Abstract
Tumor radioresistance leads to recurrence after radiation therapy. The radioresistant phenotype has been hypothesized to reside in the cancer stem cell (CSC) component of breast and other tumors and is considered to be an inherent property of CSC. In this study, we assessed the radiation resistance of breast CSCs using early passaged, patient-derived xenografts from two separate patients. We found a patient-derived tumor in which the CSC population was rapidly depleted 2 weeks after treatment with radiation, based on CD44+ CD24- lin- phenotype and aldehyde dehydrogenase 1 immunofluorescence, suggesting sensitivity to radiotherapy. The reduction in CSCs according to phenotypic markers was accompanied by a decrease in functional CSC activity measured by tumor sphere frequency and the ability to form tumors in mice. In contrast, another patient tumor sample displayed enrichment of CSC after irradiation, signifying radioresistance, in agreement with others. CSC response to radiation did not correlate with the level of reactive oxygen species in CSC versus non-CSC. These findings demonstrate that not all breast tumor CSCs are radioresistant and suggest a mechanism for the observed variability in breast cancer local recurrence.
Introduction
Radiation therapy is a mainstay of breast cancer treatment. Radiation therapy given after surgery in early stage breast cancer patients has been shown to significantly increase the probability of both local control and survival [1]. Postmastectomy irradiation in locally advanced breast cancer similarly improves local control and survival beyond both chemotherapy and antihormonal therapy [2–4]. However, tumors of a subset of patient recur locally despite best efforts. The reason why residual tumor cells escape eradication by radiation is unclear but may partially be due to intrinsic radioresistance of cancer stem cells (CSCs).
The CSC hypothesis is based on the observation that a small subset of cells obtained from a tumor (cancer stem cells) are preferentially capable of generating tumors in mouse models [5,6]. As stem cells, they are defined as being able to self-renew and are the origin of other cancer cells that contribute to the mass of the tumor. CSCs were first discovered in acutemyeloid leukemia and subsequently in solid tumors, including breast, pancreas, colon, glioblastoma, and others [7–14]. They are responsible for maintaining the tumor and have been hypothesized to lead the invasive front of the tumor and contribute to metastatic seeding.
Resistance to radiation and chemotherapy has been reported to be a defining characteristic of CSCs from various tumor types, including glioma, breast, and colon cancers [15–20]. Diehn et al. [21] report breast CSCs harbor lower levels of reactive oxygen species than the non-stem cell component, and this contributes to radioresistance of breast CSCs. A stem cell-like population of the MCF7 breast cancer cell line has been shown to be more resistant to radiation than the rest of the population [22]. Breast tumors are enriched with CD44+ CD24- CSCs in neoadjuvant chemotherapy-treated patients [20]. However, others have shown that CD44+ CD24- breast CSCs are reduced in neoadjuvant chemotherapy-treated patients [23], and we have found a similar decrease in cyclophosphamide-treated xenografts [24].
The question of whether CSCs are radiation resistant or sensitive is important given radiation's effectiveness in reducing local recurrence and improving survival. In our investigation, we find a patient-derived tumor that displays radiosensitive CSCs, in contrast to the expected radioresistance we and others define in other tumor samples. These data are based on the phenotypic and functional analysis of the CSC fraction in irradiated tumor xenografts. Our data suggest that breast CSCs are not uniform in their response to radiation, and this may account for differential chances of recurrence after radiation therapy.
Methods
Tumors and Mice
MC1 and UM2 cells have been previously described [8,25]. MC1 cells were derived from a pleural effusion and are estrogen and progesterone receptor negative and HER-2- [25]. UM2 cells were derived from an ovarian metastasis and are estrogen and progesterone receptor positive and HER-2- [25]. Both lines were maintained exclusively as xenografts in NOD.CB17-Prkdcscid/J (NOD/SCID) mouse (Jackson Laboratory, Bar Harbor, ME) mammary fat pads. Samples used in these experiments were less than 10 in vivo passages removed from original derivation.
