5% (2.5) | 4,995,332 (179,414) | 42.5% (4.2) | 929,433 (91,850) | 81.7% (2.7) | 4,087,147 (135,444) |
| LDL > 100 mg/dL % (SE) | 68.4% (3.8) | 4,632,576 (256,021) | 62.5% (5.7) | 1,366,814 (124,653) | 70.7% (4.3) | 3,426,456 (209,269) |
| LDL > 70 mg/dL % (SE) | 94.7% (1.4) | 6,420,475 (94,200) | 89.8% (2.8) | 1,963,838 (61,233) | 96.7% (1.4) | 4,684,998 (65,760) |
| ACEI/ARB non-use % (SE) | 75.1% (1.9) | 5,395,919 (134,887) | 65.6% (3.3) | 1,434,608 (72,168) | 79.2% (2.2) | 3,964,926 (109,609) |
| SBP ≥ 140 mmHg % (SE) | 45.7% (2.3) | 3,246,751 (159,757) | 48.9% (3.8) | 1,069,395 (83,102) | 44.4% (3.0) | 2,186,748 (148,910) |
| Aspirin non-use % (SE) | 64.2% (2.9) | 4,535,159 (206,899) | 44.1% (4.1) | 964,424 (89,663) | 73.0% (3.1) | 3,654,114 (153,152) |
| Not taking any anti-platelet therapy % (SE) | 61% (3.2) | 4,379,153 (228,488) | 34.2% (4.6) | 747,920 (100,597) | 72.6% (3. 1) | 3,634,990 (156,014) |
US census bureau average estimates of US adults ≥ 40 years from 1999-2004
PAD = Peripheral artery disease; CVD = Cardiovascular disease (including myocardial infarction, angina, coronary heart disease, or stroke); ACEI = angiotensin converting enzyme inhibitor; ARB = Angiotensin receptor blocker; LDL= low-density lipoprotein; SBP = systolic blood pressure
Among the 451 PAD subjects without recognized atherosclerotic CVD, 81.7% ± 2.7% were not taking a statin, 79.2% ± 4.3 were not taking an ACEI or ARB, 73% ± 3.1% were not taking aspirin, and no anti-platelet therapy was being used in 72.6% ± 3.1% (table 2). LDL cholesterol level was above 100 mg/dL in 70.7% ±4.3% of PAD subjects without CVD, and 96.7% ± 1.4% had levels above 70 mg/dL. SBP was above 140 mmHg in 44.4% ± 3% of PAD subjects without CVD and above 130 mmHg in 64.9% ± 3.2%.
Utilization of preventative therapies was significantly greater in the 196 PAD subjects with recognized CVD. Among PAD subjects with CVD, statin use was reported in 57.5% compared to only 18.3% in PAD subjects without CVD (p<0.001), ACEI/ARB therapy in 34.3% vs. 20.8% (p<0.001), and anti-platelet therapy in 65.8% vs. only 27.4% (p<0.001). Many more individuals with PAD who had recognized CVD were taking multiple (≥2) preventative therapies than individuals with PAD who did not have established CVD (55.1% vs. 16.2%, p<0.001), and conversely, significantly more subjects with PAD without CVD were taking no therapies at all (53.7% vs. 14.9%, p<0.001). In the subset of PAD subjects without CVD, only a small fraction (4.3%) reported use of all three types of medications.
Population estimates of non-use of recommended therapies in PAD
As noted above, PAD was identified in 5.9% of all adults ≥ 40 years who had ABI tested, corresponding to nearly 7.1 million US adults, and in 4.7% of ABI-tested adults without established CVD, accounting for 4.9 million (table 2). Among the PAD subjects without established CVD, an estimated 4 million were not taking a statin, 3.6 million were not taking any anti-platelet therapy, and 4.2 million were not taking an ACEI or ARB. More than 3.3 million individuals with PAD had LDL cholesterol levels above 100 mg/dL, and 4.6 million had LDL cholesterol levels above 70 mg/dL. A large number of US adults with PAD had inadequately controlled systolic blood pressure with 2.2 million who had SBP > 140 mmHg and 3.2 million with SBP > 130 mmHg.
PAD and Mortality
Among all 647 PAD subjects including those with CVD, 168 deaths were identified, for a weighted mortality rate of 22.6% over a mean follow-up period of 4.4 years. In comparison, subjects without PAD (n = 6811) had a weighted mortality rate of 5.0%. Adjusting for age, gender, and race, PAD was significantly associated with all-cause mortality (HR 2.4, 95%CI 1.9-2.9, p<0.0001). This association persisted even after multivariable adjustment (HR 1.78, 95%CI 1.4-2.3, p=0.0001) (table 3).
