Abstract
The ephrins are membrane-tethered ligands for the Eph receptor tyrosine kinases, which play important roles in patterning of the nervous and vascular systems. It is now clear that ephrins are more than just ligands and can also act as signalling-competent receptors, participating in bidirectional signalling. We have recently shown that ephrin-A5 signals within caveola-like domains of the plasma membrane upon engagement with its cognate Eph receptor, leading to increased adhesion of the cells to fibronectin. Here we show that ephrin-A5 controls sequential biological events that are consistent with its role in neuronal guidance. Activation of ephrin-A5 induces an initial change in cell adhesion followed by changes in cell morphology. Both effects are dependent on the activation of β1 integrin involving members of the Src family of protein tyrosine kinases. The prolonged activation of ERK-1 and ERK-2 is required for the change in cell morphology. Our work suggests a new role for class A ephrins in specifying the affinity of the cells towards various extracellular substrates by regulating integrin function.
Keywords: glycosylphosphatidylinositol anchor/mitogen-activated protein kinases/Src-family kinases
Introduction
Eph receptor tyrosine kinases and their membrane-bound ligands, the ephrins, are involved in multiple aspects of embryonic development, most notably the formation of spatial boundaries, tissue morphogenesis, control of angiogenesis and axonal guidance (for reviews see Frisen et al., 1999; Holder and Klein, 1999; O’Leary and Wilkinson, 1999). It has been proposed that ephrins and Eph receptors control cell position and movement through an Eph receptor-induced repulsive process activated after binding to their appropriate ligands (Gale and Yancopoulos, 1997). However, it is now clear that ephrins also play an active role in regulating cell migration and positioning during embryonic development, through the generation of a reverse signal upon interaction with their cognate receptors (Holland et al., 1996; Bruckner et al., 1997; Davy et al., 1999).
Ephrins are divided into two major classes, which differ by their mode of attachment to the plasma membrane (Gale et al., 1996). Class A ephrins are tethered to the plasma membrane by virtue of a glycosylphosphatidylinositol (GPI) anchor, whereas class B ephrins are transmembrane proteins. The importance of ephrin-induced signalling during embryonic development has been shown genetically for both classes of ephrins. In mice, a kinase-inactive form of EphB2 receptor was able to rescue the defects observed in the EphB2-deficient animals, suggesting that activation of its ligand, ephrin B1, by the mutant receptor was sufficient for proper axon guidance (Henkemeyer et al., 1996; Birgbauer et al., 2000). In Caenorhabditis elegans, ephrin homologues, which all contain a motif for a GPI modification, were shown to function in a kinase-independent VAB-1 Eph receptor pathway to control morphogenesis (Chin-Sang et al., 1999; Wang et al., 1999). There is also biochemical evidence to support a role for both ephrin B (Holland et al., 1996; Bruckner et al., 1997) and ephrin A ligands (Davy et al., 1999) in transmitting a signal upon interaction with their cognate Eph receptor.
The mechanisms by which ephrins activate downstream signalling pathways, and the consequences of these pathways at the cellular level, are still poorly understood. As is the case for many GPI-anchored proteins, the ephrin A molecules are clustered on the cell surface in discrete microdomains of the plasma membrane. These lipid rafts or caveola-like domains are characterized by their unique protein and lipid composition, including enrichment in glycosphingolipids such as gangliosides (e.g. GM1). We have recently shown that the GPI-anchored ephrin-A5 molecule is able to generate a signalling cascade that is restricted to the caveola-like domains of the plasma membrane upon binding to the extracellular domain of its cognate Eph receptor (Davy et al., 1999). Although the exact mechanism by which the signal is generated is unknown, it appears to require the activity of the Src-family kinase Fyn. The physiological consequence of such a signalling event is a change in cell adhesion in the ephrin-expressing cells (Davy et al., 1999).
The integrin family of adhesion receptors plays an essential role in embryonic development, regulating cell adhesion and migration by binding to extracellular matrix (ECM) proteins (Hynes, 1987). The integrin receptors are heterodimeric proteins composed of individual α and β subunits. The ability of integrins to bind to their respective ligands is a dynamic process regulated by conformational changes in their extracellular domains that can be regulated by way of intracellular signalling pathways, generally referred to as ‘inside-out’ signalling (Hynes, 1992; Hughes and Pfaff, 1998). In addition, integrin binding can be modulated by interactions with a number of membrane-associated proteins (Porter and Hogg, 1998). In addition to controlling physical aspects of cell adhesion, integrins are also able to activate cytoplasmic signalling cascades to regulate cell proliferation, cell survival and cell migration. Integrins are able to transduce an intracellular signal by activating various downstream effectors such as protein tyrosine kinases [e.g. focal adhesion kinase (FAK)], lipid kinases [phosphatidylinositol 3-kinase (PI3-K)] and small molecular weight GTPases (e.g. Rho family) (for review see Giancotti and Ruoslahti, 1999). All of these pathways can lead to the activation of the mitogen-activated protein kinases (MAPKs), including ERK-1 and ERK-2, which are major effectors in integrin signalling.
In order to determine how GPI-anchored ephrins control axon guidance and morphogenesis during development, we focused our attention on the mechanism by which ephrin-A5 regulates cell adhesion. Using an ectopic expression system, we showed that short-term engagement of ephrin-A5 by its cognate Eph receptor increases adhesion by modulating integrin affinity via a Src-family kinase-dependent signalling cascade. Engagement of ephrin-A5 resulted in MAPK activation, by both integrin-dependent and -independent pathways. We also demonstrated that MAPK activation is crucial for the long-term effect of ephrin-A5 engagement on cell morphology and architecture. Moreover, we were able to show that these effects of ephrin-A5 signalling are analogous in endogenously expressing retinal neurons, in sustaining neurite outgrowth, thus revealing a new role for GPI-linked ephrins in vivo.
Results
RGD-dependent integrins are required for the increased adhesion induced by ephrin-A5
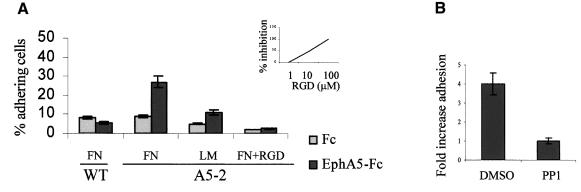
We have shown previously that the GPI-anchored protein ephrin-A5 controls cell architecture and cell adhesion through a signalling cascade involving the Src-family tyrosine kinase Fyn (Davy et al., 1999). To explore further the mechanisms by which ephrin-A5-induced signalling regulates cell adhesion, we assessed the involvement of integrins in this process. NIH 3T3 cells ectopically expressing ephrin-A5 were subjected to adhesion assays on various components of the extracellular matrix. The ephrin-A5-specific signalling cascade was activated by incubation of the cells with a chimera composed of the extracellular domain of the EphA5 receptor fused to the Fc fragment of human IgG (EphA5–Fc). As described previously, the engagement of ephrin-A5 by EphA5–Fc induces an increased adhesion of the cells expressing ephrin-A5 to fibronectin (Figure 1A). Control cells (WT) that do not express ephrin-A5 did not exhibit an increased adhesion to fibronectin (Figure 1A). When the same type of experiment was performed on laminin, cells expressing ephrin-A5 showed a more limited but significant increased adhesion after treatment with EphA5–Fc (Figure 1A). However, no increase in the adhesive properties of the cells was observed on poly-l-lysine-coated plates, suggesting that integrins were actively involved in the increased adhesion (data not shown). These experiments were reproduced several times and showed a consistent level of increased adhesion following EphA5–Fc treatment (3- to 4-fold), although the basal adhesion varied from experiment to experiment. To assess the involvement of integrins in the process activated by ephrin-A5, the effect of EphA5–Fc was monitored in the presence of soluble RGD peptide. This competitive inhibitor was capable of blocking the increased adhesion of the cells on fibronectin (Figure 1A) in a dose-dependent manner (Figure 1A, inset). This result demonstrated that the increased adhesion observed in response to ephrin-A5 engagement involves at least one RGD-dependent integrin. These experiments did not allow us to rule out the possible involvement of an RGD-independent integrin such as α4β1.
