Abstract
Many pharmacotherapies for treating cocaine dependence are aimed at reducing drug effects, alleviating craving, and/or preventing relapse. We demonstrated previously that citicoline, a compound used to repair neuronal damage in stroke and brain injury, is safe in cocaine-abusing volunteers.
Objectives
This study assessed the effectiveness of an eight-week citicoline treatment period and four-week follow-up in cocaine-dependent individuals.
Methods
Twenty-nine healthy non-treatment-seeking cocaine-dependent male and female volunteers were randomized in this double-blind, placebo-controlled study, eighteen of whom completed the treatment period of the study. Participants took citicoline (500 mg b.i.d) or matched placebo each day, and recorded measures of craving and drug use. Participants visited the laboratory twice a week for urine screens, as well as to attend weekly group therapy sessions.
Results
Citicoline had no effect on cocaine craving or total use.
Conclusions
While the current preliminary results from this small trial suggest that citicoline is not an effective treatment for heavy cocaine users, further investigation of citicoline’s efficacy as a treatment for substance dependence in other settings may be warranted.
Keywords: Citicoline, Cocaine, Alcohol, Marihuana, Pharmacotherapy
Current strategies for treating cocaine dependence are aimed at reducing or blocking the effects of the drug, mimicking the drug’s pharmacology (i.e., agonist therapy), or reducing craving. A novel strategy may be one that addresses the cerebral perfusion deficits associated with long-term cocaine use, and implements therapeutics used to target neuronal damage (1). Citicoline (cytidine-5′-diphosphocholine, CDP-choline) is an example of such a drug, as it already has been established as a promising therapy to treat stroke and brain injury (2).
The scope of citicoline’s therapeutic potential extends beyond its ability to be neuroprotective. Citicoline not only reduces neuronal free fatty acid accumulation and free radical production (3) while improving bioenergetics and increasing the rate of phospholipid turnover (4, 5), but it also enhances neurotransmission. Citicoline has been shown to increase monoaminergic levels within the central nervous system by increasing dopamine and norepinephrine production (6, 7), and its metabolites contribute to the synthesis of acetylcholine (8). Taken together, its ability to repair membranes and enhance neurotransmitter concentration suggests that citicoline might be a useful pharmacotherapy for cocaine dependence.
Studies to date that have endeavored to test this hypothesis have been relatively unsuccessful. While a preliminary pilot study from our group showed that crack cocaine users who underwent short-term (14 days) treatment with citicoline (N=6 on citicoline, N=8 on placebo) reported more control over their cocaine use and reductions in “urge for cocaine”, the statistical significance of those results is questionable due to multiple uncorrected comparisons (9). Similarly, a recent 12-week proof-of-concept trial claiming that citicoline reduced the likelihood of cocaine use in cocaine-dependent individuals with co-morbid bipolar I disorder suffered from low statistical power due to a high drop-out rate (e.g., only nine citicoline-treated and four placebo-treated individuals out of 44 completed the study; 10). Although results from those studies make it difficult to conclude definitively whether citicoline is an effective treatment for cocaine dependence, the present double-blind, placebo-controlled, 16-week pilot study was aimed at investigating the effects of treatment with citicoline in cocaine-dependent individuals.
METHODS
Participants
Participants were recruited via newspaper advertisements, and provided informed consent to participate in this study that was reviewed and approved by the McLean Hospital Institutional Review Board. Before being admitted to the study, all participants received a physical examination and a Structured Clinical Interview for the DSM-IV (SCID; 11). All participants met criteria for cocaine dependence; they were permitted to be dependent on marihuana and/or alcohol. Those individuals meeting current criteria for DSM-IV defined axis I diagnoses, including dependence on opiates or benzodiazepines, were excluded from participation. A total of 43 non-treatment-seeking cocaine-dependent individuals were accepted into the study. Of those, a total of 29 were randomized to either the placebo or drug treatment condition following the baseline period. Participant demographics are reported in Table 1. Volunteers were paid for their participation.
TABLE 1.