Table 3. Risk of all-cause mortality associated with PAD.
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Subjects without CVD (n=6385) | 4.39 (3.1-6.2) | <0.0001 | 1.93 (1.3-2.8) | 0.001 | 1.59 (1.1-2.4) | 0.02 | 1.39 (0.9-2.2) | 0.1 |
| All subjects (n=7458) | 5.28 (4.3-6.5) | <0.0001 | 2.36 (1.9-2.9) | <0.0001 | 1.9 (1.5-2.4) | <0.0001 | 1.78 (1.4-2.3) | 0.0001 |
Model 1 = Adjusted for age, gender, race/ethnicity
Model 2 = Model 1 + atherosclerotic risk factors (diabetes, hypertension, hyperlipidemia, smoking, and chronic kidney disease)
Model 3 = Model 2 + health insurance status, education level, and socioeconomic status (based on poverty-income ratio)
There were 450 patients with PAD but without existing CVD in whom mortality data was available. Among these subjects, there were 89 deaths, for a weighted death rate of 16.1%, significantly higher than the weighted death rate of 4.1% in non-PAD subjects without CVD (p<0.0001). PAD was strongly associated with all-cause mortality even after excluding subjects with previously recognized CVD (adjusted HR 1.9, 95%CI 1.3-2.8, p=0.001) (table 3). All-cause mortality remained significantly higher in PAD subjects without CVD compared to non-PAD subjects after additional adjustment for traditional atherosclerotic risk factors (HR 1.59 [1.1-2.4], p=0.02). This relationship did not persist after also accounting for socioeconomic variables, including education, insurance status, and income level (HR 1.39 [0.9-2.2]).
Secondary prevention therapies and mortality in individuals with PAD
Given the limited data on the role of preventative therapies on mortality in individuals with PAD but without previously recognized CVD, we focused on this subset for mortality analyses. Because treatment was not randomly assigned among participants in NHANES, we first explored differences in baseline characteristics based on number of treatments that might bias the association between treatment and mortality (table 4). PAD subjects on multiple treatments were more likely to be older, male, and non-Hispanic white, and had higher prevalence of diabetes, hypertension, hyperlipidemia, and chronic kidney disease. These individuals were also significantly more likely to have health insurance and be in higher income categories. In subjects with PAD without known CVD, use of multiple preventative treatments (≥2) was associated with a 65% reduction in risk of all-cause mortality compared to PAD subjects receiving no treatments (HR 0.35 [95% CI 0.2-0.86], p=0.02) after full multivariable adjustment including socioeconomic factors (table 5).
Table 4. Baseline characteristics of PAD subjects according to number of secondary prevention treatments.
| Characteristic | No treatment* (n=244) | One treatment (n=129) | Multiple treatments (n=77) |
|---|---|---|---|
| Age, years (mean) | 63.7 (1.0) | 69.8 (1.6) | 70.4 (1.8) |
| Gender (% male) | 32.2 (3.8) | 33.8 (5.5) | 45.2 (6.6) |
| Race (% Non-Hispanic white) | 70.7 (3.6) | 83.6 (2.4) | 87.9 (3.8) |
| Diabetes (%) | 15.0 (2.1) | 21.7 (4.3) | 22.1 (5.6) |
| Hypertension (%) | 59.0 (4.4) | 76.8 (5.8) | 89.7 (3.9) |
| Hyperlipidemia (%) | 43.1 (3.7) | 61.1 (5.9) | 72.4 (6.3) |
| Smoking (% current or former) | 59.2 (5.0) | 62.8 (6.6) | 68.4 (6.3) |
| Chronic kidney disease (%) | 19.8 (2.9) | 32.2 (4.6) | 45.3 (7.6) |
| Systolic blood pressure, mmHg (mean) | 138.2 (1.4) | 142.3 (3.7) | 137.1 (2.3) |
| Body mass index, kg/m2 (mean) | 28.0 (0.6) | 28.9 (0.6) | 29.8 (1.3) |
| Ankle-brachial index (mean) | 0.80 (0.01) | 0.76 (0.01) | 0.74 (0.02) |
| High school education (%) | 69.6 (3.5) | 70.1 (4.8) | 63.8 (6.6) |
| Socioeconomic status (based on PIR) | |||
| Income below poverty level (%) | 21.1 (4.0) | 11.4 (2.3) | 5.3 (1.8) |
| Income 1-2 times poverty level (%) | 29.2 (3.3) | 27.9 (5.1) | 26.9 (6.7) |
| Income 2-3 times poverty level (%) | 17.4 (4.7) | 28.2 (5.7) | 23.4 (5.9) |
| Income 3-4 times poverty level (%) | 12.4 (3.1) | 8.2 (3.5) | 8.3 (4.3) |
| Income >5 times poverty level (%) | 19.9 (5.2) | 24.2 (4.9) | 36.1 (7.9) |
| Low socioeconomic status (Income < 2 times poverty level) (%) | 50.3 (4.8) | 39.4 (5.4) | 32.2 (6.9) |
| Uninsured (%) | 13.9 (2.9) | 2.6 (1.5) | 0.8 (0.8) |
includes anti-platelet therapy, statin use, and/or ACE inhibitor or ARB;
PIR = poverty-income ratio
Table 5. Cox proportional hazards models of mortality and number of preventative therapies used among PAD subjects without recognized CVD.
