Abstract
BACKGROUND: Epithelial-to-mesenchymal transition (EMT) is a transient process occurring during developmental stages and carcinogenesis, characterized by phenotypic and molecular alterations, resulting in increased invasive and metastatic capabilities of cancer cells and drug resistance. Moreover, emerging evidence suggests that EMT is associated with increased enrichment of cancer stem-like cells in neoplastic tissues. We interrogated the molecular alterations occurring in breast cancer using proposed EMT markers such as E-cadherin, vimentin, epidermal growth factor receptor (EGFR), platelet-derived growth factor (PDGF) D, and nuclear factor κB (NF-κB) to decipher their roles in the EMT and breast cancer progression. METHODS: Fifty-seven invasive ductal adenocarcinomas of the breast were assessed for the expression of E-cadherin, vimentin, EGFR, NF-κB, and PDGF-D using immunohistochemical analysis. Tumors were categorized into three groups: A (ER+, and/or PR+, HER-2/neu-), B (ER+, and/or PR+, HER-2/neu+), and C (triple-negative: ER-, PR-, and HER-2/neu-). Immunostained slides were microscopically evaluated and scored using intensity (0, 1+, 2+, and 3+) and percentage of positive cells, and data were statistically analyzed. RESULTS: Membranous E-cadherin was positive in all 57 cases (100%), whereas cytoplasmic E-cadherin was predominantly positive in groups B and C compared with group A (21%, 7%, and 0%, respectively). All group A cases were negative for vimentin and EGFR. There was statistically significant increased expression of vimentin (P < .0002), EGFR (P < .0001), and NF-κB (P < .02) in triple-negative cases when compared with groups A and B. CONCLUSIONS: Vimentin, EGFR, and NF-κB were significantly increased in triple-negative tumors, which is consistent with the aggressiveness of these tumors. These markers could be useful as markers for EMT in breast cancers and may serve as predictive markers for designing customized therapy in the future.
Introduction
Epithelial-to-mesenchymal transition (EMT) is a transient process occurring during carcinogenesis that is characterized by alterations resulting in increased invasive and metastatic capabilities of cancer cells, and it has also been proposed to play a key role in drug resistance [1,2]. During the acquisition of EMT phenotype, the cancer cells undergo phenotypic and molecular alterations representing mesenchymal differentiation. The phenotypic changes include transformation of the cuboidal/cobblestone morphology to elongated, spindle, fibroblastic-type morphology [1,2]. The molecular changes include loss of specialized epithelial cell adhesion molecules like cadherins and acquisition of mesenchymal markers like vimentin.
The acquisition of EMT phenotype eventually leads to increased motile function of the cells consistent with invasion and metastasis. These elongated spindle-shaped cells interact with the neighboring cells only to a limited extent (and only at focal points) and display a “front-back” polarity [3]. Physiologically, EMT-like changes have been described during embryogenesis [4], in adult skin (as cells move from the inner germinal layer to the surface), and in intestine (as cells move from the crypts to the villi) [3]. However, these are coordinated and organized unlike EMT seen in cancers. EMT also plays a key role in inflammation, fibrosis, and wound healing where there is loss of cell adhesion, increased cell motility, and reversible dedifferentiation [3].
The gene regulatory programs controlling EMT regulate cell adhesion molecules and their signaling pathways and have emerged as important determinants of tumor cell invasion and metastasis [5]. EMT is reversible and is regulated by the interplay of several extracellular signals, growth factors, their effectors and transcription factors [6]. Moreover, EMT has also been proposed to play a critical role in “cancer stem cells” or cancer stem-like cells (CSCs) [7], although these cellular processes are not synonymous [6]. Recently, salinomycin has been used to selectively kill CSCs induced by treating breast cancer cells with transforming growth factor β1 documenting the acquisition of EMT phenotype [8], and these results suggest that the killing of drug-resistant EMT-type cells (CSCs) could be achieved by novel therapeutics.
Breast cancer has a diverse clinical spectrum. Despite similarities in the histologic presentation at the time of disease diagnosis, their clinical behaviors, including time to disease progression and metastasis, cannot be predicted with certainty. Therefore, there is a dire need to identify which patients would respond to therapeutic regimens and who would not. Identifying the aggressive phenotype such as the presence of EMT-type cells, one would be able to predict the behavior of cancer cells. This would likely assist in predicting the clinical behavior of tumors and, consequently, design better and customize therapy to cure breast cancer in patients with tumors for which there is no targeted therapy.
