Abstract
Metronomic chemotherapy, which is defined by the frequent, repetitive administration of chemotherapeutic drugs at relatively low doses, and without prolonged drug-free break, is an emerging strategy to fight cancer. Initially thought to act by targeting tumor angiogenesis, additional mechanisms have been recently unveiled, and metronomic chemotherapy is now considered to represent a form of multitargeted therapy. Despite representing a genuine alternative for advanced and/or high-risk cancer therapy, the development of metronomic approaches in pediatric oncology is still in the early stage. The few numbers of large-scale state-of-the-art clinical trials, issues regarding terminology and the limited understanding of the complex and intertwined mechanisms of action of metronomic treatments have limited progress in this important field of research. On March 18 and 19, 2010, the 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in Paediatric Oncology was held in Marseille, France, and brought together clinicians, basic scientists, physician-scientists, trainees, and students from all around the world. The main aim of this international meeting was to provide a unique forum to 1) reflect on the major advances that have been made in this field of research since its creation, 2) communicate results from the most recent clinical trials and preclinical studies, 3) discuss the current and future challenges of the field, and 4) set forth a solid framework for future collaborative biologic and clinical studies. The present report documents the main preclinical and clinical data that were presented in the keynote and best abstract sessions and delivers the key messages from the meeting.
Introduction
Most conventional chemotherapy regimens are based on the cyclic administration of anticancer drugs near or at the maximum tolerated dose (MTD), alternating with long periods of drug-free break to allow patient recovery from toxic adverse effects. This strategy has led to disease control in a significant number of both adult and pediatric cancer patients but is associated with significant short-term and long-term complications. In addition, despite impressive initial tumor regression or even remission, regrowth and recurrence are common events in metastatic cancer and high-risk tumors. Although the effectiveness and rationale of MTD-based chemotherapy regimens and dose-escalation strategies has been questioned for many years, especially in patient populations with poor-prognosis tumors [1], convincing preclinical data were needed to validate the potential of alternative schedules of drug administration. Such groundbreaking preclinical studies were published 10 years ago by Browder et al. [2] from Judah Folkman's laboratory and confirmed in Robert Kerbel's laboratory [3]. Using transplantable tumors [2] and xenograft models [3], both teams demonstrated that the frequent administration of low-dose chemotherapy could exert potent anticancer effects, through inhibition of angiogenesis. The first study further showed that antiangiogenic scheduling of cyclophosphamide administration was more effective than conventional schedule and could overcome drug resistance [2], whereas the second study revealed a synergism between continuous treatment with low-dose vinblastine and anti-vascular endothelial growth factor (VEGF) receptor therapy [3]. After these landmark articles, Hanahan et al. [4] coined the term metronomic to the treatment regimens defined by the frequent, repetitive administration of chemotherapeutic drugs at low doses, with no prolonged drug-free break.
As reviewed recently [5], metronomic chemotherapy has gained greater attention in the clinic and showed promising results in phase 2 clinical studies for the treatment of adult patients with various types of advanced and/or refractory tumors such as metastatic breast and prostate cancers. Several phase 3 clinical trials are also currently underway (www.clinicaltrials.gov) including for the treatment of metastatic (NCT01131195) and triple-negative (NCT01112826) breast cancer and advanced colorectal carcinoma (NCT00442637). In pediatric oncology, however, as for other antiangiogenic strategies, the clinical development of metronomic chemotherapy is still in its early stage [6]. While the mechanisms of action of metronomic chemotherapy have only recently been investigated, the use of low-dose oral chemotherapy, even after administration of MTD doses of the same drug, has been around for many decades in both adult [7] and pediatric patients [8], with excellent radiographic response rates. Likewise, the longtermadministration of oral agents in themaintenance phase of leukemia may function through a similar mechanism [9,10].
On March 18 and 19, 2010, the 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in Paediatric Oncology was held in Marseille, France. This workshop brought together clinicians (pediatric oncologists, radiologists and pharmacists), basic scientists (cell biologists, biochemists, pharmacologists, and mathematicians), physician-scientists, trainees, and students from Europe, North and South America, Africa, Israel, and Australia. The specific objectives of this international meeting, which included 16 keynote addresses, 8 oral presentations selected from the best submitted abstracts, and 3 “tumor-specific” working group sessions (i.e., brain tumors, sarcomas, and neuroblastoma), were to:
reflect on the major advances that have been made in this field of research since its inception;
increase awareness and credibility of metronomic treatments by communicating results from the most recent clinical trials and preclinical studies;
discuss the current and future challenges of the field, which include overcoming empiricism in protocol design, identifying reliable biomarkers and defining appropriate treatment end points for clinical trials; and
set forth a solid framework for future research and design innovative protocols of metronomic treatments to be investigated in multicentered clinical trials.
The present report documents the main preclinical and clinical data that were presented in the keynote and best abstract sessions, summarizes the results of the tumor-specific working groups, highlights the future challenges of the field, and delivers the key messages from the meeting.
Key Clinical Data Supporting the Development of Metronomic Treatments in Pediatric Oncology
After the publication in 2000 of the two pioneering studies that led to the emergence of this field of cancer research [2,3], the safety and efficacy of metronomic scheduling of chemotherapy has been investigated and demonstrated in several clinical trials in adult cancer patients with advanced and/or refractory tumors (reviewed in Pasquier et al. [5]). Despite a first pilot study published by Sterba et al. in 2002 [11], clinical studies performed in pediatric cancer patients currently represent less than 10% of all refereed publications on metronomic treatments. The 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in PaediatricOncology provided a much-needed opportunity to present and discuss the results from recently completed and currently ongoing clinical trials (Figure 1). In total, the results from 15 different clinical trials, ranging from small monocentered pilot studies to larger multicentered phase 2 trials and involving around 500 patients across Europe, North America, and Africa, were presented. Here, we summarize some of the key findings.
