Abstract
Sleep deprivation (SD) can have a negative impact on cognitive function, but the mechanism(s) by which SD modulates memory remains unclear. We have previously shown that astrocyte-derived adenosine is a candidate molecule involved in the cognitive deficits following a brief period of SD (Halassa et al., 2009). In this study, we examined whether genetic disruption of soluble N-ethylmaleimide-sensitive factor attached protein (SNARE)-dependent exocytosis in astrocytes (dnSNARE mice) or pharmacological blockade of A1 receptor signaling using an adenosine A1 receptor (A1R) antagonist, 8-cyclopentyl-1,3-dimethylxanthine (CPT), could prevent the negative effects of 6 h of SD on hippocampal late-phase long-term potentiation (L-LTP) and hippocampus-dependent spatial object recognition memory. We found that SD impaired L-LTP in wild-type mice but not in dnSNARE mice. Similarly, this deficit in L-LTP resulting from SD was prevented by a chronic infusion of CPT. Consistent with these results, we found that hippocampus-dependent memory deficits produced by SD were rescued in dnSNARE mice and CPT-treated mice. These data provide the first evidence that astrocytic ATP and adenosine A1R activity contribute to the effects of SD on hippocampal synaptic plasticity and hippocampus-dependent memory, and suggest a new therapeutic target to reverse the hippocampus-related cognitive deficits induced by sleep loss.
Introduction
Several studies support the idea that sleep deprivation (SD) can cause cognitive deficits, affecting multiple memory systems in animals. Indeed, SD impairs many forms of hippocampus-dependent memory in rodents, such as passive avoidance (Silva et al., 2004), spatial water maze learning (Guan et al., 2004), or contextual fear conditioning (Graves et al., 2003; Vecsey et al., 2009). Other non-hippocampus-dependent memories, such as novel object recognition, are also affected when sleep is disrupted (Palchykova et al., 2006; Halassa et al., 2009). However, the cellular and molecular mechanisms by which SD modulates memory consolidation are still unclear. It is believed that SD increases extracellular adenosine levels in basal forebrain and cortex, suggesting that adenosine may be an endogenous sleep-promoting factor (Porkka-Heiskanen et al., 1997). Indeed, infusion of an adenosine analog in the basal forebrain promotes sleep (Porkka-Heiskanen et al., 2000). Combined, these findings raise the possibility that adenosine buildup during SD contributes to memory deficits.
Like neurons, astrocytes release several chemical transmitters, called gliotransmitters, including ATP. Once released into the extracellular space, ATP is rapidly hydrolyzed to adenosine via a variety of ectonucleotidases (Dunwiddie et al., 1997; Cunha et al., 1998). Using a molecular genetic approach to disrupt soluble N-ethylmaleimide-sensitive factor attached protein (SNARE)-dependent gliotransmission (dnSNARE mice), Pascual et al. (2005) found that extracellular accumulation of adenosine is dependent on astrocytic release of ATP. One effect of astrocyte-derived adenosine is to cause heterosynaptic depression of excitatory synaptic transmission, which it does via action on presynaptic adenosine A1 receptor (A1R) (Pascual et al., 2005). In a recent study, we showed that dnSNARE mice were protected against the effects of SD on a non-hippocampus-dependent memory task (Halassa et al., 2009). The same protection against SD was observed when wild-type (WT) mice were treated with the adenosine A1R antagonist 8-cyclopentyl-1,3-dimethylxanthine (CPT). Together, these findings suggest that astrocyte-derived ATP/adenosine mediates cognitive deficits following SD.
Previously, we demonstrated that brief SD disrupts the maintenance of hippocampal late-phase long-term potentiation (L-LTP) in area CA1 and also impairs hippocampus-dependent memory (Vecsey et al., 2009). Because hippocampal function seems to be particularly susceptible to disruption by SD, we therefore examined here whether inhibition of gliotransmission or blockade of adenosine A1R signaling could prevent the effects of brief SD on hippocampal L-LTP. Because different classes of memory rely on different brain regions (e.g., novel object recognition depends on extrahippocampal regions, whereas spatial memory is highly dependent on the hippocampus) (Winters et al., 2004, 2008; Winters and Bussey, 2005; Oliveira et al., 2010), we investigated whether memory in a spatial version of the object recognition task is also affected by SD. In the present study, we used a combined neuropharmacological and genetic approach to demonstrate that astrocyte-derived adenosine and adenosine A1Rs play an important role in synaptic plasticity and memory deficits induced by SD.
Materials and Methods
Subjects
Generation of dominant-negative SNARE mice (dnSNARE) has been described previously (Pascual et al., 2005; Halassa et al., 2007, 2009). Briefly, dnSNARE mice were obtained by crossing two different mouse lines: GFAP.tTA, in which the expression of the tet-off tetracycline transactivator (tTA) is driven by the human astrocyte-specific glial fibrillary acidic protein (GFAP) promoter, and tetO.dnSNARE, in which the dnSNARE domain of the vesicle protein synaptobrevin II and the reporter enhanced green fluorescence protein (EGFP) are coexpressed under the control of the tetO promoter (Fig. 1A). Twelve- to 16-week-old male and female dnSNARE mice and littermate controls were maintained on 40 mg/kg doxycycline (Dox)-containing food (Bio-Serv) and were switched to a regular diet for 4–5 weeks before the beginning of the experiments. Transgenic mice have been backcrossed onto C57BL/6J genotype for >10 generations. All mice had ad libitum access to food and water in their holding cages. Lights were maintained on a 12 h light/dark cycle, with all behavioral testing performed during the light portion of the cycle (lights on 7:00 A.M. to 7:00 P.M.). All experiments were conducted according to the National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The investigators were blind to the genotype and/or drug treatment of the mice during behavioral or electrophysiological testing. The dnSNARE and wild-type genotypes were confirmed by PCR following behavioral and electrophysiological tests.
