Abstract
Diethylstilbestrol (DES) and bisphenol-A (BPA) are estrogen-like endocrine-disrupting chemicals that induce persistent epigenetic changes in the developing uterus. However, DES exposure in utero is also associated with an increased risk of breast cancer in adult women. Similarly, fetal exposure to BPA induces neoplastic changes in mammary tissue of mice. We hypothesized that epigenetic alterations would precede the increased risk of breast neoplasia after in utero exposure to endocrine disruptors. Enhancer of Zeste Homolog 2 (EZH2) is a histone methyltransferase that has been linked to breast cancer risk and epigenetic regulation of tumorigenesis. We examined the effect of BPA and DES on EZH2 expression and function in MCF-7 cells and in mammary glands of mice exposed in utero. DES and BPA treatment approximated human exposure. EZH2 functional activity was assessed by measuring histone H3 trimethylation. Treatment of MCF-7 cells with DES or BPA led to a 3- and 2-fold increase in EZH2 mRNA expression, respectively (p < 0.05) as well as increased EZH2 protein expression. Mice exposed to DES in utero showed a >2-fold increase in EZH2 expression in adult mammary tissue compared with controls (p < 0.05). EZH2 protein was elevated in mammary tissue of mice exposed to DES or BPA. Histone H3 trimethylation was increased in MCF-7 cells treated with BPA or DES. Similarly, mice exposed to BPA or DES in utero showed increased mammary histone H3 trimethylation. Developmental programming of EZH2 is a novel mechanism by which in utero exposure to endocrine disruptors leads to epigenetic regulation of the mammary gland.
Keywords: Endocrine disruptor, Epigenetics, Histone methylation, Breast cancer
Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous substances that alter endocrine function due to their hormone-like activity. Humans are widely exposed to these compounds, which have been implicated in disruption of normal development [1]. Exposure to estrogen-like EDCs (xenoestrogens) during critical stages of development can interfere with normal hormonal signaling and result in persistently altered gene expression. Diethylstilbestrol (DES) is a non-steroidal synthetic estrogen that is known to have teratogenic and carcinogenic effects. An estimated 10 million Americans have been exposed to DES. While DES-related genital tract abnormalities are well described, exposure in utero has also been associated with abnormalities outside of the reproductive tract. Women exposed to DES while pregnant show a modest increase in the incidence of breast cancer with relative risks (RR) ranging from 1.33 to 1.4 [2–5]. Daughters born after in utero exposure to DES have a RR of 2.5 for developing breast cancer after the age of 40 when compared with unexposed women of the same age [6]. Animal studies have also demonstrated associations between in utero DES exposure and breast cancer [7–9].
Bisphenol-A, a known EDC, is a monomer commonly used in the production of polycarbonate plastics and epoxy resins. It is present in many plastics leading to almost ubiquitous human exposure [10, 11]. Fetuses and young infants are commonly exposed to bisphenol-A (BPA) by transplacental transfer of maternal BPA and through ingestion of maternal milk or formula in BPA containing plastic bottles [12, 13]. Studies reporting BPA exposure in humans have detected plasma BPA levels of 0–2 ng/mL in non-pregnant subjects, 0.3–18.9 ng/mL in pregnant subjects, 0.2–9.2 ng/mL in fetal serum (sampled from umbilical vein), and 0.55–8.3 ng/mL in amniotic fluid [13–16]. BPA is structurally similar to DES; both are non-steroidal and have estrogenic effects (Fig. 1). BPA has been associated with adverse reproductive outcomes in both humans and animal models. Similar to DES, BPA alters expression of key developmental regulators such as HOX genes in the reproductive tract [17–19]. Perinatal exposure to BPA alters mammary gland development in mice [20]. Mice exposed to BPA in utero demonstrate altered morphology of mammary tissue, increased estradiol sensitivity, increased cell proliferation, decreased apoptosis, and altered timing of development [21]. Rats exposed in utero to BPA have an increased incidence of carcinoma in situ in the mammary glands as adults [22]. Pre-neoplastic lesions, such as intraductal hyperplasias, are also increased in rats after in utero BPA exposure. BPA exposure in utero also promotes breast neoplasms in rats after exposure to a known carcinogen at a dose that does not cause cancer in control (non-BPA treated) mice [23].
Fig. 1.
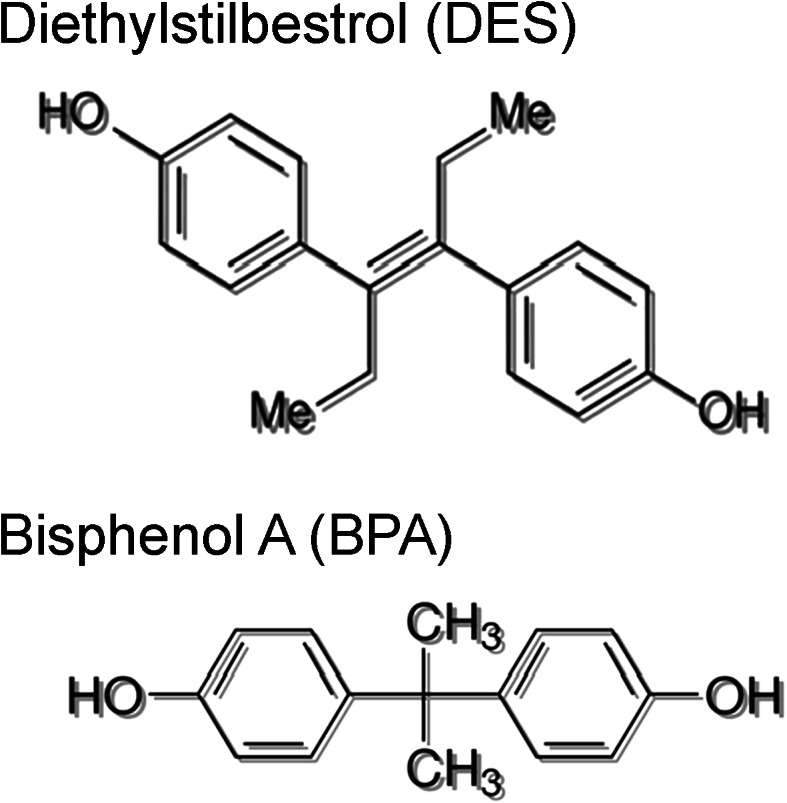
Structure of diethylstilbestrol (DES) and bisphenol-A (BPA) structure of the endocrine-disrupting compounds diethylstilbestrol and bisphenol-A.