Demographics, drug use, and psychiatric diagnoses of the participants recorded after randomization at intake (means ± SD)
| Variables | Citicolinea | Placebo |
|---|---|---|
| Demographics | ||
| Number of subjects | 15 | 14 |
| Raceb | 1/0/1/11/2 | 0/1/0/13/0 |
| Age | 38.4 ± 4.5 | 39.0 ± 5.3 |
| Weight (lbs) | 188.2 ± 32.5* | 154.9 ± 20.3 |
| Sex (Male/Female) | 11/4 | 7/7 |
| Years of Education | 12.6 ± 1.3 | 12.0 ± 1.6 |
| Age began regular use of cocaine | 22.9 ± 5.4 | 24.7 ± 7.7 |
| Years of regular use of cocaine | 15.5 ± 6.1 | 14.3 ± 6.6 |
| Drug use | ||
| Smoked cocaine (instances per week) | 3.4 ± 1.9c | 4.1 ± 1.9 |
| Snort (intranasal) cocaine (instances per week) | 2.8 ± 0.7d | 1.8 ± 1.1e |
| Alcoholic beverages (drinks per week) | 12.0 ± 14.2 | 15.2 ± 21.3 |
| Marihuana cigarettes (per week) | 4.0 ± 2.8f | 3.9 ± 3.5f |
| Tobacco cigarettes (per day) | 8.5 ± 7.3g | 9.1 ± 4.9g |
| Psychiatric diagnoses | ||
| Cocaine dependence | 15 | 14 |
| Alcohol abuse or dependence | 7 | 9 |
| Marihuana abuse or dependence | 6 | 7 |
| Substance abuse induced-mood disorder | 1 | 2 |
1,000 mg/day, p.o
Hispanic/American Indian/Cape Verdian/African-American/Caucasian
13 out of 15 study participants
6 out of 15 study participants
2 out of 14 study participants
6 study participants in each group
11 study participants in each group
p<0.05 (t test for independent samples)
Experimental Design
The study design included a two-week baseline assessment during which participants were asked to fill out daily diaries (described below) assessing a variety of aspects about their daily life. During this period, participants were asked to come into the laboratory for two visits per week to collect urine specimens. Also, hematology, pregnancy tests, adverse events, body weight, blood pressure, and pulse were recorded once during the baseline period.
At the start of the treatment phase, participants were randomly assigned to receive either citicoline or placebo twice daily for eight weeks (i.e. weeks 3 through 10 of the study). Similar to the baseline period, participants came into the laboratory for two visits per week (approximately 60 min each) for the assessments listed above. Participants were asked a series of standardized questions about any side effects they may have been experiencing. Participants also were required to attend one hour of group therapy on a weekly basis (described below). Every other week hematological and blood chemistry assessments, as well as electrocardiograms (EKGs), were performed. The final phase of the experiment was the follow-up period designed to detect any residual effects after the eight weeks of treatment with citicoline (or placebo) had been terminated. These two visits occurred at weeks 12 and 16 during which both hematology and urine were monitored.
Daily Diaries
The daily diary was a questionnaire in which the participants: 1) recorded medication use (Did you take your study medication yesterday?); 2) reported on drug use (How many tobacco cigarettes/alcohol drinks have you had in the past 24 hrs? How many times have you used cocaine/marihuana/ecstasy/hallucinogens/opiates in the past 24 hrs? What was the dollar amount of the cocaine you used? Have you used any other drugs in the past 24 hrs?); 3) provided a rating on a scale that ranged from 0 ‘none at all’ to 10 ‘extremely high’ of their desire to use cocaine; and 4) rated their mood, ability to concentrate, appetite, amount of sleep, irritability, physical tension and aggravation, and physical symptoms (headache, chills/sweating, muscle spasms, nausea/upset stomach, tremors/shakes, diarrhea) on the same scale. In the section regarding physical symptoms, participants also had the option of writing in any additional effects they had experienced. Participants were asked to fill out a diary every day upon awakening in the morning to report the patterns of the previous day. Participants brought the diaries with them on days they visited the laboratory.
Group Drug Counseling
In addition to the pharmacotherapy, weekly Group Drug Counseling (GDC) was administered throughout the treatment period. This is a supportive group program designed to educate clients about important concepts in addiction recovery, and to promote an atmosphere in which members can express feelings, discuss problems, and learn from one another (12). Participants were instructed that they would be eliminated from the study if they missed more than one session. Sessions were led by a licensed drug counselor, who was supervised by a licensed psychologist.
Citicoline
Citicoline and identical placebo capsules were provided by Grupo-Ferrer, Barcelona, Spain. Each active capsule contained 500 mg citicoline. Subjects were instructed to take one capsule twice daily (at 9:00 a.m. and 9:00 p.m.). Thus, those individuals randomized to the citicoline group took a total dose of 1,000 mg/day, which had been shown to be safe and tolerated well in our previous studies (9, 13).
Urine Analysis
Urine samples were analyzed using a commercially-available urine drug screening kit (Triage® urine screen kits: Biosite Diagnostics, San Diego, CA) for evidence of cocaine use. Samples containing greater than 300 ng/ml of the cocaine metabolite benzoylecgonine were considered positive.
Data Analysis
Study retention during the treatment periods was compared between citicoline and placebo using the log-rank test. To compare continuous outcomes (including cocaine craving) between treatment groups, we used mixed effects piecewise linear spline models (14, 15) with covariates for time, treatment, and treatment by time interaction and a single knot (or change point) fixed at study week ten. These models assumed two different linear rates of change (or slopes) in the outcomes over time for each treatment group: one for the treatment period, beginning at study week two and ending at study week 10, and one for the post-treatment period, beginning at study week 10 and ending at study week 16. The knot at week 10 allowed a sudden change in slopes at the end of treatment. To compare the probability of daily substance use between treatment groups, we fit logistic regression spline models using the method of generalized estimating equations (14, 16) with the same covariates used for the continuous outcomes and a knot at study week 10. The odds ratio was used to model the association between outcomes from the same individual as a function of the time between measurements (17).