Fig. 1. Integrins are involved in the ephrin A5-induced increase in cell adhesion. (A) NIH 3T3 cells transfected with control vector (WT) or expressing ephrin A5 (A5-2) were detached from tissue culture plates, serum starved for 2 h and processed for adhesion assays on various substrates (FN, fibronectin; LM, laminin). The results are expressed as the percentage of adhering cells on a particular substrate after treatment with the Fc control or EphA5–Fc. To test for the effect of the RGD peptide, cells were pre-incubated with the peptide before treatment with the fusion proteins. The inset shows the dose-dependent inhibition of ephrin A5-induced adhesion by RGD peptide. (B) Adhesion assays were performed with cells expressing ephrin A5 pretreated with dimethylsulfoxide (DMSO) as a control or with 10 µM PP1. The results are presented as fold increase in adhesion (the number of adhering cells in the Fc treatment divided by the number of adhering cells in the EphA5–Fc treatment). Each figure shows a representative experiment and each point represents the mean ± SEM of triplicate wells.
As mentioned above, the activity of Src-family kinases, more specifically the activity of Fyn, is necessary for the ephrin-A5-induced signalling cascade to occur. In the presence of the Src-family kinase selective inhibitor PP1, engagement of ephrin-A5 could no longer induce the formation and stabilization of focal adhesion complexes, as monitored by vinculin staining (Davy et al., 1999). To determine whether Src-family kinases were required for the activation of the integrin(s) in response to ephrin-A5 engagement, ephrin-A5 was activated in the presence of PP1. Consistent with our previous results, inhibition of Src-family kinase activity prevented the increased adhesion of the cells expressing ephrin-A5 on fibronectin, following EphA5–Fc treatment (Figure 1B).
Collectively, these results demonstrate that engagement of ephrin-A5 by the extracellular domain of its cognate receptor induced the activation of at least one RGD-dependent integrin. The rapid increase in adhesion that was observed after 5 min of plating suggests that ephrin-A5 regulates integrin function via an ‘inside-out’ signalling pathway, possibly involving the Src-family kinases.
Activated integrin β1 participates in the response downstream of ephrin-A5
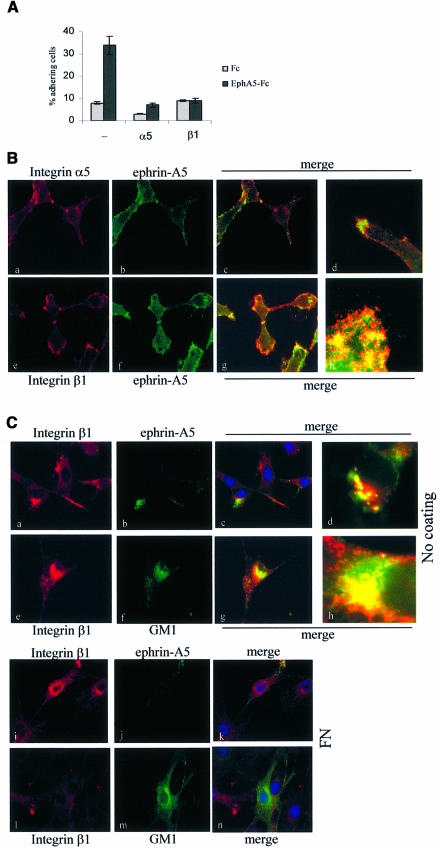
Ephrin-A5-induced increased adhesion was more potent when cells were plated on fibronectin, although an increase was also observed on laminin. Integrin α5β1 is the major fibronectin receptor and both of these integrin subunits are highly expressed in murine fibroblasts (data not shown). In order to address whether this integrin is involved in ephrin-A5 signalling, we used specific function-blocking antibodies. Antibodies specific for integrin β1 and integrin α5 were tested independently in adhesion assays performed on fibronectin. The anti-α5 integrin antibody reduced basal adhesion and ephrin-A5-induced adhesion (Figure 2A). The anti-β1 integrin antibody consistently prevented the increased adhesion after EphA5–Fc treatment. The increased cell adhesion in response to ephrin-A5 engagement was also blocked by the β1 function-blocking antibody in the neuroblastoma– glioma cell hybrid NG108-15, which endogenously expresses ephrin-A5 (data not shown). To examine further the contribution of each integrin subunit with respect to the ephrin-A5 signalling pathway, the relative distribution of these proteins at the cell surface was analysed by immunofluorescence. These experiments clearly showed that, while ephrin-A5 and α5 integrin marginally co-localized at the cell surface (Figure 2B, a–d), both ephrin-A5 and β1 integrin showed significant co-localization (Figure 2B, e–h). Ephrin A5 co-localized more strongly with the integrins at sites of dynamic cytoskeletal rearrangements, such as the ends of processes (Figure 2B, d) or leading edges (Figure 2B, h), suggesting that ephrin-A5 co-localizes preferentially with a pool of integrins that are actively involved in cytoskeletal rearrangements. This observation, as well as the results obtained with the function-blocking antibodies, strongly suggested that integrin β1 is involved in the increased adhesion induced via ephrin-A5 engagement.
Fig. 2. Activated integrin β1 supports ephrin A5-induced adhesion and co-localizes with the GPI-linked ligand. (A) Adhesion assays were performed as described in Figure 1 with NIH 3T3 cells expressing ephrin A5, in the presence of 2.5 µg/ml function-blocking antibody specific for α5 integrin (α5) or β1 integrin (β1), or in the absence of antibody (–). Each point represents the mean ± SEM of triplicate wells. (B) NIH 3T3 cells expressing ephrin A5 were analysed by immunofluorescence using EphA5–Fc as a probe to detect ephrin A5 (b and f), detected with FITC-labelled anti-human IgG and an antibody against α5 integrin (a) or β1 integrin (e), detected with a Cy3-labelled secondary antibody. Panels c, d, g and h are merged images of the two staining profiles (d and g are at a higher magnification). (C) NIH 3T3 cells expressing ephrin A5 were plated on glass cover slips left untreated (a–h) or coated with 5 µg/ml fibronectin (g–l). Cells were incubated for 45 min with anti-β1-integrin antibody and either EphA5–Fc (a–d and i–k) or EphA5–Fc and FITC-conjugated cholera toxin B-subunit to detect GM1 gangliosides (e–h and l–n). Surface-bound proteins were acid washed before fixation and permeabilization, and internalized proteins were detected using labelled secondary antibodies: FITC-conjugated anti-human IgG to detect EphA5–Fc and Cy3-conjugated anti-hamster IgG to detect the β1 integrin antibody. Panels c, g, k and n are merged images of the two staining patterns at a 63× magnification, and d and h are the merged images at higher magnification.