Epigenetic gene regulation produces heritable changes in expression of genes that are mediated through changes in DNA methylation as well as modification of histone and chromatin structure, without changes to the genomic DNA sequence. These changes modify chromatin structure and change the accessibility of DNA to transcription factors, thereby causing alterations in gene expression. CpG DNA hypermethylation within promoter regions of tumor suppressing genes can lead to tumor formation [24]. Methylation of histone lysine residues by histone methyltransferases is a common modification resulting in gene silencing associated with cancer [25, 26].
The polycomb repressive complex 2 (PRC2) enzyme complex is a histone methyltransferase known to be involved in gene silencing and tumorigenesis. It acts by methylating lysine-27 of histone H3 (H3-K27). PRC2 can add up to three methyl groups to the lysine side chain, and the trimethylated form of H3-K27 is known as histone H3 (tri methyl K27) [27]. The catalytic subunit of the PRC2 enzyme complex, enhancer of Zeste homolog 2 (EZH2), provides the methyltransferase activity [28, 29]. Over-expression of EZH2 has been described in a number of human cancers, including breast, prostate, lymphoma, myeloma, bladder, colon, skin, liver, uterine, lung, and stomach [30–33]. In breast cancer, elevated EZH2 levels are associated with aggressive forms of disease [32, 34, 35]. Engineered over-expression of EZH2 leads to increased invasiveness of cells in vivo and tumorigenesis in mice [32, 36]. EZH2 may also act to link two epigenetic systems of gene silencing, specifically histone methylation (by Polycomb group enzyme activity) and DNA methylation (by DNA methyltransferases) [37–39]. Histone methylation by EZH2 leads to chromatin alterations that “mark” the DNA for methylation by DNA methyltransferases. EZH2 over-expression in mammary tissue also impairs DNA repair mechanisms by decreasing expression of RAD51 paralogs known to function in homologous recombination, a process by which double-strand DNA breaks are repaired [40].
EZH2 may be a marker for increased risk of breast cancer development. Women without breast cancer with breast biopsies demonstrating increased EZH2 expression were more likely to subsequently develop breast cancer than women with biopsies with low expression of EZH2 [41]. However, EZH2 expression is not increased in all pre-invasive lesions of the breast.
We have recently shown that in utero exposure to DES or BPA causes hypermethylation of HOXA10 in the endometrium [42, 43]. We hypothesized that epigenetic changes may similarly occur in the mammary gland after exposure to DES and other estrogen-like endocrine-disrupting chemicals, such as BPA. We examined the expression of known epigenetic regulators in mammary tissue after exposure to DES or BPA in utero. As elevated EZH2 is associated with human breast cancers and is known to decrease expression of DNA repair mediators, we specifically examined EZH2 expression after DES or BPA exposure. We also assessed the histone methyltransferase activity of EZH2 after DES and BPA exposure by examining expression of trimethylated histone H3 at lysine 27(histone H3 tri methyl K27).
Materials and Methods
Cell Culture and In Vitro DES and BPA Exposure
MCF-7 cells, a well-differentiated breast cancer cell line known to express estrogen receptor α, estrogen receptor β, and progesterone receptor, were obtained from the American Type Collection (Rockville, MD, USA) [44, 45]. MCF-7 cells were cultured in phenol-free Eagle’s MEM (Life Technologies, Inc, Gathersburg, MD, USA) containing 10% (v/v) charcoal-stripped fetal bovine serum and supplemented with penicillin/streptomycin (100 µg/mL), l-glutamine (2 mM), and sodium pyruvate (1 mM). Cells were grown to confluence in plastic flasks (75 cm2, Falcon, Franklin Lakes, NJ, USA) and maintained at 37°C in a humidified atmosphere (5% CO2 in air). The 70–80% confluent monolayers were harvested by trypsinization, seeded in a six well plate, and maintained in serum-free medium for 24 h. The cells were subsequently treated with five concentrations of DES (5 × 10−6, 5 × 10−7, 5 × 10−8, 5 × 10−9, or 5 × 10−10 M; Sigma, St. Louis, MO, USA), five concentrations of BPA (2.5 × 10−4, 2.5 × 10−5, 2.5 × 10−6, 2.5 × 10−7, or 2.5 × 10−8 M; Sigma), or dimethyl sulfoxide (control) for 48 h. RNA was isolated using the RNEasy Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. RNA samples were stored at −80°C until use. Each experiment was repeated three times and performed in triplicate.
Animal Care
CD1 mice were obtained from Charles River Laboratories (Wilmington, MA, USA). The mice were housed in standard polypropylene cages in a temperature controlled room (22°C) with a 14-h light, 10-h dark cycle. Food (Purina Chow, Purina Mills, Richmond, IN, USA) and water were provided ad libitum. Eight-week-old CD-1 female mice were bred to male mice of the same strain and examined every 12 h until the presence of a vaginal plug was detected. Detection of a vaginal plug was considered day 0 of pregnancy. Pregnant mice were the housed individually and treated with intraperitoneal injection of DES (N = 15) (Sigma) in sesame oil at a dose of 10 µg/kg, BPA (N = 15) (Sigma) in sesame oil at a dose of 5 mg/kg, or sesame oil alone (control, N = 15) on days 9–26 of gestation. These doses have been previously described and lead to alterations in the uterus of exposed mice [17, 18, 46]. Each experiment consisted of an N of five animals and was conducted in triplicate resulting in an N of 15 for each exposure. All experiments were conducted in accordance with Yale University Animal Care Committee Guidelines.
Female offspring with in utero exposure were euthanized by cervical dislocation under CO2 inhaled anesthesia at 6 weeks after birth. Inguinal mammary glands were removed. Mammary tissue was divided, with half being used for mRNA analysis and the other half being used for protein analysis. The mammary tissue intended for mRNA analysis was then placed in 1 mL TRIzol per 100 mg tissue (Invitrogen, Carlsbad, CA, USA). Tissue was homogenized on ice, and total RNA was isolated using methodology described by the manufacturer.
Measurement of Plasma BPA Levels
Pregnant mice were treated with daily intraperitoneal injections of BPA (Sigma) in sesame oil at a dose of 5 mg/kg or sesame oil alone (control) daily beginning at day 9 of gestation. On day 13, blood samples were obtained by cardiac puncture using 25 gauge needles. Mice had blood drawn at 1, 6, 12, and 18 h after their last dose of BPA or control (sesame oil). N = 8 (four controls and four BPA-treated) at each time point. Blood samples (50 µL) were diluted with an equal volume of methanol and subjected to centrifugation. A volume of 50 µL of solution was transferred to a 1.5-mL tube, and 50 µL acetonitrile was added followed by a second centrifugation. Separation was then performed on an Agilent 1200 Series rapid resolution liquid chromatography machine (Agilent Technologies, Santa Clara, CA, USA) with a Waters YMC (C18) Column (3 µM, 4.6 × 150 mm) (Waters, Milford, MA) and a 26-min gradient of water to methanol. Determinations of plasma BPA levels were performed on an Applied Biosystems 4000 Q Trap Mass-spectrometer (Applied Biosystems, Foster City, CA, USA) in negative mode. Stock solutions of BPA (Sigma) were prepared for use as internal standards. Each blood sample was measured in triplicate.