Tumors were produced in the mammary fat pad of NOD/SCID mice by injecting 5 x 105 cells, or numbers as indicated, in a 1:1 solution of Matrigel (BD Biosciences, San Jose, CA) and serum-free Dulbecco modified Eagle medium. Single-cell suspensions of tumors were made by mincing the tumor and incubating in 300 U/ml collagenase, 100 U/ml hyaluronidase (Stem Cell Technologies, Vancouver, Canada) in medium 199 for 15 minutes at 37°C, followed by triturating through a 16-gauge needle/syringe. The digestion was stopped by addition of fetal bovine serum (FBS) to 5% volume, cells were filtered through a 100-µm cell strainer (BD Biosciences) and centrifuged, and the pellet was resuspended with Hanks balanced salt solution and 5% FBS and passed a second time through a 100-µm cell strainer. Cells were pelleted and then resuspended in 15% dimethyl sulfoxide in FBS for storage in liquid nitrogen unless analyzed immediately. Tumor volume was calculated using the equation (π/6)ab2, where a = the long dimension and b = the short dimension of the tumor. All animal experiments were performed in accordance with University Committee on Use and Care of Animals principles and guidelines.
Irradiation
Cells and tumors were irradiated with a Philips 250 orthovoltage unit at approximately 2.5 Gy/min in the Irradiation Core of the University of Michigan Cancer Center. Dosimetry was carried out using an ionization chamber connected to an electrometer system, which is directly traceable to a National Institute of Standards and Technology calibration. Mice were either placed in a Lucite restrainer or anesthetized with ketamine/xylazine and positioned such that the apex of each tumor is at the center of a 2.4-cm aperture in the secondary collimator and irradiated with the rest of the mouse being shielded from radiation.
Flow Cytometry
For analysis of the CSC phenotype based on CD44 and CD24, 106 unfixed cells were washed and resuspended in 100 µl of PBS and 2% bovine serum albumin (BSA). We added fluorescently labeled anti-CD44 and CD24 antibodies together with anti-H-2kd antibody and a lineage cocktail composed of anti-CD3, CD10, CD16, CD18, and CD140b antibodies (BD Biosciences); the cells were incubated at 4°C for 15 minutes; and then the cells were washed with PBS, 2% BSA before resuspending in PBS, 2% BSA, and 0.25 µg/ml propidium iodide (PI). PI+ cells (dead cells) were gated out before analysis. Samples for analysis were run on a BD Biosciences FACSCalibur instrument, and data were analyzed using FCS Express software (De Novo Software, Los Angeles, CA). Cell sorting was done on a BD Biosciences FACSAria or FACSVantage SE instrument.
The ALDEFLUOR assay was performed according to the kit manufacturer's instructions and as previously described [25] (Stem Cell Technologies).
Immunofluorescence
Eight-micrometer-thick sections were cut from formalin-fixed tumors and deparaffinized, and the aldehyde dehydrogenase 1 (ALDH1) epitope was unmasked by incubating in citrate buffer at 95°C for 20 minutes. Sections were blocked with Tris-buffered saline, 1% BSA, 10% normal goat serum for 1.5 hours before incubating in 1:100 anti-ALDH1 antibody (BD Biosciences) overnight at 4°C. Washed slides were then incubated in 1:1000 AlexaFluor-488-conjugated secondary antibody (Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Washed slides were mounted with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole dihydrochloride (Invitrogen) for staining nuclei.
Reactive Oxygen Species
Reactive oxygen species (ROS) were detected in cells using 5-(and 6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate (H2DFFDA; Molecular Probes, Eugene, OR). A single-cell suspension of MC1 or UM2 cells was incubated in 10 nM H2DFFDA for 30 minutes at 37°C. Cells were then washed in 2% BSA and PBS and labeled with antibodies for the detection of CD44+ CD24- CSC as previously mentioned, excluding PI staining. Flow cytometry was performed, and gates were set based on the negative control (incubation without H2DFFDA). A positive control was produced by incubating cells in 100 µMH2O2 and H2DFFDA.