Activated and inactivated integrins co-exist at the cell surface. Recycling of activated integrins seems to be necessary for their function (Ng et al., 1999). To test whether such a transport of activated integrins occurred in relation to ephrin-A5-induced signalling, we performed immunofluorescence studies to monitor β1 integrin internalization. Because integrin β1 co-localized with ephrin-A5 at the cell surface and ephrin-A5 is clustered within caveola-like domains at the plasma membrane, we postulated that integrin β1 could be internalized through a caveola-mediated endocytic pathway. We used GM1 ganglioside as a marker for this pathway, since GM1 is enriched in these specific microdomains and its endocytic route is well described (Nambiar et al., 1993; Parton, 1994). The anti-β1-integrin antibody was used to follow integrin internalization, while cholera toxin was used to follow GM1 internalization. All experiments were carried out in the presence of EphA5–Fc. In the absence of fibronectin engagement, β1 integrin was internalized in two distinct populations. One pool co-localized with EphA5–Fc (Figure 2C, a–d), which is presumably internalized after binding to ephrin-A5, while the other pool co-localized with cholera toxin internalized after binding to GM1 (Figure 2C, e–h). Ephrin A5-positive and GM1-positive internal compartments never co-localized (data not shown). When the experiment was carried out with cells plated onto fibronectin, internalized β1 integrin– GM1 co-localization was rarely detected (Figure 2C, l–n), although β1 integrin and ephrin-A5 were still detected within the same internal compartment (Figure 2C, i and k). These results indicate that β1 integrin can be sorted differently whether it is bound to its ligand or not. Ligand-unoccupied β1 integrin seems to be sorted with GM1, while engaged β1 integrin is internalized and/or sorted with ephrin-A5, and could represent a recycling pathway.
These results strongly suggest that ephrin-A5 and β1 integrin are localized in close proximity at the cell surface, thus allowing functional interaction leading to the modulation of β1 integrin function in response to ephrin-A5 engagement.
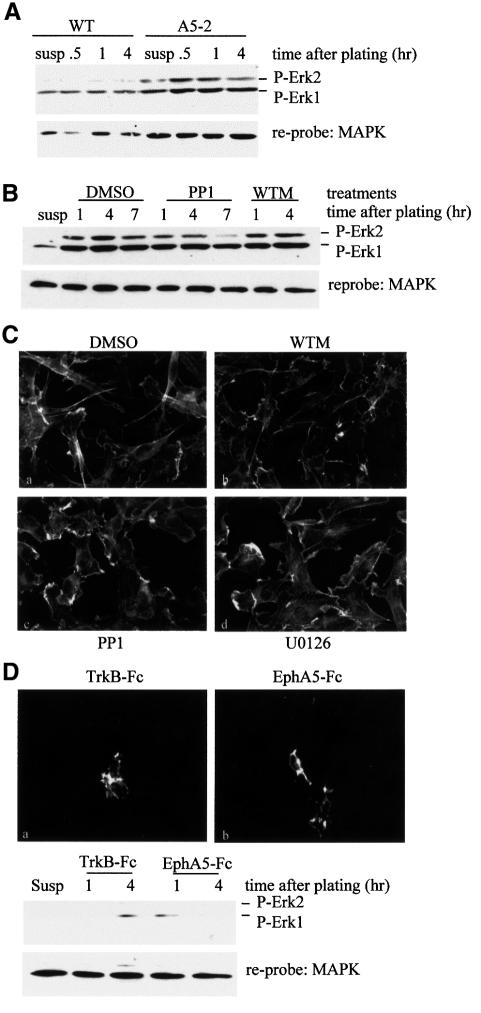
Activation of β1 integrin is required, but not sufficient, for the physiological response induced by ephrin-A5
From the results presented above, it is clear that ephrin-A5 can modulate the ability of β1 integrin to bind to fibronectin. In order to test whether ephrin-A5-induced signalling could activate β1 integrin in a fibronectin-independent manner, we used the EphA5–Fc chimera as a plating substrate and monitored downstream effectors of integrin activation, specifically FAK, ERK-1 and ERK-2. Plating of serum-starved cells expressing ephrin-A5 kept in suspension induced FAK tyrosine phosphorylation, which often correlates with integrin activation (Figure 3A). Importantly, the tyrosine phosphorylation of FAK was more potent when cells were plated onto EphA5–Fc, as compared with cells plated onto TrkB–Fc, especially at the earlier time point (1 h) (Figure 3A). This result is consistent with the hypothesis that ephrin-A5 engagement by its cognate receptor results in the activation of β1 integrin. To demonstrate that β1 integrin was activated in a specific manner following ephrin-A5 engagement, we monitored the activation status of MAPKs (ERK-1 and ERK-2), which are known downstream effectors for integrins. Plating of serum-starved cells expressing ephrin-A5 on fibronectin induced phosphorylation of both MAPKs (Figure 3B). Similarly, plating of the cells on EphA5–Fc resulted in phosphorylation of MAPKs, but the activation appeared more robust and prolonged. Pre-incubation of the cells with a function-blocking antibody specific for β1 integrin before plating on EphA5–Fc slightly reduced the level of MAPK phosphorylation (Figure 3C), indicating that only part of the MAPK activation seen in response to ephrin-A5 engagement was due to a signalling cascade downstream of integrin β1. Plating of the cells onto TrkB–Fc, a control fusion protein, did not result in robust activation of ERK-1 or ERK-2, although the cells were able to spread on this substrate, demonstrating that attachment and spreading alone are not sufficient to activate the MAPKs (Figure 3B and C). These results indicate that ephrin-A5 engagement is capable of inducing integrin activation in the absence of integrin engagement by fibronectin. They also demonstrate that ephrin-A5-induced signalling is not recapitulated entirely by integrin signalling, since the strength and duration of MAPK activation varied in both signalling cascades. To confirm further that engagement of ephrin-A5 resulted in activation of MAPKs through a mechanism distinct from integrin engagement alone, an immunofluorescence study was performed to visualize the cellular distribution of activated MAPKs. Two hours after plating, phosphorylated MAPKs could be detected only in cells plated onto EphA5–Fc (Figure 3D). Consistent with the western blot experiments, cells plated on EphA5–Fc in the presence of the function-blocking antibody exhibited an attenuated staining of phosphorylated MAPKs (Figure 3D, d). Similarly, cells plated on fibronectin, although more spread than cells plated onto either chimera, did not present a significant level of activated MAPKs at the 2 h time point, consistent with the kinetics of MAPK activation seen by western blot analysis (Figure 3D, c).