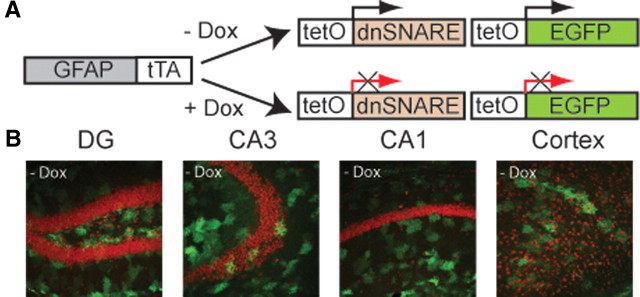
Figure 1.
Dominant-negative SNARE transgenes are expressed in hippocampal and cortical astrocytes. A, Schematic of the generation of the bitransgenic mice expressing dnSNARE in glia. Two different lines of transgenic mice have been previously described by Pascual et al. (2005). One is a line of mice in which the human GFAP promoter drives the expression of tTA. The second, tetO.dnSNARE contains a tet operator (tetO)-regulated dnSNARE domain and EGFP reporter gene. By crossing these two lines, the dnSNARE domain is expressed only in astrocytes. All mice were maintained on +Dox until the beginning of the experiments. Three to 4 weeks before experiments, mice were put off doxycycline food (−Dox) to induce dnSNARE expression. B, Confocal images showing expression of the reporter transgene EGFP (green) throughout the different hippocampal subfields [dentate gyrus (DG), CA3, and CA1] and cortex after 4–5 weeks off doxycycline food (−Dox). Propidium iodide (red) was used as a neuronal marker. Magnification ×20.
Surgical procedures and drug infusion
For the electrophysiological studies, we used the same surgical protocol and drug infusion method as reported previously by Halassa et al. (2009). Briefly, C57BL/6J adult male mice (12–16 weeks of age) were deeply anesthetized with isoflurane, and a brain cannula (the Alzet brain infusion kit 3; DURECT Corporation) was stereotaxically placed into the left lateral ventricle at the following coordinates: −0.8 mm posterior to bregma, ±1.0 mm lateral to the midline, and −2.0 mm ventral to the skull surface. One mini-osmotic pump (model 1002, Alzet), connected to the brain cannula by micro medical tubing (Scientific Commodities Inc.), was implanted subcutaneously between the shoulder blades. Intracerebroventricular injection was used for electrophysiological experiments to avoid damage to hippocampal slices that can result from direct intrahippocampal injections. For the behavioral memory task, two Alzet mini-osmotic pumps were implanted subcutaneously between the shoulders blades. Pumps were connected to bilateral chronic indwelling cannulae (3280PD, osmotic pump connect, 28 GA, Plastics One) aimed at the dorsal hippocampi (−1.70 mm posterior to bregma, ±1.50 mm lateral to the midline, and −2.3 mm ventral to the skull surface). All the mini-osmotic pumps were allowed to equilibrate in 0.9% saline for 24 h at 37°C before implantation. Implanted mini-pumps were filled with either CPT (4 mm), a specific adenosine A1R antagonist, or 50% DMSO in 0.9% saline [vehicle (VEH)], and administered in solution at a rate of 0.25 μl/h. After 7 d post-implantation, all mice had fully recovered from surgery and were submitted to either electrophysiological analysis or behavioral study (Fig. 2A). Cannula placements were determined by examination of serial coronal sections stained with cresyl violet (Fig. 2B).
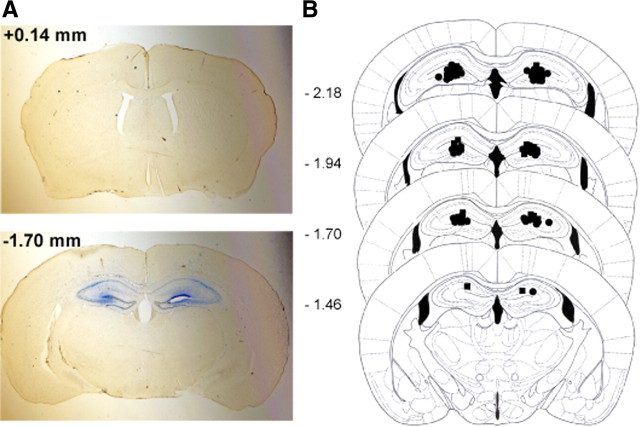
Figure 2.