Recent advances in the treatment of breast cancer have led to a significant improvement in survival of patients diagnosed with hormone receptor- and/or HER-2-positive disease. However, tumors that are lacking the expression of estrogen receptor (ER), progester one receptor (PR), and HER-2, the so-called triple-negative subgroup have a relatively aggressive clinical course, with early development of visceral metastases and a poor long-term prognosis [9]. As a group, the triple-negative breast carcinoma cases have a poor prognosis. Hence, they are an area of interest for the development of novel targeted agents, although specific targets have not been fully validated.
By uncovering the molecular mechanisms that govern EMT in breast cancer, one could prevent metastasis by converting an invasive phenotype to a noninvasive one using target-specific therapeutics. Thus, inactivation of EMT signaling pathways by novel approaches could potentially be useful for preventing cancer progression in the near future [10]. A better understanding of the biologic mechanism of these EMT molecular changes may help to identify new targets for breast cancer therapy, which would be useful for designing personalized medicine. The aim of the present study was to investigate the expression patterns of proteins associated with the EMT to elucidate intracellular cell signaling in the tumor milieu and provide novel diagnostic and therapeutic markers.
Materials and Methods
The study was conducted in the Department of Pathology, Karmanos Cancer Institute at Wayne State University School of Medicine, Detroit, MI. At the outset, an institutional review board approval and a waiver of consent for a retrospective review of archived material were obtained.
Study Cohort
On retrospective review of archived paraffin-embedded invasive ductal carcinomas of the breast, a total of 57 cases were selected for the study. Tumors were categorized into three groups: A (ER+, and/or PR+, HER-2/neu-), B (ER+, and/or PR+, HER-2/neu+), and C (triple-negative: ER-, PR-, HER-2/neu-).
Histologic Evaluation
In each case, the histopathologic slides were reviewed microscopically, and a representative tumor block was selected. In each block, 4-µm-thick tumor tissue sections were obtained from positively charged glass slides and interrogated for EMT markers including E-cadherin, vimentin, nuclear factor κB (NF-κB; nuclear and activated p65 subunit), epidermal growth factor receptor (EGFR), and platelet-derived growth factors (PDGF) D.
Immunohistochemical Analysis
Tissue sections were immunohistochemically stained using specific antibodies for E-cadherin, vimentin, NF-κB, EGFR, and PDGF-D. These markers were chosen based on existing evidence from our laboratory, documenting the role of these markers in the acquisition of EMT [11]. Standard laboratory protocols according to the laboratory manual were established using the avidin-biotin complex staining procedure. Initial trials used the manufacturer's suggested specimen preparation and staining conditions. Each protocol was optimized for antigen retrieval, antibody dilution, and incubation conditions as outlined in Table 1. A known positive tissue for the antigen of interest was used to titer the antibody and, subsequently, was stained with each investigative case for the current study. Immunohistochemical staining was performed as follows: tissue sections were deparaffinized, hydrated to phosphate-buffered saline buffer (pH 7.4) and pretreated with hydrogen peroxide (3%) for 10 minutes to remove endogenous peroxidase. This was followed by antigen retrieval through steam bath for 20 minutes with EDTA. The slides were then incubated with the primary antibody at ambient temperature and washed with phosphate-buffered saline followed by incubation with biotin-labeled secondary antibody for 30 minutes at room temperature. Finally, slides were developed with 0.05% 3′,3-diaminobenzidine tetrahydrochloride and then counterstained with Mayer hematoxylin, dehydrated, and mounted.
Table 1.
Antibody Details for Immunohistochemistry.
| Antibody | Source | Dilution |
| E-cadherin | Zymed | 1:50 |
| Vimentin | Ventana | Prefilled |
| EGFR | Zymed | Automated |
| PDGF-D | Zymed | 1:200 |
| NF-κB | Cell Signaling | 1:10 |
Slide Evaluation
Immunostained slides were blindly evaluated by a pathologist under a transmission light microscope. Areas of highest staining density were identified for evaluating the expression in tumors.