Fig. 3. Ephrin-A5 activates β1 integrin independently of fibronectin binding. (A) NIH 3T3 cells expressing ephrin A5 were trypsinized and serum starved for 2 h. Cells were either kept in suspension and lysed (‘susp’) or plated on six-well plates coated with the fusion proteins (TrkB–Fc or EphA5–Fc) as indicated. FAK was immunoprecipitated at various times after plating and its tyrosine phosphorylation status was monitored by western blotting using the monoclonal antibody 4G10. The membrane was reprobed with a monoclonal antibody specific for FAK to control for the levels of FAK in each sample. (B) NIH 3T3 cells expressing ephrin A5 were trypsinized and serum starved for 2 h. Cells were either kept in suspension and lysed (‘susp’) or plated on six-well plates coated with the fusion proteins (TrkB–Fc or EphA5–Fc) or fibronectin (FN), as indicated. One hour and 4 h after plating, cells were lysed and cell-free lysates were subjected to SDS–PAGE. In the experiments shown in the top panel, activated ERK-1 and ERK-2 were detected by western blotting using an antibody specific for phosphorylated MAPKs. The membranes were then reprobed with an anti-MAPK antibody to detect total MAPK levels as indicated in the bottom panel. (C) Serum-starved cells expressing ephrin A5 were left untreated (–) or were pre-incubated with 2.5 µg/ml anti-β1-integrin function-blocking antibody and plated for various times on EphA5–Fc or TrkB–Fc. Lysates were subjected to SDS–PAGE and western blot analysis using an antibody specific for phosphorylated MAPKs. Membranes were reprobed with an anti-MAPK antibody as a loading control. (D) NIH 3T3 cells expressing ephrin-A5 were plated onto cover slips coated with TrkB–Fc, EphA5–Fc or fibronectin as indicated, in the absence (a–c) or presence of a function-blocking antibody specific for β1 integrin (d). Two hours after plating, cells were fixed and processed for immunofluorescence using a monoclonal antibody recognizing total MAPKs (detected by a FITC-conjugated anti-mouse IgG) and a polyclonal antibody specific for phosphorylated MAPKs (detected by a Cy3-conjugated anti-rabbit IgG).
Prolonged activation of the ephrin-A5 signalling pathway induces changes in cell morphology
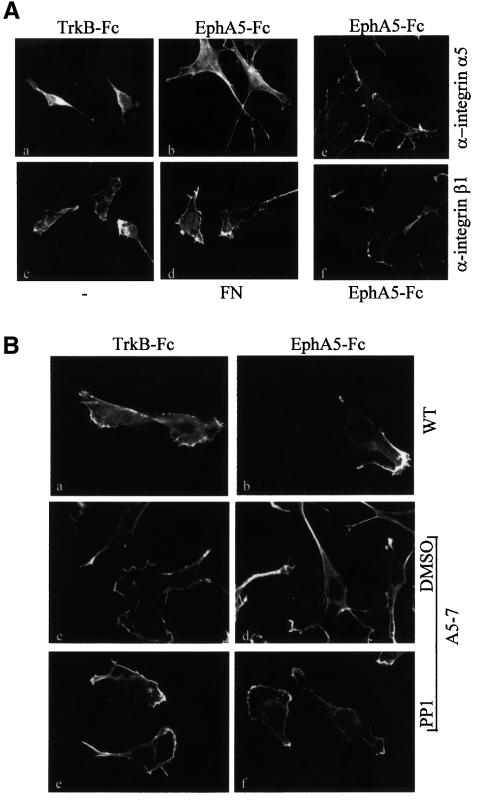
In the experiments described so far, we have focused on the short-term effects induced following ephrin-A5 engagement by its cognate Eph receptor. In vivo, interactions between Eph receptors and ephrins are likely to occur over more extended periods. To examine the long-term consequences of ephrin-A5-induced signalling, cells expressing ephrin-A5 were cultured overnight on various substrates, including fibronectin and EphA5–Fc, and then stained with phalloidin to visualize the actin cytoskeleton as an indicator of their morphology. Cells plated onto the EphA5–Fc chimera were morphologically distinct from those plated on fibronectin or control substrates: they were characterized by the extension of processes that were often branched and by the formation of numerous filopodia (Figure 4A). The change in morphology was quantified by assessing the percentage of total cells with more than three processes and/or branched processes (see Materials and methods). Among the cells plated on fibronectin or the control TrkB–Fc chimera, 6.5 and 9.2% bore processes, whereas plating onto EphA5–Fc induced a 3-fold increase (to 28%) in the proportion of process-bearing cells. Importantly, this response could be inhibited by pre-incubating the cells with function-blocking antibodies against β1 integrin (Figure 4A, f). The function-blocking antibody against α5 integrin showed little inhibition of the process extension (Figure 4A, e), raising the question of the involvement of α5 integrin downstream of ephrin-A5. Alternatively, the antibody used might not be a potent function-blocking agent. In accordance with the results obtained for MAPK activation, these results demonstrate that β1 integrin is required downstream of ephrin-A5, but that engagement of integrins with fibronectin is not sufficient to mimic ephrin-A5-induced physiological response.
Fig. 4. β1 integrin and Src-family kinases are involved in the morphological changes induced by ephrin A5 engagement. (A) Cells expressing ephrin A5 were plated on glass cover slips coated with various substrates as indicated (a, b, d) or not coated (c). After 16 h, cells were fixed and subjected to immunofluorescence staining using rhodamine-conjugated phalloidin. An analogous experiment was performed on cover slips coated with EphA5–Fc, in the presence of 2.5 µg/ml function-blocking antibodies specific for α5 integrin (e) or β1 integrin (f). (B) NIH 3T3 cells transfected with the control vector (WT), and cells expressing ephrin-A5 (A5-7) were plated on glass cover slips coated with TrkB–Fc (a, c, e) or EphA5–Fc (b, d, f). Before plating, cells were pretreated with DMSO (c, d) or 10 µM PP1 (e, f), as indicated. After 16 h, cells were fixed and processed for phalloidin staining. Magnification: ×63.
These results demonstrate that, over the long term, ephrin-A5 promotes process extension via a mechanism involving β1 integrin. Since the loss of Src-family kinase activity has been shown to block early events induced by ephrin-A5, it was important to address their role in this long-term physiological process. Cells transfected with the vector alone were used as a control, and showed no morphological differences when plated on TrkB–Fc (7%) or EphA5–Fc (8.6%) (Figure 4B, a and b). As described above, cells expressing ephrin-A5 extended processes when plated onto EphA5–Fc (28%). This response was not observed in the presence of PP1 (4.2%), demonstrating the requirement for Src-family kinase activity in this process (Figure 4B, e and f).
