Abstract
Human amniotic fluid–derived stem (AFS) cells possess several advantages over embryonic and adult stem cells, as evidenced by expression of both types of stem cell markers and ability to differentiate into cells of all three germ layers. Herein, we examine endothelial differentiation of AFS cells in response to growth factors, shear force, and hypoxia. We isolated human AFS cells from amniotic fluid samples (1–4 cc/specimen) obtained from patients undergoing amniocentesis at 15–18 weeks of gestation (n = 10). Isolates maintained in nondifferentiating medium expressed the stem cell markers CD13, CD29, CD44, CD90, CD105, OCT-4, and SSEA-4 through passage 8. After 3 weeks of culture in endothelial growth media-2 (EGM-2), the stem cells exhibited an endothelial-like morphology, formed cord-like structures when plated on Matrigel, and uptook acetylated LDL/lectin. Additionally, mRNA and protein levels of CD31 and von Willebrand factor (vWF) significantly increased in response to culture in EGM-2, with further up-regulation when stimulated by physiological levels (12 dyne/cm2) of shear force. Culture in hypoxic conditions (5% O2) resulted in significant expression of vascular endothelial growth factor (VEGF) and placental growth factor (PGF) mRNA. This study suggests that AFS cells, isolated from minute amounts of amniotic fluid, acquire endothelial cell characteristics when stimulated by growth factors and shear force, and produce angiogenic factors (VEGF, PGF, and hepatocyte growth factor [HGF]) in response to hypoxia. Thus, amniotic fluid represents a rich source of mesenchymal stem cells potentially suitable for use in cardiovascular regenerative medicine.
Introduction
Numerous stem and progenitor cell types demonstrate the potential to differentiate into cardiovascular cell-like phenotypes, particularly those derived from the bone marrow, cord and peripheral blood, and adipose tissue [1–7]. Presently, human adult bone marrow is the most common source of mesenchymal stem cells for use in regenerative medicine purposes. Unfortunately, the frequency and potential of cells derived from human adult bone marrow decreases with donor age and morbidity [8–10], potentially limiting their use in certain patient populations.
Recently, several groups have isolated pluripotent stem cells from human amniotic fluid [11–15]. These stem cells possess several potential advantages over both embryonic and adult stem cells. Considered to be at an intermediate stage between these two basic stem cell types, amniotic fluid–stem (AFS) cells express both types of stem cell markers, proliferate rapidly, and differentiate into cells of all three embryonic germ layers [16–20]. Kaviani et al. demonstrated that these cells proliferate more rapidly than comparable fetal and adult cells in culture and attach firmly to polyglycolic acid polymer [17]. This study suggested that small amounts of amniotic fluid yield enough stem cells to create a tissue-engineered construct ready for implantation immediately after the birth of the child. Due to their higher proliferative capacity and ability to maintain pluripotency at higher passage numbers, these cells may prove to be a readily available source for large numbers of cells for other types of cardiovascular regenerative medicine applications.
Both endothelial growth factors and shear force mediate endothelial differentiation and vascular development. Recently, the effect of shear on embryonic stem, endothelial progenitor, and mesenchymal stem cells has been investigated [21–24]. Shear force increases proliferation, differentiation, capillary tube formation, vascular endothelial growth factor (VEGF) receptor expression, and production of tissue plasminogen activator and nitric oxide in these cells [25–28]. Currently, the effect of shear on amniotic fluid-derived stem cell differentiation into vascular cells is unknown. Understanding this effect may provide us with an additional stimulus to use in developing the clinical potential for this unusual stem cell.
The purpose of this study is to further evaluate the potential use of amniotic fluid stem cells for use in cardiovascular regeneration strategies. Specifically, we evaluate the isolation of these cells from minute amounts of amniotic fluid and their subsequent acquisition of endothelial cell characteristics after stimulation with both endothelial growth factors and shear force. Additionally, since hypoxia modulates proangiogenic cytokine production, we also evaluate the effect of hypoxia on the function of AFS cells in this setting.
Materials and Methods
Human AFS cell isolation and culture
Under an IRB-approved protocol, human amniotic fluid samples (1–4 cc/specimen) were obtained from patients undergoing amniocentesis for clinical indications at 15–18 weeks of gestation (n = 10). The procedure was described by Kim et al. [11] with some modifications. After centrifugation the AFS cells were plated in a 24-well culture dishes and grown in AmnioMAX II Complete (Gibco, Grand Island, NY) medium at 37°C with 5% CO2 atmosphere. Non-adherent cells were removed after 5 days and the medium was subsequently replaced twice weekly. When the cells reached confluence, they were subcultured in T-25 cm2 flasks containing expansion medium (M199 media with HEPES buffer, heparin (7.5 U/mL), and 10% of FBS). For experiments involving hypoxic culture conditions (5% O2), a hypoxia workstation (Invivo2, Ruskinn Technology, Cleveland, OH) was utilized, allowing for accurate and uninterrupted hypoxic conditions. All cells used for experimentation were between passages 4 and 8.
Confirmation of multipotency
While the purpose of this study is to further define the endothelial differentiation of AFS cells, the following experiments were performed to demonstrate that our cultures were multipotent, as suggested by previous literature.
Adipogenic differentiation. The culture-expanded AFS cells at 5 × 104/mL were induced in NH AdipoDiff Medium (Miltenyi Biotec, Auburn, CA) for 3 weeks. The cells were incubated with cooled methanol for 5 min and stained with fresh Oil Red-O solution for 20 min to identify lipid droplets.
Osteogenic differentiation. The culture-expanded AFS cells at 3 × 104/mL were induced in NH OsteoDiff Medium (Miltenyi Biotec) for 3 weeks. Then the cells were stained for alkaline phosphatase (AP) activity with NBT substrate (Sigma, St. Louis, MO).