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| N | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| No preventative therapies* | 244 | ref | ref | ref | |||
| One preventative therapy | 129 | 1.12 (0.6-2.1) | 0.7 | 1.15 (0.6-2.2) | 0.7 | 1.24 (0.6-2.5) | 0.5 |
| Multiple preventative therapies (≥ 2) | 77 | 0.79 (0.4-1.7) | 0.5 | 0.63 (0.2-1.7) | 0.3 | 0.35 (0.2-0.86) | 0.02 |
Therapies include statin, anti-platelet therapy, and/or ACEI/ARB.
Model 1 = Adjusted for age, gender, race/ethnicity
Model 2 = Model 1 + atherosclerotic risk factors (diabetes, hypertension, hyperlipidemia, smoking, and chronic kidney disease)
Model 3 = Model 2 + health insurance status, education level, and socioeconomic status (based on poverty-income ratio)
Discussion
Using the nationally representative NHANES database, we estimate that millions of US adults with PAD do not receive secondary prevention treatments that may reduce the risk of adverse cardiovascular events. PAD subjects remain at significantly increased risk of all-cause death compared to those without PAD. Consistent with prior studies, we found a significantly increased risk of mortality in patients with PAD, with 2-fold increased risk of all-cause mortality after multivariable adjustment, even among individuals without recognized CVD at baseline, 16% of whom died during an average follow-up of 4.4 years. Thus, patients with PAD remain at high risk of all-cause mortality even in the absence of established CVD.
These mortality rates in individuals with PAD may have important implications for the relative benefit that secondary prevention treatments can afford in this population. Our observational data found that treatment with two or more preventative therapies (including aspirin, statin, and/or ACEI/ARB) is associated with a 65% reduced risk of all-cause mortality in individuals with PAD who do not have previously established cardiovascular disease. Yet, the role of ABI screening for the presence of PAD is controversial. The ABI is a simple, non-invasive test with high diagnostic accuracy for PAD,9 and several studies have shown a strong association with lower ABI values and increased mortality.10, 11 Multiple therapies are known to reduce cardiovascular risk in individuals with established CVD. However, no studies have evaluated whether initiation of multiple secondary prevention therapies based on a low ABI in individuals without otherwise recognized CVD can indeed improve outcomes. The lack of existing data has been acknowledged by the USPSTF which has assigned ABI screening an ‘I’ recommendation as a novel cardiovascular risk marker, indicating insufficient information to support its use in general clinical practice.15
Preventative Therapies in PAD
Although it is well-recognized that individuals with PAD are inadequately treated with secondary prevention therapies,3, 4 no prior study has estimated the absolute number of individuals in the US with PAD who are not receiving therapies that may reduce the risk of myocardial infarction, stroke, or death. The complex sampling methodology used in NHANES allows for the calculation of nationally representative population estimates. Based on these NHANES data, we estimate that approximately 5.0 million adults with PAD are not taking a statin, 5.4 million are not taking an ACEI/ARB, and 4.4 million are not receiving anti-platelet therapy. This indicates that there are millions of adults in the US with PAD with or without co-existing CVD who stand to benefit from secondary prevention treatments. Treatment utilization was significantly higher in PAD subjects with recognized CVD compared to those without previously established CVD. This supports the notion that identification of atherosclerotic vascular disease by ABI screening is likely to result in greater use of secondary prevention therapies.