Quantitative RT-PCR
Total RNA (500 ng) was reverse transcribed in 20 μl of reaction mixture using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The reaction mix was incubated for 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C using the Eppendorf Mastercycler (Eppendorf North America). Quantitative real time RT-PCR reactions were prepared using the iQ SYBR Green Supermix (Bio-Rad). Each PCR reaction consisted of the following: 1 μl of cDNA template, 1 μl of forward primer (1 µM), 1 μl of reverse primer (1 μM), 9.5 μl of nuclease-free H2O, and 12.5 μl of iQ SYBR Green Supermix. EZH2 intron-spanning primers were designed using PerlPrimer version 1.1.14. Primers designed for human EZH2 yielded a 156-bp amplicon. Primers designed for murine EZH2 yielded a 168-bp amplicon. β-Actin was used as a housekeeping gene. β-Actin primers have been described previously [18].
EZH2 Primers
Human: Forward—5′-TAATGTGCTGGAATCAAAGG-3′
Reverse—5′-TGGCTTCATCTTTATTGGTG-3′
Mouse: Forward—5′-TCCGAATAACAGTAGCAGAC-3′
Reverse—5′-ACACCGAGAATTTGCTTCAG-3′
The Bio-Rad iCycler iQ system (Bio-Rad) was used to quantify fluorescence of PCR products during amplification. RT-PCR reactions were performed for 45 cycles at 95°C for 2 s and 60°C for 5 s, and 72°C for 18 s. Melting curve data were collected for analysis. Relative gene expression was determined by analyzing data using the 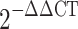 method to adjust for expression of β-actin [47]. Specificity of the amplified products and the absence of primer-dimmers were confirmed via melt curve analysis. All products obtained yielded the predicted melting temperature. All experiments were conducted in triplicate. Samples without cDNA template were used as negative controls.
method to adjust for expression of β-actin [47]. Specificity of the amplified products and the absence of primer-dimmers were confirmed via melt curve analysis. All products obtained yielded the predicted melting temperature. All experiments were conducted in triplicate. Samples without cDNA template were used as negative controls.
Western Blot
MCF-7 cells were treated with DES (5 × 10−8 M), BPA (2.5 × 10−6 M), or dimethyl sulfoxide (control) for 48 h. Pregnant CD1 mice were treated with intraperitoneal injection of DES (Sigma) in sesame oil at a dose of 10 µg/kg, BPA (Sigma) in sesame oil at a dose of 5 mg/kg, or sesame oil alone (control) on days 9–26 of gestation. Protein was extracted from MCF-7 cells and homogenized murine mammary gland tissue using the Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s protocol for whole-cell protein extraction. The protein was quantified by the Bradford method using a protein assay kit (Bio-Rad). Aliquots (60 µg) were loaded on to a 6% SDS polyacrylamide gel, size fractioned, and transferred to a nitrocellulose membrane using a transblot apparatus (Bio-Rad). The membrane was incubated in blocking buffer (1× TBS, 0.1% Tween-20 with 5% w/v nonfat dry milk) at room temperature for 1 h, washed for 5 min × 3 in TBS/T (1× TBS, 0.1% Tween-20) and then incubated overnight with primary antibody at 4°C. The primary antibody for EZH2 was a 1:1,000 dilution of mouse polyclonal EZH2 antibody (Cell Signaling Technology, Danvers, MA, USA). The primary antibody for histone H3 was a 1:250 dilution of rabbit monoclonal histone H3 antibody (Abcam, Cambridge, UK). The primary antibody for histone H3 trimethylated at lysine 27 (histone H3 tri methyl K27) was a 1:5,000 dilution of mouse monoclonal histone H3 (tri methyl K27) antibody (Abcam). The primary antibody for β-actin is a 1:500 dilution of mouse monoclonal β-actin antibody (Abcam). After incubation with primary antibody, the membrane was washed with TBS for 5 min at room temperature and incubated for 1 h with a 1:2,500 dilution of secondary antibody. Donkey anti-mouse IgG-horseradish peroxidase (Santa-Cruz, San Francisco, CA, USA) was the secondary antibody used for EZH2, histone H3 (tri methyl K27), and β-actin Western blotting. Donkey anti-rabbit IgG-horseradish peroxidase (Santa-Cruz, San Francisco, CA, USA) was used for histone H3 Western blotting. Following treatment with secondary antibody, the membrane was then washed twice in TBS for 5 min at room temperature and immersed in a horseradish peroxidase color developer buffer (Bio-Rad) for 30 min. Photographs were taken immediately following color development. β-Actin was used as a loading control. All experiments were conducted in triplicate. Densitometry was performed on scanned images using ImageJ (NIH). Mean gray values and areas were measured for each band. Background measurements were also obtained. Mean gray values were subtracted from background and multiplied by band area. Band values were normalized to β-actin to obtain relative densitometric intensity.
Statistical Analysis
Differences in EZH2 mRNA expression between treatment groups and the control were compared using Student’s t test. Relative densitometric intensities of Western blot data were also compared using Student’s t tests. p < 0.05 was considered statistically significant.
Results
DES and BPA Exposure In Vitro Alter EZH2 Expression in Mammary Cells
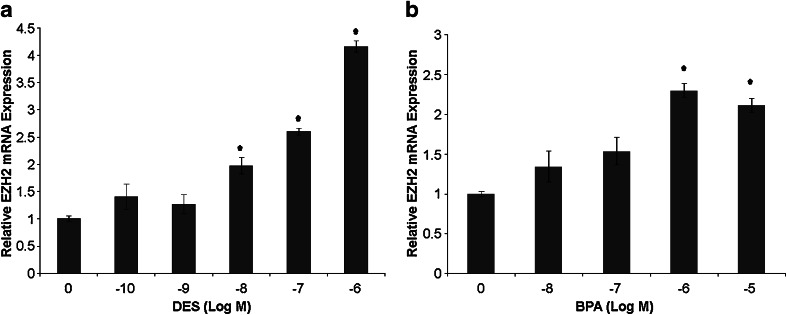
To define the effect of DES and BPA on adult human breast cells, the effects of these compounds on EZH2 expression were evaluated in MCF-7 cells. Cells were treated with DES in five concentrations ranging from 5 × 10−10 to 5 × 10−6, BPA in five concentrations from 2.5 × 10−8 to 2.5 × 10−4 M, or dimethyl sulfoxide (control) for 48 h. EZH2 mRNA levels (normalized to β-actin levels) were measured by quantitative RT-PCR (Fig. 2).