A representative brain section showing an intrahippocampal administration of dye solution using osmotic mini-pumps. A, A 0.02% Evans blue solution in 50% DMSO was chronically infused into the dorsal hippocampus for a week. At the bottom is a picture showing the diffusion only into the hippocampus without affecting other brain areas like the striatum (top). Striatum and dorsal hippocampal slices were collected at +0.14 mm and −1.70 mm from bregma, respectively. B, Schematic representations of the tip of infusion tracts for the CPT-treated (black circle) and vehicle-treated (black square) mice. Pictures were modified from the work of Paxinos and Franklin (2001).
Electrophysiology
Hippocampal slice preparation.
At the beginning of the light phase (8:00 A.M.), mice were sleep deprived for 6 h by gentle handling or were left undisturbed. At the completion of the 6 h, mice were killed by cervical dislocation and hippocampi were rapidly dissected in iced, oxygenated artificial CSF (ACSF) (in mm: 124 NaCl, 4.4 KCl, 1 NaH2PO4, 26 NaHCO3, 2.5 CaCl2, 1.3 MgSO4, 10 glucose). Transverse hippocampal slices (400 μm thick) were placed in an interface recording chamber (Fine Science Tools) (Matthies et al., 1997) and continuously perfused with oxygenated ACSF while they equilibrated for at least 1.5 h at 30°C before starting electrophysiological recordings.
Hippocampal slice electrophysiology.
A stimulating electrode (A-M Systems; 0.002-inch-diameter nichrome wire) placed in the stratum radiatum was used to elicit action potentials. Extracellular recordings of field EPSPs (fEPSPs) were made using an ACSF-filled glass microelectrode (A-M Systems; 1.5 mm × 0.85 mm), with a resistance between 3 and 5 MΩ, that was placed in the stratum radiatum region of CA1. Data were acquired using Clampex 8.2 (Molecular Devices) and analyzed using Clampfit 8.2 (Molecular Devices). Peak fEPSP amplitudes from the stimulator were required to be at least 3 mV, and stimulus intensity was set to produce 40% of the maximal response. Baseline responses were recorded for 20 min, and L-LTP was then induced by applying four trains of stimuli at 100 Hz for 1 s, with a 5 min intertrain interval. Recordings continued for 150 min after LTP induction. Initial fEPSP slopes were normalized against the average of the 20 baseline traces, and were expressed as percentage. Input–output characteristics were examined by recording fEPSPs in CA1 resulting from stimuli of increasing intensity. Initial fEPSP slopes were plotted against the corresponding presynaptic fiber volley amplitudes, and the resulting plots were fit with linear regressions. Paired-pulse facilitation (PPF), a short-term form of synaptic plasticity, was measured in slices from sleep-deprived and control mice. Pairs of stimuli were delivered with varying delays (300, 200, 100, 50, 25 ms) between the two stimuli, and the initial fEPSP slope from the second stimulus was plotted relative to the slope from the first stimulus.
Spatial object recognition task
Mice were handled for 1 min each day, for three consecutive days leading up to experimentation. The spatial object recognition task was conducted in a gray rectangular box (40 × 30 × 30 cm) built of polyvinyl chloride plastic. At the beginning of the light phase, mice were placed in the empty box for 6 min. Subjects were then removed and placed back in the home cage. After 3 min, mice were placed in the box with two different objects (a 100 ml glass bottle and a metallic rectangular tower) for three consecutive 6 min training sessions, separated by a 3 min interval, during which the animals were returned to the holding cage. At the completion of the training sessions, mice were sleep deprived for 6 h by gentle handling or were left undisturbed in their home cage. Eighteen hours later, mice were submitted to a single testing session, in which one of the two objects was repositioned, thereby changing the spatial configuration of the objects in the box. Mice were allowed to explore the objects for 6 min. The time exploring the displaced object during the test session was scored as an index to determine whether mice preferentially reexplored the displaced object during the testing session. A reduction in time spent exploring the displaced object was indicative of a memory impairment.
Data analysis
ANOVAs were performed in all experiments using SPSS software (version 13.0) and SigmaStat software (version 3.5). To evaluate differences in input–output characteristics, ANOVAs were performed comparing the average linear regression slopes for each group. For paired-pulse facilitation, repeated-measures ANOVAs were used to compare the ratio of the initial fEPSP slopes elicited by the two stimuli across different interstimulus intervals. For LTP experiments, the maintenance of LTP during the last 20 min of the recording was analyzed using repeated-measures ANOVAs. Simple planned comparisons were made using Student's t tests.
Results
dnSNARE transgene is expressed in the hippocampus and cortex
As indicated in Figure 1B, dnSNARE and EGFP transgenes are coexpressed in the cortex and hippocampus in the absence of doxycycline food (−Dox), and are not expressed when animals are fed 40 mg/kg doxycycline food (+Dox) (Pascual et al., 2005). Mice were bred in the presence of +Dox food until they reached 3–4 months of age. Before starting experiments, +Dox food was replaced by −Dox food for 3–4 weeks to initiate the expression of the transgene. Only mice fed with −Dox food were used for our studies. As has been indicated in previous studies, astrocytes express the dnSNARE transgene in different brain areas including the hippocampus and the cortex (see, for example, Pascual et al., 2005, their Fig. 1). We previously demonstrated that dnSNARE animals do not express the transgene when they are maintained on +Dox food throughout development (Halassa et al., 2009) and adulthood (Fellin et al., 2009).