Endothelial growth factors and shear force treatments
Amniotic fluid–stem cells (passages 3–6) were kept in expansion media as control or cultured in endothelial growth media-2 (EGM-2; Clonetics, Chicago, IL) with SingleQuots containing VEGF, hFGF-b, epidermal growth factor, insulin-like growth factor-1, heparin, ascorbic acid and with 10% FBS. The medium was exchanged with fresh medium every 3 days.
Shear stress experiments were performed using an orbital shaker as described by Dardik et al. [29]. AFS cells cultured in EGM-2 for 2 weeks were seeded onto six-well plates at 5 × 104 cells/cm2, seated on an orbital shaker (Bellco Biotechnology, Vineland, NJ) located in an incubator maintained at 37°C, 5% CO2. The shaker was rotated for 48 h at 210 cycles/min to produce 12 dynes at the periphery of the wells. Cells kept under static conditions were used as controls.
Functional assays of endothelial differentiation
Uptake ac-LDL and lectin. AFS cells cultured in EGM-2 for 2 weeks were incubated with DiI-labeled acetylated low-density lipoprotein (ac-LDL; 10 µg/mL; Sigma) at 37°C for 2 h, fixed and labeled with FITC-conjugated human lectin (10 mg/mL; Sigma) for 1 h.
In vitro cord formation assay. Trypsinized cells were plated on top of Matrigel substrate (BD Biosciences, San Jose, CA) and incubated at 37°C in a 5% CO2 for up to 24 h. Formation of cord-like structures was visualized by phase contrast microscopy. NIH Image software was used to determine the total length of the tube-like structure in images captured [25 ].
Flow cytometric analysis
The specific cell surface antigen of AFS cells in the cultures were characterized by flow cytometry. The cells were detached with trypsin (0.25% with 0.1% EDTA in HBSS) and suspended in cold staining buffer. Approximately 5 × 105 cells were incubated with fluorescence-conjugated antibodies for 30 min. The antibodies used were CD13 (APC-conjugated; BD Biosciences), CD29 (PE-Cy5-conjugated; BD Biosciences), CD31 (PE-conjugated; BD Biosciences), CD34 (PE-Cy5-conjugated; BD Biosciences), CD44 (APC-conjugated; eBioscience, San Diego, CA), CD45 (FITC-conjugated; BD Biosciences), CD90 (FITC-conjugated; BD Biosciences), CD105 (PE-conjugated; R&D, Minneapolis, MN), CD133 (PE-conjugated; Miltenyi Biotec, Minneapolis, MN), SSEA-4 (APC-conjugated; R&D), OCT-3/4 (FITC-conjugated; R&D), and vWF (BD Biosciences). Isotype identical antibodies served as controls to exclude nonspecific binding. Quantitative analysis was performed using FACSCalibur flow cytometer (BECKMAN) and FlowJo software.
Real-time RT-PCR analysis
Total RNA was extracted through RNeasy mini columns (QIAGEN, Valencia, CA). Gene transcripts were measured by TaqMan real-time reverse transcription PCR with the 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). PCR primers targeting human platelet/endothelial cell adhesion molecule (PECAM, CD31), von Willebrand factor (vWF), endothelial nitric oxide synthase (eNOS), VEGF, placental growth factor (PGF), hepatocyte growth factor (HGF), and TaqMan probes were obtained from Applied Biosystems. PCR conditions were 48°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The housekeeping gene GAPDH was amplified in separate tubes to normalize for variance in input RNA. The level of target mRNA in samples was estimated by the relative standard method with a series of dilution of RNA from human vascular cells.
Measurement of the effect of hypoxia on growth factors
RNA was isolated from AFS cells cultured in normoxia or hypoxia condition for 48 h. To detect the specific gene expression, RT-PCR was used with the following designed primers: basic fibroblast growth factor (bFGF): 5′-primer (5′-GGTGAAACCCCGTCTCTACA-3′) and 3′-primer (5′-TCTGTTGCCTAGGCTGGACT-3′); hepatocyte growth factor (HGF): 5′-primer (5′-ATCAAATG TCAGCCCTGGAG-3′) and 3′-primer (5′-TCGATAACTCTC CCCATTGC-3′); placental growth factor (PGF): 5′-primer (5′-TGCCTTCAACAACGTGAGAG-3′) and 3′-primer (5′-AGGATCCGCATCCCTACTTT-3′); angiopoietins-1 (Ang-1): 5′-primer (5′-GAAGGGAACCGAGCCTATTC-3′) and 3′-primer (5′-GGGCACATTTGCACATACAG-3′); endothelial nitric oxide synthase (eNOS): 5′-primers (5′-ATCCCC CAGAACTCTTCCTT-3′) and 3′-primer (5′-CTCATTCTCCA GGTGCTTCA-3′). Amplified PCR products were electrophoresed on 2% agarose gels with ethidium bromide and visualized with a UV transilluminator.
Enzyme-linked immunosorbent assay (ELISA)
VEGF concentration within the cell culture supernatant was quantified using a quantitative sandwich ELISA kit (R&D systems) per the manufacturer’s instructions. Samples were evaluated on the ELISA plate reader at 450 nm with corrections at 570 nm.
Statistical analysis
Data represented are mean ± standard error from three independent experiments (from different donors) for each group. Student’s t-test was used for two group comparisons. Multigroup comparisons were determined by one-way ANOVA with Bonferroni correction. A value of P < 0.05 was considered significant.
Results
Isolation and cultivation of human amniotic fluid-derived stem cells
We successfully isolated human amniotic fluid-derived stem cells from each of 10-min samples of amniotic fluid (1–4 mL each). After discarding nonadherent cells 5 days after initial isolation, colonies of mesenchymal-like stem cells appeared by the 10th day of culture in AmnioMaxII complete medium (Fig. 1A). This population of cells, which exhibited a fibroblast-like morphology, expanded in vitro for over 10 passages with expansion medium (Fig. 1B).