MAPKs are essential effectors of the ephrin-A5-induced change in morphology
As shown in Figure 3A, plating of NIH 3T3 cells expressing ephrin-A5 on EphA5–Fc induced prolonged MAPK activation. In Figure 4 we showed that Src-family kinases were involved in the morphological changes observed after plating on this substrate. In an attempt to describe the relative contribution of these signalling molecules in the pathways activated in response to ephrin-A5 engagement, the same type of plating experiment was performed in the presence of various inhibitors over a 7 h time course, and the status of MAPK activation was monitored. Control cells that do no express ephrin-A5 showed only a slight increase in the phosphorylation status of MAPK after plating onto EphA5–Fc (Figure 5A). Consistent with the previous result, plating of cells expressing ephrin-A5 onto EphA5–Fc resulted in a potent and prolonged activation of the MAPKs (Figure 5A). Unexpectedly, inhibition of the Src-family kinases did not completely block MAPK activation induced by plating of the cells onto EphA5–Fc. However, the level and duration of activation were attenuated in the presence of PP1 (Figure 5B). MAPK activation was unaffected by wortmannin, an inhibitor of PI3-K, suggesting that this kinase is not involved in the pathway downstream of ephrin-A5 (Figure 5B). These results indicate that there might be two distinct pathways leading to MAPK activation following ephrin-A5 engagement: one dependent on Src-family kinases and possibly involving β1 integrin, and the other being independent of these proteins.
Fig. 5. Ephrin A5 activates two distinct pathways to induce prolonged MAPK activation, a process required for morphological changes. (A) NIH 3T3 cells transfected with the vector control (WT) and cells expressing ephrin A5 (A5-2) were serum starved for 2 h and plated on six-well plates coated with EphA5–Fc. Cells were lysed at various times after plating and the status of MAPK activation was monitored by western blotting. Membranes were reprobed with an anti-MAPK antibody. (B) The same type of experiment as described above was performed with NIH 3T3 cells expressing ephrin A5, in the presence of DMSO, 10 µM PP1 or 100 nM wortmannin (WTM), plated on EphA5–Fc. (C) NIH 3T3 cells expressing ephrin A5 were plated overnight on EphA5–Fc-coated cover slips (a–d), in the presence of various inhibitors as indicated, and stained with phalloidin–rhodamine. (D) NG108-15 cells were plated on TrkB–Fc or EphA5–Fc as indicated and either grown overnight and stained with phalloidin–rhodamine (top) or lysed after the indicated times (in hours) after plating for western blot analysis of MAPK activation (bottom). Magnification: 63×.
The contribution of the MAPK pathway in the long-term effect of ephrin-A5-induced signalling was demonstrated using the MEK inhibitor U0126. In the presence of this inhibitor, cells expressing ephrin-A5 adhered to EphA5–Fc, similarly to the DMSO-treated control cells although no activation of MAPKs could be detected (data not shown). However, overnight treatment with U0126 prevented the cells from extending processes when plated on EphA5–Fc, analogous to the PP1-treated cells (Figure 5C, c and d). The activity of PI3-K was not required for this physiological response, since treatment with a PI3-K inhibitor, wortmannin, had no effect on the ability to extend processes (Figure 5C, b). However, it should be noted that wortmannin treatment affected cell morphology independently of the substrate used for plating and thus the exact involvement of PI3-K downstream of ephrin-A5 remains equivocal. The role of MAPKs in the extension of cellular processes observed in response to ephrin-A5 engagement was further confirmed using NG108-15 cells. As previously shown, NG108-15 cells are responsive to ephrin-A5-induced increased adhesion on fibronectin (Davy et al., 1999); however, after plating on EphA5–Fc, these cells were unable to spread and extend processes (Figure 5D, top panel), even though they express β1 integrin (data not shown). Correlating with this lack of response, NG108-15 cells did not exhibit any increase in MAPK activation following plating on EphA5–Fc (Figure 5D, bottom panel). Collectively, these results suggest that the prolonged MAPK activation after ephrin-A5 engagement is essential for the morphological changes observed.
Engagement of ephrin-A5 induces neurite outgrowth on primary retinal neurons
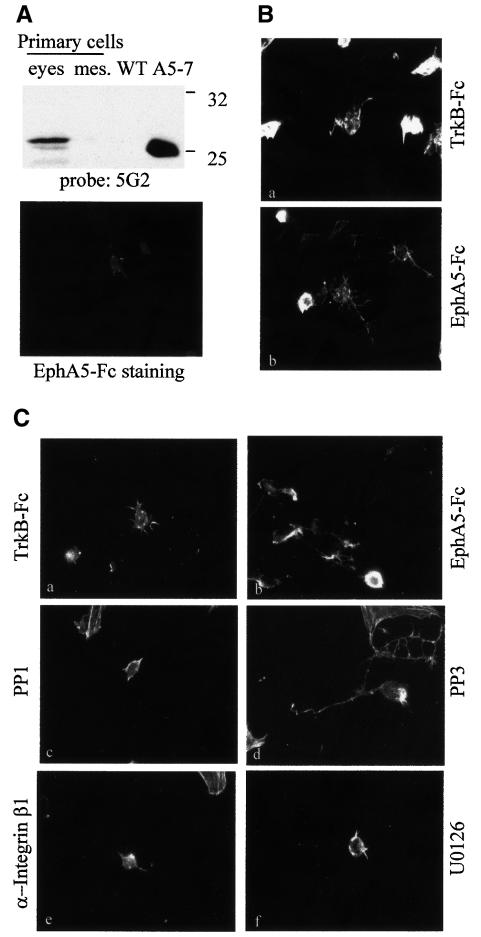
It is clear from the work presented here that engagement of ephrin-A5 by its cognate Eph receptor has a profound effect on cell morphology. In order to determine whether a similar physiological response can be observed with primary cells expressing ephrin-A5, cells were isolated from embryonic retinas and primary cultures were established. The expression of ephrin-A5 in these primary cells was confirmed by western blotting and immunofluorescence (Figure 6A). Among the various primary cell types obtained, only retinal neurons (as characterized by morphological features and β-tubulin reactivity) seemed affected by the substrate on which they were growing. The retinal neurons plated onto EphA5–Fc extended neurites and consistently appeared less refractile than those growing on TrkB–Fc (Figure 6B). Treatment of these cells with the Src-family kinase selective inhibitor PP1, the function-blocking antibody specific for β1 integrin or the MEK inhibitor U0126 all led to a decrease in the number of retinal ganglion neurons extending neurites (Figure 6C). However, treatment with a chemical compound analogous to PP1, but inactive for Src-family kinases (PP3), did not prevent the neurite extension observed with these neurons (Figure 6C, d). These results suggest that, as in the case for ephrin-A5-expressing fibroblasts, Src-family kinases, integrins and MAPKs appear essential for ephrin-A5-induced physiological responses in primary cells.