Fig. 2.
DES and BPA exposure in vitro induce EZH2 mRNA expression in human mammary cells. a Quantitative RT-PCR performed on MCF-7 cells treated with DES at five concentrations. Treatment with 5 × 10−8 and 5 × 10−7 M DES resulted in a greater than 2-fold increase in EZH2 mRNA compared with controls. Treatment with 5 × 10−6 M DES caused a 4-fold increase in EZH2 mRNA expression. Results are representative of three independent experiments performed in triplicate. *p < 0.05. b Quantitative RT-PCR performed on MCF-7 cells treated with four concentrations of BPA. Treatment with 2.5 × 10−6 and 2.5 × 10−5 M BPA resulted in greater than 2-fold increases in EZH2 mRNA when compared with controls. Results are representative of three independent experiments performed in triplicate. *p < 0.05.
Treatment with DES at 5 × 10−10 and 5 × 10−9 M did not significantly alter EZH2 expression. Expression of EZH2 was increased 2-fold in cells treated with DES at 5 × 10−8 M (p < 0.05), increased 2.5-fold in cells treated with DES at 5 × 10−7 M (p < 0.05), and increased 4-fold in cells treated with DES at 5 × 10−6 M (p < 0.05), when compared with controls.
Treatment with BPA at 2.5 × 10−8 and 2.5 × 10−7 M did not significantly alter EZH2 expression. EZH2 expression increased over 2-fold in cells treated with BPA at 2.5 × 10−6 (p < 0.05) and 2.5 × 10−5 M (p < 0.05) when compared with controls. Treatment of cells with BPA at a concentration of 5 × 10−4 M was toxic to cells.
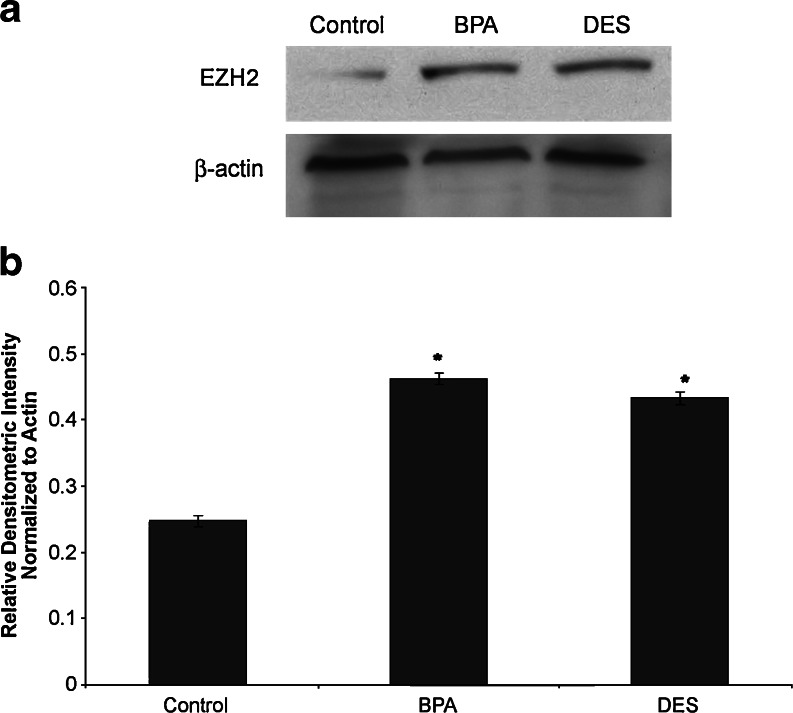
We also investigated whether treatment of MCF-7 cells with DES and BPA would alter EZH2 protein. Western blot analysis (Fig. 3) was performed to measure EZH2 protein expression. Densitometry was then performed to quantify changes in protein expression. Cells treated with vehicle control demonstrated low, but detectable, EZH2 protein expression. DES treatment resulted in increased EZH2 protein expression when compared with control cells (p < 0.05). Treatment with BPA also increased EZH2 protein expression when compared with controls (p < 0.05). There were no changes in β-actin associated with exposure.
Fig. 3.
DES and BPA exposure in vitro increase EZH2 protein expression in human mammary cells. a Western blotting demonstrates that BPA and DES treatment of MCF-7 cells for 48 h results in increases in EZH2. EZH2 was present in low, but detectable, amounts in vehicle-treated control cells. DES-treated cells showed an increase in EZH2. BPA-treated cells also showed an increase in EZH2. β-Actin was used to as a loading control. b Densitometric analysis of Western blots confirm increased EZH2 expression in BPA and DES-treated MCF-7 cells. Values were normalized to β-actin to obtain relative densitometric intensity. *p < 0.05.
DES and BPA Exposure In Vitro Alters Net EZH2 Functional Activity in Mammary Cells
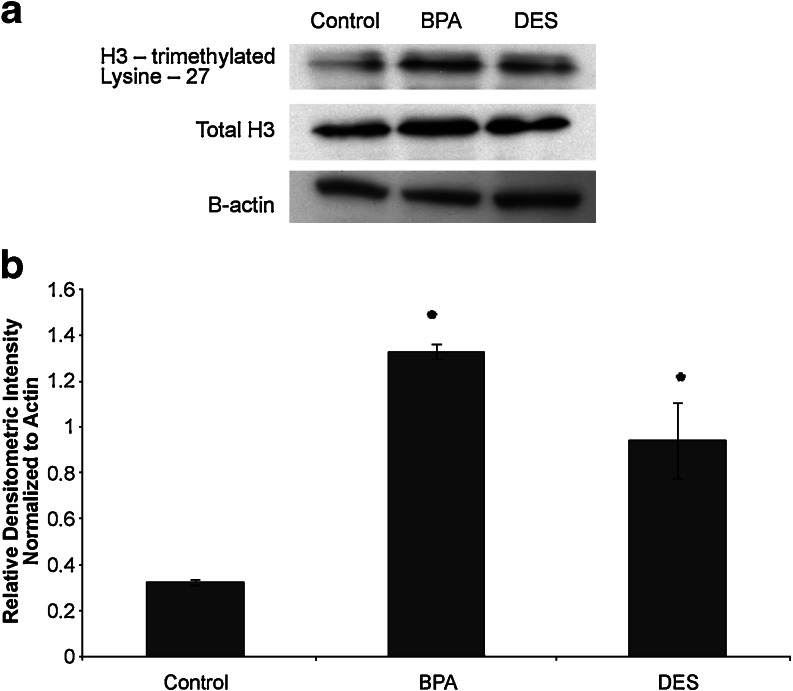
In order to assess functional consequences resulting from changes in EZH2 expression in MCF-7 cells in response to in vitro treatment with DES or BPA, we examined the expression of histone H3 (tri methyl K27) in these cells. Western blots were also performed for total histone H3. Western blot data from MCF-7 cells treated with DES, BPA, and control is shown in Fig. 4. Cells treated with vehicle control showed low but detectable histone H3 (tri methyl K27) expression. Cells exposed to DES for 48 h had increased expression of histone H3 (tri methyl K27) when compared with control cells (p < 0.05). Treatment with BPA also increased expression of histone H3 (tri methyl K27) when compared with control cells (p < 0.05). Total histone H3 expression and β-actin expression did not change after treatment with either DES or BPA.