Most prior studies of secondary prevention have only included individuals with recognized and/or symptomatic disease enrolled on the basis of symptoms of intermittent claudication or by a history of prior lower extremity revascularization.5, 7, 8, 21, 22 For example, the Heart Protection Study (HPS), the Heart Outcomes Prevention Evaluation (HOPE) study, and the Antithrombotic Trialists' Collaboration meta-analysis, evaluating statins, ACEIs, and anti-platelet therapy respectively, predominantly included symptomatic PAD subjects with either claudication or prior peripheral vascular intervention.5, 7, 8 Whether the benefits of these agents would be applicable to individuals with asymptomatic PAD, recognized solely on the basis of an abnormal ABI, remains to be determined. Furthermore, the combined effect of multiple preventative therapies to reduce mortality in PAD has not been previously studied.
The role of anti-platelet therapy, and aspirin in particular, in reducing cardiovascular risk in individuals with newly detected PAD remains controversial. The Aspirin for Asymptomatic Atherosclerosis (AAA) trial evaluated asymptomatic, otherwise healthy, individuals with previously unrecognized PAD and found that aspirin therapy alone did not reduce the risk of adverse cardiovascular events.15 In addition, the POPADAD study found no benefit of aspirin in asymptomatic PAD subjects, all of whom had co-existing diabetes.14 Several factors may have contributed to the apparent lack of benefit in these two studies, including the use of higher ABI thresholds (0.95 and 0.99, respectively) which may have reduced the specificity for detecting patients with PAD and lowered the overall mortality rate, thereby limiting the potential to see a therapeutic benefit. However, the findings are consistent with a recent meta-analysis by Berger and colleagues that included both symptomatic and asymptomatic individuals with PAD. This meta-analysis also showed no significant benefit of aspirin in reducing all-cause or cardiovascular mortality, although the largest study included in the meta-analysis, the POPADAD study, exclusively enrolled individuals with diabetes, a population in which the role of aspirin therapy remains unclear.12, 14, 23 These data stand in contrast to an earlier meta-analysis from the Antiplatelet Trialists' Collaboration which has supported a role of anti-platelet therapy in secondary prevention in all patients with atherosclerotic vascular disease.8
The use of statins for the prevention of cardiovascular events has been extensively studied in individuals with recognized and established vascular disease, including PAD.5, 6, 24 Specifically, the Heart Protection study demonstrated a 22% relative risk reduction with simvastatin compared to placebo of a first major vascular event.5 However, the Heart Protection Study included only symptomatic PAD, and statin therapy has not specifically been evaluated in individuals with asymptomatic or previously unrecognized PAD. The HOPE study showed that ACE inhibitors reduce cardiovascular events by approximately 25% in patients with symptomatic PAD.7 Although ABI (determined by palpation of the foot pulse) was measured in HOPE, patients were not enrolled solely on the basis of a low ABI.25
Taken together, our data suggest that combination therapy with multiple risk-modifying therapies may be associated with clinical benefit in a population of individuals defined solely by an abnormal ABI. In the end, these data are hypothesis-generating and underscore the need for a definitive clinical trial to determine whether cardiovascular risk-modifying therapies alone or in combination can in fact reduce mortality and cardiovascular events in subjects identified as being high risk based on a screening ABI examination.
Limitations
The limitations of our study warrant consideration. First, despite the power of NHANES to allow population estimates for the United States, the mortality analyses included only the 450 individuals with PAD who did not have established CVD. This limited our power to observe effects of any individual treatments on outcomes in PAD, especially because only a small number of individuals were taking these therapies. Second, it is unknown whether a new diagnosis of PAD may have altered an individual's medical management; data in NHANES are collected at only a single time point and no follow-up information is available regarding initiation of medications after the study visit. Third, the diagnosis of cardiovascular disease was based on participant self-report and not verified by medical record. However, we would expect any resulting misclassification to bias towards a null result. Finally, with respect to measurement of ABI, blood pressure was measured in only one arm and only in the posterior tibial artery, another factor that might raise the potential for misclassification.