The primary goal of the analyses was to compare change in self-reported cocaine craving and probability of self-reported daily cocaine use between citicoline- and placebo-treated participants during the treatment period. We also performed secondary comparisons of change between treatment groups during the post-treatment period and change from the start of the treatment period to the end of the study. A model with the probability of a positive urine test as the outcome assessed the sensitivity of our results to our decision to use self-report of cocaine use rather than positive urine test as a primary outcome, and models with probability of non-zero cocaine craving and number of daily cocaine uses as the outcomes assessed the sensitivity of these results to our decision to model cocaine craving as a continuous outcome and to dichotomize daily cocaine use respectively. Due to the high percentage of missing urine measurements, we fit the model for probability of a positive urine test both excluding missing urine measurements and treating missing urine tests as positive to help assess the potential impact of missing measurements on the results. To assess for side-effects of citicoline, we compared self-reported ratings of concentration, appetite, sleep, irritability, physical symptoms, tension, mood, and anxiety between treatment groups using the methods for continuous variables. Exploratory analyses similar to those for probability of daily cocaine use compared alcohol, tobacco, and marihuana use between treatment groups. The analysis for marihuana use was restricted to the treatment period due to small sample size (i.e., the number of participants who reported having used marihuana).
With the exception of the models for alcohol, tobacco, and marihuana use, which used data only for those participants who reported any use of the corresponding substances during the baseline period, all analyses used all available data collected beginning from the treatment start day. Models were fit using PROC LIFETEST (study retention), PROC MIXED (continuous outcomes), and PROC GENMOD (all other outcomes) in SAS (version 9.1.3, SAS Institute, Cary, NC). The piecewise linear spline models were fit by including covariates for both study week and study week minus 10 weeks (coded zero for measurements taken during the treatment period), or an equivalent method. Statistical significance for each test required two-tailed p ≤ 0.05. Ninety -five percent confidence intervals are reported in brackets.
RESULTS
Safety Assessments
No serious adverse events were reported by participants in either treatment group. However, side effects such as headache, chills/sweating, muscle spasms, nausea/upset stomach, tremors/shakes, and/or diarrhea were reported by participants in both treatment groups over the course of the study. During the baseline period 50% of placebo-treated and 53% citicoline-treated participants reported physical symptoms, while during the treatment phase 50% of placebo-treated reported physical symptoms compared to 27% of citicoline-treated participants. Side effects were reduced for both groups during the follow-up period as only 21% of placebo-treated and 27% of citicoline-treated participants reported physical symptoms. Full hematology and blood chemistry, as well as urinalysis tests were performed multiple times during the study. All blood work and urinalyses were within clinical limits.
Participant retention declined as the study progressed (Fig. 1). The largest rate of attrition occurred prior to randomization during the baseline period (weeks 0–2; 14 participants did not complete the baseline period). Retention decreased 37.9% more over the eight-week medication phase of the study, but there was no significant difference in retention between the two treatment groups during the treatment period (log-rank χ2 = 0.10, p = 0.75).
FIGURE 1.
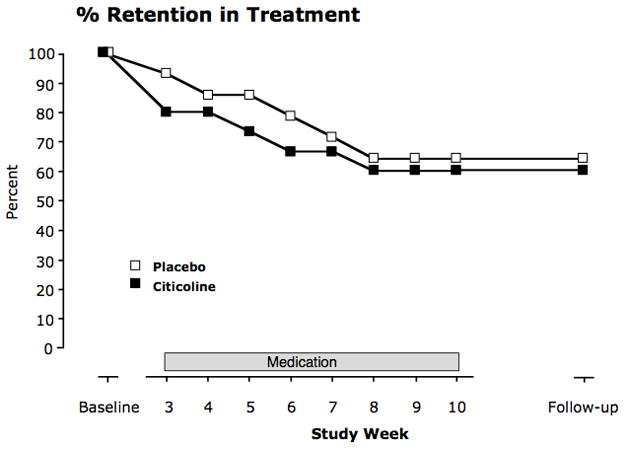
Retention rate throughout the entire 16-week study. Shown is the percent of the randomized participants (the 29 participants who completed the 2-week baseline period) who were remaining at the end of each week during the study (Placebo N=14; Citicoline N=15).