Fig. 6. Engagement of ephrin A5 stimulates neurite outgrowth on retinal neurons. (A) Primary cells isolated from E14 eyes and mesencephalon (mes) were lysed and analysed by western blotting with an antibody specific for ephrin A5 (5G2) (top). Caveola-like domains of NIH 3T3 cells transfected with the vector control (WT) and cells expressing ephrin A5 (A5-7) were used as negative and positive controls, respectively. The expression of ephrin A5 by retinal neurons was monitored by immunofluorescence using EphA5–Fc as a probe (bottom). (B) Primary cells isolated from E14 eyes were plated on glass cover slips coated with TrkB–Fc (a) or EphA5–Fc (b) as indicated, and cultured for 2 days. Cells were then fixed and stained with rhodamine-conjugated phalloidin. (C) An analogous experiment was performed in the presence of DMSO (a, b), 3 µM PP1 (c), 5 µM PP3 (d), 2.5 µg/ml function-blocking antibody specific for β1 integrin (e) or 10 µM U0126 (f). Cells were then fixed and stained with rhodamine-conjugated phalloidin. The figure shows cells representative of each treatment, after plating onto TrkB–Fc (a) or EphA5–Fc (b–f). Magnification: 63×.
Discussion
We have shown here that the GPI-linked ephrin-A5 is capable of activating β1 integrin rapidly, presumably via an ‘inside-out’ signalling pathway involving at least one member of the Src-family kinases. In retinal ganglion neurons, activation of integrin β1 downstream of ephrin-A5 was sufficient to sustain neurite outgrowth, mimicking the effect of plating these neurons on laminin or, to a lesser extent, on fibronectin (Rogers et al., 1983; Smalheiser et al., 1984). In the nervous system, integrin β1, in combination with the α6 subunit, forms the major laminin receptor expressed on retinal ganglion neurons. This integrin is also involved in laminin-induced neurite outgrowth in vitro (Cohen et al., 1987). Growing retinal axons navigate on a substrate of laminin to reach their target in the central nervous system (CNS). Before entering the CNS, retinal axons encounter a critical crossroads, the optic chiasm, where they ‘decide’ whether to continue straight across to the opposite side of the brain or to stay in the side of the brain on which they started. Although the molecules regulating the decision are still unknown, a recent study demonstrated that overexpression of ephrin A ligands on retinal axons perturbs their navigational choices at the optic chiasm (Dutting et al., 1999). In this study, it was shown that, after overexpression of ephrin-A5, more stable ipsilateral projections were observed, suggesting that the presence of ephrin-A5 on these axons favoured growth in conditions that are not permissive in the normal situation. These results could be consistent with an activation of β1 integrin downstream of ephrin-A5 in the axons overexpressing the ligand, and leading to the stabilization of ipsilateral projections. At the end of the optic tract, axons leave the laminin-containing basal lamina to enter their target lacking detectable laminin. The ability of retinal axons to adapt their growth to different substrates probably results from a change in integrin function or expression that axons undergo during development. It is known, for instance, that the laminin receptor is downregulated once retinal axons have reached their target (Cohen et al., 1986). Interestingly, we have observed the upregulation of β3 integrin in fibroblasts expressing ephrin-A5 (data not shown). This may reflect one of the developmental changes permitting retinal axons to switch substrate when they reach their target. In support of this hypothesis, β3 integrin expression by retinal neurons has been reported (Reichardt, 1991).
As has been described for Eph receptors (Huynh-Do et al., 1999; Zhou et al., 1999; Becker et al., 2000; Miao et al., 2000), we have shown that ephrin-A5 can modulate integrin function through ‘inside-out’ signalling. One possibility is that ephrin-A5 usurps the Eph receptors already known to influence integrin function (Huynh-Do et al., 1999; Zhou et al., 1999; Miao et al., 2000). However, this is very unlikely, since the transduction pathway activated downstream of ephrin-A5 did not result in a change in the tyrosine phosphorylation status of EphA2 (the Eph receptor that is endogenously expressed in NIH 3T3 cells). In addition, engagement of ephrin-A5 resulted in an increase in phosphorylation of FAK, whereas activation of the EphA2 receptor was shown to have the opposite effect (Miao et al., 2000). We are convinced that the phenotypes reported herein are due to ephrin-A5-induced signalling independent of an Eph receptor tyrosine kinase. It is possible that ephrin A ligands and integrins interact directly, as has been shown for another GPI-linked receptor, urokinase plasminogen activator receptor (uPAR) (Wei et al., 1996; Chapman et al., 1999). The uPAR– integrin interaction was dependent on the presence of caveolin, one of the structural proteins of caveolae, which has also been shown to modulate integrin function (Wary et al., 1998; Wei et al., 1999). The role of caveolin in the modulation of β1 integrin function by ephrin-A5 has not been evaluated, in part because we have never been able to demonstrate clearly a physical interaction between β1 integrin, ephrin-A5 and caveolin. In addition, although ephrin-A5 and β1 integrin clearly co-localize at the cell surface, we have not been able to show that caveolin and ephrin-A5 are present in the same microdomains at the plasma membrane (data not shown). Moreover, although β1 integrin partitions in a detergent-insoluble cellular compartment, this locale differs from caveola-like microdomains in its buoyant density, so the detergent-dependent purification procedure might disrupt the β1 integrin– ephrin-A5 interactions.
Activation of MAPKs is implicated in various biological processes, including proliferation, differentiation and neurite outgrowth in cells of neuronal origin. It has been proposed that the duration of MAPK activation is crucial for determining the decision between proliferation and differentiation (Traverse et al., 1992; Marshall, 1995). ERK-1 and ERK-2 can be activated by different pathways, the most common being the Ras–Raf–MEK cascade, but other signalling cascades involving FAK or small GTPases of the Rho family have also been shown to activate ERK-1 and ERK-2 (Lewis et al., 1998). However, the relative contribution of each pathway in the transient versus sustained activation of MAPKs is still poorly understood. It has recently been shown that sustained MAPK activation in PC12 cells in response to nerve growth factor involves two distinct pathways: initial activation involves Ras, while Rap1 (a related small molecular weight GTPase) is responsible for the prolonged MAPK activation that leads to neuronal differentiation (York et al., 1998). Similarly, we also found that sustained activation of ERK-1 and ERK-2 in response to ephrin-A5 engagement correlated with process extension, and that MAPK activation involved at least two pathways: one pathway was dependent on Src-family kinases, while the other one was independent of these kinases. Both pathways were independent of PI3-K. Src-family kinases could be involved in sustaining the activation of MAPKs, since, in the presence of the Src-family kinase inhibitor, the duration of MAPK activation was dramatically reduced. The components of the second independent pathway are unclear, but FAK could be involved.
Despite the important role for Src-family kinases in ephrin-A5-induced signalling and activation of β1 integrin, this study demonstrates the simultaneous activation of a parallel pathway independent of Src-family kinases in response to ephrin-A5 engagement. Both pathways cooperate to induce prolonged MAPK activation leading to profound morphological changes. These long-term morphological changes, together with the short-term modulation of adhesion, certainly contribute to the pleiotropic effects of ephrins in regulating morphogenesis and axon guidance. This study also emphasizes that both ephrins and Eph receptors are able to induce changes in the adhesive properties of the cells, thus lending further support to the notion that both classes of molecule are involved in bidirectional signalling for pattern formation and remodelling during embryogenesis.
Materials and methods
Reagents and cell lines
The EphA5–Fc and control TrkB–Fc fusion proteins were prepared as described previously (Davis et al., 1994; Gale et al., 1996). The monoclonal antibody specific for ephrin-A5, the fibroblast cell lines expressing ephrin-A5 and the neuroblastoma cell line expressing ephrin-A5 (NG108-15) were as described (Davy et al., 1999). All cell lines were maintained in Dulbecco’s modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS; Canadian Life Technologies, Gibco-BRL). All chemical inhibitors were from Calbiochem.