FIG. 1.
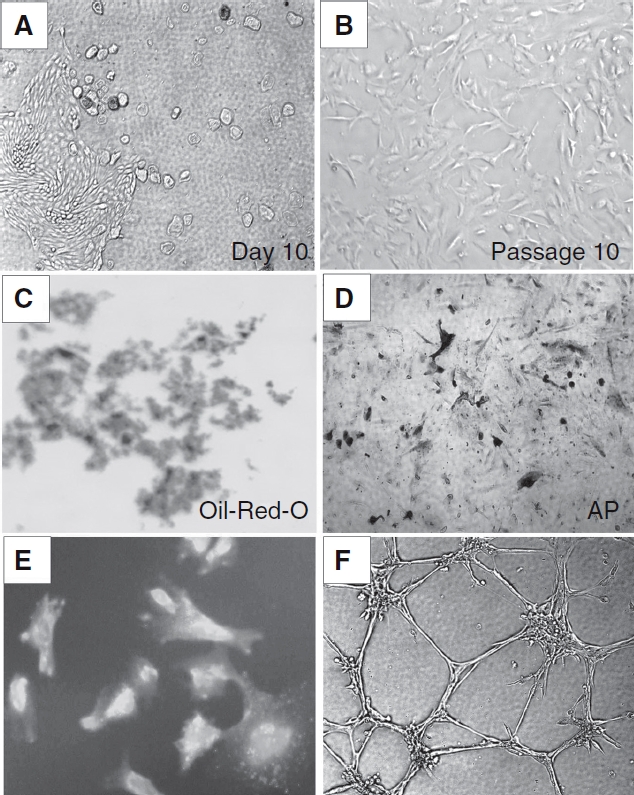
Morphology and differentiation of amniotic fluid-derived stem (AFS) cells. (A) Isolation and culture of AFS cells. After discard of nonadherent cells 5 days after initial isolation, colonies of mesenchymal-like stem cells appeared by the 10th day of culture in AmnioMaxII complete medium. (B) This population exhibited a fibroblast-like morphology in vitro for over 10 passages with expansion medium. (C and D) Adipogenic and osteogenic differentiation of AFS as demonstrated by Oil Red-O staining and alkaline phosphatase (AP) activity assay after 3 weeks of culture in adipocyte and osteogenic induction media, respectively, indicates the isolated stem cells are multipotent. (E) Characteristics of AFS cells differentiated into endothelial-like cells. Uptake of DiI-labeled ac-LDL followed by staining with FITC-labeled human lectin revealed AFS cells cultured in endothelial growth media-2 media for 2 weeks stained positive for both markers. (F) Formation of capillary-like structures by AFS cells plated onto Matrigel after 2 weeks of culture in endothelial differentiation.
Phenotype of isolated AFS cells
The surface marker expression of the culture-expanded cells at passages 5–8 from human amniotic fluid was analyzed by flow cytometry (Fig. 2). The amniotic fluid-derived stem cells were negative for CD14, CD34, and CD45, known markers for monocyte/macrophage and hematopoietic progenitor cells. The isolated AFS cells were strongly positive for CD13, CD29, CD44, CD90, and CD105, weakly positive for CD133, SSEA-4, and OCT-4, having an immunophenotype similar to that of bone marrow mesenchymal stem cells.
FIG. 2.
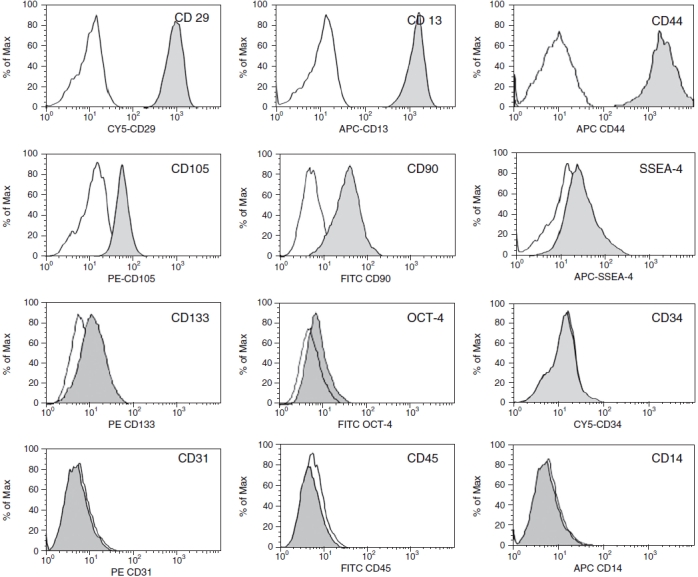
Immunophenotype characterization of amniotic fluid-derived stem (AFS) cells. AFS cells (passage 5) were labeled with antibodies specific for either the molecule indicated (filled histograms) or isotype controls (open histograms). Flow cytometry analysis revealed that expression of surface antigens CD29, CD90, CD13, CD44, and CD105 was strongly positive, while SSEA-4, CD133, and OCT-4 were low positive, and CD34, CD14, CD45, and CD31 were negative. A representative example of four amniotic fluid samples is shown.
Differentiation potential of AFS cells
The differentiation potential of the isolated cells was confirmed by adipogenic and osteogenic induction. Cultured AFS cells at passages 6–8 were treated with adipogenic or osteogenic media. We observed that nearly all the cells cultured in adipocyte induction medium for 3 weeks contained numerous Oil Red-O-positive lipid droplets (Fig. 1C). Similarly, after culture in osteogenic induction medium for 3 weeks, most cells exhibited high levels of alkaline phosphatase (Fig. 1D). Non-induced control cultures of AFS cells did not show either of these two phenomena.
