Abstract
Background
2009 H1N1 influenza A disproportionately affected pregnant and postpartum women compared to the general population with higher rates of hospitalization and severe illness. The purpose of this study was to examine the use and pharmacokinetics of intravenous peramivir in the treatment of a newly postpartum patient with severe influenza.
Case
A 28-year-old, 37 week pregnant (G4,P3) woman presented to the hospital with severe respiratory symptoms. 2009 H1N1 influenza RT-PCR returned positive and intravenous peramivir was started. She showed rapid improvement and was discharged 29 days following admission.
Conclusion
The pharmacokinetic parameters for this case patient were unexpected based on previously reported pharmacokinetic parameters from phase 1 trials. These differences highlight the need for additional pharmacokinetic reporting of peramivir in pregnant and postpartum patients.
Introduction
On April 26, 2009, the United States Secretary of Health and Human Services declared a public health emergency due to the 2009 H1N1 influenza. On June 11, 2009, the World Health Organization declared an influenza pandemic. The 2009 outbreak disproportionately targeted healthy adults between the ages of 25 to 49 (1).
Pregnant women infected by 2009 H1N1 influenza experienced higher rates of severe illness and hospitalization than the general population (1). According to the Food and Drug Administration (FDA), an estimated five percent of confirmed 2009 H1N1 influenza deaths in the United States from April 14 to August 21, 2009 were pregnant women. While there is no evidence of pregnant women being more susceptible to influenza infection, the complication rate among infected pregnant women is higher than that of non-pregnant women (1, 2). The hospital admission rate for 2009 H1N1 influenza infection was 0.32 per 100 000 for pregnant women compared to 0.076 per 100 000 for the general population, a nearly five-fold increase (1).
Due to the lack of FDA-approved intravenous antiviral agents for use in critically ill patients, in April 2009 FDA requested BioCryst Pharmaceuticals to provide peramivir, an investigational IV neuraminidase inhibitor under FDA Emergency Investigational New Drug (eIND) regulations (21 CFR 312.56). Subsequently in October 2009, this drug was made available through the Centers for Disease Control and Prevention (CDC) by FDA Emergency Use Authorization (EUA). Peramivir is a cyclopentane analogue that binds to influenza neuraminidase, thereby preventing virus release from infected cells and halting viral replication. Peramivir is able to inhibit all known strains of human influenza A and influenza B viruses in vitro (3). To date, there are limited data available on the use of peramivir in pregnancy or the postpartum period.
Case
A 28-year-old otherwise healthy woman in her 37th week of pregnancy (G4,P3) presented on September 20th, 2009 with a 3 day history of fever, myalgia, cough and progressive dyspnea (see Table 1). Her examination was significant for bilateral diffuse rales and progressive, severe tachypnea and hypoxemia. The fetus was near full term, viable and thought to be in no distress. The patient had no leukocytosis and a nasopharyngeal swab for rapid influenza antigen A and B was negative. Blood and sputum cultures were obtained. A chest x-ray revealed bilateral diffuse interstitial infiltrates.
Table 1.
Timeline of events and clinical status changes
| Day | Patient Status and Treatment Course* | Laboratory/Clinical Values* |
|---|---|---|
| Day 0 |
|
SrCr = 0.78 mg/dL Wt = 100.8 kg CrCl = 93 mL/min |
| Day 1 |
Over course of next 3 days:
|
SrCr = 0.8 mg/dl CrCl = 90 mL/min FB: + 896 mL (C-section) |
| Day 2 | SrCr = 0.76 mg/dl Wt = 100.8 kg CrCl = 95 mL/min FB: + 2,975 mL |
|
| Day 3 |
|
SrCr = 0.61 mg/dl Wt = 103.7 kg CrCl = 119 mL/min FB: + 280 mL |
| Day 4 | SrCr = 0.6 mg/dl Wt = 103.8 kg CrCl = 120 mL/min FB: − 262 mL |
|
| Day 5 |
|
SrCr = 0.47 mg/dl CrCl = 154 mL/min FB: + 695 |
| Day 6 | SrCr = 0.48 mg/dl Wt = 103.9 kg CrCl = 151 mL/min FB: − 19 mL |
|
| Day 7 |
|
SrCr = 0.52 mg/dl Wt = 104.3 kg CrCl =139 mL/min FB: + 1,092 mL |
| Day 15 |
|
|
| Day 22 |
|
|
| Day 29 |
|
CrCl: calculated using Cockroft-Gault methodology; FB: fluid balance (intake-output); Wt: patient weight
Over the next 12 hours, the patient received oseltamivir 150 mg twice daily (typical dose is 75 mg BID), broad spectrum antibiotics and supportive care. Despite this treatment, the patient’s condition continued to deteriorate rapidly. She went into respiratory failure requiring intubation and mechanical ventilation. An emergency caesarian section was performed and a healthy newborn was delivered on hospital day 1 without complications.