Figure 1.
The 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in Paediatric Oncology.
In 2005, Kieran et al. [12] reported the results from a clinical study on the feasibility and efficacy of a four-drug metronomic treatment regimen, involving continuous oral thalidomide and celecoxib with alternating metronomic etoposide and cyclophosphamide in 20 consecutive patients with relapsed, incurable cancer of various tumor types. Although the disease of eight patients rapidly progressed, suggesting that metronomic treatments may be more effective in minimal residual disease setting, 10 patients remained on therapy for a longer period than their prior remission. Furthermore, three partial responses and seven stable disease for more than 6 months were observed, leading to an overall clinical benefit of 50%. After this relative clinical success and the recent demonstration of the potent antiangiogenic properties of peroxisome proliferator-activated receptor a agonists [13], fenofibrate was added to the subsequent protocol, and the safety and efficacy of the resulting five-drug metronomic chemotherapy regimen was investigated in 101 consecutive patients with relapsed, incurable disease of various tumor types in eight different strata, and results of this combination were presented and are being readied for publication (M.W.K., personal communication). The antiangiogenic mechanism of the combination of celecoxib, fenofibrate, and oral etoposide or cyclophosphamide has recently been demonstrated in preclinical murine models [14]. Thalidomide is, however, not tested in preclinical murine models because of differences in metabolism when compared with humans.
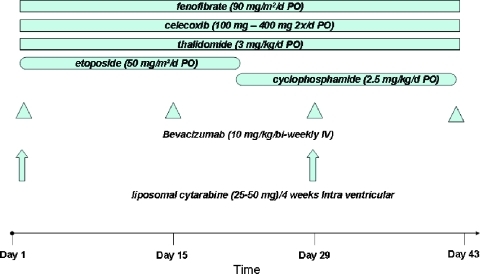
An adapted five/six-drug protocol was subsequently investigated by Peyrl et al. [15] in 13 patients with recurrent embryonal or ependymal brain tumors. The sophisticated treatment regimen consisted of continuous oral thalidomide, celecoxib, and fenofibrate with alternating metronomic etoposide and cyclophosphamide, in combination with bi-weekly low-dose bevacizumab, with or without intrathecal chemotherapy (etoposide and/or liposomal cytarabin). Very impressive results were reported with excellent 2-year event-free survival (EFS) and 3-year overall survival (OS) in a study population with a very dismal prognosis.
Following a similar gradual development strategy, during the meeting, Dr Sterba reported on the results and recent evolution of the COMBAT (Combined Oral Maintenance Biodifferentiating and Antiangiogenic Therapy) protocol. The first COMBAT regimen, which was based on continuous administration of celecoxib in combination with retinoic acid and alternating metronomic etoposide and temozolomide, was investigated in 22 patients with refractory, relapsing, or “high risk of relapse” tumors of various types [16]. This study reported a response rate of 32% and a clinical benefit of 77%, with 3 complete response, 4 partial response, and 10 stable disease for more than 6 months. However, long-term clinical outcome remained dismal, with a 2-year EFS of less than 10% and a 2-year OS of 30%. In an attempt to increase the long-term efficacy of COMBAT, new agents were incorporated into the protocol based on preclinical and clinical findings, including the peroxisome proliferator-activated receptor α agonist fenofibrate, vitamin D (COMBAT II), and anti-VEGF antibody bevacizumab (COMBAT III). A multicentered clinical trial was designed to investigate the efficacy of COMBAT II and III in patients with relapsed or high risk of relapse tumors of various types, including brain tumors, neuroblastoma, and sarcomas. A total of 81 patients were recruited across four different institutions in Czech Republic, France, and Slovakia. After a median follow-up of 1 year, a promising response rate was reported (J.S., personal unpublished data). Interestingly, the efficacy of COMBAT II and III is proving to be superior to the first COMBAT protocol. Surprisingly, an effect of sex was observed on the clinical outcome, with male patients responding significantly better than female patients in terms of both EFS and OS. Finally, nonrandomized retrospective comparison of the subgroup of patients with relapsed high-grade sarcomas revealed improved overall survival with metronomic treatment over MTD-based chemotherapy. This promising result was in accordance with a recent study by Klingebiel et al. [17] showing that metronomic-based oral maintenance (i.e., daily trophosphamide and etoposide, alternating with daily trophosphamide and idarubicin) was more effective than high-dose chemotherapy in children with metastatic soft tissue sarcoma (5-year OS of 52% and 15%, respectively; P = .001).
The preliminary results of a phase 2 clinical trial of a combination of weekly vinorelbine and daily cyclophosphamide in recurrent or refractory pediatric tumors were also presented during the meeting (O.O., personal unpublished data). This study, which recruited 114 patients with rhabdomyosarcoma (RMS), soft tissue sarcoma, bone sarcoma, neuroblastoma, and medulloblastoma, reported mixed results according to the tumor type. For instance, the response rate and clinical benefit for all evaluable patients were 18% and 36%, respectively, whereas they were 34% and 52%, respectively, in the subgroup of patients with RMS. The 6-month progression-free survival (PFS) was 28% and the 1-year OS was 29% for all patients. For RMS patient only, the 6-month PFS was 40% and the 1-year OS was 34%. This study thus confirms the results previously reported by Casanova et al. [18,19], showing that vinorelbine, both alone or in combination with low-dose metronomic cyclophosphamide, can be effective in the treatment of recurrent or refractory RMS. On the basis of the results of these pilot studies, the EpSSG (European Soft Tissue Sarcoma Study Group) is currently evaluating the benefit of “maintenance chemotherapy” with the association of vinorelbine and cyclophosphamide for high-risk RMS patients in a randomized trial.