Induction of human AFS cells into endothelial cells
Morphological change. We induced endothelial differentiation by culturing AFS cells in EGM-2 containing several endothelial growth factors (VEGF, basic fibroblast growth factor (hFGF-b), epidermal growth factor, insulin-like growth factor-1, heparin, ascorbic acid plus 10% FBS). After 1 week of culture, AFS cells acquired a clear endothelial-like morphology (Fig. 3A ).
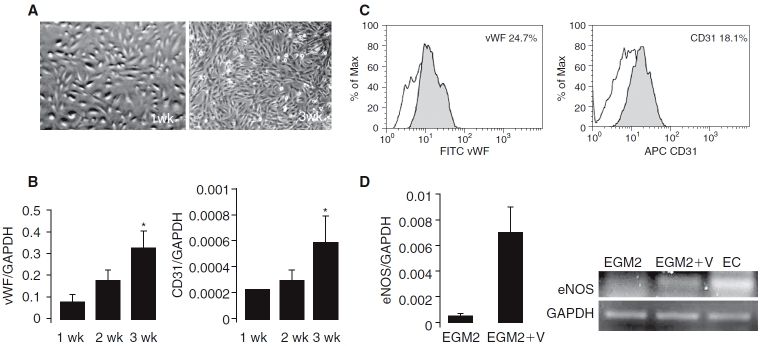
FIG. 3.
Phenotypic characteristics and expression of endothelial cell (EC)-specific markers on differentiated amniotic fluid-derived stem (AFS) cells. (A) Morphology of AFS cells after cultivation in endothelial growth media (EGM-2) for 1 and 3 weeks. (B) Time course of transcript levels of EC-specific genes determined by TaqMan real-time quantitative RT-PCR. AFS cells were cultured in EGM-2 for 1 and 3 weeks to induce endothelial differentiation. Endothelial-specific transcript levels increased by approximately 3-fold (CD31) and 4-fold (vWF) by week 3 compared with week 1. Values of mRNA amount were normalized to GAPDH expression (*P < 0.05, n = 3). (C) CD31 and vWF protein expression in the differentiated AFS cells. Flow cytometry revealed the presence of CD31 (18.1%) and vWF (24.7%) in cells cultured in EGM-2 for 3 weeks (filled peaks). The open peaks show the isotype-matched antibody control. (D) Vascular endothelial growth factor (VEGF)-induced expression of eNOS in differentiated AFS cells. Cells were cultured in EGM-2 alone or EGM-2 supplemented with VEGF (50 ng/mL) for 2 weeks. AFS cells expressed eNOS mRNA only when VEGF was added to the culture medium.
Endothelial marker expression. We examined the time course of the expression of EC-specific genes and proteins in the differentiating AFS cells from 1 to 3 weeks of differentiation. Quantitative RT-PCR analysis showed a consistent increase with time in the transcription of EC-specific markers including platelet/endothelial cell adhesion molecule (PECAM, CD31) and vWF during AFS cell differentiation in EGM-2. Compared with week 1, the expression of vWF mRNA increased 4-fold and CD31 increased 3-fold by week 3 (Fig. 3B ). Additionally, flow cytometry revealed the presence of vWF (24.7%) and CD31 (18.1%) after culture in EGM-2 for 3 weeks (Fig. 3C ).
We did not observe endothelial nitric oxide synthase (eNOS) expression after 2 weeks in EGM-2 induction medium alone. However, eNOS was expressed following the addition of VEGF (50 ng/mL) to the EGM-2 culture medium (EMG2+V, Fig. 3D).
Functional characteristics. After 2 weeks, fluorescent immunocytochemical study revealed that the AFS cells stained double positive for DiI-ac-LDL and human lectin (Fig. 1E), confirming their ability to engulf acetylated LDL.
Differentiated AFS cells were evaluated for cord formation in vitro. After 1 week of differentiation in EGM-2, subsequent culture on Matrigel revealed that the cells form small but seldom interconnected cords. After 2 weeks of differentiation, however, clear cord structures were seen (Fig. 1F). These changes confirm the potential for angiogenic behavior within the differentiated cells.
Effects of shear stress on the endothelial differentiation of AFS cells
Morphological change. Exposure of differentiated AFS cells (those cultured in EGM-2 for 2 weeks) to physiological levels of shear stress (12 dynes) resulted in realignment in the direction of flow after 48 h (Fig. 4A ), a change characteristic of endothelial cells in response to shear.
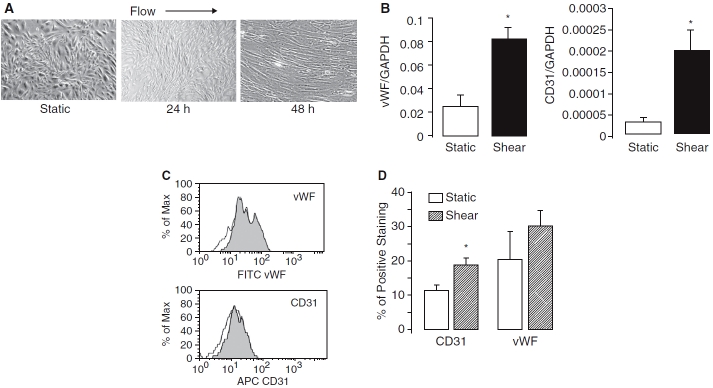
FIG. 4.
Effects of shear force on amniotic fluid-derived stem (AFS) cells differentiation. (A) Phase contrast photomicrographs of AFS cells cultured under static conditions or exposed to shear stress (12 dyne) for 24 and 48 h. (B) Up-regulation of endothelial cell (EC)-specific markers by shear force. Relative CD31 and vWF mRNA expression in AFS cells cultured in static conditions or exposed to shear stress for 48 h. Values of mRNA amounts were normalized to GAPDH expression and expressed relative to its static control for that experimental condition (n = 3). (C) CD31 and vWF protein expression as determined by flow cytometry analysis. Representative histograms for CD31 and vWF expression in cells exposed to shear stress (filled peaks) versus static control groups (open peaks). (D) Mean number of cells positive for CD31 or vWF in shear stress and control groups (*P < 0.05 vs. static control, n = 3).