Postoperatively, over the next three days the patient had a volatile course including the need for high FiO2 and positive end expiratory pressure (PEEP) on mechanical ventilation, vasopressors and the development of paralytic ileus. Her low-grade fever continued. H1N1 RT-PCR from her admission nasopharyngeal swab returned positive on hospital day 3 and oseltamivir was continued through the nasogastric tube. Broad spectrum antibiotics were withdrawn (repeat blood and sputum cultures remained negative).
On day 4, the patient’s condition had not improved. Because this patient was critically ill with a paralytic ileus, the absorption of oral oseltamivir was a major concern and hence, intravenous peramivir was requested from Biocryst Pharmaceuticals under eIND regulations. Peramivir 600mg was started on hospital day 5 after obtaining written consent and was infused intravenously over 30 minutes daily for the next 10 days. Oseltamivir was discontinued.
The patient began showing clinical and radiological improvement on the 3rd day of peramivir infusion (hospital day 7). She was discharged from the hospital on hospital day 22 to a long term acute care facility. There, she had rapid improvement, was completely weaned from the ventilator and was discharged to home on day 29.
Peramivir Analysis
Quantitative determination of peramivir in human plasma was performed at BioCryst Pharmaceuticals, Inc. by use of solid phase extraction (SPE) and high-performance liquid chromatography with tandem mass spectrometry detection (HPLC-MS/MS) as described elsewhere (4). Deuterated (D3) peramivir analog (D3-1812) was used as the internal standard. Peramivir and internal standard were extracted from 250 μL plasma, using a Varian BondElut™ C18 SPE cartridge. Extracts were analyzed by reverse phase chromatography at 50 °C (±10 °C), using a BETASIL™ Phenyl column under isocratic conditions. Column effluent was analyzed by multiple reaction monitoring using the triple quadruple mass spectrometer (Applied Biosystems/MDS Sciex® API 3200) equipped with Turbo V Ion Spray® in positive ion mode. The lower limit of quantification of peramivir in plasma was 1ng/mL and the upper limit of quantification was 50,000ng/mL.
Pharmacokinetics
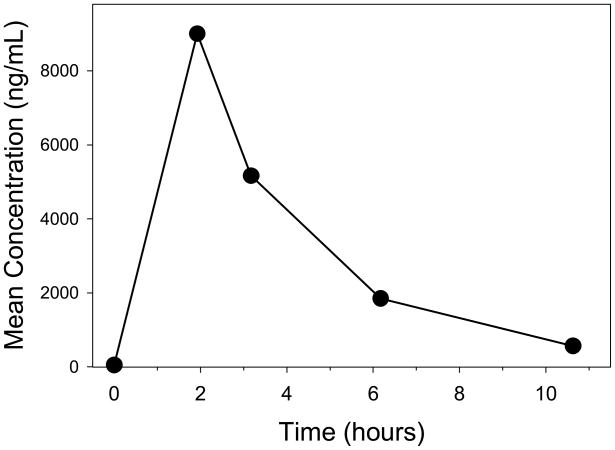
On day 3 of peramivir treatment, multiple blood samples (Figure 1) were drawn for pharmacokinetic analysis at 2, 3, 6 and 10.5 hours post-dose. Data were modeled by noncompartmental analysis (IV bolus) using WinNonLin Version 5.2.1 (Pharsight Corporation, Cary, North Carolina). The following peramivir pharmacokinetic parameters were recovered from the modeling: half-life (t½) 2.4 hours (Ke = 0.29 hr−1), AUC0–12 hour 32,659 ng•hr/mL, AUC0-∞ of 34,590 ng•hr/mL, clearance 17 L/hour, volume of distribution 59 L.