Results from other pilot, phase 1 and 2 clinical trials, were also presented during the workshop. Dr Kivivuori presented data from the AngioComb trial (i.e., antiangiogenic combination therapy for pediatric tumors in the brainstem) from NOPHO (Nordic Society of Paediatric Haematology and Oncology). Eight patients with diffuse intrinsic pontine glioma were treated with a combination of radiotherapy and the radiosensitizer topotecan, followed by metronomic etoposide and thalidomide in combination with celecoxib. Comparison with eight historic matching control patients revealed that the AngioComb therapy resulted in a significant benefit in time to clinical progression (11 vs 6.8 months, P = .04) [20]. Moreover, the OS at 12 months was 63% in this patient population with very poor prognosis. Therapy was well tolerated in all patients, with very few toxic adverse effects except for neutropenia, and resulted in a significant improvement in the quality of life. A total of 24 patients have now been enrolled in an expanded study across the different NOPHO countries (i.e., Finland, Sweden, Norway, Denmark and Iceland), and there are plans also to extend AngioComb therapy to other pediatric malignancies, including glioblastoma multiform and ependymoma.
Dr Baruchel presented the results of several pilot and phase 1 pharmacokinetic studies of metronomic chemotherapy [21–23] and antiangiogenic therapy [24] in pediatric patients with recurrent and/or refractory tumor of various types. Although the results of these studies were quite disappointing in terms of clinical response, they provided essential pharmacokinetic data for some of the main anticancer agents currently used in metronomic and antiangiogenic treatments in children (i.e., celecoxib, vinblastine, cyclophosphamide, temozolomide, and bevacizumab) and investigated numerous potential biomarkers to monitor treatment activity (see section o Biomarker). Elsewhere, safety results were reported for the ongoing New Approaches to Neuroblastoma Therapy's (NANT's) clinical trial of low-dose metronomic cyclophosphamide in combination with the biphosphonate, zoledronic acid, in children with recurrent or refractory neuroblastoma. Treatment was well tolerated with only few toxic adverse effects, and clinical and biologic responses were observed [25]. The preliminary data of a phase 1 study organized by the Children's Oncology Group (COG) also showed the feasibility of combining metronomic vinblastine and celecoxib to standard therapy for the treatment of Ewing sarcoma patients, with only three dose-limiting toxicities reported (J.L. Felgenhauer and S.B., personal unpublished data).
Finally, the preliminary results of the first pilot study of metronomic chemotherapy performed in Africa were also presented during the meeting [26]. In low- and middle-income countries, although the real incidence of cancer remains unknown due to the lack of accurate cancer registers and very poor survival rates, it is estimated that around 200,000 children are diagnosed with cancer each year and only approximately 25% survive [27]. Several issues prevent the development of an efficient management of children with cancer in developing countries: the availability of drugs and treatment facilities, delayed diagnosis, cost, lack of follow-up, compliance with treatment, prior consultation of traditional practitioners, and cultural barrier. Themanagement of children with relapsed, progressive, and/or very advanced disease is particularly challenging in these countries because second-line intensive or experimental treatments with new expensive drugs are not realistic options. Therefore, metronomic chemotherapy regimens, based on inexpensive off-patent anticancer drugs administered orally, in an outpatient basis, represent a genuine alternative strategy for children with cancer in low-income countries. The efficacy of a metronomic protocol, consisting of weekly vincristine, daily low-dose cyclophosphamide, and twice-weekly methotrexate, was evaluated in a small prospective study performed in Mali (Metro-Mali-01) in the Bamako Center, which is part of the French-African Group of Paediatric Oncology [26]. Twelve children with relapsing or refractory nephroblastoma, retinoblastoma, or neuroblastoma were enrolled in the study. Treatment was well tolerated with no grade 3 and only two grade 4 (anemia and neutropenia) toxicities reported. The best response was disease stabilization observed in seven patients (58%), including three patients for more than 6 months after treatment completion, and after a median follow-up of 39 weeks, six patients (50%) were alive. This pilot study demonstrates that metronomic chemotherapy can be delivered at low cost, with minimal toxicity, and can be efficacious in children and young adults with cancer and provides a strong rationale for the rapid development of further clinical trials in middle- and low-income countries [28].
Upcoming Clinical Trials
During the meeting, it was also noted that numerous clinical trials of metronomic and/or antiangiogenic therapy in pediatric cancer patients are currently ongoing across Europe, North America, and South America. In addition to the ongoing clinical trials detailed above (i.e., COMBAT II and III, CCLG-EpSSG-RMS-2005, AngioComb), the clinical studies whose results are most anticipated include
the NANT 2007-02 study investigating the toxicity and feasibility of the combination of chemoswitch cyclophosphamide (i.e., 28-day cycles of high-dose cyclophosphamide on day 1 followed by continuous low-dose cyclophosphamide) and zoledronic acid, with and without bevacizumab, in children with refractory or recurrent high-risk neuroblastoma
the SFCE-Metro-01 (French Society for Childhood Cancer) phase 2 trial of low-dose metronomic cyclophosphamide alternating with low-dose methotrexate and in combination with continuous celecoxib and weekly vinblastine in children and young adults with relapsed or refractory solid tumors (NCT01285817)
the Metro-Mali-02 (French-African Group of Paediatric Oncology) phase 2 trial of daily low-dose cyclophosphamide in combination with valproic acid, weekly vincristine, and twice-weekly methotrexate for the treatment of relapsing or refractory pediatric solid tumors
the GLATO 2006 (Latin-American Group for the Treatment of Osteosarcoma) randomized phase 3 trial investigating the efficacy of metronomic cyclophosphamide and methotrexate in combination with conventional chemotherapy and surgery in metastatic osteosarcoma patients
the randomized phase 2 trial of bevacizumab in combination with standard chemotherapy in children and adolescents with metastatic RMS and other soft tissue sarcoma (NCT00643565)
the pilot study of bevacizumab in combination with radiotherapy and temozolomide in children and young adults with newly diagnosed high-grade glioma and diffuse intrinsic pontine glioma (NCT00890786)
the phase 1 study of bevacizumab and sorafenib in combination with low-dose cyclophosphamide in children and young adults with refractory solid tumors and leukemia performed at the St Jude Children's Research Hospital (Memphis, TN).