Fig. 4.
DES and BPA exposure in vitro increase net EZH2 functional activity in mammary cells. a Western blot demonstrates that in vitro BPA and DES exposure results in increases in EZH2 protein function, assessed by blotting with antibody specific to histone H3 (tri methyl), the target of EZH2 methyltransferase activity. Histone H3 (tri methyl K27) was low, but detectable, in vehicle-treated control cells. Treatment with DES increased histone H3 (tri methyl K27). BPA-treated cells also showed an increase in histone H3 (tri methyl K27). Total histone H3 protein expression was not changed by treatment with DES or BPA. b Densitometric analysis of Western blots confirm increased histone H3 (tri methyl K27) in BPA and DES-treated MCF-7 cells. Values were normalized to β-actin to obtain relative densitometric intensity. *p < 0.05.
Plasma BPA Levels After Treatment of Pregnant Mice
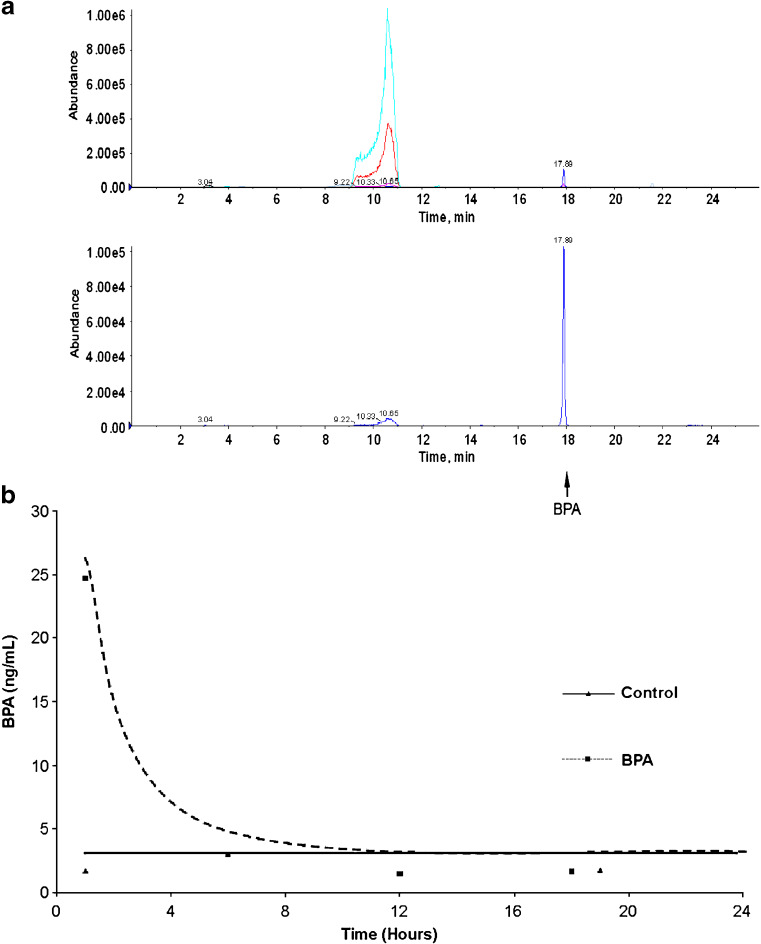
While DES administration has been well established, BPA dose and resultant serum levels are not well characterized. BPA levels were determined in pregnant mice treated with intraperitoneal injections of BPA at a dose of 5 mg/kg on days 9–13 of gestation. Plasma BPA levels were measured 1–18 h after receiving an intraperitoneal dose of BPA, or control vehicle, on day 13 of pregnancy (Fig. 5). One hour after treatment, animals administered BPA had mean plasma BPA levels of 24.69 ng/mL, while control mice had mean plasma BPA levels of 1.70 ng/mL. Mice had mean plasma BPA levels of 3 ng/mL 6 h after treatment with BPA. By 18 h after intraperitoneal administration of BPA, plasma BPA levels in treated animals (1.67 ng/mL) were indistinguishable from those of controls (1.77 ng/mL). These levels approximate the mean levels reported in plasma from pregnant women sampled between 32 and 41 weeks of gestation (ranging 0.3 to 18.9 ng/mL, mean 3.1 ng/mL) [13].
Fig. 5.
Plasma BPA concentration in treated animals and controls as determined by mass spectroscopy. a Representative LC/MS chromatography from untreated and BPA-treated mice sampled 1 h after administration of BPA. Top panel demonstrates quantification of BPA in control mice. Bottom panel shows quantification of BPA after treatment. b Plasma BPA concentration was determined after administration of BPA or vehicle control to pregnant mice. Plasma was obtained at 1, 6, 12, and 18 h after BPA administration.