Average self-reported ratings of concentration (F1,24 = 8.91, p = 0.007) and appetite (F1,46 = 12.91, p < 0.001), and sleep (F1,34 = 22.01, p < 0.001) between the citicoline and placebo groups increased significantly during the treatment period, whereas average ratings of irritability (F1,42 = 6.50, p = 0.01) and physical symptoms (F1,27 = 18.80, p < 0.001) decreased significantly during the post-treatment period. The decrease in physical symptoms in the post-treatment period was significantly greater for the placebo group (F1,27 = 6.85, p = 0.009). There were no significant changes in mood, anxiety, or tension during either the treatment or post-treatment periods, and there were no significant differences in changes between citicoline and placebo during the treatment period for any ratings.
The percentage of diaries returned to study personnel was similar between treatment groups. The individuals randomized to the placebo group returned 93.7% (range 81.9–99%) of their diaries, while those individuals in the citicoline group returned 95.7% (range 85.7–100%) of their diaries. Overall, there was a 94.7% return rate for the daily diaries from participants who did not drop out of the study.
Cocaine Use and Craving
Cocaine use and cocaine craving decreased during the treatment period for both treatment groups (Figs. 2, 3, and 4). However, there was no association between treatment with citicoline and cocaine-related outcomes.
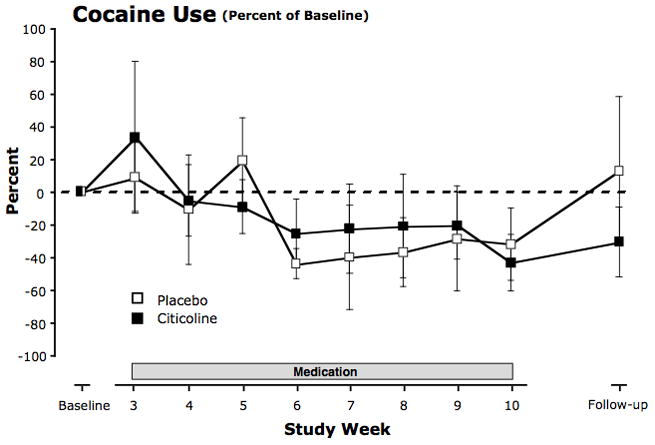
FIGURE 2.
Percent change in cocaine use during the eight treatment weeks and during the six-week follow-up period. Cocaine use (number of times used per day) was measured by self-report in the daily diaries. Participants recorded their cocaine use each morning for the previous 24 hours. For each participant, percent daily changes in numbers of times used from baseline were averaged over each week of the treatment period and over the six-week follow-up period. Means across participants were then calculated for each timepoint. Values are means ± SEM.
FIGURE 3.
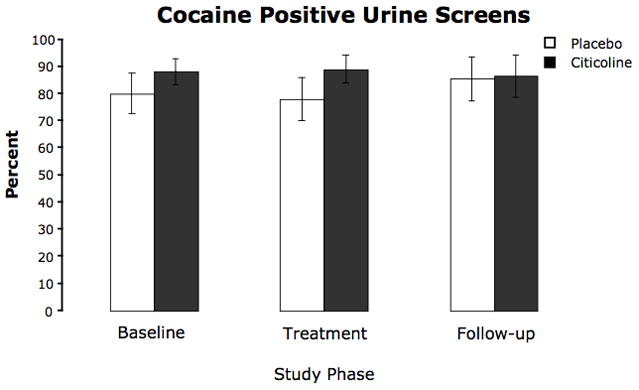
Percent of urine screens that were positive for cocaine, represented according to study phase. Urines were collected twice per week. Values are means ± SEM.
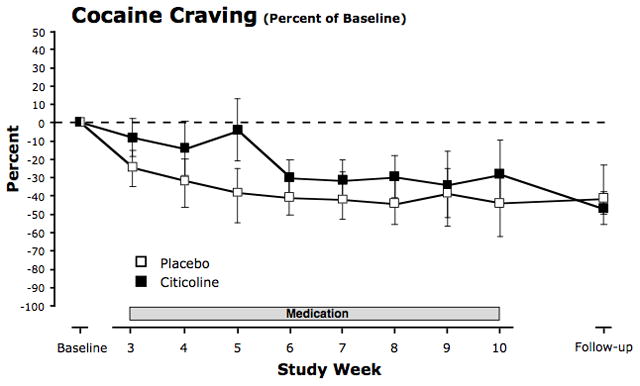
FIGURE 4.
Percent change in craving for cocaine during the eight treatment weeks and during the six-week follow-up period. Craving was reported in the daily diary on a 10-point Likert-type scale ranging from 0 (none at all) to 10 (extremely high). Each recording occurred in the morning and reported on craving during the previous 24 hours. For each participant, percent changes in daily craving ratings from baseline were averaged over each week of the treatment period and over the six-week follow-up period. Means across subjects were then calculated for each timepoint. Values are means ± SEM.