Tumor Sphere Formation
Tumor spheres were grown on ultra-low-attachment six-well plates at 2 x 104 and 1 x 105 cells per well in 1:1 Dulbecco modified Eagle medium-F-12 (Hyclone, Logan, UT) containing 5% FBS, 2 mM glutamine, 4 µg/ml heparin (Stem Cell Technologies), 20 ng/ml epidermal growth factor (R&D Systems, Minneapolis, MN), 20 ng/ml basic fibroblast growth factor (R&D Systems), and B-27 (Invitrogen). Cells were incubated for 2 weeks at 37°C in 5% CO2 before counting resulting tumor spheres.
Statistics
Error bars and P values were generated using GraphPad Prism 5 software (GraphPad Software for Science, Inc, San Diego, CA). Error bars represent SEM. Two-tailed Student's t test was used for P value calculations unless otherwise noted.
Results
We assessed the effect of radiation on the content of CSC and non-CSC populations of two patient-derived breast tumors (MC1 and UM2) in an in vivo model with the hypothesis that radioresistant CSC would be enriched by radiation, whereas radiosensitive CSC would be depleted. Breast CSCs have been shown to be enriched in the CD44+ CD24- lin- population of a tumor, according to Al-Hajj et al. [8], and more recently, Ginestier et al. [25] have used the enzymatic-based ALDEFLUOR assay to identify CSCs with ALDH enzyme activity.
Depletion of CSCs in Breast Xenografts by Radiation
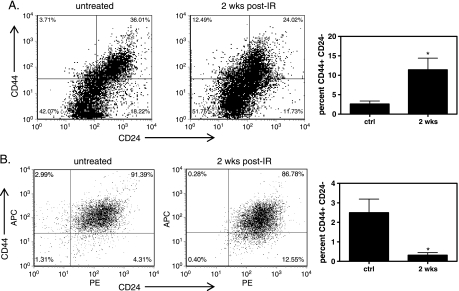
MC1 and UM2 breast tumor xenografts were given 8 Gy as a single dose to elicit a response that would result in decreased tumor volume but was not expected to be a curative dose. Irradiated tumors were removed 2 weeks after treatment for analysis. In UM2 tumors, we found an increase in the proportion of CD44+ CD24- lin- cells, from 2.6% ± 0.8% in untreated controls to 11% ± 3% in irradiated tumors (P < .05; Figure 1A). These data suggest UM2 CSCs are relatively resistant to radiation compared with the bulk population of cells, in agreement with other data on breast CSC [21,22,26].
Figure 1.
Effect of radiation on CSCs. MC1 and UM2 xenografts were treated with radiation and the proportion of CSCs analyzed after 2 weeks using flow cytometry. (A) Flow cytometric detection of CD44+ CD24- cells (upper left quadrant) in untreated and treated UM2 xenografts. (B) Flow cytometric detection of CD44+ CD24- cells in untreated and treated MC1 xenografts. *P < .05 compared with untreated, n = 2–15.
We next analyzed MC1 tumors for CSC content after radiation. We found a rapid and progressive decrease in the proportion of CSCs in irradiated MC1 tumors (Figure 1B). Control MC1 tumors had an average of 2.5% ± 0.7% CD44+ CD24- lin-. Two weeks after 8-Gy treatment, the proportion of CD44+ CD24- lin- cells dropped to 0.31% ± 0.14% (P < .05; Figure 1B). The loss of cells was progressive, with 0.84% ± 0.14% (P < .05, analysis of variance) CD44+ CD24- lin- present in MC1 tumors 1 day after radiation (not shown). There was also a decrease in ALDEFLUOR-positive MC1 cells (not shown). These data suggest MC1 tumor CSC are sensitive to radiation compared with the non-CSC population.