Results from the Metronomic Working Groups
With the assembly of experts in metronomic therapy at the 2nd International Workshop, a number of participants met separately to investigate the possible development of an international protocol for the treatment of specific tumor types. Three working groups were constituted to propose a protocol for the treatment of brain tumors, neuroblastoma, and sarcomas.
The brain tumor committee had a number of different proposals including those for ependymoma, brainstem glioma, and medulloblastoma. After extensive discussion and preliminary feasibility assessment, plans for a recurrent medulloblastoma protocol were approved in principle (Figure 2). Members of this ad hoc committee followed up by e-mail and conference calls to fine-tune the protocol proposal. Approximately 20 centers throughout Europe and the United States have been identified for participation. Ethical approval has been obtained in Austria with plans to begin accrual in approximately 6 months.
Figure 2.
Metronomic multitarget antiangiogenic protocol for relapsing medulloblastoma.
Similarly, the neuroblastoma and sarcoma committees developed different proposals based on recent clinical data and sound preclinical rationale. After extensive discussions to assess feasibility and anticipate potential toxicities, a metronomic treatment protocol for recurrent neuroblastoma patients was approved in principle. This proposal will be finalized in the next few months and regulatory approval and funding application processes will be initiated.
Future Challenges
Terminology
In the original publication from Browder et al. [2], the regimen consisting of weekly administration of cyclophosphamide was termed antiangiogenic scheduling as angiogenesis inhibition was shown to be responsible for the observed antitumor effect. Soon after, Hanahan et al. [4] coined the term metronomic to the concept of antiangiogenic chemotherapy. Nevertheless, as foreseen by Hanahan et al. [4] and Gasparini [29], the important finding behind low-dose antiangiogenic chemotherapy was very likely to be the new schedule of drug administration [28]. Although metronomic and/or antiangiogenic chemotherapy remain the preferred terminology, it does not always reflect the underlying mechanisms of action or the complexity of the treatment regimens. In several metronomic studies, nonchemotherapeutic drugs per se such as thalidomide, Cox-2 inhibitors, retinoic acid, and, more recently, fenofibrate or zoledronic acid have been used in combination with conventional chemotherapeutic drugs, making the term chemotherapy not perfectly adapted to these combinatorial strategies. In addition, recent findings have shown that the anticancer activity of metronomic chemotherapy does not solely rely on antiangiogenic effects. Instead, this type of treatment may represent a form of multitargeted therapeutic strategy [5,30], making the term antiangiogenic therapy probably too restrictive. It is also important to realize that not all chemotherapeutic agents administered in a long-term, repetitive, oral manner will have an antiangiogenic mechanism of action, despite the frequent use of this terminology. Lastly, nonchemotherapeutic drugs used in metronomic protocols are sometimes administered at higher doses than when used for nonanticancer purposes. This is, for instance, the case with Cox inhibitors [12,16,21,31], retinoic acid [16], and fluvastatin [32]. Thus, the term low dose is not always appropriate either. In the case of complex multimodal metronomic protocols, we recommend to use broader terms such as metronomic treatment or metronomic scheduling of anticancer treatment (MSAT) [30].
Anticipating Long-term Toxicities
In all the different clinical trials discussed during the meeting, predominantly mild toxicities were reported. The most common adverse effects observed were hematological toxicities, such as neutropenia, lymphopenia, and anemia, and were usually grade 1 or 2. As expected, adverse events were more frequent when numerous drugs were combined and episodes of peripheral neuropathy, anorexia, ataxia, fatigue, and infections were encountered (M.W.K. and A.P., personal unpublished observation).
Although normal growth and development was maintained in children who were treated with alternating metronomic etoposide and cyclophosphamide in combination with continuous oral thalidomide and celecoxib for up to 2 years [12] and despite the overall good tolerance of metronomic chemotherapy in children, potential long-term toxicities still warrant caution and require further investigations. It is important to note, for instance, the high incidence of secondary leukemia that has been reported after frequent intravenous etoposide administration in children and young adults [33], requiring careful observation in patients treated with the oral formulation for extended periods. In addition, deleterious effects of cyclophosphamide on spermatogenesis have been recently reported [34]. Although investigating the effects of MTD-based chemotherapy, this study showed that ifosfamide was associated with a lower risk of gonadal damage than cyclophosphamide, suggesting that ifosfamide may be a safer drug to use in metronomic treatments. Finally, potential radiosensitization issues as evidenced by episodes of hemorrhagic cystitis and radiation pneumonitis have also been noted in the ongoing COG study of metronomic vinblastine and celecoxib in combination with standard therapy in children with Ewing sarcoma (J.L. Felgenhauer and S.B., personal unpublished data).
Elsewhere, recent evidence shows that the administration of certain anticancer agents, including chemotherapeutics, vascular-disrupting agents, and antiangiogenic drugs, can induce rapid systemic elevation in circulating endothelial progenitor cells (EPCs). These cells home to the treated tumor site and can induce angiogenesis and subsequent tumor cell repopulation and tumor regrowth [35,36]. These host effects, which were documented in both non-tumor-bearing and tumorbearing mice after treatment with anticancer drugs can compromise some of the antitumor effects of the drug used [35,36]. The combination of antiangiogenic drugs, and to some extent metronomic chemotherapy, with the anticancer agents that induced rapid systemic host effects resulted in a reduction in tumor cell repopulation, thus preventing tumor regrowth [36–38]. However, it is important to note that, in addition to cytotoxic drugs, some antiangiogenic agents can also induce systemic host responses. It has been demonstrated that the administration of escalating doses of the receptor tyrosine kinase inhibitor, sunitinib malate, to non-tumor-bearing mice resulted in elevated plasma levels of multiple growth factors, cytokines, and chemokines (e.g., G-CSF, SDF-1) [39]. These effects could explain the provocative results of preclinical studies, which demonstrated that tumor invasiveness and metastasis are being promoted by both tumor and host cells in response to treatment with certain antiangiogenic drugs [40,41]. Interestingly, it is plausible that such responses may be minimal or absent when using low-dose metronomic chemotherapy (Y.S., personal communication). However, these systemic effects need to be further investigated in the context of combinatorial metronomic treatments to anticipate any potential long-term adverse effects.