Microscopic Scoring of Expression
Expression was scored for each antibody separately and semi-quantitatively by assessing the stain localization, intensity, and the percentage of stained cells in the tumors. Stain localization was classified as nuclear, cytoplasmic, and membranous. Staining intensity was scored as 0 (no staining), 1+ (weak), 2+ (medium), or 3+ (strong). The percentage of stained cells was categorized into the following: 1, 0% to 10% stained cells; 2, 11% to 50% stained cells; 3, 50% stained cells or greater. The final score was obtained by multiplying the two scores. Cases with a score of 0 to 4 were classified as negative, and those with a final score of 5 to 9 were classified as positive. Statistical analyses were performed with the SPSS for Windows software (version 13.0; SPSS, Inc, Chicago, IL).
Results and Discussion
Membranous E-cadherin expression was positive in all 57 cases (100%), whereas cytoplasmic E-cadherin staining was more prevalent in the more aggressive B and C groups with 21%(5/24) and 7% (1/14) cytoplasmic positive staining, respectively. Of all group A cases (n = 19), none (0%) were negative for vimentin and EGFR, 15 (79%) were positive for PDGF-D, and 6 (32%) were positive for nuclear NF-κB (Figure 1; Table 2). In all group B cases (n = 24), 18 (75%) were positive for PDGF-D, 10 (42%) were positive for NF-κB, 6 (25%) were positive for vimentin, and 2 (8%) were positive for EGFR. In group C cases (triple-negative; n = 14), there was increased expression of vimentin in 9 cases (64%), of EGFR in 14 cases (100%), of NF-κB in 11 cases (79%), and of PDGF-D in 9 cases (64%). We found increased expression of vimentin (P < .0002), EGFR (P < .0001), and NF-κB in triple-negative breast cancer specimens, which was statistically highly significant (P < .02) when compared with groups A and B.
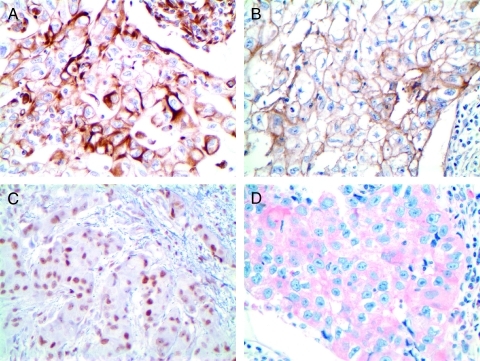
Figure 1.
Microphotographs of representative examples of immunohistochemical staining of EMT markers in breast cancer cases showing (A) vimentin cytoplasmic positivity in group C, (B) EGFR membranous and cytoplasmic positivity in group C, (C) NF-κB nuclear positivity in group A, and (D) PDGF-D cytoplasmic positivity in group C. Original magnification, x400.
Table 2.
EMT Markers in Breast Cancer Cases.
| Tumor Class | n | Vimentin | EGFR | NF-κB | PDGF-D | Cytoplasmic E-cadherin |
| Group A | 19 | 0 (0%) | 0 (0%) | 6 (32%) | 15 (79%) | 0 (0%) |
| Group B | 24 | 6 (25%) | 2 (8%) | 10 (42%) | 18 (75%) | 5 (21%) |
| Group C | 14 | 9 (64%) | 14 (100%) | 11 (79%) | 9 (64%) | 1 (7%) |
| P | <.0002 | <.0001 | <.02 | <.62 | .04 |
Breast cancer is a heterogeneous disease that consists of multiple molecular subtypes. The presence of hormone receptors ER and PR and overexpression of human EGFR-2 (HER-2) is of central importance in the therapeutic decision-making process for patients with breast cancer. Apart from predicting response to therapy, these factors may also determine the likelihood of disease relapse. Hormone receptor-positive tumors have been considered to have favorable outcome because of their response to endocrine manipulations such as tamoxifen, aromatase inhibitors, or ovarian ablation. Triple-negative breast cancer (TNBC) is a term that has been used to describe a biologically diverse group of breast tumors that are lacking in the expression of ER, PR, and HER-2. Tumors with a triple-negative phenotype tend to have poor prognosis and, unlike their hormone receptor or HER-2-positive counterparts, lack targeted therapeutics. As a result, the interest in this aggressive TNBC from both clinicians and scientists has grown exponentially.