Brief sleep loss does not impair the maintenance of L-LTP in dnSNARE mice
We first examined the effects of dnSNARE expression and/or sleep deprivation on the properties of synaptic transmission and plasticity at CA1 synapses. To determine whether basal synaptic transmission at hippocampal CA1 synapses was altered by the expression of the dnSNARE transgene in the astrocytes and/or brief sleep deprivation, field potentials in the CA1 region of hippocampi from transgenic mice and WT littermates were elicited with bipolar stimulating electrodes placed in the Schaffer collaterals and characterized with respect to input–output relationship. Figure 3A illustrates the EPSP slope plotted against the fiber volley amplitude for dnSNARE (n = 12) and wild-type hippocampal slices (n = 12) to form input/output (I/O) curves. The EPSP-to-fiber volley slopes were similar between dnSNARE and wild-type mice for the SD and non-sleep deprivation (NSD) conditions (dnSNARE-NSD: y = 0.27 x, R2 = 0.67; dnSNARE-SD: y = 0.27 x, R2 = 0.78; WT-NSD: y = 0.24 x, R2 = 0.63; WT-SD: y = 0.29, R2 = 0.62; genotype × condition interaction, F(1,25) = 0.00285, p = 0.958).
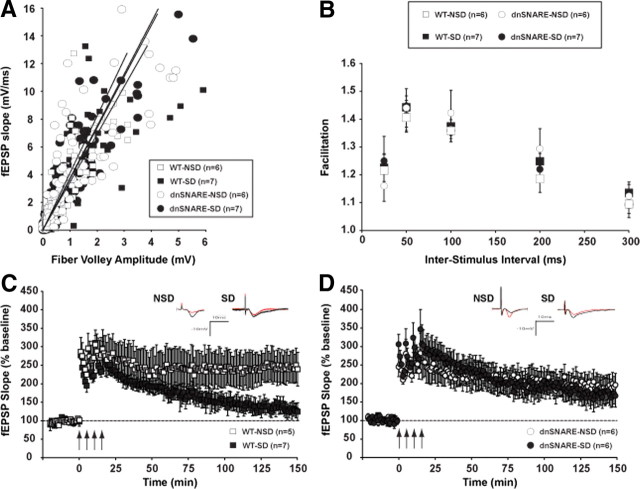
Figure 3.
SD alters baseline electrophysiological properties and LTP maintenance in the CA1 region of wild-type mice but not in dnSNARE mice. Both dnSNARE and wild-type mice were deprived of sleep for 6 h by gentle handling or were left undisturbed in their home cages. A, The strength of the synaptic input, as measured by the presynaptic fiber volley amplitude, was gradually increased, and the resulting postsynaptic output was plotted. The resulting input/output curves showed no difference in basal excitability between groups. B, Pairs of stimuli were delivered with interstimulus intervals of 25, 50, 100, 200, or 300 ms. Plotted is the ratio of the slopes of the resulting fEPSPs. PPF was similar across groups at each interval tested. C, Following 6 h of SD by gentle handling, LTP was induced in hippocampal slices by application of four 100 Hz, 1 s duration trains of stimuli to the Schaffer collateral pathway, with a 5 min interstimulus interval (indicated by arrows). Wild-type SD mice showed an impaired L-LTP compared with NSD littermates. D, In hippocampal slices from dnSNARE mice, L-LTP is maintained after SD at the same level as in NSD mice. Insets show representative recordings from an animal from each group taken during the first and last 5 min of the recording. Values are means ± SEM and n represents the number of animals.
To determine whether dnSNARE expression and/or sleep deprivation alter presynaptic function, we examined PPF, a short-term enhancement of synaptic efficacy following delivery of two closely spaced stimuli that is sensitive to alterations in presynaptic function (Manabe et al., 1992). As shown in Figure 3B, PPF was not significantly different between the genotype (dnSNARE and wild-type) and the experimental condition (sleep deprived and non-sleep deprived) at interpulse intervals from 25 to 300 ms (F(1,22) = 0.577; p = 0.455). These findings suggested that basal synaptic transmission and short-term presynaptic plasticity were normal in dnSNARE transgenic mice and wild-type littermates under the conditions of these experiments (see Discussion).
Previous studies showed that neuronal plasticity in the rodent hippocampus is reduced after 12–72 h sleep deprivation (Campbell et al., 2002; Davis et al., 2003; McDermott et al., 2003; Ruskin et al., 2004). More recently, we demonstrated that 5 h of SD disrupts the maintenance of hippocampal CA1 LTP in mice (Vecsey et al., 2009). Consistent with the literature, slices from sleep-deprived wild-type mice stimulated with a 4-train stimulation protocol showed a marked reduction in potentiation during the last 20 min of LTP (139.5 ± 7.1% of baseline) relative to undisturbed controls (247.7 ± 42.6%) (F(1,10) = 11.047; p < 0.010) (Fig. 3C). Interestingly, the maintenance of the late phase of LTP was not altered in slices from sleep-deprived dnSNARE mice (169.6 ± 30.7% of baseline) relative to undisturbed transgenic mice (190.0 ± 19.2%) (F(1,10) = 0.383; p = 0.550) (Fig. 3D), indicating that astrocyte-derived ATP release contributes to the sleep loss-induced hippocampal synaptic plasticity deficit. Our data also suggest that L-LTP is not altered by the expression of the dnSNARE transgene in NSD mice compared with WT-NSD controls (F(1,9) = 2.133; p = 0.178).