Endothelial marker expression. Shear force also led to significant up-regulation in the expression of CD31 (approximately 7-fold) and vWF (approximately 3-fold) mRNA levels when compared with static controls by real-time RT-PCR analysis (Fig. 4B ). Flow cytometry showed the levels of protein expression of CD31 increased approximately 2-fold and vWF increased approximately 3-fold after exposure to shear stress for 48 h (Fig. 4C and D). No significant expression of eNOS could be demonstrated in AFS cultured in EGM-2, with or without the addition of shear stress (data not shown).
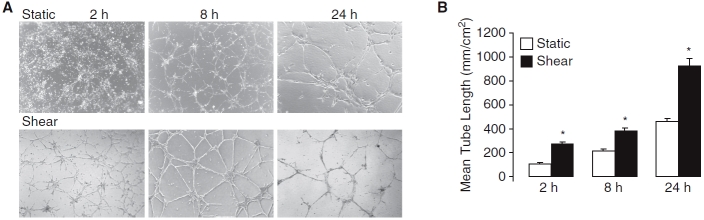
Functional characteristics. To investigate whether shear altered the angiogenic behavior of the AFS cells, differentiated cells exposed to shear stress (12 dynes) were observed on Matrigel over a 24-h period. AFS cells cultured without shear formed cords 8 h after plating on Matrigel, while those exposed to shear began this process as early as 2 h (Fig. 5A ). Quantitative analysis revealed that the total length of the cords at 2, 8, 24 h was significantly greater in shear-stressed AFS cells compared to static controls (Fig. 5B), suggesting that shear enhances the potential angiogenic behavior of the AFS cells.
FIG. 5.
Comparison of the amniotic fluid-derived stem (AFS) cells ability to formation of capillary-like structures in Matrigel between static and shear stress by in vitro angiogenesis assay. (A) Representative images captured after plating on Matrigel for 2, 8, and 24 h. AFS cells cultured in endothelial growth media-2 for 2 weeks and subsequently incubated under static conditions or exposed to shear stress (12 dyne for 2, 8, or 24 h) were seeded onto Matrigel. Capillary-like structures appeared at 8 h in static AFS cell cultures, while this process began as early as 2 h in shear-stressed cells. (B) Quantification of tube formation. National Institutes of Health image was used to determine the total length of the tube-like structures in images of cells exposed to shear stress for 2, 8, and 24 h (*P < 0.01 vs. static control, n = 3).
Hypoxia increases expression of VEGF, PGF, and HGF in AFS cells
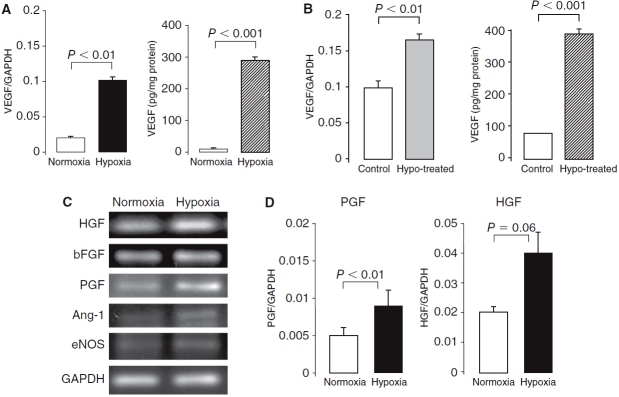
We analyzed VEGF mRNA expression in AFS cells after 48 h of culture under hypoxic conditions (5% O2) by real-time RT-PCR. The hypoxia-conditioned cells expressed higher levels (5-fold increase) of VEGF mRNA than cells cultured under normoxic conditions, and released higher levels (30-fold increase) of VEGF into the medium by ELISA (Fig. 6A). The hypoxic pretreatment of AFS cells (5% O2, 48 h) subsequently cultured in endothelial differentiation medium for 1 week resulted in significantly higher expression of VEGF mRNA and protein levels over non-pretreated cells (Fig. 6B). Lastly, we used RT-PCR to analyze the expression of an additional five angiogenesis-related genes in AFS cells under hypoxic conditions. AFS cells were positive for HGF, bFGF, and PGF but did not express the genes of Ang-1 and eNOS (Fig. 6C). Real-time RT-PCR confirmed increase in the expression of PGF and HGF mRNA in AFS cells under hypoxic conditions (Fig. 6D). Ang-1 and eNOS showed very low expression, and bFGF expression was not changed by hypoxia treatment.
FIG. 6.
Effect of hypoxia on angiogenic factor expression in amniotic fluid-derived stem (AFS) cells. AFS cells were cultured in hypoxic conditions (5% O2) for 48 h then induced into endothelial cells by cultured in endothelial growth media-2 for 1 week. (A) Vascular endothelial growth factor (VEGF) mRNA level was significantly increased in response to hypoxia as measured by real-time RT-PCR. AFS cells produce low levels VEGF as measured by ELISA; however, AFS cells cultured in hypoxia significantly increased VEGF production. (B) After 1 week of culture in endothelial differentiation medium, the hypoxia-pretreated AFS cells (48 h, 5% O2) showed up-regulation of VEGF in both mRNA and protein levels compared to the cells untreated with hypoxia. (C) Characterization of additional angiogenic cytokine expression in AFS cells using RT-PCR. AFS cells were positive for HGF, bFGF, and PGF, but did not express the genes of Ang-1 and eNOS. (D) Real-time RT-PCR confirmed the PGF and HGF mRNA levels were increased in hypoxia culture. The Ang-1 and eNOS showed very low expression, and the expression of bFGF was not changed by hypoxia treatment.