Primary cells
Eyes and mesencephalon from CD1 embryos at embryonic day 14 (E14) were dissected and incubated in phosphate-buffered saline (PBS) containing 0.2% trypsin and 50 µg/ml DNase to dissociate primary cells. The dissociated cells were plated on poly-ornithine or on fusion proteins (see below) and grown in DMEM supplemented with 0.5% FCS.
Biochemical analysis of MAPK and FAK activation
Cells were trypsinized and serum starved for 3 h at 37°C. Cells were left untreated or pre-incubated for 15 min with either vehicle alone (DMSO), 10 µM PP1, 100 nM wortmannin or 2.5 µg/ml anti-β1-integrin antibody (Pharmingen). Aliquots of the cell suspension were then plated in six-well plates coated with various Fc fusion proteins. Coating of the plates was performed by a 1 h incubation with PBS containing 100 µg/ml anti-human IgG, followed by a 3 h incubation (at 4°C) with 2.5 µg/ml purified Fc fusion proteins diluted in PBS, or in conditioned medium from COS-7 cells transiently transfected with the cDNAs coding for the individual fusion proteins. Cells were lysed in 1% NP-40 lysis buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 1 mM Na3VO4, 1% NP-40) at various times after plating and analysed by western blotting as described previously (Robbins et al., 1995), using a phospho-specific polyclonal antibody that recognizes both ERK-1 and ERK-2 (MAPKs) (Promega) and a monoclonal anti-MAPK antibody that was not activation specific (Zymed). For FAK immunoprecipitation, cell lysates were pre-cleared for 30 min at 4°C with protein A–Sepharose. Pre-cleared lysates were incubated overnight with 5 µg of anti-FAK monoclonal antibody (Transduction Laboratories). Immunocomplexes were precipitated using protein A–Sepharose and washed extensively in 1% NP-40 lysis buffer. Samples were analysed by western blotting using a monoclonal antibody specific for phosphotyrosine (4G10; Upstate Biotechnology Inc.). The membrane was reprobed with the FAK monoclonal antibody.
Immunofluorescence microscopy analysis
NIH 3T3 cells expressing ephrin-A5 or primary cells were plated on glass cover slips coated with Fc fusion proteins as described above and grown for 16 h in DMEM/10% FCS. Cells were fixed in 2% paraformaldehyde (PFA) for 10 min at room temperature and permeabilized in 0.1% Triton X-100 for 3 min. Cells were then incubated for 30 min in rhodamine-conjugated phalloidin (Molecular Probes) as recommended by the supplier. Changes in cell morphology were quantified by determining the proportion of cells bearing more than three processes and/or extending branched processes. Other morphological differences, such as the length of processes or the presence of filopodia, were not counted since they were difficult to evaluate. Each experimental condition was repeated three times and ≥100 cells per experiment were analysed. The percentage of process-bearing cells is the mean value from these three independent experiments. Surface staining for ephrin-A5 was performed on non-permeabilized cells using 4 µg/ml EphA5–Fc as a probe for ephrin-A5 and staining for integrin subunits was carried out using 2.5 µg/ml anti-α5-integrin (Pharmingen) or anti-β1-integrin antibody. Bound antibodies were detected by incubation with secondary antibodies [fluorescein isothiocyanate (FITC)-conjugated anti-human and Cy3-conjugated anti-hamster (Jackson ImmunoResearch Laboratories)]. For analysis of MAPK activation, cells were plated onto cover slips coated with various substrates, in the absence or presence of 2.5 µg/ml anti-β1-integrin function-blocking antibody. Two hours after plating, cells were fixed and permeabilized as described above, and incubated for 1 h at room temperature in a solution containing the polyclonal antibody specific for the phosphorylated forms of ERK-1 and ERK-2 (Promega; 1/100 dilution in PBS containing 1% bovine serum albumin) and the non-activation-specific monoclonal antibody recognizing ERK-1 and ERK-2 (Zymed; 10 µg/ml). Primary antibodies were detected with an anti-rabbit antibody conjugated with Cy3 and an anti-mouse antibody conjugated with FITC, respectively. Images were acquired on a Leica DMRB microscope and cells were photographed at 63× or 100× magnification as indicated.
Internalization studies
Cells were plated on cover slips coated with 5 µg/ml fibronectin or plated on uncoated cover slips. The next day, cells were incubated with various soluble reagents: 4 µg/ml EphA5–Fc; 2.5 µg/ml anti-β1-integrin antibodies; 4 µg/ml FITC-conjugated cholera toxin B-subunit (Sigma) for 45 min at 37°C. Cells were chilled on ice and subjected to an acid wash (0.5 mM NaCl, 0.2 mM acetic acid, pH 2) for 15 min to remove cell-bound proteins. After rinsing with ice-cold PBS, cells were permeabilized as described above and incubated for 1 h with secondary antibodies.
Adhesion assays
Fibroblasts stably transfected with ephrin-A5 cDNA or with the vector control were detached from the tissue culture plates using PUCKS-EDTA (5 mM KCl, 130 mM NaCl, 3 mM NaHCO3, 5 mM d-glucose, 10 mM HEPES pH 7.3, 1 mM EDTA) to preserve the integrity of the extracellular proteins. Cells were resuspended in DMEM containing 0.5% Cosmic calf serum (Hyclone) and incubated for 30 min at 37°C. Cells were left untreated or pre-incubated for 15 min with soluble RGD peptide (100 µM), anti-β1 antibody (5 µg/ml), PP1 (10 µM) or DMSO. Aliquots of cell suspension (0.25 × 106 cells/ml) were plated on tissue culture wells that had been pre-coated with 5 µg/ml fibronectin or 5 µg/ml laminin and the plates were immediately spun at 800 r.p.m. for 3 min. The medium was then supplemented with 8 µg/ml Fc or EphA5–Fc. After 5 min at 37°C, non-adhering cells were removed by extensive washing with PBS. The remaining adhering cells were then trypsinized and counted using a haemocytometer. The data, from three independent experiments performed in triplicate, are displayed as the percentage of adhering cells.
Acknowledgments
Acknowledgements
We wish to thank Dr Nick Gale (Regeneron, Tarrytown, NY) for providing the TrkB–Fc and EphA5–Fc cDNAs. We would also like to thank Laurie Roberston of the Alberta Cancer Board funded FACS Facility at the University of Calgary for her expert assistance. This work was supported by a grant from the Medical Research Council of Canada (MT-15647) and funds from the University of Calgary, Faculty of Medicine Neuro-degenerative Diseases Research Endowment. A.D. is a recipient of an Alberta Cancer Board Research Fellowship and S.M.R. is a scholar of the Alberta Heritage Foundation for Medical Research.