A specific adenosine A1R antagonist treatment rescues the synaptic plasticity deficit induced by 6 h of SD
To ask whether a chronic absence of adenosine A1R activation underlies the dnSNARE phenotype, we chronically treated wild-type mice in vivo via intracerebroventricular delivery of the adenosine A1R antagonist CPT and VEH for a week and then submitted mice to 6 h of SD. Immediately following SD, slices from CPT- and VEH-treated mice were collected for electrophysiological recordings.
I/O curves did not show any difference between CPT- and VEH-treated mice across the two different sleep conditions (CPT-NSD: y = 0.25 x, R2 = 0.71; CPT-SD: y = 0.27 x, R2 = 081; VEH-NSD: y = 0.26 x, R2 = 0.81; VEH-SD: y = 0.26, R2 = 0.73; treatment × condition interaction, F(1,27) = 0.00299, p = 0.957) (Fig. 4A). A lack of an effect of drug treatment or SD on PPF suggested that short-term presynaptic function was unaltered between groups (F(1,18) = 1.700; p = 0.209) (Fig. 4B).
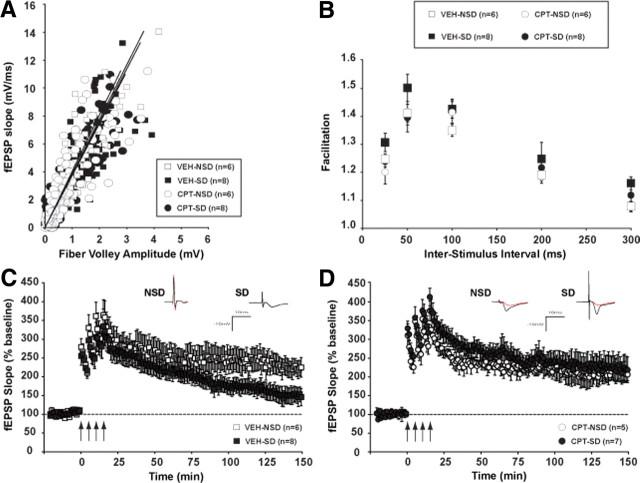
Figure 4.
Hippocampal adenosine A1R inactivation prevents LTP deficits produced by SD. A, Input/output curves relating presynaptic fiber volley amplitude to the initial slope of the resulting fEPSP are not different across groups. B, Paired-pulse facilitation is unaffected across groups at any of the intervals examined. C, L-LTP was induced as in Figure 1. SD mice treated with vehicle showed a L-LTP impairment in CA1 compared with the undisturbed controls (NSD). D, The L-LTP impairment is prevented in SD mice treated with a chronic infusion of the adenosine A1R antagonist CPT. CPT treatment alone does not affect the maintenance of LTP (CPT-NSD vs VEH-NSD). Insets show representative recordings from an animal from each group taken during the first and last 5 min of the recording. Values are means ± SEM and n represents the number of animals.
We tested L-LTP by recording field potentials in hippocampal slices from vehicle- and CPT-treated mouse brains after SD. The maintenance of L-LTP was reduced in sleep-deprived mice treated with vehicle (VEH-SD, 148.0 ± 21.7% of baseline) during the last 20 min of the recording, compared with the undisturbed group (VEH-NSD, 222.9 ± 23.7%, F(1,12) = 6.201; p < 0.05) (Fig. 4C). The inactivation of A1Rs by CPT treatment prevented the L-LTP CA1 deficit induced by sleep loss (CPT-SD, 223.0 ± 33.4%, CPT-NSD, 211.5 ± 18.7%, F(1,10) = 0.084; p = 0.778) (Fig. 4D). It is also important to note that chronic CPT treatment alone did not affect the maintenance of L-LTP (VEH-NSD vs CPT-NSD, F(1,9) = 0.163; p = 0.696).
Overall, blocking the adenosine A1R activity during the SD period mimics the dnSNARE phenotype by rescuing the SD-induced synaptic plasticity deficit. These results also suggest a role of the hippocampus and, more particularly, its adenosine A1Rs in the effect of SD on the synaptic plasticity functions.