Biomarker
One of the major limitations of metronomic treatments is empiricism. Powerful and reliable biomarkers (i.e., diagnostic, predictive, and surrogate markers) are crucially needed to predict which patients are most likely to benefit from MSAT and to monitor treatment activity. Furthermore, the complex mechanisms of action of the different agents used in combination still need to be completely unraveled to define the best drugs to use according to the clinical setting, tumor type, and patient population.
Considerable efforts have been made recently, both at the preclinical and clinical level, to try and identify markers of activity for metronomic chemotherapy and antiangiogenic therapies. The first obvious candidates are serum levels of proangiogenic and antiangiogenic growth factors and cytokines, although their predictive potential has been poorly investigated in pediatric cancer patients to date. During the meeting, Dr Baruchel presented the results of pharmacodynamic analyses performed in several completed and ongoing phase 1 trials of metronomic chemotherapy in children with relapsed or refractory tumors. No significant change in serum levels of VEGF, basic fibroblast growth factor, soluble Vascular Cell Adhesion Protein 1 (sVCAM-1), thrombospondin-1 (TSP-1), and endostatin were observed in pediatric patients treated with low-dose metronomic vinblastine or cyclophosphamide in combination with celecoxib [21]. In a recent multicenter pilot study, a trend toward a greater decrease in VEGF plasma levels associated with longer EFS was observed in pediatric patients with recurrent brain tumors treated with low-dose metronomic temozolomide [23]. In the four-drug antiangiogenic clinical trial of thalidomide, celecoxib, oral etoposide, and oral cyclophosphamide, elevated thrombospondin levels at baseline were correlated with event-free survival [12], and a similar analysis of the five-drug strategy is underway (M.W.K., personal communication).
After robust preclinical studies by Bertolini, Shaked, and others (reviewed in Bertolini et al. [42] and Pasquier and Dias [43]), circulating endothelial cells (CECs) and EPC have emerged as promising surrogate markers to monitor the activity of metronomic chemotherapy and antiangiogenic therapies. In mice, suppressed levels of viable EPCs have been shown to correlate with the optimal biologic dose of antiangiogenic drugs or metronomic antiangiogenic treatment strategy [44,45]. In clinical studies, breast cancer patients treated with metronomic cyclophosphamide and thalidomide-increased levels of apoptotic CECs were reported, but only in patients responding to the therapy [46]. In addition, in breast cancer patients treated with metronomic chemotherapy and bevacizumab, higher baseline CEC levels were associated with clinical response and improved PFS [47,48]. Although a significant correlation between apoptotic CECs and clinical outcome has been recently reported in children treated with bevacizumab [24], the pediatric clinical data addressing the predictive value of CECs and EPCs remain sparse. These cells are currently under evaluation in numerous COG phase 1 studies of various metronomic and antiangiogenic treatments including sunitinib, sorafenib, pazopanib, metronomic etoposide, and bevacuzimab in combination with metronomic chemotherapy. Similarly, future clinical trials will need to include more biologic investigations to validate these potential predictive and surrogate markers to better monitor metronomic treatments.
Mechanisms of Action
Another issue fueling the empiricism associated with these treatments is the lack of complete understanding of the mechanisms of action. During the past decade, sustained research efforts have helped decipher the different mechanisms involved in the activity of antiangiogenic therapies: i) normalization of tumor vasculature that may improve drug delivery and efficacy of radiotherapy, ii) prevention of rapid tumor cell repopulation after MTD-based chemotherapy, and iii) potentiation of the antivascular activity of chemotherapy [49,50]. Similar in-depth biologic studies are now needed to unravel the complex mechanisms of action of metronomic treatments. During the meeting, it was suggested to try and implement state-of-the-art imaging technologies, such as dynamic contrast-enhanced magnetic resonance imaging, in clinical trials to investigate potential changes in blood flow and tumor perfusion during metronomic treatment. Additional mechanisms recently unveiled were also thoroughly discussed. These include restimulation of the anticancer immune response [51], direct effects on cancer cells [52–54], and potential induction of senescence in vascular endothelial cells (E.P., personal unpublished data). Given the combinatorial aspect of metronomic chemotherapy regimens, it is most likely that more mechanisms are yet to be discovered, thus calling for further studies. For instance, the recent demonstration of the key role played by putative cancer stem cells in the establishment of the tumor vasculature [55] warrants the investigation of the effects of metronomic chemotherapy on cancer stem cells.
Rational Design of Clinical Trials and Relevant End Points
Several questions still need to be addressed to help design optimal clinical trials. Numerous anticancer drugs fit the requirements that define a potential candidate for metronomic treatments: i) orally available, ii) nonoverlapping and low toxicity, iii) antiangiogenic and/or immunostimulant properties, and iv) low probability of inducing drug resistance (Table 1). Similarly, numerous types of high-risk tumors, including brain tumors (i.e., high-grade glioma, ependymoma, medulloblastoma), neuroblastoma, and sarcomas, could potentially benefit from these treatments. As discussed previously, the common utilization of daily oral therapy for the maintenance phase of leukemia may represent an old example of metronomic chemotherapy. However, strong rationales are still needed to determine the best drug candidates and drug combinations according to the tumor type and clinical setting. In the ongoing clinical trial in high-risk RMS patients, for instance, cyclophosphamide was chosen to be combined with vinorelbine, based on i) its proven activity in RMS, ii) its bioavailability when given by mouth allowing low-dose continuous administration on an outpatient basis, and iii) its documented efficacy when used at low dose in other clinical settings.