The EMT phenomenon is triggered by the interplay of several extracellular signals; many secreted soluble factors, growth factors, their effectors, and many transcription factors including PDGF, Notch, and NF-κB [6]. The translational relevance of these EMT markers in evaluating aggressiveness of human breast cancers specimens has not been well investigated, and thus in the present study, we interrogated EMT markers such as E-cadherin, vimentin, NF-κB, EGFR, and PDGF-D in breast cancer specimens. Cases were divided based on their prognosis and aggressiveness into the highly aggressive triple-negative tumors versus the relatively innocuous ER-, PR-, HER-2-positive tumors. EMT markers (vimentin, EGFR, and NF-κB) were found to be significantly higher in triple-negative breast tumors (known aggressive tumors) but not PDGF-D, and our findings are consistent with similar findings reported in the literature [12].
Vimentin is a cytoplasmic intermediate filament protein found to be the major component of the cytoskeleton. Our findings of its association with clinically aggressive behavior of breast tumors are also consistent with previous studies [13–15]. Conversely, Heatley et al. [16] have demonstrated that vimentin expression did not inversely predict patient survival. The association of vimentin with clinically aggressive behavior of tumors has been explained based on the correlation of vimentin's expression with lack of steroid receptors and poor differentiation of cancer [9]. During EMT, the cell intermediate filament status changes from a keratin-rich network, which connects to adherens junctions and hemidesmosomes, to a vimentin-rich network connecting to focal adhesions [17]. Sarrio et al. [18] have suggested that vimentin-positive cells may have a phenotypic plasticity prone to undergo EMT, and although transient, they involve modulation of a number of EMT genes.
The EGFR is a member of the ErbB family of receptor tyrosine kinases. The high incidence of EGFR expression in triple-negative tumors in our study is similar to findings by other authors [19,20]. The EGFR positivity has been related to a less favorable response to chemotherapy and poorer survival, indicating that it can serve as a valuable tool for selecting appropriate treatment regimens for patients with TNBC [19]. The activation of EGFR leads to the activation of NF-κB, which could be useful for predicting tumor aggressiveness. NF-κB is a transcription factor that is well known to contribute to the acquisition of EMT and tumor cell invasion [3]. High NF-κB expression found in the triple-negative breast cancer cases in our study is similar to those of previous authors [21,22]. Genes induced by the NF-κB activation could serve as therapeutic targets of triple-negative breast cancers, and to that end, inactivation of NF-κB pathway by many natural agents has been reported [23–26], including recent findings of plumbagin, suggesting that natural agents could be useful for the prevention and/or treatment of aggressive breast cancer especially the TNBCs [27].
PDGF-D is an important regulator of cell proliferation, transformation, invasion, and metastasis in human cancer [10]. It has been linked with several human malignancies [28] and has also been shown to play an important role in the processes of EMT [29,30] by causing changes in cellular morphology concomitant with loss of E-cadherin, gain of vimentin, increase in tumor growth, and increased cancer cell invasion and angiogenesis [10]. Moreover, forced overexpression of PDGF-D in PC prostate cancer cells showed the acquisition of stem cell characteristics, suggesting the role of PDGF-D in self-renewal and tumor cell aggressiveness [31]. In the present study, we found that PDGF-D was not significantly higher in triple-negative breast tumors when compared with the other prognostic groups. A review of literature reveals that similar studies have not been previously done in human breast cancer tissues; however, in breast cancer cell lines, PDGF-D has been demonstrated to play an important role in tumor aggressiveness [28]. Several reasons might be behind the lack of correlation between tumor aggressiveness and PDGF-D positivity in our study. First, the use of IHC to evaluate protein levels does not always reflect the structure or functionality of the protein. Second, the small size of the patient cohort would introduce an element of bias in this evaluation. Third, technical aspects such as the clone of antibody used and antigen retrieval methods might have played a part in this disparity and needs further clarification in future IHC studies.
In summary, based on our findings, we conclude that the expression of vimentin, EGFR, and NF-κB were significantly increased in human TNBCs. The higher expression of these markers seen in triplenegative tumors may explain the different biologic behavior of these tumor types. Therefore, vimentin, EGFR, and NF-κB may have the potential to be used as markers for EMT in breast cancers, and targeted inactivation of these markers could be useful for designing personalized medicine, which would be the future to make an impact on improving the overall survival of patients diagnosed with TNBCs.
