Abstract
External glucose stimulates transcription of several genes including ptsG encoding IICBGlc, a membrane component of the phosphotransferase system (PTS), by relieving the negative regulation of a global repressor Mlc in Escherichia coli. We investigate here how glucose modulates Mlc action. The Mlc-mediated repression is eliminated by a ptsI mutation, while Mlc is constitutively active in a ptsG mutant. We show that IICBGlc-FLAG interacts physically with Mlc in crude extracts prepared from cells in which IICBGlc is supposed to exist as the non-phosphorylated form. The IICBGlc–Mlc interaction is no longer observed when IICBGlc is phosphorylated. Exogenously added purified Mlc binds to purified IICBGlc-FLAG. We also demonstrate that Mlc is associated with membrane when IICBGlc is dephosphorylated while it is in the cytoplasm when IICBGlc is phosphorylated or absent. We conclude that IICBGlc regulates the cellular localization of Mlc, depending on its phosphorylation state, which is determined by the availability of external glucose. Thus, glucose induces the transcription of Mlc-regulated promoters by sequestering Mlc to the membrane through dephosphorylation of IICBGlc.
Keywords: glucose transporter/membrane sequestration/Mlc/PTS/spatial regulation
Introduction
How Escherichia coli cells respond to external glucose is a classical paradigm in bacterial signal transduction and global gene regulation. Early studies on the glucose effect led to important concepts on gene regulation. Subsequent studies have revealed that the phosphoenolpyruvate (PEP)-dependent carbohydrate phosphotransferase system (PTS) is primarily responsible for various effects of glucose on cellular activities (reviewed by Meadow et al., 1990; Postma et al., 1996; Saier et al., 1996). The PTS consists of two common cytoplasmic proteins, enzyme I and HPr, and an array of sugar-specific enzyme II complexes (EIIs). The phosphoryl group from PEP is sequentially transferred to enzyme I, to HPr, to EIIs and finally to the substrate as it is translocated across the membrane. The glucose-specific EII (glucose transporter) consists of cytoplasmic protein IIAGlc and membrane receptor IICBGlc.
Recent genetic and biochemical studies have revealed several new aspects of glucose signaling in E.coli. For example, we found that the inhibitory effect of glucose on the utilization of lactose is due primarily to the inhibition of lactose uptake (inducer exclusion) by non-phosphorylated IIAGlc and therefore the well-known cAMP signaling model is no longer true (Inada et al., 1996). In addition, one of the real roles of cAMP in the preferential utilization of glucose over lactose was shown to support inducer exclusion by activating the expression of the ptsG gene (Kimata et al., 1997).
Another new aspect of cellular response to glucose is the induction of gene expression by glucose. It is now established that glucose stimulates transcription from certain promoters. For example, the expression of the pts operon, composed of ptsH, ptsI and crr encoding HPr, enzyme I and IIAGlc, respectively, is known to be stimulated by external glucose, and one of the two major ptsH promoters, ptsH P0, is subject to this glucose induction (De Reuse and Danchin, 1988, 1991; De Reuse et al., 1992; Fox et al., 1992; Ryu and Garges, 1994). A more dramatic inducible effect of glucose was observed with the ptsG in which a major promoter is the target of glucose induction (Kimata et al., 1998; Plumbridge, 1998b). An important finding concerning the mechanism of glucose induction was that the ptsG and ptsH P0 promoters are under the negative control of a global repressor, Mlc, and that mutations in the mlc gene lead to constitutive expression of the ptsG and ptsH promoters (Kimata et al., 1998; Plumbridge, 1998b, 1999; Kim et al., 1999; Tanaka et al., 1999). Mlc also regulates the expression of manXYZ, encoding three proteins of the mannose PTS, malT, encoding the activator of maltose regulon, and mlc itself (Decker et al., 1998; Plumbridge, 1998a).