Figure 1. Peramivir concentration time-profile.
Multiple blood samples were drawn from the case patient on day three of the peramivir treatment. These blood samples were drawn at 2, 3, 6, and 10.5 hours post-dose and peramivir concentrations were quantified as described.
Comment
Data from Phase 1 trials have determined that peramivir displays a linear relationship between dose and drug exposure (AUC and Cmax). Although our patient did not receive the same dose as those reported from the phase 1 trials, some comparisons of drug exposure can be made. In brief, the peramivir half-life for our patient was shorter, clearance was more rapid, and overall drug exposure was lower than previously reported in phase 1 trials. Our patient received a dose of 5.8 mg/kg once daily with an AUC0-∞ of 34,590 ng•hr/mL. In the previously reported trials, six non-pregnant subjects received an IV dose of 4 mg/kg once daily, with a mean AUC0-∞ of 49,902 ng•hr/mL (2). Additionally, the calculated half-life in our patient was 2.4 hours, whereas the half-life is reported to range from 7.5 to 20.8 hours in the phase 1 patients (2). Our patient’s clearance of peramivir was 0.16 L/hr/kg, compared to the reported clearance of peramivir (0.11 L/hr/kg) in influenza-infected patients in phase 1 trials (3). Notably, since the half-life of peramivir was 2.4 hours, by the third dose she would have reached steady-state with respect to peramivir pharmacokinetics, assuming stable renal clearance at that time. Also, with such a short half-life, she would have had minimal accumulation of peramivir.
There are several plausible explanations for the pharmacokinetic parameter differences between this patient case and the parameters previously reported in clinical trials. Pregnant and postpartum patients typically display an increased renal plasma flow as well as an increase in plasma volume (5, 6). Peramivir is primarily eliminated unchanged via the kidney, accounting for approximately 90% of total clearance (3). Comparing the reported peramivir clearance (0.11 L/hr/kg) to the average creatinine clearance for a 70 kg adult (0.10 L/hr/kg) suggests that peramivir is excreted mainly by glomerular filtration, with a possible contribution of net tubular secretion, given the minimal plasma protein binding (<30%) (3). The glomerular filtration rate (GFR) is known to increase by approximately 50% in the first trimester of pregnancy (5). The GFR continues to increase throughout the remainder of the pregnancy and is elevated compared to 8-week postpartum values (5). In our patient, the creatinine clearance (CrCl, as calculated by the Crockcoft-Gault equation) increased over the first few days postpartum (see Table 1), potentially related to improvements in renal function. Her CrCl was variable over the duration of hospitalization, peaking on day 5 (peramivir day 1) and reaching 139 mL/min on day 7 when pharmacokinetic sampling occurred. This supranormal rate could have contributed to the peramivir clearance of 0.16 L/hr/kg which is higher than what was previously reported in phase 1 trials (3).
Pregnant women also have an increase in plasma volume which may result in an increase in the apparent volume of distribution of peramivir (6). Plasma volume in a recently postpartum patient could be rapidly changing, causing further fluctuations in apparent volume of distribution of drugs. We were unable to find any reports of the volume of distribution of peramivir, so a direct comparison to our patient’s volume of distribution (59 L) cannot be made, although this value clearly exceeds blood volume. Although changes in plasma protein binding may occur post-partum, the plasma protein binding of peramivir is minimal (<30%), so even a two-fold decrease would not meaningfully increase this patient’s total peramivir clearance (3).
Comparing the CrCl (0.087 L/hr/kg) to the peramivir clearance (0.16 L/hr/kg) of this patient suggests that her increased clearance would be consistent with either an increase in net renal tubular secretion, or an increase in non-renal clearance. Consistent with its relative hydrophilicity, peramivir is not extensively metabolized (3). As a result, this patient’s rapid clearance may likely involve one or more transporters for renal tubular secretion. The effects of infection or inflammation on drug transport mechanisms are not clearly established; however, they appear to differ by tissue, cytokine, transporter, and species. Since peramivir is zwitterionic at pH 7.4, it is difficult to predict the transport mechanism, which may include renal organic anion or organic cation transporters.