Discussion
Amniotic fluid is a stem cell source that yields cells shown to have high self-renewal capability and broad pluripotency [13]. The findings from this study indicate that these multipotent stem cells can differentiate into cells with endothelial-like morphology, marker expression, and function. In an in vitro experimental model, endothelial differentiation induced by growth factors was augmented by shear stress. Further, hypoxia stimulates secretion of VEGF by AFS cells. Our data confirm that these cells can be easily obtained and culture-expanded from small amounts of human amniotic fluid in an ethically acceptable fashion. Taken together, these results suggest that human AFS cells represent a potent source of stem cells with potential therapeutic uses for cardiovascular disease.
In the present report, we provide further in vitro evidence that human AFS cells can acquire several important endothelial cell characteristics. A number of studies have shown that embryonic and adult mesenchymal stem cells can differentiate into endothelial cells (ECs) in the presence of VEGFs [1,15,30–33]. De Coppi et al. (2007) observed endothelial differentiation (as measured by expression of CD31) of amniotic fluid-derived cells when the cells were cultured in EGM-2 supplemented with recombinant human bFGF for 8 days [13,15]. Schmidt et al. [34] and Perin et al. [35] reported that culturing amniotic fluid-derived stem cells in EGM-2 with addition of VEGF or hFGF resulted in CD31 and vWF expression after 2 weeks. In our differentiation system, AFS cells express both of these molecular markers as early as 1 week, and continued to increase expression 3-fold over the next 2 weeks.
When cultured in EGM-2 (which includes VEGF 10 ng/mL) for up to 3 weeks, AFS cells did not express endothelial nitric oxide synthase (eNOS). However, measurable expression of eNOS appeared with the addition of VEGF (50 ng/mL) to EGM-2 for 2 weeks. Endothelial NOS is essential in the signaling for VEGF, which itself is required for the development and function of endothelial cells [36]. Several previous investigators have used VEGF as a key stimulus for endothelial differentiation of AFS cells [13,15,37]. This growth factor is also an important part of cocktails used to differentiate in vitro endothelial progenitor cells and mesenchymal progenitor cells into endothelial cells [5,38–40]. Recently, Liu et al. demonstrated that eNOS was expressed in bone marrow stem cells during the induction of differentiation with VEGF [41]. In this study, we found that expression of eNOS during AFS cell differentiation appeared dependent on elevated VEGF concentration.
In addition, our study shows for the first time that endothelial-induced AFS cells also have the ability to engulf ac-LDL and form capillary-like structures, both typical functions of endothelial cells. Along with the expression of key endothelial molecular markers (vWF, CD31, and eNOS), these data implicate stem cells derived from amniotic fluid are potential new candidates in cell therapy for vascular diseases.
An additional aim of this study was to assess the combined effects of biochemical and mechanical stimuli (shear stress) on the endothelial differentiation of AFS cells. It is well known that shear stress modulates gene expression of many functional proteins in endothelial cells and increases the proliferation, differentiation, and capillary tube formation of endothelial progenitor cells [25,42]. Prior studies involving shear stress demonstrate induction of mature endothelial cell-specific markers such as CD31 and vWF at both the mRNA and protein levels in a murine embryonic mesenchymal progenitor cell line [22]. Wu et al. showed that shear stress increases CD31, vWF, and Flk-1 expression in human placenta-derived multipotent cells [43]. Our results demonstrate that the expression of CD31 and vWF at both the messanger RNA and protein levels in AFS cells were up-regulated by exposure to physiological levels of shear stress, supporting the hypothesis that shear is an important mediator in endothelial differentiation.
In addition to an increased expression of the molecular markers, shear stress caused a morphological change in the stem cells (realignment in the direction of flow similar to endothelial cells) and further promoted their ability to form capillary-like structures in response to extracellular matrix proteins. An important function of endothelial cells is the formation of new vasculature (angiogenesis). To begin to assess this endothelial function in the differentiated AFS cells, we investigated their formation of tube-like structures on Matrigel. Our results showed that culturing AFS cells in EGM-2 with exposure to shear stress significantly increased tube-like structure formation on Matrigel compared with cells cultured in growth media without shear. These results are consistent with others demonstrating an effect of shear stress on inducing differentiation of mesenchymal progenitor cells to an EC phenotype with functional capability [22]. In the present study, we provide evidence for the first time that AFS cells display angiogenic potential and may therefore be considered new candidates for cell therapy to treat vascular diseases.
Previous work demonstrated that amniotic fluid-derived MSCs proliferate more quickly in culture than bone marrow-derived MSCs [20]. In our study, a 1–2 mL amniotic fluid sample yielded several hundred million cells in 4–5 weeks, and expansion in undifferentiating medium was possible at least through passage 10. As such, our data support previous work suggesting that the proliferation capacity of these cells is high. Importantly, we demonstrate that the AFS cells remain phenotypically and genetically stable through this time period. At late passage, the cells continued to express surface markers consistent with mesenchymal stem cells derived from bone marrow, maintain a consistent morphology, and retain their osteogenic and adipogenic potentials. These properties make amniotic fluid a rich source of MSC suitable for banking when large amounts of cells or repeated infusion are required.
Hypoxia is a well-known stimulus of stem cell differentiation into the phenotype of injured tissue, repopulating the diseased organ with healthy cells, and a major stimulus of angiogenesis [44–46]. Hypoxia increases VEGF expression and endothelial differentiation of stem cells, contributing to their therapeutic value in promoting angiogenesis after implantation [47,48]. The prototypical angiogenic cytokine increased by hypoxia is VEGF; however, other angiogenic factors like PGF, hepatocyte growth factor (HGF), and basic fibroblast growth factor (bFGF) are also regulated by hypoxia [49,50]. We observed that in vitro hypoxia stimulated AFS cell expression of VEGF at mRNA and protein levels. We also found that PGF and HGF are also up-regulated by hypoxia in these cells. These data suggest that pre-culturing AFS cells in hypoxic conditions may prove important to their potential use in promoting angiogenesis.