While glucose induces the expression of target genes apparently by relieving Mlc-mediated repression, how glucose modulates Mlc action was not known. Several lines of genetic evidence suggested that PTS proteins are involved in the signal transduction pathway from glucose to Mlc. First, the ΔptsHI crr, ΔptsI crr or ptsI mutation causes constitutive expression of both ptsG and ptsH (De Reuse and Danchin, 1991; Plumbridge, 1999). Secondly, the constitutive expression of ptsG and ptsH no longer occurs when the ptsG gene has been further disrupted (Plumbridge, 1999). Thirdly, mutation in mlc leads to constitutive expression of ptsG and ptsH in all strains tested (Plumbridge, 1999). Fourthly, in addition to glucose several other PTS sugars could also induce the expression of ptsG and ptsH to various extents (De Reuse and Danchin, 1991; Plumbridge, 1999). However, the actual roles of PTS proteins in glucose induction and/or in modulation of Mlc action remain to be studied.
An attractive model for the mechanism of Mlc regulation by glucose has been proposed in which IICBGlc interacts physically with Mlc depending on its phosphorylation state to sequester Mlc away from the DNA (Plumbridge, 1999). Here, we present evidence that IICBGlc interacts physically with Mlc when IICBGlc exists as non-phosphorylated form. More importantly, we demonstrate that the cellular localization of Mlc varies depending on the phosphorylation state of IICBGlc. Our data clearly indicate that the Mlc protein shuttles between the cytoplasm, where it binds operators to repress the transcription of target promoters, and the membrane, where it presumably fails to access DNA. Thus, glucose induces the transcription of Mlc-regulated promoters by sequestering Mlc to the membrane through dephosphorylation of IICBGlc. This is the first instance in which a membrane component of PTS is involved in spatial regulation of a transcription factor via protein–protein interaction depending on its phosphorylation state.
Results
Effect of external glucose on Mlc expression
There are several possible mechanisms by which glucose induces transcription from Mlc-repressible promoters. First, glucose could stimulate the target promoters by reducing the Mlc level. In fact, the mlc gene appears to be under the positive control of CRP–cAMP (Decker et al., 1998). This raises a possibility that glucose downregulates Mlc expression through the catabolite repression mechanism although the situation is complicated because mlc is also negatively regulated by Mlc itself (Decker et al., 1998). Thus, glucose could affect Mlc expression in principle simultaneously through two control circuits, positive regulation mediated by CRP–cAMP and negative regulation mediated by Mlc as in other Mlc-repressible operons. The net effect of glucose on Mlc expression must be tested experimentally. Thus, we analyzed the effect of external glucose on Mlc expression in wild-type cells (PP6) by western blotting using anti-Mlc antibody. The Mlc level in cells grown in the presence of glucose was essentially equivalent to that in cells grown in the absence of glucose (Figure 1, lanes 2 and 3). Thus, external glucose does not significantly affect the Mlc level no matter how two control circuits are involved in mlc regulation. We conclude that fluctuation in Mlc level can not be responsible for the inducible effect of glucose on target promoters.
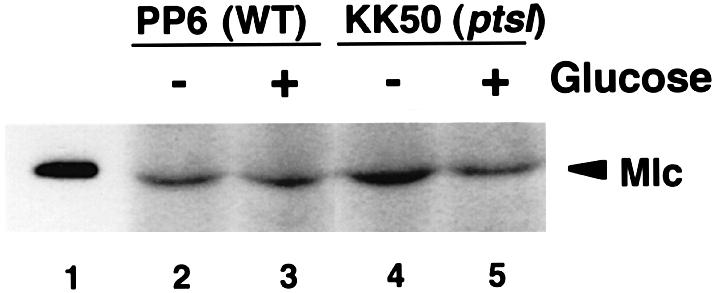
Fig. 1. Effect of glucose on the expression of Mlc protein. Crude extracts equivalent to an OD600 of 0.1, prepared from E.coli PP6 (lanes 2 and 3) or KK50 (lanes 4 and 5) grown in LB medium with and without 1% glucose, were analyzed by western blotting using anti-Mlc antibody as described in Materials and methods. Lane 1 corresponds to 5 ng of purified Mlc.