Spatial object memory retrieval in dnSNARE and CPT-treated mice is not affected after sleep disturbances
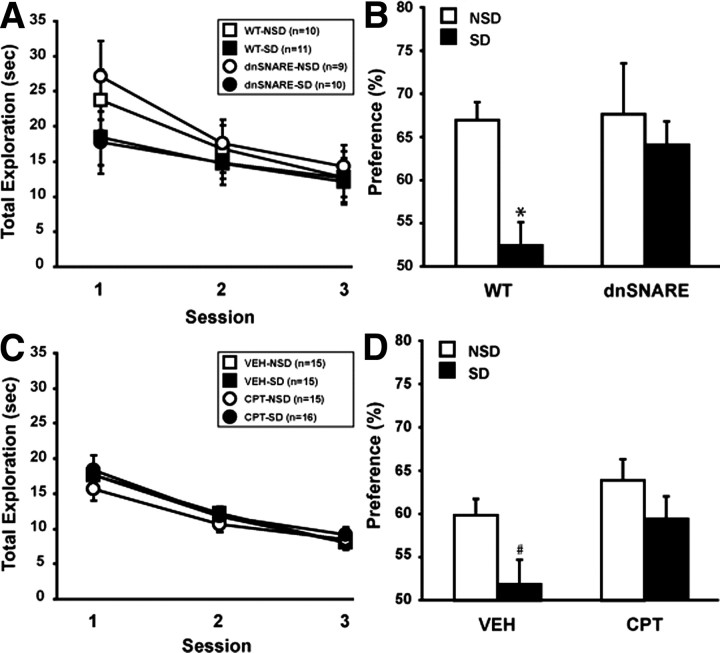
Because the maintenance of hippocampal LTP was normal after sleep deprivation in both dnSNARE and CPT-treated mice, we hypothesized that a hippocampus-dependent task, like spatial object recognition, would be unaffected by sleep loss in the same groups. To specifically block the adenosine A1R response in the hippocampus for our behavioral experiments, CPT and vehicle were directly and chronically infused into the dorsal hippocampus before mice were submitted to the spatial object recognition task. Figure 5A shows the total time of contact across the training sessions with the two objects. No major differences in overall object exploration were observed between genotypes (dnSNARE vs wild-type) and experimental conditions (NSD vs SD) in training sessions 1, 2, and 3. A repeated-measures ANOVA revealed a significant session effect (F(2,72) = 13.187; p < 0.001) and no genotype × condition × session effect (F(2,72) = 1.431; p = 0.239), demonstrating that dnSNARE and wild-type mice had no difference in the total time exploring the objects over the three training sessions. Moreover, dnSNARE and wild-type mice showed a similar decrease in the total time exploring the objects over the sessions. A repeated-measures ANOVA revealed only a significant session effect (F(2,76) = 12.794; p < 0.001) but no genotype (F(1,38) = 0.086; p = 0.772) and no interaction between session and genotype (F(2,76) = 0.028; p = 0.973). In wild-type mice, SD reduced the overall time spent exploring the displaced object during the 24 h test, indicating impaired memory (t(19) = −4.222, p < 0.05) (Fig. 5B). To the contrary, blocking gliotransmission prevented the effect of sleep deprivation on spatial object memory. Within dnSNARE mice, SD and NSD mice spent equivalent amounts of time exploring the displaced object (t(17) = −0.570, p = 0.576). To determine whether an absence of adenosine A1R activation underlies the dnSNARE behavioral phenotype, we chronically antagonized the adenosine A1R in wild-type mice via intrahippocampal delivery of CPT for a week. Similar to dnSNARE mice, chronic CPT infusion did not affect the exploration time, and no differences among drug (CPT vs VEH) and experimental condition (NSD vs SD) were observed with regard to overall object exploration across the three consecutive training sessions (Fig. 5C). There was a significant session effect (F(2,114) = 74.934; p < 0.001) but no effect on the drug × condition × session interaction (F(1,114) = 0.203; p = 0.817). While vehicle-treated mice showed a deficit in the time spent exploring the displaced object after SD (t(28) = −2.340, p < 0.05), chronic infusion of the adenosine A1R antagonist CPT in wild-type mice protected against the memory-degrading effects of SD (t(29) = −1.269, p = 0.214) (Fig. 5D).
Figure 5.
Purinergic gliotransmission contributes to spatial object memory impairment following SD. In the spatial object recognition paradigm, mice were trained through three consecutive training sessions to recognize two different objects. A, No difference of total exploration time for the objects between dnSNARE mice and wild-type littermates was observed. Immediately after training, mice were either left undisturbed (NSD) or were sleep deprived for 6 h (SD). Twenty-four hours later, mice were tested for the ability to detect the spatial change of one of the objects. B, SD impairs spatial memory for objects in wild-type but not in dnSNARE mice during the 24 h test. C, Similar to WT and dnSNARE mice, hippocampal treatment with adenosine A1R antagonist CPT in wild-type mice did not alter the exploration of the objects during the three training sessions. D, SD impairs spatial memory for objects in vehicle-treated mice but not in mice receiving chronic intrahippocampal delivery of CPT for a week before experimentation. #p < 0.05, *p < 0.001. Values are means ± SEM and n represents the number of animals.
Discussion
In this study, we first investigated whether disrupting transmitter release from astrocytes using a transgenic mouse model might be able to prevent the effects of SD on hippocampal function. Our results clearly show that hippocampal L-LTP is preserved after a brief period of SD when gliotransmission is attenuated. We found the same phenotype when we pharmacologically antagonized adenosine A1Rs with CPT treatment, suggesting a role of adenosine A1Rs in the impairments of hippocampal synaptic plasticity induced by SD. Because chronic intrahippocampal or intracerebroventricular infusions of the adenosine A1R antagonist had similar effects on basal synaptic activity and LTP, we have suggested a specific role of the hippocampus, and more particularly, hippocampal adenosine A1Rs, in generating the observed sleep loss-induced deficits in synaptic plasticity. We next asked whether the same adenosine A1R mechanism could account for deficits in hippocampus-dependent spatial long-term memory. Hence, we submitted dnSNARE and CPT-treated mice to a spatial object memory test 24 h after brief SD. We found that both dnSNARE mice and wild-type mice treated with a specific adenosine A1 antagonist, CPT, showed no memory deficit 24 h after induction of SD. Our results show for the first time a role of the hippocampal purinergic system in sleep loss-induced spatial memory deficits.