Finally, it is understood that the most appropriate use of these cells may be as an autologous source of stem cells, thereby avoiding possible issues with allogenicity. Potential paradigms for use include isolation and culture of the cells in preparation for cardiovascular tissue engineering needs for newborns with congenital heart defects, or storage of these cells in a tissue bank for future use, similar to cord blood banks.
Conclusions
In summary, the present study suggests that AFS cells grown in culture with growth factors acquire several important endothelial characteristics such as expression of endothelial molecular markers (vWF, CD31, and eNOS), uptake of acetylated LDL, and capacity to participate in angiogenesis. We also provide new information about the up-regulation of these endothelial characteristics in response to physiological shear stress. In addition, we found that hypoxia modulated VEGF, PGF, and HGF expression by AFS cell. Finally, we confirm that AFS cells can be isolated relatively easily and proliferate quickly under standard culture conditions. These observations support the promise that AFS cells have a future role in cardiovascular regenerative therapies.
Contributor Information
Ping Zhang, Departments of Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania..
Jason Baxter, Departments of Obstetrics and Gynecology, Thomas Jefferson University, Philadelphia, Pennsylvania..
Kateki Vinod, Departments of Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania..
Thomas N. Tulenko, Departments of Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania.
Paul J. Di Muzio, Departments of Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania..
Acknowledgments
This work was supported by the National Institutes of Health (grants K08 HL076300-02 and AHA Beginning Grant-in-Aid 0565454U to P.J.D.) and the American Vascular Association Lifeline Foundation (P.J.D.).
References
- 1.Li ZJ, Wu JC, Sheikh AY, Kraft D, Cao F, Xie XY, Patel M, Cambhir SS, Robbins RC, Cooke JP, Wu JC. Differentiation, survival, and function of embryonic stem cell-derived endothelial cells for ischemic heart disease. Circulation. (2007);116:46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H, Fauza DO. Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials. J Pediatr Surg. (2007);42:974–980. doi: 10.1016/j.jpedsurg.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Feng B, Liu YG, Xie N, Feng K, Son LF, Zhau XD. Construction of tissue-engineered homograft bioprosthetic heart valves in vitro. ASAIO J. (2006);52:303–309. doi: 10.1097/01.mat.0000206125.81406.02. [DOI] [PubMed] [Google Scholar]
- 4.Xu Q. Progenitor cells in vascular repair. Curr Opin Lipidol. (2007);18:534–539. doi: 10.1097/MOL.0b013e3282a66082. [DOI] [PubMed] [Google Scholar]
- 5.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. (2004);22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 6.Adams B, Xiao Q, Xu Q. Stem cell therapy for vascular disease. Trends Cardiovasc. (2007);17:246–251. doi: 10.1016/j.tcm.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhang RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. (2005);332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 8.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. (1999);14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 9.Doetscheman T, Shull M, Kier A, Coffin JD. Embryonic stem cell model systems for vascular morphogenesis and cardiac disorders. Hypertension. (1993);22:618–629. doi: 10.1161/01.hyp.22.4.618. [DOI] [PubMed] [Google Scholar]
- 10.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. (2004);109((3)):1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, Cho DJ, Kang SG, You J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. (2007);40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel N, Rosner M, Hanneder M, Freilinger A, Hengstschlager M. Human amniotic fluid stem cells: a new perspective. Amino Acids. (2008);35((2)):291–293. doi: 10.1007/s00726-007-0593-1. [DOI] [PubMed] [Google Scholar]
- 13.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. (2007);25((10)):100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 14.Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. (2004);19:1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- 15.Delo DM, Coppi PD, Bartsch G, Atala A. Amniotic Fluid and Placental stem cells. Methods Enzymol. (2006);419:426–438. doi: 10.1016/S0076-6879(06)19017-5. [DOI] [PubMed] [Google Scholar]
- 16.Cipriani S, Boniela D, Marchina E, Balgkouranidou I, Caimi L, Zucconi GG, Barlati S. Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain. Cell Biol Int. (2007);31:845–850. doi: 10.1016/j.cellbi.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler JM, Fauza DO. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg. (2001);36:1662–1665. doi: 10.1053/jpsu.2001.27945. [DOI] [PubMed] [Google Scholar]
- 18.Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Hist. (2007);38:405–403. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- 19.Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschlager M. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol. (2004);191:309–314. doi: 10.1016/j.ajog.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Kunisaki SM, Fuchs JR, Steigman SA, Fauza DO. A comparative analysis of cartilage engineered from different perinatal mesenchymal progenitor cells. Tissue Eng. (2007);13:2633–2644. doi: 10.1089/ten.2006.0407. [DOI] [PubMed] [Google Scholar]