References
- 1.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 2.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavert N, Ben-Ze'ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 5.Emadi Baygi M, Soheili ZS, Schmitz I, Sameie S, Schulz WA. Snail regulates cell survival and inhibits cellular senescence in human metastatic prostate cancer cell lines. Cell Biol Toxicol. 2010;26:553–567. doi: 10.1007/s10565-010-9163-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prindull G. Hypothesis: cell plasticity, linking embryonal stem cells to adult stem cell reservoirs and metastatic cancer cells? Exp Hematol. 2005;33:738–746. doi: 10.1016/j.exphem.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantinidou A, Jones RL, Reis-Filho JS. Beyond triple-negative breast cancer: the need to define new subtypes. Expert Rev Anticancer Ther. 2010;10:1197–1213. doi: 10.1586/era.10.50. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Ahmad A, Li Y, Kong D, Azmi AS, Banerjee S, Sarkar FH. Emerging roles of PDGF-D signaling pathway in tumor development and progression. Biochim Biophys Acta. 2010;1806:122–130. doi: 10.1016/j.bbcan.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010;3(1):90–99. [PMC free article] [PubMed] [Google Scholar]
- 12.Sousa B, Paredes J, Milanezi F, Lopes N, Martins D, Dufloth R, Vieira D, Albergaria A, Veronese L, Carneiro V, et al. P-cadherin, vimentin and CK14 for identification of basal-like phenotype in breast carcinomas: an immunohistochemical study. Histol Histopathol. 2010;25:963–974. doi: 10.14670/HH-25.963. [DOI] [PubMed] [Google Scholar]
- 13.Kusinska RU, Kordek R, Pluciennik E, Bednarek AK, Piekarski JH, Potemski P. Does vimentin help to delineate the so-called “basal type breast cancer”? J Exp Clin Cancer Res. 2009;28:118. doi: 10.1186/1756-9966-28-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zajchowski DA, Bartholdi MF, Gong Y, Webster L, Liu HL, Munishkin A, Beauheim C, Harvey S, Ethier SP, Johnson PH. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 2001;61:5168–5178. [PubMed] [Google Scholar]
- 15.Raymond WA, Leong AS. Co-expression of cytokeratin and vimentin intermediate filament proteins in benign and neoplastic breast epithelium. J Pathol. 1989;157:299–306. doi: 10.1002/path.1711570406. [DOI] [PubMed] [Google Scholar]
- 16.Heatley MK, Ewings P, Odling SW, Maxwell P, Toner PG. Vimentin expression does not assist in predicting survival in ductal carcinoma of the breast. Pathology. 2002;34:230–232. doi: 10.1080/00313020220131273. [DOI] [PubMed] [Google Scholar]
- 17.Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer—observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 18.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 19.Nogi H, Kobayashi T, Suzuki M, Tabei I, Kawase K, Toriumi Y, Fukushima H, Uchida K. EGFR as paradoxical predictor of chemosensitivity and outcome among triple-negative breast cancer. Oncol Rep. 2009;21:413–417. [PubMed] [Google Scholar]
- 20.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Ito T, Shimizu T, Ishida T, Semba K, Watanabe S, Yamaguchi N, Inoue JI. Epigenetic alteration of the NF-κB-inducing kinase (NIK) gene is involved in enhanced NIK expression in basal-like breast cancer. Cancer Sci. 2010;101:2391–2397. doi: 10.1111/j.1349-7006.2010.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman KM, Sarkar FH, Banerjee S, Wang Z, Liao DJ, Hong X, Sarkar NH. Therapeutic intervention of experimental breast cancer bone metastasis by indole-3-carbinol in SCID-human mouse model. Mol Cancer Ther. 2006;5:2747–2756. doi: 10.1158/1535-7163.MCT-06-0221. [DOI] [PubMed] [Google Scholar]
- 24.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar FH, Li Y. NF-κB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–2959. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar FH, Li YW. Targeting multiple signal pathways by chemopreventive agents for cancer prevention and therapy. Acta Pharmacol Sin. 2007;28:1305–1315. doi: 10.1111/j.1745-7254.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad A, Banerjee S, Wang Z, Kong D, Sarkar FH. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-κB and Bcl-2. J Cell Biochem. 2008;105:1461–1471. doi: 10.1002/jcb.21966. [DOI] [PubMed] [Google Scholar]
- 28.Ahmad A, Wang Z, Kong D, Ali R, Ali S, Banerjee S, Sarkar FH. Platelet-derived growth factor-D contributes to aggressiveness of breast cancer cells by up-regulating Notch and NF-κB signaling pathways. Breast Cancer Res Treat. 2011;126:15–25. doi: 10.1007/s10549-010-0883-2. [DOI] [PubMed] [Google Scholar]
- 29.Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, Kim HR, Cher ML, Sarkar FH. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]