Compared to previously reported pharmacokinetic parameters (in non-pregnant patients), the parameters in our patient were unexpected. Our patient had decreased drug exposure compared to patients in phase 1 clinical trials, despite the fact that she received a higher dose than the patients from reported studies. Our patient did receive peramivir at the dose that is currently recommended for adults with influenza. However because of the shorter half-life observed here, one could suggest the need for an increased dose (or decreased dosing interval) in pregnant and postpartum patients.
Incorrect or ineffective dosing of drugs in pregnant and postpartum patients could lead to subtherapeutic treatment, harm to the mother and fetus and the development of drug resistance (7). Because our patient had lower drug exposure than would have been predicted, this case supports the growing viewpoint that more studies for the treatment of 2009 H1N1 influenza in pregnant and postpartum patients are needed in order to adequately assess whether these patients have altered pharmacokinetics that would lead to the need for an increase in dosage (8). The results herein were unexpected and raise the question of whether the differences are due to inter-patient variability or the postpartum status of the patient. The authors recognize the limitations of generalizations made from a single case report. Additional data obtained as a condition of the eIND from use of peramivir in pregnant and postpartum patients should include pharmacokinetics whenever possible, analyzed and published to aid physicians and pharmacists involved in the management of this at risk population. Finally, the clearance mechanism(s) for peramivir need to be further characterized.
Acknowledgments
The authors acknowledge the assistance of BioCryst in providing the medication within 24-hours of request as well as the details regarding their LC-MS/MS drug assay methodology; Christopher Rathbun, Pharm.D., BCPS, FCCP and Michael Burton, Pharm.D. of the University of Oklahoma and Alan Glaros, Ph.D. of Kansas City University of Medicine and Biosciences for their review of this manuscript. Partial salary support for Dr. Gerk was funded by NIH MD 002256.
Footnotes
Financial disclosures: All authors have no financial conflicts of interest.
Contributor Information
Patrick G. Clay, Kansas City University of Medicine and Biosciences, Kansas City, MO.
Raghavendra Adiga, Liberty Hospital, Liberty MO.
Tracey A.H. Taylor, Kansas City University of Medicine and Biosciences, Kansas City, MO.
Rachael Alsup, St. Louis College of Pharmacy, Affton, MO.
Phillip M. Gerk, Virginia Commonwealth University, Richmond, VA
MaryPeace McRae, Kansas City University of Medicine and Biosciences, Kansas City, MO.
References
- 1.Jamieson DJ, Honein MA, Rasmussen SA, Jennifer L, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 2.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. New Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. EUA Fact Sheet for Health Care Providers. 2009. [Google Scholar]
- 4.Lindegardh N, Hanpithakpong W, Phakdeeraj A, Singhasivanon P, Farrar J, Hien TT, et al. Development and validation of a high-throughput zwitterionic hydrophilic interaction liquid chromatography solid-phase extraction-liquid chromatography-tandem mass spectrometry method for determination of the anti-influenza drug peramivir in plasma. J Chromatogr. 2008;1215:145–51. doi: 10.1016/j.chroma.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop W. Serial changes in renal haemodynamics during normal human pregnancy. Brit J Obstet Gyn. 1981;88:1–9. doi: 10.1111/j.1471-0528.1981.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 7.Goldkind SF, Sahin L, Gallauresi B. Enrolling pregnant women in research-lessons from the H1N1 influenza pandemic. New Engl J Med. 2010;362:2241–3. doi: 10.1056/NEJMp1003462. [DOI] [PubMed] [Google Scholar]
- 8.Saleeby E, Chapman J, Morse J, Bryant A. H1N1 influenza in pregnancy: cause for concern. Obstet Gynecol. 2009;114:885–91. doi: 10.1097/AOG.0b013e3181bb44bb. [DOI] [PubMed] [Google Scholar]