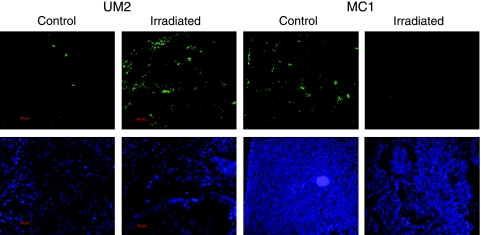
Flow cytometry results were confirmed by using immunofluorescence on histologic sections to detect ALDH1, one enzyme active in the ALDEFLUOR assay [27]. ALDH1 was detected in untreated control tumors as widely distributed, with no discernable histologic pattern (Figure 2). On treatment with radiation, the number of ALDH1+ cells in UM2 tumors increased, in agreement with flow cytometry results showing enrichment of the CSC population. In contrast, the number of ALDH1+ cells in MC1 tumors was substantially decreased 2 weeks after radiation.
Figure 2.
Immunofluorescent detection of ALDH1. Control and irradiated UM2 and MC1 tumor sections were stained for ALDH1 2 weeks after treatment. ALDH1 staining is in green on the upper panels, and DAPI staining of nuclei is in blue on the lower panels. Irradiated tumors displayed fewer ALDH1-stained cells than untreated tumors.
These data suggest that breast CSCs derived from MC1 are sensitive to the effects of radiation compared with the non-CSC population. Radiation caused preferential loss of CSCs according to surface phenotype, ALDH activity, and ALDH immunofluorescent staining. In contrast, CSCs in UM2 cells were enriched by treatment with radiation and thus radiation resistant compared with non-CSCs.
Functional CSC Activity in Irradiated Tumors
Only a subset of marker-positive cells have the capability to produce tumors in mice; therefore, the discordance in phenotypic markers after irradiation does not necessarily mean that there was a functional decrease in tumor-initiating activity. One measure of functional CSC activity is by the ability of cells to form tumor spheres in vitro. Mammary tumor spheres retain tumorigenic potential and maintain similarities to CSC [28]. To determine whether there was a loss of CSC activity in irradiated MC1 and UM2 tumors, we measured the tumor sphere frequency.
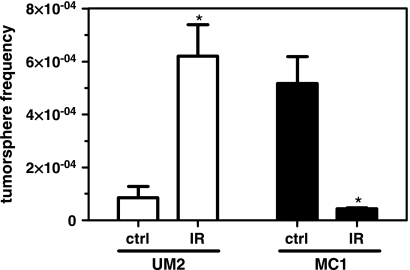
Control UM2 tumors had a tumor sphere frequency of 8.5 ± 4.3 x 10-5, which was increased 7-fold to 6.2 ± 1.2 x 10-4 in irradiated tumors (P < .01; Figure 3). In MC1 tumors, tumor sphere frequency was reduced 12-fold to 4.3 ± 0.3 x 10-5 after radiation compared with 5.2 ± 1.0 x 10-4 in control tumors (P < .01). Thus, radiation caused a decrease in tumor sphere frequency in MC1 tumors, but an increase in UM2 cells, in accordance with the effect seen on CSC phenotypic markers in Figures 1 and 2.
Figure 3.
Tumor spheres in control and irradiated tumors. The frequency of tumor sphere-forming cells in control or irradiated UM2 and MC1 xenografts was determined. *P < .01, n = 3–4.
We then chose further analysis of MC1 functional CSC activity in a robust in vivo tumor initiation model to verify that loss of CSCs occurred. To assess the functional state of tumor-initiating activity in irradiated MC1 tumors compared with controls, we injected serial dilutions of unsorted tumor cells from treated and untreated tumors into mice. If stem cell activity is reduced in a treated tumor compared with an untreated tumor, then the time required for tumor formation should be delayed. Conversely, if treatment enriched for stem cell activity, then recurrent tumors should appear sooner than untreated controls.
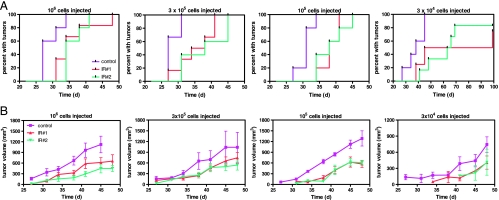
In mice injected with cells from radiation-treated tumors, median time to formation of recurrent tumors was delayed up to 33 days compared with controls (Figure 4A). In untreated controls, 100% of mice (18/18, all cell doses combined) developed tumors within 45 days of cell injection. Several mice (2/4 and 2/6 from tumor IR no. 1 and IR no. 2, respectively, at the 3 x 104 cell dose) injected with irradiated tumor-derived cells failed to produce tumors up to 100 days after injection. The difference between the frequency of tumor formation between control and treated groups was significant (P < .05) at all cell doses except IR no. 1 at 3 x 104, which showed the same trend (P = .09). Differences in the time to grow a tumor were not due to injection of nonviable cells because PI staining and flow cytometry revealed that all samples were of equivalent viability, 85% to 92% (not shown).