While the demographic data presented in Table 1 suggest that the two groups were well-matched on the basis of their self-reported cocaine use at the start of the study, several participants randomized to the citicoline treatment increased their use during the baseline period. Participants randomized to the citicoline group reported using cocaine an average of 1.78 times per day during the two-week baseline period, and participants randomized to the placebo group used cocaine an average of 0.59 times per day. However, using our models for cocaine craving and daily probability of cocaine use, we found no significant differences between daily probability of cocaine use (χ2 = 1.31, p = 0.25) or mean cocaine craving (F1,28 = 1.83, p = 0.19) between citicoline and placebo at the start of the treatment period.
There were no significant differences in change in daily probability of cocaine use between citicoline and placebo for the treatment (χ2 = 0.01, p = 0.91), post-treatment (χ2 = 0.15, p = 0.70), or the total 14-week post-baseline study (χ2 = 0.10, p = 0.75) period (Fig. 2, and Fig. 5, top left panel). Estimated odds ratios for daily cocaine use comparing daily odds of cocaine use at the end of the eight-week treatment period to daily odds of cocaine use at the start of the treatment period were 0.58 [0.35, 0.97] for citicoline and 0.56 [0.31, 0.99] for placebo, and estimated odds ratios comparing daily odds of use at the end of the post-treatment period to the beginning of the treatment period were 0.62 [0.38, 1.00] for citicoline and 0.71 [0.36, 1.41] for placebo. Similar results were obtained using the model for daily number of cocaine uses.
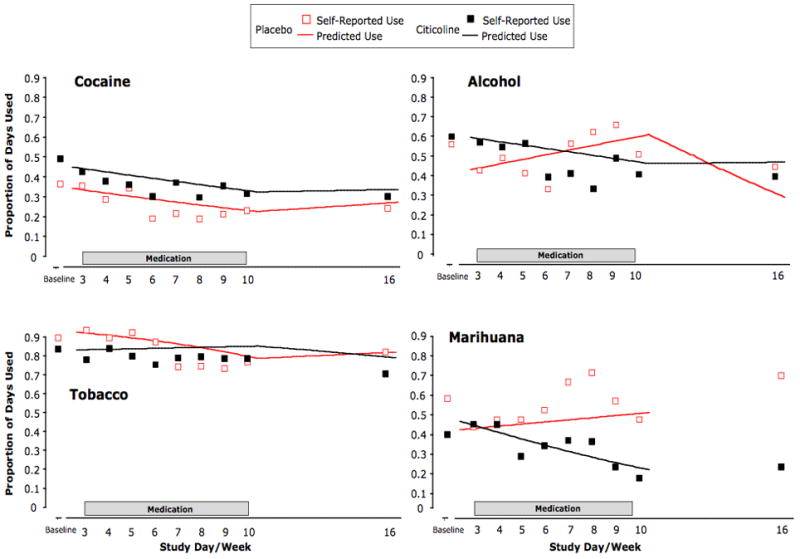
FIGURE 5.
Citicoline’s effect on cocaine, alcohol, tobacco, and marihuana use plotted as self-reported percent days of use for each week of the treatment and post-treatment period (total days of use divided by total days of follow-up for each study week). Lines represent estimated daily probabilities of cocaine, alcohol, tobacco, and marihuana use as predicted by logistic regression for the treatment period (all substances) and follow-up period (cocaine, alcohol, and tobacco only). Significant or nearly significant differences in changes in the probability of use during the treatment period were observed for alcohol (p=0.02) and marihuana (p=0.051) but not tobacco (p=0.18). Not all participants used all drugs during the study (see text for details).
Urine samples were analyzed for evidence of recent cocaine use. While there was a total of 18 possible post-baseline samples for each participant (to be collected twice-weekly over the eight treatment weeks, once at study week 12, and once at study week 16), not all samples were obtained even prior to study dropout. During study enrollment, 23.1% (45/195) were missing from the placebo group, while 20.1% (38/189) were missing from the citicoline group. There were no significant differences between treatment groups in the change in the probability of a positive urine test for the treatment (χ2 = 0.08, p = 0.78), post-treatment (χ2 = 2.25, p = 0.13), or total study period (χ2 = 2.07, p = 0.15), and there was no significant change in the probability of a positive test during the treatment period for citicoline (χ2 = 0.65, p = 0.42) or placebo (χ2 = 2.13, p = 0.14). Results did not change qualitatively when the missing samples were assumed to represent positive tests.
There also were no significant differences in mean craving changes between citicoline and placebo during the treatment (F1,26 = 0.40, p = 0.53) or post-treatment (F1,52 = 0.46, p = 0.50) periods, and there was no significant difference in model-predicted changes between the beginning of the treatment period and the end of the study period (F1,18 = 0.00, p = 1.00; Fig. 5). Model-estimated mean decreases in cocaine craving were 1.25 [0.08, 2.43] points for citicoline and 0.74 [-0.43, 1.91] points for placebo for the eight-week treatment period and 0.90 [-1.06, 2.86] points for citicoline and 0.90 [-1.05, 2.84] points for placebo for the full 14-week post-baseline period. Similar results were obtained from the model for probability of positive cocaine craving.