- 21.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. (2002);105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. (2005);25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Nakayama Y, Qin K, Yamamoto K, Ando J, Yamashita J, Itoh H, Kanda K, Yakn H, Okamoto Y, Nemoto Y. Differentiation from embryonic stem cells to vascular cells under in vitro pulsatile flow loading. J Artif Organs. (2005);8:110–118. doi: 10.1007/s10047-005-0291-2. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto K, Sokaaki T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. (2005);288:1915–1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. (2003);95:2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Tao J, Wang JW, Tu C, Xu MG, Wang Y, Pan SR. Shear stress contributes to t-PA mRNA expression in human endothelial progenitor cells and nonthrombogenic potential of small diameter artificial vessels. Biochem Biophys Res Commun. (2006);342:577–584. doi: 10.1016/j.bbrc.2006.01.172. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, Wang JW, Wang LC. In vitro shear stress modulates antithrombogenic potentials of human endothelial progenitor cells. J Thromb Thrombolysis. (2007);23:121–127. doi: 10.1007/s11239-006-9045-0. [DOI] [PubMed] [Google Scholar]
- 28.Tao J, Yang Z, Wang JM, Tu C, Pan SR. Effect of fluid shear stress on mRNA expression and NO production in human endothelial progenitor cells. Cardiology. (2006);106((2)):82–88. doi: 10.1159/000092636. [DOI] [PubMed] [Google Scholar]
- 29.Dardik A, Chen L, Frattini J, Asada H, Aziz F, Kudo FA, Sumpio BE. Differential effects of orbital and laminar shear stress on endothelial cells. J Vasc Surg. (2005);41:869–880. doi: 10.1016/j.jvs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Liu JW, Dunoyer-Geindre S, Serre-Beinier V, Mai G, Lambert JF, Fish RJ, Pernod G, Buehler L, Bounameaux H, Kruithof EKO. Characterization of endothelial-like cells derived from human mesenchymal stem cells. J Thromb Haemost. (2006);5:826–834. doi: 10.1111/j.1538-7836.2007.02381.x. [DOI] [PubMed] [Google Scholar]
- 31.Kestendjieva S, Kyurkchiev D, Tsvetkova G, Mehandjiev T, Dimitrov A, Nikolov A, Kyurkchiev S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol Int. (2008);32:724–732. doi: 10.1016/j.cellbi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SJ, Zhang H, Hou M, Zheng Z, Zhou J, Su W, Wei Y, Hu S. Is it possible to obtain “True endothelial progenitor cells” by in vitro culture of bone narrow mononuclear cells? Stem Cells Dev. (2007);16:683–690. doi: 10.1089/scd.2006.0062. [DOI] [PubMed] [Google Scholar]
- 33.Lin H, Shabbir A, Molnar M, Yang J, Marion S, Canty JM, Jr, Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulates bone marrow-derived mesenchymal stem cells. J Cell Physiol. (2008);214((2)):434–441. doi: 10.1002/jcp.21414. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt D, Achermann J, Odermatt B, Breymann C, Mol A, Genoni M, Zund G, Hoerstrup SP. Prenatally fabricated autologous human living heart valves based on amniotic fluid-derived progenitor cells as single cell source. Circulation. (2007);116:I64–I70. doi: 10.1161/CIRCULATIONAHA.106.681494. [DOI] [PubMed] [Google Scholar]
- 35.Perin L, Sedrakyan S, Da Sacco S, De Filippo R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol. (2008);86:85–99. doi: 10.1016/S0091-679X(08)00005-8. [DOI] [PubMed] [Google Scholar]
- 36.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res. (1999);43:532–541. doi: 10.1016/s0008-6363(99)00094-2. [DOI] [PubMed] [Google Scholar]
- 37.Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. (2006);30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Jackson L, Jones DR, Scotting P, Sottile V. Adult mesenchymal stem cells: differentiation potential and therapeutic applications. J Postgrad Med. (2007);53:121–127. doi: 10.4103/0022-3859.32215. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Liu X, Jiang Y, Chu L, Hao H, Liu Z, Verfaillie C, Zweier J, Gupta K, Liu Z. MAPK/ERK signaling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med. (2008);12((6A)):2395–2406. doi: 10.1111/j.1582-4934.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Huang L, Zhou Q, Song Y, Li A, Jin J, Cui B. Mesenchymal stem cells participating in ex vivo endothelial repair and its effect on vascular smooth muscle cells growth. Int J Cardiol. (2005);105:274–282. doi: 10.1016/j.ijcard.2004.12.090. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Jiang Y, Hao H, Gupta K, Xu J, Chu L, McFalls E, Zweier J, Verfaillie C, Bache RJ. Endothelial nitric oxide synthase is dynamically expressed during bone marrow stem cell differentiation into endothelial cells. Am J Physiol Heart Circ Physiol. (2007);293:H1760–H1765. doi: 10.1152/ajpheart.01408.2006. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez P, Bourget C, Bareille R, Daculsi R, Bordenave L. Gen response in endothelial cells cultured on engineered surfaces is regulated by shear stress. Tissue Eng. (2007);13:1607–1614. doi: 10.1089/ten.2006.0399. [DOI] [PubMed] [Google Scholar]
- 43.Wu CC, Chao YC, Chen CN, Chien S, Chen YC, Chien CC, Chiu JJ, Yen BL. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech. (2008);41:813–821. doi: 10.1016/j.jbiomech.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Avouac J, Wipff J, Goldman O, Ruiz B, Couraud PO, Chiocchia G, Kahan A, Boileau C, Uzan G, Allanore Y. Angiogenesis in systemic sclerosis: impaired expression of vascular endothelial growth factor receptor 1 in endothelial progenitor-derived cells under hypoxic conditions. Arthritis Rheum. (2008);58((11)):3550–3561. doi: 10.1002/art.23968. [DOI] [PubMed] [Google Scholar]
- 45.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. (2008);26((8)):2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. (2007);358((3)):948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 47.Taosheng L, Kimikazu H, Kazuhiko S, Hiroshi I, Nobuya Z, Masunori M. Improved angiogenic potency by implantation of ex vivo hypoxia pre-stimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol. (2002);283:H468–H473. doi: 10.1152/ajpheart.00261.2002. [DOI] [PubMed] [Google Scholar]
- 48.Ramirez-Bergeron DL, Simon MC. Hypoxia inducible factor and development of stem cells of the cardiovascular system. Stem Cells. (2001);19((4)):279–286. doi: 10.1634/stemcells.19-4-279. [DOI] [PubMed] [Google Scholar]
- 49.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. (2003);93((11)):1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 50.Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxia induction of an HIF-1alpha-depender bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. (2006);107((7)):2705–2712. doi: 10.1182/blood-2005-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]