Adenosine A1R: a purinergic receptor involved in the deficits in hippocampal plasticity and memory induced by SD
Adenosine A1Rs are highly expressed in the CNS, including the hippocampus and cortex (Fredholm, 1995). SD is widely known to cause a progressive increase in extracellular adenosine in the basal forebrain and neocortex (Porkka-Heiskanen et al., 1997; Basheer et al., 1999). Interestingly, a recent study showed an increase in levels of ATP in the hippocampus following an adenosine infusion into the basal forebrain designed to increase sleep need (Dworak et al., 2010). Because extracellular ATP is rapidly hydrolyzed to adenosine, we asked whether the hippocampal adenosine buildup from SD could be responsible for the deficits in hippocampal synaptic plasticity. Adenosine acting on A1Rs during SD might inhibit hippocampal neurons, by inducing or modulating ionic currents postsynaptically, and by reducing transmitter release presynaptically (see, for example, Longordo et al., 2009). With the two strategies used in this study, we first prevented the accumulation of astrocyte-derived adenosine (by using the dnSNARE mice), and then blocked the action of adenosine A1Rs (by using intrahippocampal or intracerebroventricular treatment with CPT). Nevertheless, release of other gliotransmitters could be affected by the expression of the dnSNARE transgene. Indeed, in addition to ATP, astrocytes can release several other neuroactive molecules, such as glutamate (Parpura et al., 1994; Bezzi et al., 1998), d-serine (Wolosker et al., 1999), nitric oxide (Murphy, 2000; Ikeda and Murase, 2004), and S100β (Nishiyama et al., 2002), which have already been described to play a role in synaptic plasticity and LTP modulation. Here, we have observed a reduction of the effect of SD on hippocampal synaptic plasticity and spatial memory both in the dnSNARE mice and after adenosine A1Rs blockage by CPT, providing indirect evidence that dnSNARE mice are impaired in their adenosine response to SD. Direct measure of extracellular adenosine will be a helpful future step to give more direct evidence for the relationship between adenosine and hippocampal function during brief SD.
Intracellular signaling pathways regulated by astrocyte-derived ATP release and hippocampal adenosine A1Rs: effects on the cAMP/PKA/ERK signaling pathway
ATP/adenosine from hippocampal astrocytes activates adenosine A1Rs at the presynaptic level, where they are usually coupled to inhibitory G-proteins (i.e., Gi/Go), which inhibit adenylyl cyclase and thus decrease the level of cAMP (Gilman, 1987) and extracellular signal-regulated kinase (ERK) phosphorylation (Guan et al., 2004). ERK1/2 activation is necessary for L-LTP maintenance (Sweatt, 2004), and phospho-ERK1/2 expression is also decreased by paradoxical sleep deprivation in the dorsal hippocampus (Ravassard et al., 2009). In a recent study, we showed that mice submitted to 5 h of SD immediately after contextual fear conditioning, another hippocampal-dependent task, had impaired long-term memory compared with undisturbed mice (Vecsey et al., 2009). This study revealed that cAMP signaling was affected in the hippocampal CA1 subfield, mediated by an increase in phosphodiesterase (PDE) 4 activity following SD. Together, these studies suggest that the cAMP/PKA (protein kinase A)/ERK signaling pathway is disrupted by SD. Therefore, if astrocytic ATP release and resulting adenosine A1R activation contribute to the disruption of cAMP/PKA/ERK signaling, we would expect dnSNARE and CPT-treated mice to be resistant to this effect. A recent study showed that a chronic treatment of caffeine (known to be a nonspecific adenosine receptor antagonist) prevents the harmful effect of SD on cognitive properties (Alhaider et al., 2010). Furthermore, caffeine is well known to inhibit cAMP PDE activity (Fredholm et al., 1999) and increase cAMP level in brain (Wu et al., 2009). Here, we suggest that blocking adenosine receptors and, more particularly, A1R, could prevent the decrease in cAMP that follows a brief period of SD. These last two studies support the idea that purinergic receptor activation and cAMP PDE activity play an important role in synaptic plasticity and memory deficits during SD.