Alcohol, Tobacco, and Marihuana Use
While in this study, participants recorded their daily use of drugs other than cocaine, including alcohol, tobacco cigarettes, and marihuana. During baseline, among those study participants with study data from the start of the treatment period or later, 12 participants assigned to citicoline and 12 participants assigned to placebo reported alcohol use (of whom eight in each contributed alcohol use data past the end of the treatment period), 11 participants assigned to citicoline and 12 participants assigned to placebo reported tobacco cigarette use (of whom six and eight respectively contributed cigarette use data past the end of the treatment period), and six citicoline participants and four placebo participants reported marihuana use (of whom four and three respectively contributed marihuana use data past the end of the treatment period). One of these citicoline participants (alcohol and tobacco only) and one of these placebo participants (alcohol, tobacco, and marihuana) had only one non-missing measurement. Exploratory analyses compared changes in daily usage rates for these substances between placebo and citicoline among participants reporting baseline use.
There was a significant difference in changes in alcohol but not tobacco use between the citicoline and placebo groups during the treatment period (Fig. 5). Model-estimated daily probability of alcohol use declined during the treatment period for citicoline but increased for placebo. We also found a difference in change in marihuana use during the treatment period that approached statistical significance, though the validity of this result is questionable due to the small number of participants who contributed data to the analysis.
Estimated odds ratios comparing daily odds of alcohol use at the end of the eight-week study period to the beginning of the study period were 0.58 [0.35,0.96] for citicoline and 2.09 [0.93,4.66] for placebo. The change during the treatment period was significantly different between the two groups (χ2 = 5.12, p = 0.02). In contrast, neither the post-treatment changes (χ2 = 2.21, p = 0.14) nor the 14-week changes (χ2 = 0.02, p = 0.89) were significantly different between treatment groups.
There were no significant differences in changes in daily odds of tobacco cigarette use between citicoline and placebo for the treatment period (χ2 = 1.78, p = 0.18), post-treatment period (χ2 = 1.51, p = 0.22), or the 14-week post-baseline period (χ2 = 0.69, p = 0.41). The difference in change in daily odds of marihuana use during the treatment period approached statistical significance (χ2 = 3.82, p = 0.051). Estimated odds ratios comparing daily odds of marihuana use at the end of the eight-week study period to the beginning of the study period were 0.32 [0.10, 1.01] for citicoline and 1.42 [0.93, 2.17] for placebo.
DISCUSSION
This small trial investigated the effects of eight weeks of treatment with citicoline (500 mg b.i.d.) on cocaine dependence in non-treatment-seeking, cocaine-dependent volunteers. This study aimed to determine primarily if citicoline would reduce cocaine craving and use, while secondary outcome measures included reductions in the use of other drugs such as alcohol, tobacco, and marihuana. Although citicoline was safe and tolerated well, it was not better than placebo on measures for the treatment of cocaine dependence. However, exploratory results suggest that citicoline may be useful for reducing the concomitant use of alcohol, and possibly marihuana, in this population.
The incidence of any side effects resulting from citicoline treatment was low. Safety measures indicated no differences between treatment groups in terms of general health, blood chemistry, urinalysis, or vital signs, and there were no serious adverse events. Furthermore, both treatment groups had similar retention rates. Previously, citicoline has been demonstrated to be safe during short-term treatment (i.e., 14 days; 9), and to reduce scores on the Somatic Symptom Scale during a longer trial (i.e., 12 weeks; 10). We also demonstrated previously that citicoline had no effect on the acute subjective, cardiovascular, or pharmacokinetic effects of cocaine (13). All together, these results confirm that citicoline is safe, even when participants are exposed to cocaine.
Citicoline had no effect on cocaine use or self-reported craving for cocaine relative to placebo. Although the twice-weekly urine specimens tested positive for cocaine consistently, analysis of the daily self-reports indicated that both groups reduced their cocaine consumption over the course of treatment. This overall reduction in cocaine use in both treatment groups may have resulted from the influence of frequent contact with study personnel or the mandatory weekly GDC sessions. With respect to cocaine craving, the present results are in agreement with a previous report in which pretreatment with citicoline twice daily over four days did not alter the desire to use cocaine either before or after intra-nasal cocaine challenge (13). Although many pharmacologic treatments for substance abuse disorders have been aimed at reducing craving (18, 19), the significance of craving in terms of subsequent relapse still is unclear (20, 21).