Figure 4.
Functional assay for CSC activity. (A) MC1 cells from control and two different irradiated tumors (2 weeks after irradiation) were injected into the mammary fat pad of mice at the indicated cell quantities. The number of days required for tumor formation was recorded and plotted. (B) Measurement of MC1 tumor growth. There was no statistical difference between the growth rate of each group (P < .05).
These data not only show that radiation treatment resulted in a decrease in marker-positive cells in tumors but also that this was reflected as a decrease in functional CSC activity, providing confirmation that stem cells were lost to radiation treatment. Thus, radiation was preferentially detrimental to MC1 CSCs compared with non-CSCs.
Characterization of Recurrent Tumors
Recurrent MC1 tumors arising from injection of cells from treated, primary tumors, were examined for abnormal growth rates and whether the proportion of CSCs remained reduced or returned to an equilibrium state similar to the original untreated, control tumors. Measurement of tumor volume showed that the rate of growth of recurrent tumors derived from treated primary tumors was equivalent to those derived from untreated tumors (Figure 4B; P < .05). Thus, once tumors were established, there was no defect in growth.
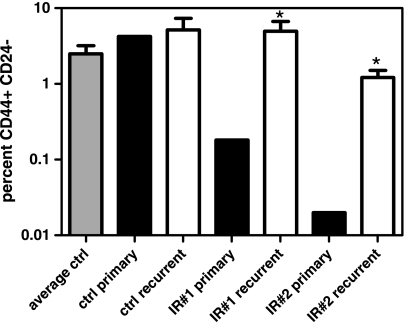
We then examined the CSC content of recurrent tumors from treated primary tumors to determine whether they returned to a state equivalent to untreated controls (Figure 5). The proportion of CD44+ CD24- lin- cells in IR no. 1 and IR no. 2 recurrent tumors was 4.9% ± 1.7% and 1.2% ± 0.3%, respectively. This is significantly increased from the proportion of CSCs initially infused of 0.2% in IR no. 1 and 0.02%in IR no. 2 (Figure 5; P < .05). Furthermore, the proportion of CSCs in IR no. 1 and IR no. 2 was not different from the average number found in control tumors of 2.5% ± 0.7% (P > .05). Although the proportion of CSCs in IR no. 2 trended lower, it was not significant. Neither was there a significant difference in the proportion of CSCs between any recurrent tumor group (P > .05). Control tumors displayed 5.2% ± 2.2% CSCs, similar to the proportion injected of 4.2%. Taken together, these data show that recurrent tumors do not have a growth defect and reestablish the baseline proportion of CSCs found in untreated tumors.
Figure 5.
Analysis of CSC in recurrent tumors. Recurrent tumor xenografts derived from control or two irradiated primary xenografts (IR#1 and IR#2) were subjected to flow cytometric detection of CD44+ CD24- CSC. *P < .05 compared with control.
Reactive Oxygen Species
One potential mechanism contributing to radiation resistance of CSC is the level of ROS in the cell [21]. Low levels of ROS are associated with increased expression of free radical scavengers and radiation resistance. We measured basal ROS in MC1 and UM2 cells using a flow cytometric method to determine whether it was consistent with the radiation sensitivity and resistance observed in MC1 and UM2 cells, respectively.