Our results demonstrate that dnSNARE mice have normal basal synaptic transmission as shown by input/output curves determined across a range of input current (Fig. 3A), as well as normal hippocampal synaptic plasticity in area CA1 (Fig. 3C,D). These findings are in contrast to a previous report (Pascual et al., 2005), which found increased excitability and reduced theta-burst LTP in the same line of mice. However, there are several differences between these studies that may account for these differences, including the conditions of slice incubation (submersion or interface) (Capron et al., 2006), as well as the time of day the animals are killed and slice recordings are made. All the LTP data shown in the present study have been obtained using interface chamber recordings. In our interface-slice preparation, hippocampal slices are partially submerged in ACSF solution with the top surface of the slice exposed to a humidified atmosphere of 95% O2 and 5% CO2. In this condition, the hippocampal slices get oxygen that is diffused through the very thin film of liquid covering the slices. This preparation can optimize oxygen supply to the slices, affecting the basal activity of the hippocampal slices (Bingmann and Kolde, 1982). In contrast, in the submerged-slice preparation used in the study by Pascual et al. (2005), hippocampal slices are completely submerged in ACSF and the oxygen supply to the slices is provided by the oxygen dissolved in the ACSF. This difference in oxygenation could affect both neurotransmission and gliotransmission. In fact, Kukley et al. (2005) demonstrated that enhancement of the mossy fiber fEPSP by a high-affinity adenosine A1R antagonist can be increased in submerged recording chambers. This suggests that the type of recording chamber can have an impact on the sensitivity to detect different molecular mechanisms underlying LTP, which may explain the differences between the present findings and those of Pascual et al. (2005). An additional potential reason for this discrepancy is that recent studies have shown a strong time-of-day dependence on the adenosine-mediated gliotransmission. In agreement with in vivo data (Halassa et al., 2009), SNARE-dependent A1R-mediated presynaptic inhibition of synaptic transmission is sensitive to time of day: at Zeitgeber time 6, when recordings were made in this study, we found limited SNARE-dependent A1R gliotransmission (Schmitt et al., unpublished observations) in slices obtained from undisturbed mice.
In summary, the present study proposes a new role of glial release of ATP acting via adenosine on A1Rs in the hippocampus in the genesis of memory deficits following SD. Furthermore, these data provide the first evidence that the hippocampal purinergic system is responsible for the effects of sleep loss on hippocampal synaptic plasticity and hippocampus-dependent memory. Together, these results suggest a new therapeutic target to reverse the cognitive dysfunction induced by sleep disturbance, a major health problem in our modern society.
Footnotes
This work was supported by National Institutes of Health (NIH) P50 Grant AG017628 (to T.A.), NIH Training Grant HL07953 (to C.G.V., A. I. Pack, principal investigator), and Grants R01 NS037585 and R01 NS043142 (to P.G.H.). We thank Karuna Meda for her help with the sleep deprivation experiments.
References
- Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Caffeine prevents sleep loss-induced deficits in long-term potentiation and related signaling molecules in the dentate gyrus. Eur J Neurosci. 2010;31:1368–1376. doi: 10.1111/j.1460-9568.2010.07175.x. [DOI] [PubMed] [Google Scholar]
- Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Brain Res Mol Brain Res. 1999;73:1–10. doi: 10.1016/s0169-328x(99)00219-3. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bingmann D, Kolde G. PO2-profiles in hippocampal slices of the guinea pig. Exp Brain Res. 1982;48:89–96. doi: 10.1007/BF00239575. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Capron B, Sindic C, Godaux E, Ris L. The characteristics of LTP induced in hippocampal slices are dependent on slice-recovery conditions. Learn Mem. 2006;13:271–277. doi: 10.1101/lm.135406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Sebastião AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci U S A. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Purinoceptors in the nervous system. Pharmacol Toxicol. 1995;76:228–239. doi: 10.1111/j.1600-0773.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Murase K. Glial nitric oxide-mediated long-term presynaptic facilitation revealed by optical imaging in rat spinal dorsal horn. J Neurosci. 2004;24:9888–9896. doi: 10.1523/JNEUROSCI.2608-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Schwan M, Fredholm BB, Dietrich D. The role of extracellular adenosine in regulating mossy fiber synaptic plasticity. J Neurosci. 2005;25:2832–2837. doi: 10.1523/JNEUROSCI.4260-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longordo F, Kopp C, Lüthi A. Consequences of sleep deprivation on neurotransmitter receptor expression and function. Eur J Neurosci. 2009;29:1810–1819. doi: 10.1111/j.1460-9568.2009.06719.x. [DOI] [PubMed] [Google Scholar]
- Manabe T, Renner P, Nicoll RA. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992;355:50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- Matthies H, Schulz S, Thiemann W, Siemer H, Schmidt H, Krug M, Höllt V. Design of a multiple slice interface chamber and application for resolving the temporal pattern of CREB phosphorylation in hippocampal long-term potentiation. J Neurosci Methods. 1997;78:173–179. doi: 10.1016/s0165-0270(97)00149-0. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29:1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Nishiyama H, Knopfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99:4037–4042. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Meerlo P, Dürr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85:263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scoté-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19:3121–3124. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- Silva RH, Chehin AB, Kameda SR, Takatsu-Coleman AL, Abílio VC, Tufik S, Frussa-Filho R. Effects of pre- or post-training paradoxical sleep deprivation on two animal models of learning and memory in mice. Neurobiol Learn Mem. 2004;82:90–98. doi: 10.1016/j.nlm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, Kim SS, Chen T, Shang YZ, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:4243–4251. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine racemase: biosynthesis of the neuromodulator d-serine. Proc Natl Acad Sci U S A. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci. 2009;29:11029–11037. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]