Unlike the null effects of citicoline on cocaine use, alcohol use appeared to be reduced by citicoline over the course of treatment. All participants reported the use of alcohol at study intake, and 55% met DSM-IV criteria for either abuse or dependence. This is in accordance with many reports indicating that cocaine and alcohol are used together frequently (see references in 22). In contrast, only a small subset of participants reported using marihuana, but there was a notable suggestion of a citicoline-induced reduction in marihuana use (p = 0.051). These results should be interpreted with caution because the study was not designed to assess for reductions in alcohol and marihuana use among cocaine-dependent individuals. In addition, we note that we performed individual significance tests for our primary and secondary outcomes with test-wise alpha levels of 0.05. As a result, our global Type I error rate across primary and secondary outcomes exceeded five percent. However, the present results warrant further examination, given that both sub-populations tend to be more likely to relapse to cocaine following treatment (23, 24, 25).
Although alcohol and marihuana use may be reduced by citicoline, treatment did not affect the other a priori outcome measures (i.e., cocaine use and/or craving). Limiting factors in this study include the small sample size, a relatively short treatment period, and the possibility that the daily dose was too low. While 43 participants were admitted initially, those numbers diminished over the course of the 16-week study due to participants’ inability to commit the time to the trial or to remain compliant with the study protocol. Furthermore, because these were non-treatment-seeking individuals, they had little motivation to quit using cocaine. Since medication was dispensed over eight weeks of the 16-week study, the duration of this trial could be considered relatively short. However, citicoline has been shown to improve memory in early-onset Alzheimer’s disease patients after only one month of daily treatment (26), and older adults with slight memory impairments showed improvement after two months of treatment with citicoline (27). Similarly, a meta-analysis of a heterogeneous collection of clinical studies determined that citicoline had beneficial effects on memory and behavior following relatively short-term treatment (range of 20 days-3 months; 28). Altogether these data suggest that the onset of neuronal effects may occur rapidly. Moreover, in the interest of facilitating the rapid screening and marketing of potentially efficacious medications for treating drug dependence, trials of a similar duration are sponsored by the National Institute of Drug Abuse’s Clinical Research Efficacy Screening Trial program (29). Finally, the daily dose administered in this study may not have been sufficient to alter the more unyielding drug taking behavior that defines crack cocaine use (30, 31). Consistent with our previous studies, participants took a total of 1,000 mg/day (9, 13). However, recent evidence from a preliminary trial investigating the safety and efficacy of citicoline in the treatment of human intra-cerebral hemorrhaging has demonstrated that a total dose of 2,000 mg/day may be a more effective oral dose (32). In the future, larger studies should be aimed at investigating the effect of a higher dose of citicoline either alone or as an adjunct pharmacotherapy for cocaine-dependent individuals who abuse alcohol and/or marihuana.
Acknowledgments
This work was supported by the National Institute on Drug abuse grants DA011098 (SEL), T32 DA015036 (SEL), K24DA15116 (PFR), and K05DA00343 (SEL).
The authors thank Grupo Ferrer (Barcelona, Spain) for donating the citicoline and matched placebo caplets. Also, the authors thank Dr. Garrett Fitzmaurice for helpful discussion regarding the statistical analyses.
Footnotes
Clinical Trial Identification Number: NCT00158249
References
- 1.Kosten TR. Pharmacotherapy of cerebral ischemia in cocaine dependence. Drug Alcohol Depend. 1998;49:133–144. doi: 10.1016/s0376-8716(97)00158-0. [DOI] [PubMed] [Google Scholar]
- 2.Conant R, Schauss AG. Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature. Altern Med Rev. 2004;9:17–31. [PubMed] [Google Scholar]
- 3.Rao AM, Hatcher JF, Dempsey RJ. CDP-choline: neuroprotection in transient forebrain ischemia of gerbils. J Neurosci Res. 1999;58:697–705. [PubMed] [Google Scholar]
- 4.Babb SM, Wald LL, Cohen BM, et al. Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study. Psychopharmacology. 2002;161:248–254. doi: 10.1007/s00213-002-1045-y. [DOI] [PubMed] [Google Scholar]
- 5.Silveri MM, Dikan J, Ross AJ, et al. Citicoline enhances frontal lobe bioenergetics as measured by phosphorous magnetic resonance spectroscopy. NMR Biomed. 2008 doi: 10.1002/nbm.1281. [DOI] [PubMed] [Google Scholar]