Table 1.
Drugs That Can Be Used in MSAT.
| ??Chemotherapeutic Drugs | Nonchemotherapeutic Drugs |
| Alkylating agents | Anti-VEGF agents |
| Cyclophosphamide | Bevacizumab |
| Temozolomide | Cox inhibitors |
| Trophosphamide | Celecoxib |
| Ifosfamide | HDAC inhibitors |
| Antimetabolites | Valproic acid |
| Methotrexate | Tyrosine kinase inhibitors |
| Antimicrotubule agents | Sunitinib |
| Vinblastine | Sorafenib |
| Vinorelbine | Imatinib |
| Vincristine | Dasatinib |
| Anthracyclines | Nilotinib |
| Idarubicin | Others |
| Topoisomerase inhibitors | Fenofibrate |
| Etoposide | Fluvastatin |
| Topotecan | Retinoic acid |
| Thalidomide | |
| Zoledronic acid | |
Elsewhere, the best treatment end points remain to be determined to fully appreciate the potential clinical benefits of metronomic treatments. This again depends on the clinical setting. In phase 2 trials, response rates and 6-month EFS are still the most commonly used end points. However, it is increasingly accepted that the Response Evaluation Criteria in Solid Tumors (RECIST) may not be well suited for the evaluation of metronomic and antiangiogenic treatments. Furthermore, the capacity of metronomic chemotherapy to induce disease stabilization, associated with a good quality of life, is to be regarded as a desirable and relevant clinical end point. This is particularly the case in maintenance and palliative care settings. Therefore, an effort should be made to develop reliable methods to objectively assess the impact of these treatments on patient quality of life. Duration of clinical benefit compared to previous treatments may also be included to assess the efficacy of these treatments. Finally, it should be noted that time to best response is usually longer with metronomic versus conventional treatments, thus suggesting that later assessments of clinical end points may be preferable.
When and how to stop metronomic therapy also represents a crucial issue that needs to be addressed. The long median time to response suggests that treatment should not be terminated too early, even in the absence of objective clinical response. Furthermore, a recent case report of rapid and fatal relapse soon after treatment completion also warrants caution as to when to discontinue therapy in children who achieved remission after long-term metronomic treatment [56]. The best strategy regarding time to stop therapy remains to be determined for each tumor type and clinical setting. Should long-term metronomic treatments be stopped abruptly? Or inversely, should it be stopped gradually through de-escalation strategy? Future preclinical studies and clinical trials will need to address these questions.
Key Messages from the Meeting
Metronomic treatments constitute an alternative strategy that is gaining increasing interest in both adult and pediatric oncology. It represents a promising therapeutic option particularly in patients with high-risk refractory and/or relapsed cancer as well as in heavily pretreated patient populations. Although complete responses remain rare, these treatments often lead to long-term disease stabilization and significant improvement of the quality of life of patients. Despite recent clinical success in adults [5], the implementation of metronomic treatments in pediatric oncology is still in its early stage as a result of the lack of state-of-the-art clinical studies clearly demonstrating efficacy. Strong international clinical and biologic collaborations are therefore needed to facilitate the development of metronomic scheduling to treat children with cancer, and these international meetings have become an important venue for bringing a diverse array of experts together to address these problems. With the next meeting planned for 2012 in Israel, we have the opportunity to begin to systematically evaluate this treatment modality in pediatric patients.
One of the main limitations in this field is the current lack of powerful and reliable biomarkers to overcome the empiricism associated with the design of treatment regimens and clinical trial protocols. Future biologic investigations will need to use more relevant models and tools, integrating cutting-edge technologies. These may include advanced imaging technology to better understand the effects of metronomic treatments on blood flow and tumor perfusion, state-of-theart flow cytometry to detect and quantify bone marrow-derived and other circulating proangiogenic cells, ELISA and multiplex cytokine assays to identify markers of activity, and pharmacogenomics and proteomic analyses to perform molecular profiling and predict treatment response and clinical outcome. In addition, innovative preclinical models taking into account tumor heterogeneity, as well as the contribution of tumor stroma and cancer stem cells, are also crucially needed. Finally, large multicentered clinical studies, integrating biomarker analyses, will need to investigate and validate the best treatment combinations for each tumor type and patient population.
Acknowledgments
The authors thank all the speakers and attendees for making the meeting such an exciting and successful event. In particular, the authors thank all the presenters of the “best abstract” session as well as Atout.com for their precious help with organizing the meeting.