DES and BPA Exposure In Utero Alter EZH2 Expression in Adult Mammary Tissue
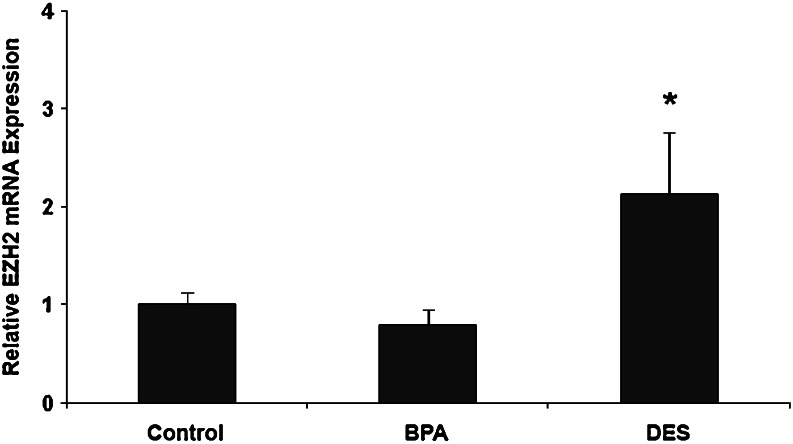
To evaluate whether in utero DES or BPA exposure would alter EZH2 expression in mammary tissue, pregnant mice were treated with DES (10 µg/kg, n = 15), BPA (5 mg/kg, n = 15), or empty vector (sesame oil, n = 15) on days 9–26 of gestation. The female offspring of the treated mice were killed at 6 weeks of age, and EZH2 mRNA and protein expression were examined in inguinal mammary glands. DES exposure in utero led to a 2-fold increase in EZH2 mRNA expression (p < 0.05). BPA exposure did not have an effect on EZH2 mRNA (Fig. 6).
Fig. 6.
DES, but not BPA, exposure in utero increases EZH2 mRNA expression in adult murine mammary tissue. Quantitative RT-PCR was performed in mammary tissue from 6-week-old mice after in utero exposure to DES or BPA during days 9–26 of gestation. DES treatment resulted in a 2-fold increase in EZH2 mRNA expression when compared with control mice. BPA treatment did not alter expression of EZH2 mRNA. Results are representative of three independent experiments using five animals in each treatment group in each experiment. *p < 0.05.
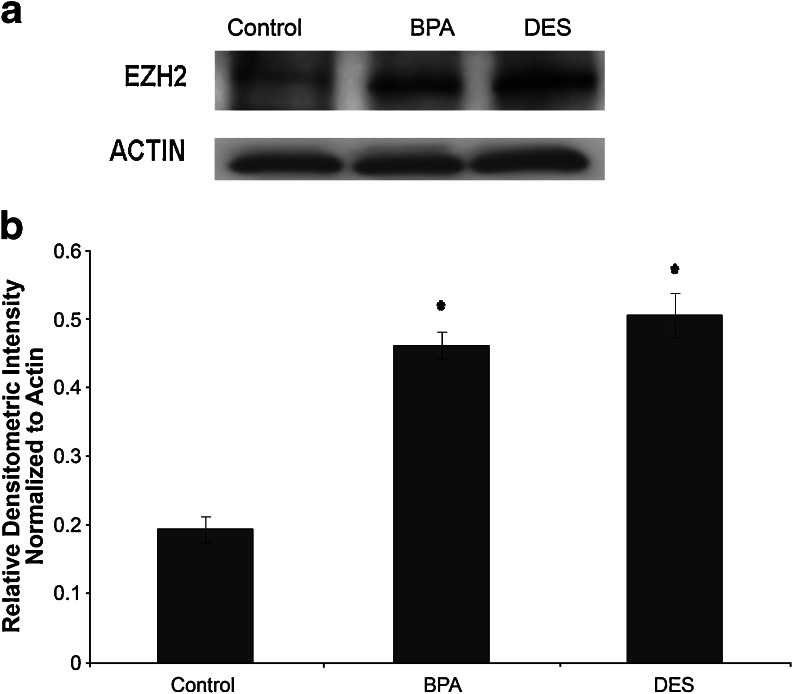
EZH2 protein levels were measured by Western blot analysis in mammary tissue of female mice with in utero exposure to DES, BPA, or control (Fig. 7). Control-treated mice had low, but detectable, EZH2 protein expression. Exposure to DES in utero resulted in increased EZH2 protein (p < 0.05). Similarly, BPA exposure in utero led to increased EZH2 protein expression (p < 0.05). There were no changes in β-actin.
Fig. 7.
DES and BPA exposure in utero increases EZH2 protein levels in adult murine mammary tissue. a Western blot demonstrating that in utero BPA or DES exposure during days 9–26 of gestation increases EZH2 protein within mammary tissue of 6-week-old female mice. EZH2 was present in low, but detectable, amounts in control animals. DES-treated animals showed an increase in EZH2. BPA-treated animals also showed an increase in EZH2. b Densitometric analysis of Western blots confirm increased EZH2 expression in adult murine mammary tissue subsequent to in utero BPA or DES exposure. Values were normalized to β-actin to obtain relative densitometric intensity. *p < 0.05.
DES and BPA Exposure In Utero Alters Net EZH2 Functional Activity in Adult Mammary Tissue
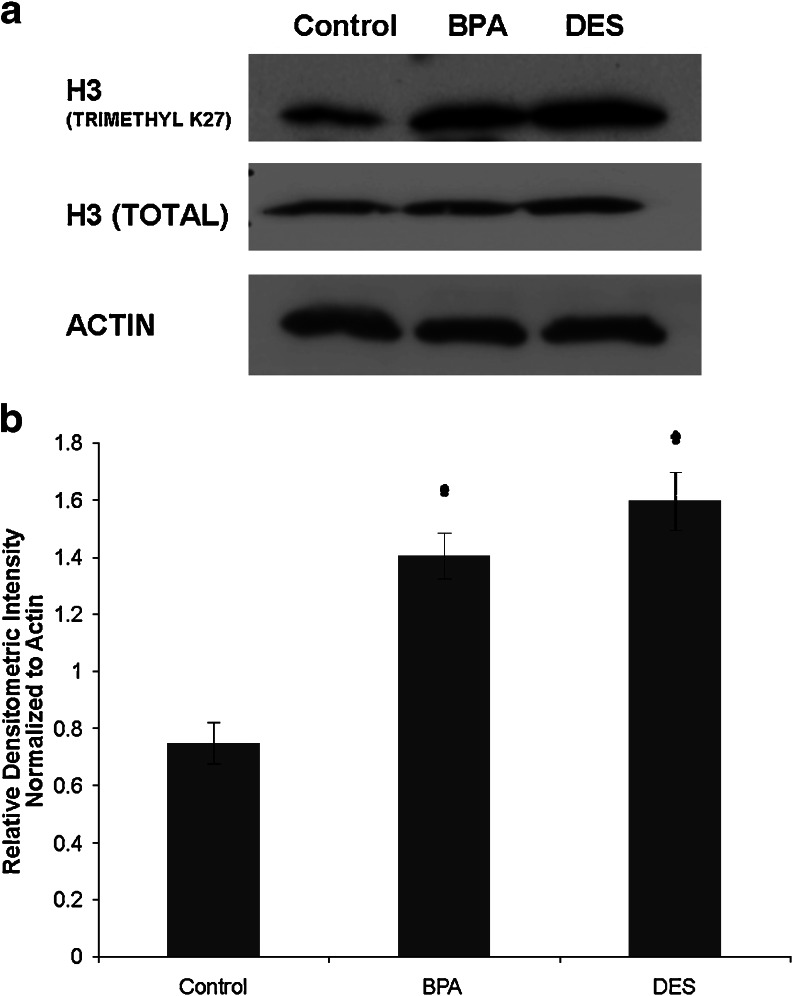
To evaluate the effect of in utero exposure to the endocrine-disrupting chemicals DES and BPA on EZH2-mediated histone methyltransferase activity, we treated pregnant female mice with DES (10 µg/kg, n = 15), BPA (5 mg/kg, n = 15), or vehicle control (sesame oil, n = 15) on days 9–26 of gestation. The female offspring of the treated mice were killed at 6 weeks of age, and Western blots were performed to assess the effect on expression of trimethylated histone H3 (histone H3 tri methyl K27). Western blots were performed with primary antibodies against histone H3 (tri methyl K27). Western blots were also performed for total histone H3. Western blot results from female mice with in utero exposure to DES, BPA, or control vehicle are shown in Fig. 8. Mice treated with vehicle control had detectable levels of histone H3 (tri methyl K27). DES exposure in utero increased histone H3 (tri methyl K27) when compared with control mice (p < 0.05). BPA exposure in utero also increased histone H3 (tri methyl K27) (p < 0.05). In utero exposure to DES or BPA did not change the amount of total histone H3.