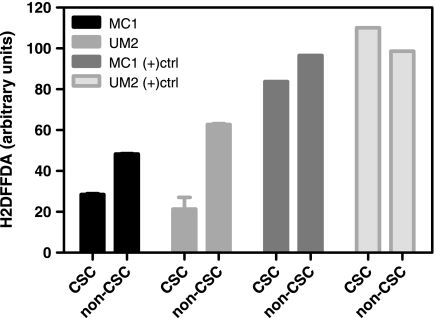
CSCs contained lower levels of ROS than non-CSCs in MC1 and UM2 cells (P < .05; Figure 6). In MC1 cells, the ROS levels of the CSC population were 59% the level of non-CSCs, whereas in UM2 cells, the ROS levels of the CSC population were proportionally lower, at 34% the level of non-CSCs. There was no significant difference in ROS levels between MC1 and UM2 CSCs or between MC1 and UM2 non-CSCs (P > .05). These data are consistent with those of Diehn et al. [21] in that the CSC populations had lower ROS; however, in our samples, there was no correlation between ROS levels in vitro and relative radiation resistance determined in vivo.
Figure 6.
Reactive oxygen species in CSC. MC1 and UM2 cells were stained with H2DFFDA to detect ROS levels by flow cytometry. CSC had statistically lower ROS levels than non-CSC (P < .05, N = 2). (+)ctrl signifies positive controls treated with H2O2.
Discussion
In this study, we have found that breast cancers can contain either sensitive or resistant CSCs relative to the bulk tumor population. More specifically, early in vivo passage MC1 tumors contain CSCs with relative sensitivity to radiation, whereas UM2 xenografts displayed radioresistant CSCs compared with the rest of the tumor. When MC1 xenografts were exposed to radiation, the proportion of CSCs based on two phenotypic definitions (CD44+ CD24- lin- flow cytometry and ALDH1 immunofluorescence) preferentially decreased as early as 1 day after treatment and to a greater degree 2 weeks after treatment. In contrast, CSCs in UM2 xenografts were preferentially enriched 2 weeks after radiation treatment. Importantly, the loss of CSCs in MC1 xenografts was accompanied by a functional defect in the ability of cells derived from treated tumors to produce tumor spheres or recurrent tumors in secondary NOD/SCID mice. Thus, the effect observed on phenotypic markers correlated with functional activity. Recurrent MC1 tumors grew at a similar rate to controls and reestablished baseline proportions of CSCs. ROS levels were lower in CSC than in non-CSC, in agreement with Diehn et al. [21], but the magnitude of difference was greater in the radioresistant sample (UM2).
There is a general perception that CSCs are inherently resistant to radiation, extending the hypothesis that this is a general property of cancer stem cells [17,22,26]. However, the data supporting this conclusion are limited. In a glioma xenograft model, radiation therapy resulted in enrichment of CD133+ glioma CSCs [17]. Radiation resistance was attributed to increased activity of the DNA damage checkpoint response. Of note is that gliomas are clinically far more resistant to radiation than breast cancer, so a difference between these tumor types may not be surprising. In breast cancer, in vitro work with the MCF-7 cell line has shown that radiation enriches for the CD44+ CD24- fraction of floating cells but not adherent cells [22]. Furthermore, MCF-7 mammospheres displayed greater survival and less expression of γH2AX than adherent cultures exposed to radiation. This important early study was limited to the breast cancer cell line, MCF-7, and, to a lesser extent, MDA-MB-231, without explicit validation that the cell phenotypes analyzed possessed cancer stem cell activity, a question of continuing controversy in cell lines [19,29–32]. In addition, the behavior of cells in culture may be different from that of a tumor [33]. Similar findings were reported by Woodward et al. [26], using side population (SP cells) as a phenotypic definition of CSCs in MCF-7 cells. Our analysis of UM2 xenografts extends theses studies by showing enrichment of CSCs after in vivo irradiation using an early-passage xenograft that has not been culture-adapted. MC1 xenografts, however, supports the hypothesis that breast CSCs are not universally radioresistant. Therefore, we feel our data do not contradict other findings but may have produced different results because of a different cell type and a more stringent model system.