References
- 1.Weitman SD, Glatstein E, Kamen BA. Back to the basics: the importance of concentration x time in oncology. J Clin Oncol. 1993;11:820–821. doi: 10.1200/JCO.1993.11.5.820. [DOI] [PubMed] [Google Scholar]
- 2.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 3.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 6.Samuel DP, Wen PY, Kieran MW. Antiangiogenic (metronomic) chemotherapy for brain tumors: current and future perspectives. Expert Opin Investig Drugs. 2009;18:973–983. doi: 10.1517/13543780903025752. [DOI] [PubMed] [Google Scholar]
- 7.Haim N, Ben-Shahar M, Epelbaum R. Prolonged daily administration of oral etoposide in lymphoma following prior therapy with adriamycin, an ifosfamide-containing salvage combination, and intravenous etoposide. Cancer Chemother Pharmacol. 1995;36:352–355. doi: 10.1007/BF00689054. [DOI] [PubMed] [Google Scholar]
- 8.Ashley DM, Meier L, Kerby T, Zalduondo FM, Friedman HS, Gajjar A, Kun L, Duffner PK, Smith S, Longee D. Response of recurrent medulloblastoma to low-dose oral etoposide. J Clin Oncol. 1996;14:1922–1927. doi: 10.1200/JCO.1996.14.6.1922. [DOI] [PubMed] [Google Scholar]
- 9.Kieran MW, Billett A. Antiangiogenesis therapy. Current and future agents. Hematol Oncol Clin North Am. 2001;15:835–851. vii. doi: 10.1016/s0889-8588(05)70254-9. [DOI] [PubMed] [Google Scholar]
- 10.Andre N, Pasquier E, Gentet JC, Kamen BA. Looking at the seemingly contradictory role of vinblastine in anaplastic large-cell lymphoma from a metronomic perspective. J Clin Oncol. 2011;29:e90–e91. doi: 10.1200/JCO.2010.32.2883. [DOI] [PubMed] [Google Scholar]
- 11.Sterba J, Pavelka Z, Slampa P. Concomitant radiotherapy and metronomic temozolomide in pediatric high-risk brain tumors. Neoplasma. 2002;49:117–120. [PubMed] [Google Scholar]
- 12.Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, Klement G, Laforme A, Gordon A, Thomas A, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 13.Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnes CM, Fannon M, Laforme AM, Chaponis DM, Folkman J, Kieran MW. PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panigrahy D, Kaipainen A, Butterfield CE, Chaponis DM, Laforme AM, Folkman J, Kieran MW. Inhibition of tumor angiogenesis by oral etoposide. Exp Ther Med. 2010;1:739–746. doi: 10.3892/etm.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peyrl A, Azizi AA, Reismueller B, Kieran MW, Heinrich M, Czech T, Dieckmann K, Slavc I. Antiangiogenic metronomic chemotherapy for patients with recurrent embryonal and ependymal brain tumors. Neuro-Oncol. 2010;12:ii44. [Google Scholar]
- 16.Sterba J, Valik D, Mudry P, Kepak T, Pavelka Z, Bajciova V, Zitterbart K, Kadlecova V, Mazanek P. Combined biodifferentiating and antiangiogenic oral metronomic therapy is feasible and effective in relapsed solid tumors in children: single-center pilot study. Onkologie. 2006;29:308–313. doi: 10.1159/000093474. [DOI] [PubMed] [Google Scholar]
- 17.Klingebiel T, Boos J, Beske F, Hallmen E, Int-Veen C, Dantonello T, Treuner J, Gadner H, Marky I, Kazanowska B, et al. Treatment of children with metastatic soft tissue sarcoma with oral maintenance compared to high dose chemotherapy: report of the HD CWS-96 trial. Pediatr Blood Cancer. 2008;50:739–745. doi: 10.1002/pbc.21494. [DOI] [PubMed] [Google Scholar]
- 18.Casanova M, Ferrari A, Spreafico F, Terenziani M, Massimino M, Luksch R, Cefalo G, Polastri D, Marcon I, Bellani FF. Vinorelbine in previously treated advanced childhood sarcomas: evidence of activity in rhabdomyosarcoma. Cancer. 2002;94:3263–3268. doi: 10.1002/cncr.10600. [DOI] [PubMed] [Google Scholar]
- 19.Casanova M, Ferrari A, Bisogno G, Merks JH, De Salvo GL, Meazza C, Tettoni K, Provenzi M, Mazzarino I, Carli M. Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: pilot study for the upcoming European Rhabdomyosarcoma Protocol. Cancer. 2004;101:1664–1671. doi: 10.1002/cncr.20544. [DOI] [PubMed] [Google Scholar]
- 20.Kivivuori SM, Riikonen P, Valanne L, Lonnqvist T, Saarinen-Pihkala UM. Antiangiogenic combination therapy after local radiotherapy with topotecan radiosensitizer improved quality of life for children with inoperable brainstem gliomas. Acta Paediatr. 2011;100:134–138. doi: 10.1111/j.1651-2227.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- 21.Stempak D, Gammon J, Halton J, Moghrabi A, Koren G, Baruchel S. A pilot pharmacokinetic and antiangiogenic biomarker study of celecoxib and low-dose metronomic vinblastine or cyclophosphamide in pediatric recurrent solid tumors. J Pediatr Hematol Oncol. 2006;28:720–728. doi: 10.1097/01.mph.0000243657.64056.c3. [DOI] [PubMed] [Google Scholar]
- 22.Baruchel S, Diezi M, Hargrave D, Stempak D, Gammon J, Moghrabi A, Coppes MJ, Fernandez CV, Bouffet E. Safety and pharmacokinetics of temozolomide using a dose-escalation, metronomic schedule in recurrent paediatric brain tumours. Eur J Cancer. 2006;42:2335–2342. doi: 10.1016/j.ejca.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Sharp JR, Bouffet E, Stempak D, Gammon J, Stephens D, Johnston DL, Eisenstat D, Hukin J, Samson Y, Bartels U, et al. A multi-centre Canadian pilot study of metronomic temozolomide combined with radiotherapy for newly diagnosed paediatric brainstem glioma. Eur J Cancer. 2010;46:3271–3279. doi: 10.1016/j.ejca.2010.06.115. [DOI] [PubMed] [Google Scholar]
- 24.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 25.Russel HV, Groshen SG, Ara T, Declerck YA, Hawkins R, Jackson HA, Daldrup-Link HE, Marachelian A, Skerjanec A, Park JR, et al. A phase I study of zoledronic acid and low-dose cyclophosphamide in recurrent/refractory neuroblastoma: a new approaches to neuroblastoma therapy (NANT) study. Pediatr Blood Cancer. 2010 doi: 10.1002/pbc.22821. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fousseyni T, Diawara M, Pasquier E, André N. Children treated with metronomic chemotherapy in a low-income country:METRO-MALI-01. J Pediatr Hematol Oncol. 2011;33:31–34. doi: 10.1097/MPH.0b013e3182018ab8. [DOI] [PubMed] [Google Scholar]