Summary
In this nationally representative sample, population-based ABI measurement identified millions of high-risk US adults with PAD who were not receiving guideline-recommended secondary prevention therapies. Individuals with PAD, and notably those without recognized CVD, were at a high risk of mortality. Treatment with multiple secondary prevention therapies was associated with reduced risk of all-cause mortality in this population. These observational findings highlight the critical need for a large-scale clinical trial to determine if implementation of secondary prevention therapies in high-risk individuals identified by ABI screening as having PAD can reduce mortality and cardiovascular events.
Clinical perspective.
Cardiovascular disease remains a major cause of morbidity and mortality in the United States. Peripheral artery disease (PAD) is a manifestation of systemic atherosclerosis that confers a significantly increased risk of myocardial infarction, stroke, and death. Whether cardiovascular risk can be reduced by implementation of secondary prevention therapies (such as anti-platelet therapy, statins, or angiotensin converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB)) in individuals with PAD identified by a screening ankle-brachial index (ABI) measurement is unknown. Using data from the National Health and Nutrition Examination Survey (NHANES), we demonstrate that millions of high-risk US adults with PAD (ABI ≤ 0.90) were not receiving guideline-recommended secondary prevention therapies. All-cause mortality was significantly higher in Individuals with PAD, including those without previously recognized CVD. Furthermore, treatment with multiple secondary prevention therapies was associated with significantly reduced risk of all-cause mortality in this population. Given the conflicting literature about the use of secondary prevention therapies, and aspirin in particular, in patients with PAD, these observational findings underscore the importance of a large-scale clinical trial to determine if implementation of multiple secondary prevention therapies specifically in high-risk individuals identified by ABI screening as having PAD can indeed reduce cardiovascular morbidity and mortality.
Acknowledgments
Sources of Funding: Dr. Pande and Dr. Perlstein have received support from a Research Career Development Award (K12 HL083786) from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Pande has also received funding from an American Heart Association Scientist Development Grant (#10SDG4200060). This work was also supported by grant R01 HL075771 from the NHLBI. Dr. Creager is the Simon C. Fireman Scholar in Cardiovascular Medicine at Brigham and Women's Hospital.
Footnotes
Disclosures: No authors have any relevant conflicts of interest to disclose. Dr. Pande had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45 S:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 4.Cacoub PP, Abola MT, Baumgartner I, Bhatt DL, Creager MA, Liau CS, Goto S, Rother J, Steg PG, Hirsch AT. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Atherosclerosis. 2009;204:e86–92. doi: 10.1016/j.atherosclerosis.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–654. doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 6.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 8.Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. Bmj. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 9.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 10.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 12.Berger JS, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301:1909–1919. doi: 10.1001/jama.2009.623. [DOI] [PubMed] [Google Scholar]
- 13.Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. Jama. 2010;303:841–848. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 14.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O'Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. Bmj. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2009;151:474–482. doi: 10.7326/0003-4819-151-7-200910060-00008. [DOI] [PubMed] [Google Scholar]
- 16.Pande RL, Perlstein TS, Beckman JA, Creager MA. Association of insulin resistance and inflammation with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999 to 2004. Circulation. 2008;118:33–41. doi: 10.1161/CIRCULATIONAHA.107.721878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:166–172. doi: 10.1161/ATVBAHA.107.153262. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Available at: http://www.cdc.gov/nchs/data/datalinkage/nh99+_mortality_matching_methodology_final.pdf. Accessed July 22, 2010.
- 20.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feringa HH, van Waning VH, Bax JJ, Elhendy A, Boersma E, Schouten O, Galal W, Vidakovic RV, Tangelder MJ, Poldermans D. Cardioprotective medication is associated with improved survival in patients with peripheral arterial disease. Journal of the American College of Cardiology. 2006;47:1182–1187. doi: 10.1016/j.jacc.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 22.Hoeks SE, Scholte op Reimer WJ, van Gestel YR, Schouten O, Lenzen MJ, Flu WJ, van Kuijk JP, Latour C, Bax JJ, van Urk H, Poldermans D. Medication underuse during long-term follow-up in patients with peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2009;2:338–343. doi: 10.1161/CIRCOUTCOMES.109.868505. [DOI] [PubMed] [Google Scholar]
- 23.Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care. 2003;26:3264–3272. doi: 10.2337/diacare.26.12.3264. [DOI] [PubMed] [Google Scholar]
- 24.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 25.Ostergren J, Sleight P, Dagenais G, Danisa K, Bosch J, Qilong Y, Yusuf S. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24. doi: 10.1016/j.ehj.2003.10.033. [DOI] [PubMed] [Google Scholar]