Fig. 8.
DES and BPA exposure in utero increase net EZH2 functional activity in adult murine mammary tissue. a Western blot demonstrates that in utero BPA and DES exposure during days 9–26 of gestation increases EZH2 protein function, assessed by blotting with antibody specific to histone H3 (tri methyl), the target of EZH2 methyltransferase activity. Histone H3 (tri methyl K27) was low, but detectable, in control mammary glands. Treatment with DES increased histone H3 (tri methyl K27). BPA-treated mice also showed an increase in histone H3 (tri methyl K27). Total histone H3 protein expression was not changed by treatment with DES or BPA. b Densitometric analysis of Western blots confirm increased histone H3 (tri methyl K27) in adult murine mammary tissue subsequent to in utero BPA or DES exposure. Values were normalized to β-actin to obtain relative densitometric intensity. *p < 0.05.
Discussion
DES and BPA are endocrine-disrupting chemicals with well-known developmental effects. Many of their effects are mediated through their estrogen-agonist activity. However, many of the effects of these endocrine disruptors persist well after exposure, with lasting changes in gene expression that cannot be explained simply as a result of their direct estrogenic effects [48–50]. We have previously shown that DES or BPA exposure causes epigenetic changes in HOXA10 expression in the reproductive tract by altered DNA methylation [42, 43]. Epigenetic changes as a result of exposure to EDC may predispose to malignancies in adulthood. In women, exposure to DES in utero is associated with an increased incidence of breast cancer as an adult [5, 6]. Rodent studies have shown that BPA exposure in utero causes molecular changes in mammary tissue, altering estrogen sensitivity and predisposing to mammary ductal hyperplasia and an increase in carcinoma in situ of the breast [21, 22, 51]. Here we examine the mechanisms by which DES and BPA exert these epigenetic effects in mammary tissue.
Recently, epigenetic changes involving DNA methylation and chromatin remodeling as a result of histone modifications (methylation, acetylation, etc.) have been implicated in the process of carcinogenesis. Interestingly, nearly half of the tumor suppressor genes known to cause certain cancer syndromes can be activated by DNA promoter hypermethylation in sporadic cancers [52]. As described above, histone methylation by EZH2 is a known epigenetic modifier in breast cancers.
Because of the possible role of EZH2 in the development of breast cancer, we investigated the effects of exposure to two well described EDCs, DES and BPA, on expression of EZH2. DES was administered to pregnant women at high dose, while BPA is a ubiquitous environmental contaminant. Here, plasma BPA concentrations in treated mice were found to be similar to those commonly reported in pregnant women [19–23]. Exposure to either DES or BPA in human MCF-7 cells increased EZH2 mRNA and protein expression. After in utero exposure to DES or BPA, mice demonstrated lasting increases in EZH2 expression in the adult. DES exposure in utero caused an increase in EZH2 mRNA and protein levels. Interestingly, after in utero exposure to BPA, EZH2 protein expression was increased, while there was no apparent change in EZH2 mRNA in mice. This suggests that BPA increases EZH2 protein by increasing translation or decreasing protein degradation. BPA may decrease microRNAs specific to EZH2 mRNA, thus increasing protein translation without any significant changes in mRNA expression. Alternatively, BPA might stabilize EZH2 protein by a mechanism-decreasing protein catabolism.
We also assessed the functional significance of increased EZH2 protein levels by examining the expression of histone H3 (tri methyl K27). We found increases in histone H3 (tri methyl K27) in MCF-7 cells treated with DES or BPA. Mice exposed to DES or BPA in utero also showed increased histone H3 (tri methyl K27) levels. Because the epigenetic effect of EZH2 is mediated largely due to its histone methyltransferase activity at lysine 27 of histone H3, our data confirm that both DES exposure and BPA exposure in utero cause epigenetic alterations in mammary tissue.
In breast cells, an interesting target of EZH2-mediated histone methyltransferase activity is p57 (CDKN1C), a-cyclin dependent kinase inhibitor that functions in the maintenance of the cell cycle, which has recently been shown to be repressed by EZH2-mediated methylation of histone H3 at lysine 27 in breast cancer cells [53]. Repression of this cell cycle regulator may lead to the increase in cell proliferation seen in breast tumors with elevations in EZH2 [33, 34]. Another important target gene, repressed by EZH2-mediated histone methylation, is E-cadherin. E-Cadherin is important in cell–cell adhesion, and its disruption has been associated with increased invasiveness and metastases of cancers [54]. E-Cadherin is transcriptionally silenced by EZH2 by methylation of histone H3 at lysine 27 [55]. The increased invasiveness seen in breast cancers with elevated EZH2 levels could therefore involve repression of E-cadherin.
In conclusion, we have demonstrated a novel mechanism by which endocrine-disrupting chemicals regulate developmental programming in the breast. Exposure to DES or BPA in utero alters mammary tissue expression of EZH2, a histone methyltransferase with known associations to tumorigenesis. EZH2 function, measured by examination of histone H3 (tri methyl K27), also increases as a result of exposure to DES or BPA. Increased expression of EZH2 within the breast, even in morphologically normal appearing tissue, may prove to be a marker of increased breast cancer risk [41]. The increase in EZH2 expression and function shown here in mice after in utero exposure to these chemicals is a potential mechanism for the increased risk of breast cancer as a result of exposure to these EDCs. This study also generates important safety concerns about exposures to environmental endocrine disruptors such as BPA and suggests a potential need to monitor women exposed to these chemicals for the development of breast lesions as adults.