- 27.Kellie SJ, Howard SC. Global child health priorities: what role for paediatric oncologists? Eur J Cancer. 2008;44:2388–2396. doi: 10.1016/j.ejca.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Klement GL, Kamen BA. Nontoxic, fiscally responsible, future of oncology: could it be beginning in the Third World? J Pediatr Hematol Oncol. 2011;33:1–3. doi: 10.1097/MPH.0b013e3182024918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–740. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 30.Andre N, Padovani L, Pasquier E. Metronomic scheduling of anticancer treatment: the next generation of multitarget therapy? Future Oncol. 2011;7:385–394. doi: 10.2217/fon.11.11. [DOI] [PubMed] [Google Scholar]
- 31.Andre N, Rome A, Coze C, Padovani L, Pasquier E, Camoin L, Gentet JC. Metronomic etoposide/cyclophosphamide/celecoxib regimen given to children and adolescents with refractory cancer: a preliminary monocentric study. Clin Ther. 2008;30:1336–1340. doi: 10.1016/s0149-2918(08)80059-8. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Aguilar E, Sepulveda-Vildosola AC, Betanzos-Cabrera Y, Rocha-Moreno YG, Gascon-Lastiri G, Rivera-Marquez H, Wanzke-del-Angel V, Cerecedo-Diaz F, de la Cruz-Yanez H. Phase II study of metronomic chemotherapy with thalidomide, carboplatin-vincristine-fluvastatin in the treatment of brain stem tumors in children. Arch Med Res. 2008;39:655–662. doi: 10.1016/j.arcmed.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Le Deley MC, Vassal G, Taibi A, Shamsaldin A, Leblanc T, Hartmann O. High cumulative rate of secondary leukemia after continuous etoposide treatment for solid tumors in children and young adults. Pediatr Blood Cancer. 2005;45:25–31. doi: 10.1002/pbc.20380. [DOI] [PubMed] [Google Scholar]
- 34.Ridola V, Fawaz O, Aubier F, Bergeron C, de Vathaire F, Pichon F, Orbach D, Gentet JC, Schmitt C, Dufour C, et al. Testicular function of survivors of childhood cancer: a comparative study between ifosfamide- and cyclophosphamide-based regimens. Eur J Cancer. 2009;45:814–818. doi: 10.1016/j.ejca.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Shaked Y, Tang T, Woloszynek J, Daenen LG, Man S, Xu P, Cai SR, Arbeit JM, Voest EE, Chaplin DJ, et al. Contribution of granulocyte colony-stimulating factor to the acute mobilization of endothelial precursor cells by vascular disrupting agents. Cancer Res. 2009;69:7524–7528. doi: 10.1158/0008-5472.CAN-09-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M, Daenen LG, Man S, Xu P, Emmenegger U, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 38.Daenen LG, Shaked Y, Man S, Xu P, Voest EE, Hoffman RM, Chaplin DJ, Kerbel RS. Low-dose metronomic cyclophosphamide combined with vascular disrupting therapy induces potent antitumor activity in preclinical human tumor xenograft models. Mol Cancer Ther. 2009;8:2872–2881. doi: 10.1158/1535-7163.MCT-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumorindependent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 43.Pasquier E, Dias S. Endothelial progenitor cells: hope beyond controversy. Curr Cancer Drug Targets. 2010;10:914–921. doi: 10.2174/156800910793358041. [DOI] [PubMed] [Google Scholar]
- 44.Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 46.Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 48.Calleri A, Bono A, Bagnardi V, Quarna J, Mancuso P, Rabascio C, Dellapasqua S, Campagnoli E, Shaked Y, Goldhirsch A, et al. Predictive potential of angiogenic growth factors and circulating endothelial cells in breast cancer patients receiving metronomic chemotherapy plus bevacizumab. Clin Cancer Res. 2009;15:7652–7657. doi: 10.1158/1078-0432.CCR-09-1493. [DOI] [PubMed] [Google Scholar]
- 49.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;12:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 50.Shaked Y, Kerbel RS. Antiangiogenic strategies on defense: on the possibility of blocking rebounds by the tumor vasculature after chemotherapy. Cancer Res. 2007;67:7055–7058. doi: 10.1158/0008-5472.CAN-07-0905. [DOI] [PubMed] [Google Scholar]
- 51.Ghiringhelli F, Apetoh L, Housseau F, Kroemer G, Zitvogel L. Links between innate and cognate tumor immunity. Curr Opin Immunol. 2007;19:224–231. doi: 10.1016/j.coi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Choijamts B, Naganuma Y, Nakajima K, Kawarabayashi T, Miyamoto S, Tachibana K, Emoto M. Metronomic irinotecan chemotherapy combined with ultrasound irradiation for a human uterine sarcoma xenograft. Cancer Sci. 2011;102:452–459. doi: 10.1111/j.1349-7006.2010.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merritt WM, Danes CG, Shahzad MM, Lin YG, Kamat AA, Han LY, Spannuth WA, Nick AM, Mangala LS, Stone RL, et al. Anti-angiogenic properties of metronomic topotecan in ovarian carcinoma. Cancer Biol Ther. 2009;8:1596–1603. doi: 10.4161/cbt.8.16.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emmenegger U, Francia G, Chow A, Shaked Y, Kouri A, Man S, Kerbel RS. Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia. 2011;13:40–48. doi: 10.1593/neo.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sterba J, Pavelka Z, Andre N, Ventruba J, Skotakova J, Bajciova V, Bronisova D, Dubska L, Valik D. Second complete remission of relapsed medulloblastoma induced by metronomic chemotherapy. Pediatr Blood Cancer. 2010;54:616–617. doi: 10.1002/pbc.22382. [DOI] [PubMed] [Google Scholar]