References
- Becker E., Huynh-Do,U., Holland,S., Pawson,T., Daniel,T.O. and Skolnik,E.Y. (2000) Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol. Cell. Biol., 20, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgbauer E., Cowan,C.A., Stretavan,D.W. and Henkemeyer,M. (2000) Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development, 127, 1231–1241. [DOI] [PubMed] [Google Scholar]
- Bruckner K., Pasquale,E.B. and Klein,R. (1997) Tyrosine phosphoryl ation of transmembrane ligands for Eph receptors. Science, 275, 1640–1643. [DOI] [PubMed] [Google Scholar]
- Chapman H.A., Wei,Y., Simon,D.I. and Waltz,D.A. (1999) Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb. Haemost., 82, 291–297. [PubMed] [Google Scholar]
- Chin-Sang I.D., George,S.E., Ding,M., Moseley,S.L., Lynch,A.S. and Chisholm,A.D. (1999) The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell, 99, 781–790. [DOI] [PubMed] [Google Scholar]
- Cohen J., Burne,J.F., Winter,J. and Bartlett,P.F. (1986) Retinal ganglion cells lose response to laminin with maturation. Nature, 322, 465–467. [DOI] [PubMed] [Google Scholar]
- Cohen J., Burne,J.F., McKinlay,C. and Winter,J. (1987) The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev. Biol., 122, 407–418. [DOI] [PubMed] [Google Scholar]
- Davis S., Gale,N.W., Aldrich,T., Maisonpierre,P.C., Lhotak,V., Pawson,T., Goldfarb,M. and Yancopoulos,G.D. (1994) Ligands for Eph-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science, 266, 816–819. [DOI] [PubMed] [Google Scholar]
- Davy A., Gale,N.W., Murray,E.W., Feuerstein,C., Klinghoffer,R., Soriano,P. and Robbins,S.M. (1999) Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev., 13, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutting D., Handwerker,C. and Drescher,U. (1999) Topographical targeting and pathfinding errors of retinal axons following overexpression of ephrin A ligands on retinal ganglion cells. Dev. Biol., 216, 297–311. [DOI] [PubMed] [Google Scholar]
- Frisen J., Holmberg,J. and Barbacid,M. (1999) Ephrins and Eph receptors: multitalented directors of embryonic development. EMBO J., 18, 5159–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N.W. and Yancopoulos,G.D. (1997) Ephrins and their receptors: a repulsive topic? Cell Tissue Res., 290, 227–241. [DOI] [PubMed] [Google Scholar]
- Gale N.W. et al. (1996) Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron, 17, 9–19. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. and Ruoslahti,E. (1999) Integrin signaling. Science, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M., Orioli,D., Henderson,J.T., Saxton,T.M., Roder,J., Pawson,T. and Klein,R. (1996) Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell, 86, 35–46. [DOI] [PubMed] [Google Scholar]
- Holder N. and Klein,R. (1999) Eph receptors and ephrins: effectors of morphogenesis. Development, 126, 2033–2044. [DOI] [PubMed] [Google Scholar]
- Holland S.J., Gale,N.W., Mbamalu,G., Yancopoulos,G.D., Henkemeyer,M. and Pawson,T. (1996) Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature, 383, 722–725. [DOI] [PubMed] [Google Scholar]
- Hughes P.E. and Pfaff,M. (1998) Integrin affinity modulation. Trends Cell Biol., 8, 359–364. [DOI] [PubMed] [Google Scholar]
- Huynh-Do U., Stein,E., Lane,A.A., Liu,H., Cerreti,D.P. and Daniel,T.O. (1999) Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through αvβ3 and αvβ1 integrins. EMBO J., 18, 2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. (1987) Integrins: a family of cell surface receptors. Cell, 48, 549–554. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1992) Integrins: versatility, modulation and signaling in cell adhesion. Cell, 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Lewis T.S., Shapiro,P.S. and Ahn,N.G. (1998) Signal transduction through MAP kinase cascades. Adv. Cancer Res., 74, 49–139. [DOI] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Miao H., Burnett,E., Kinch,M., Simon,E. and Wang,B. (2000) Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nature Cell Biol., 2, 62–69. [DOI] [PubMed] [Google Scholar]
- Nambiar M.P., Oda,T., Chen,C., Kuwazuru,Y. and Wu,H.C. (1993) Involvement of the Golgi region in the intracellular trafficking of cholera toxin. J. Cell Physiol., 154, 222–228. [DOI] [PubMed] [Google Scholar]
- Ng T., Shima,D., Squire,A., Bastiaens,P.I.H., Gschmeissner,S., Humphries,M.J. and Parker,P.J. (1999) PKCα regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J., 18, 3909–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary D.D. and Wilkinson,D.G. (1999) Eph receptors and ephrins in neural development. Curr. Opin. Neurobiol., 9, 65–73. [DOI] [PubMed] [Google Scholar]
- Parton R.G. (1994) Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem., 42, 155–166. [DOI] [PubMed] [Google Scholar]
- Porter J.C. and Hogg,N. (1998) Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol., 8, 390–396. [DOI] [PubMed] [Google Scholar]
- Reichardt L.F. (1991) Extracellular matrix molecules and their receptors: Functions in neural development. Annu. Rev. Neurosci., 14, 531–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins S.M., Quintrell,N.A. and Bishop,J.M. (1995) Myristoylation and differential palmitoylation of the HCK protein-kinases govern their attachment to membranes and association with caveolae. Mol. Cell. Biol., 15, 3507–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S.L., Letourneau,P.C., Palm,S.L., McCarthy,J.B. and Furcht,L.T. (1983) Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev. Biol., 98, 212–220. [DOI] [PubMed] [Google Scholar]
- Smalheiser N.R., Crain,S.M. and Reid,L.M. (1984) Laminin as a substrate for retinal axons in vitro. Brain Res., 314, 136–140. [DOI] [PubMed] [Google Scholar]
- Traverse S., Gomez,N., Paterson,H., Marshall,C. and Cohen,P. (1992) Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J., 288, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Roy,P.J., Holland,S.J., Zhang,L.W., Culotti,J.G. and Pawson,T. (1999) Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol. Cell, 4, 903–913. [DOI] [PubMed] [Google Scholar]
- Wary K.K., Mariotti,A., Zurzolo,C. and Giancotti,F.G. (1998) A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell, 94, 625–634. [DOI] [PubMed] [Google Scholar]
- Wei Y., Lukashev,M.E., Simon,D.I., Bodary,S.C., Rosenberg,S., Doyle,M.V. and Chapman,H.A. (1996) Regulation of integrin function by the urokinase receptor. Science, 273, 1551–1555. [DOI] [PubMed] [Google Scholar]
- Wei Y., Yang,X., Liu,Q., Wilkins,J.A. and Chapman,H.A. (1999) A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol., 144, 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York R.D., Yao,H., Dillon,T., Ellig,C.L., Eckert,S.P., McCleskey,E.W. and Stork,P.J. (1998) Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature, 392, 622–626. [DOI] [PubMed] [Google Scholar]
- Zhou J.X., Wang,B., Kalo,M.S., Zisch,A.H., Pasquale,E.B. and Ruoslahti,E. (1999) An Eph receptor regulates integrin activity through R-Ras. Proc. Natl Acad. Sci. USA, 96, 13813–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]