- 6.Martinet M, Fonlupt P, Pacheco H. Effects of cytidine-5’-diphosphocholine on norepinephrine, dopamine, and serotonin synthesis in various regions of the rat brain. Arch Int Pharmacodyn. 1979;239:52–56. [PubMed] [Google Scholar]
- 7.Petkov VD, Stancheva SL, Tocuschieva L, et al. Changes in brain biogenic monoamines induced by the nootropic drugs adafenoxate and meclofenoxate by citicholine (experiments on rats) Gen Pharmacol. 1990;21:71–75. doi: 10.1016/0306-3623(90)90598-g. [DOI] [PubMed] [Google Scholar]
- 8.Ulus IH, Wurtman RJ, Mauron C, et al. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–227. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]
- 9.Renshaw PF, Daniels S, Lundahl LH, et al. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report. Psychopharmacology. 1999;142:132–138. doi: 10.1007/s002130050871. [DOI] [PubMed] [Google Scholar]
- 10.Brown ES, Gorman AR, Hynan LS. A randomized, placebo-controlled trial of citicoline add-on therapy in outpatients with bipolar disorder and cocaine dependence. J Clin Psychopharmacol. 2007;27:498–502. doi: 10.1097/JCP.0b013e31814db4c4. [DOI] [PubMed] [Google Scholar]
- 11.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-I/P, vers 2.0) New York: Biometrics Research Department; 1995. [Google Scholar]
- 12.Mercer D, Carpenter G, Daley D, et al. Addiction Recovery Manual. Vol. 2. Philadelphia: University of PA, Treatment Research Unit; 1994. [Google Scholar]
- 13.Lukas SE, Kouri EM, Rhee C, et al. Effects of short-term citicoline treatment on acute cocaine intoxication and cardiovascular effects. Psychopharmacology. 2001;157:163–167. doi: 10.1007/s002130100824. [DOI] [PubMed] [Google Scholar]
- 14.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 15.Laird NM, Ware JH. Random-effects model for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 16.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 17.Carey V, Zeger SL, Diggle P. Modelling multivariate binary data with alternating logistic regressions. Biometrika. 1993;80:517–526. [Google Scholar]
- 18.Kosten TR. Can cocaine craving be a medication development outcome? Am J Addict. 1992;1:230–239. [Google Scholar]
- 19.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien CP, Childress AR, Ehrman R, et al. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 21.Pickens RW, Johanson C-E. Cravings: consensus of status and agenda for future research. Drug Alcohol Depend. 1992;30:127–131. doi: 10.1016/0376-8716(92)90017-7. [DOI] [PubMed] [Google Scholar]
- 22.Gossop M, Manning V, Ridge G. Concurrent use of alcohol and cocaine: differences in patterns of use and problems among users of crack cocaine and cocaine powder. Alcohol Alcohol. 2006;41:121–125. doi: 10.1093/alcalc/agh260. [DOI] [PubMed] [Google Scholar]
- 23.Aharonovich E, Liu X, Samet S, et al. Postdischarge cannabis use and its relationship to cocaine, alcohol, and heroin use: a prospective study. Am J Psychiatry. 2005;162:1507–1514. doi: 10.1176/appi.ajp.162.8.1507. [DOI] [PubMed] [Google Scholar]
- 24.McKay JR, Alterman AI, Rutherford MJ. The relationship of alcohol use to cocaine relapse in cocaine dependent patients in an aftercare study. J Stud Alcohol. 1999;60:176–180. doi: 10.15288/jsa.1999.60.176. [DOI] [PubMed] [Google Scholar]
- 25.Mengis MM, Maude-Griffin PM, Delucchi K, et al. Alcohol use affects the outcome of treatment for cocaine abuse. Am J Addict. 2002;11:219–227. doi: 10.1080/10550490290087992. [DOI] [PubMed] [Google Scholar]
- 26.Franco-Maside A, Caamaõo J, Gómez MJ, Cacabelos R. Brain mapping activity and mental performance after chronic treatment with CDP-choline in Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994;16:597–607. [PubMed] [Google Scholar]
- 27.Spiers PA, Myers D, Hochanadel GS, Lieberman HR, Wurtman RJ. Citicoline improves verbal memory in aging. Arch Neurol. 1996;53:441–448. doi: 10.1001/archneur.1996.00550050071026. [DOI] [PubMed] [Google Scholar]
- 28.Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev. 2005;2:CD000269. doi: 10.1002/14651858.CD000269.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelick DA, Gardner EL, Xi Z-X. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- 30.Gossop M, Griffiths P, Powis B, et al. Cocaine: patterns of use, route of administration, and severity of dependence. Br J Psychiatry. 1994;164:660–664. doi: 10.1192/bjp.164.5.660. [DOI] [PubMed] [Google Scholar]
- 31.Ferri CP, Gossop M. Route of cocaine administration: patterns of use and problems among a Brazilian sample. Addict Behav. 1999;24:815–821. doi: 10.1016/s0306-4603(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 32.Secades JJ, Alvarez-Sabin J, Rubio F, et al. Citicoline in intracerebral haemorrhage: a double blind, randomized, placebo-controlled, multi-centre pilot study. Cererbrovasc Dis. 2006;21:380–385. doi: 10.1159/000091547. [DOI] [PubMed] [Google Scholar]