Acknowledgments
Disclosures
The authors have nothing to disclose in this paper.
References
- 1.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.2307/3431890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg ER, Barnes AB, Resseguie L, Barrett JA, Burnside S, Lanza LL, Neff RK, Stevens M, Young RH, Colton T. Breast cancer in mothers given diethylstilbestrol in pregnancy. N Engl J Med. 1984;311:1393–1398. doi: 10.1056/NEJM198411293112201. [DOI] [PubMed] [Google Scholar]
- 3.Colton T, Greenberg ER, Noller K, Resseguie L, Van Bennekom C, Heeren T, Zhang Y. Breast cancer in mothers prescribed diethylstilbestrol in pregnancy. Further follow-up. JAMA. 1993;269:2096–2100. doi: 10.1001/jama.269.16.2096. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Mervis CA, Thun MJ, Rodriguez C, Wingo PA, Heath CW., Jr Diethylstilbestrol and risk of fatal breast cancer in a prospective cohort of US women. Am J Epidemiol. 1996;144:645–652. doi: 10.1093/oxfordjournals.aje.a008976. [DOI] [PubMed] [Google Scholar]
- 5.Palmer JR, Hatch EE, Rosenberg CL, Hartge P, Kaufman RH, Titus-Ernstoff L, Noller KL, Herbst AL, Rao RS, Troisi R, Colton T, Hoover RN. Risk of breast cancer in women exposed to diethylstilbestrol in utero: prelimiinary results (United States) Cancer Causes Control. 2002;13:753–758. doi: 10.1023/A:1020254711222. [DOI] [PubMed] [Google Scholar]
- 6.Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Herbst AL, Noller KL, Hyer M, Hoover RN. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1509–1514. doi: 10.1158/1055-9965.EPI-06-0109. [DOI] [PubMed] [Google Scholar]
- 7.Lopez J, Ogren L, Verjan R, Talamantes F. Effects of perinatal exposure to a synthetic estrogen and progestin on mammary tumorigenesis in mice. Teratology. 1988;38:129–134. doi: 10.1002/tera.1420380205. [DOI] [PubMed] [Google Scholar]
- 8.Rothschild TC, Boylan ES, Calhoon RE, Vonderhaar BK. Transplacental effects of diethylstilbestrol on mammary development and tumorigenesis in female ACI rats. Cancer Res. 1987;47:4508–4516. [PubMed] [Google Scholar]
- 9.Walker BE. Tumors in female offspring of control and diethylstilbestrol-exposed mice fed high-fat diets. J Natl Cancer Inst. 1990;82:50–54. doi: 10.1093/jnci/82.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/en.132.6.2279. [DOI] [PubMed] [Google Scholar]
- 11.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:110–115. doi: 10.1016/j.jchromb.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 13.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 15.Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, Sata F, Kishi R, Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16:735–739. doi: 10.1016/S0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 16.Engel SM, Levy B, Liu Z, Kaplan D, Wolff MS. Xenobiotic phenols in early pregnancy amniotic fluid. Reprod Toxicol. 2006;21:110–112. doi: 10.1016/j.reprotox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 18.Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007;21:239–246. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 20.Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol. 2007;23:383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Munoz-de-Toro M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg962. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Cao R, Wang L, Jones RS. Mechanism of Polycomb group gene silencing. Cold Spring Harb Symp Quant Biol. 2004;69:309–317. doi: 10.1101/sqb.2004.69.309. [DOI] [PubMed] [Google Scholar]
- 26.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 27.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 30.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 31.Croonquist PA, Van Ness B. The polycomb group protein enhancer of zeste homolog 2 (EZH 2) is an oncogene that influences myeloma cell growth and the mutant ras phenotype. Oncogene. 2005;24:6269–6280. doi: 10.1038/sj.onc.1208771. [DOI] [PubMed] [Google Scholar]
- 32.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 34.Collett K, Eide GE, Arnes J, Stefansson IM, Eide J, Braaten A, Aas T, Otte AP, Akslen LA. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12:1168–1174. doi: 10.1158/1078-0432.CCR-05-1533. [DOI] [PubMed] [Google Scholar]
- 35.Raaphorst FM, Meijer CJ, Fieret E, Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RA, Otte AP, van Diest PJ. Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia. 2003;5:481–488. doi: 10.1016/s1476-5586(03)80032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- 37.Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben-Shushan E, Reubinoff BE, Bergman Y, Simon I, Cedar H. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007;39:232–236. doi: 10.1038/ng1950. [DOI] [PubMed] [Google Scholar]
- 38.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 39.Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin SB. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeidler M, Varambally S, Cao Q, Chinnaiyan AM, Ferguson DO, Merajver SD, Kleer CG. The Polycomb group protein EZH2 impairs DNA repair in breast epithelial cells. Neoplasia. 2005;7:1011–1019. doi: 10.1593/neo.05472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding L, Erdmann C, Chinnaiyan AM, Merajver SD, Kleer CG. Identification of EZH2 as a molecular marker for a precancerous state in morphologically normal breast tissues. Cancer Res. 2006;66:4095–4099. doi: 10.1158/0008-5472.CAN-05-4300. [DOI] [PubMed] [Google Scholar]
- 42.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–3382. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bromer J, Zhou Y, Taylor M, Doherty L, Taylor H (2010) Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. PMID: 20181937 [DOI] [PMC free article] [PubMed]
- 44.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 45.Brooks SC, Locke ER, Soule HD. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973;248:6251–6253. [PubMed] [Google Scholar]
- 46.Bromer JG, Zhou Y, Taylor MB, Taylor HS. Bisphenol-A (BPA) exposure in utero leads to epigenetic changes and altered developmental programming. Washington: The Endocrine Society; 2009. [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- 49.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–S17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 50.Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK. Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog. 2007;46:783–796. doi: 10.1002/mc.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 52.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 53.Yang X, Karuturi RK, Sun F, Aau M, Yu K, Shao R, Miller LD, Tan PB, Yu Q. CDKN1C (p57) is a direct target of EZH2 and suppressed by multiple epigenetic mechanisms in breast cancer cells. PLoS One. 2009;4:e5011. doi: 10.1371/journal.pone.0005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, Mehra R, Laxman B, Cao X, Kleer CG, Varambally S, Chinnaiyan AM. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–7284. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]