The sensitivity of CSC to chemotherapy has also shown variability. In one study, CD44+ CD24- cells in HER2- tumors were enriched during the course of therapy with docetaxel or doxorubicin plus cyclophosphamide [20]. However, CD44+ CD24- cells in HER2+ tumors were decreased during treatment with lapatinib, an inhibitor of epidermal growth factor receptor/HER2. We and others have found a reduction in CD44+ CD24- breast CSC after chemotherapy in laboratory and clinical analyses [23,24]. In a subset of glioblastoma tumors, temozolomide treatment results in depletion of CSC, but in colon cancer, chemotherapy enriches CSC [18,34]. Taken together, these studies suggest that the relative resistance or sensitivity of CSCs to anti-cancer therapy is a more complex question than originally thought.
The analysis of ROS levels indicates that CSCs contain lower levels than non-CSCs do, suggesting increased an expression of free radical scavengers that limit the impact of radiation damage. These data suggest a possible contribution to the radiation response, but other mechanisms are likely to have equal or greater impact. For example, we also detected a difference in PCNA expression after irradiation in UM2 versus MC1 cells (not shown) and cannot exclude cell cycle as playing a role in the radiation response.
Elucidation of additional mechanisms for MC1 radiation sensitivity, as well as the frequency and extent of this phenomenon in the patient population, is an important avenue of continued study that could impact individualized therapies and new approaches aimed at radiosensitization. The overall conclusion is that breast CSCs are not universally radiation resistant but can respond uniquely to therapy, and this should be a consideration in future work.
Acknowledgments
The authors thank G. Dontu, C. Ginestier, and J. Dutcher for helpful discussion and technical expertise.
Footnotes
S. Zielske was supported by a LUNGevity Foundation - American Cancer Society Postdoctoral Fellowship in Lung Cancer and the Elsa Pardee Foundation. This work was supported by the Flow Cytometry and Histology Core facilities of the UM Comprehensive Cancer Center.
References
- 1.Zhao L, Wang L, Ji W, Wang X, Zhu X, Hayman JA, Kalemkerian GP, Yang W, Brenner D, Lawrence TS, et al. Elevation of plasma TGF-β1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: a combined analysis from Beijing and Michigan. Int J Radiat Oncol Biol Phys. 2009;74:1385–1390. doi: 10.1016/j.ijrobp.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Sun Y, Chen S, Roy K, Price BD. The FATC domains of PIKK proteins are functionally equivalent and participate in the Tip60-dependent activation of DNA-PKcs and ATM. J Biol Chem. 2006;281:15741–15746. doi: 10.1074/jbc.M513172200. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton JP, Sato F, Greenwald BD, Suntharalingam M, Krasna MJ, Edelman MJ, Doyle A, Berki AT, Abraham JM, Mori Y, et al. Promoter methylation and response to chemotherapy and radiation in esophageal cancer. Clin Gastroenterol Hepatol. 2006;4:701–708. doi: 10.1016/j.cgh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 5.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 6.Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neoplasma. 2005;52:435–440. [PubMed] [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 12.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 13.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 14.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 15.Ishii H, Iwatsuki M, Ieta K, Ohta D, Haraguchi N, Mimori K, Mori M. Cancer stem cells and chemoradiation resistance. Cancer Sci. 2008;99:1871–1877. doi: 10.1111/j.1349-7006.2008.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 18.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 21.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 23.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zielske SP, Spalding AC, Lawrence TS. Loss of tumor-initiating cell activity in cyclophosphamide-treated breast xenografts. Transl Oncol. 2010;3:149–152. doi: 10.1593/tlo.09307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong L, Stojkovic M, Dimmick I, Ahmad S, Stojkovic P, Hole N, Lako M. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- 28.Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res. 2007;67:8671–8681. doi: 10.1158/0008-5472.CAN-07-1486. [DOI] [PubMed] [Google Scholar]
- 29.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 31.Pries R, Witrkopf N, Trenkle T, Nitsch SM, Wollenberg B. Potential stem cell marker CD44 is constitutively expressed in permanent cell lines of head and neck cancer. In Vivo. 2008;22:89–92. [PubMed] [Google Scholar]
- 32.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 34.Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U, Trampe-Kieslich A, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–5715. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]