Effects of pts mutations on Mlc action
Secondly, glucose and/or its metabolites could act as an allosteric effector to downregulate Mlc activity. However, this model also appears to be less likely because glucose and its related compounds had no effect on Mlc action in vitro, and attempts to identify a direct inducer for Mlc was unsuccessful (Decker et al., 1998; Kimata et al., 1998; Plumbridge, 1998b, 1999). Instead, previous studies have suggested that PTS proteins are involved in the modulation of Mlc action (De Reuse and Danchin, 1991; Plumbridge, 1999).
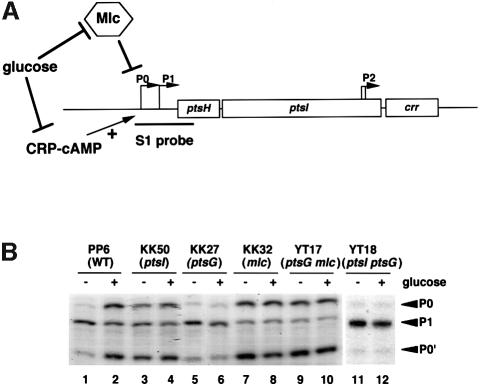
We examined the effects of pts mutations on ptsH mRNA expression by S1 nuclease analysis. We found that the ptsH P0 promoter, which is both negatively and positively regulated, by Mlc and CRP–cAMP, respectively (Kim et al., 1999; Plumbridge, 1999; Tanaka et al., 1999), was constitutively expressed in ptsI cells (KK50) regardless of the availability of glucose (Figure 2B, lanes 3 and 4) as in the case of mlc cells (KK32) (Figure 2B, lanes 7 and 8). The P0 mRNA level was slightly lower in the ptsI cells than in the mlc cells. This is presumably due to the reduced cAMP level caused by the ptsI mutation (Takahashi et al., 1998). In contrast, the ptsH P0 promoter was little expressed in ptsG cells (KK27) (Figure 2B, lanes 5 and 6). These results are consistent with earlier gene fusion studies (De Reuse and Danchin, 1991; Plumbridge, 1999) and strongly suggest that PTS proteins affect the Mlc-dependent negative regulatory pathway.
Fig. 2. (A) Schematic representation of the pts operon and its regulation. The open bars represent the coding region for ptsH, ptsI and crr. P0 and P1 are two major promoters. Transcription from P0 is regulated both negatively and positively, by Mlc and CRP–cAMP, respectively. Glucose affects P0 transcription through two regulatory pathways. The DNA fragment used for S1 nuclease assay is shown below the map. (B) Effect of pts mutations on pts mRNA expression. The indicated strains were grown in LB medium with and without 1% glucose. Total RNAs (30 µg) were subjected to S1 analysis. P0 and P1 transcripts along with the presumed processed RNA (P0′) are shown by arrowheads.
How does the ptsI mutation cause the constitutive expression of ptsH P0? This could happen again if the Mlc level were reduced by the ptsI mutation. We found, however, that this is not the case because the ptsI mutation caused a slight (in the absence of glucose, Figure 1, lane 4) or no (in the presence of glucose, Figure 1, lane 5) increase in the Mlc level. This implies that the derepression of P0 promoter in the ptsI mutant is not due to a reduction in Mlc level. Thus, the ptsI mutation may inhibit the repressor activity of Mlc by some unknown mechanism(s).
Implication of IICBGlc in glucose–Mlc signaling
The membrane component of glucose transporter IICBGlc is obviously important for glucose signaling because it acts as a sensor for external glucose. The IICBGlc catalyzes PEP-dependent phosphorylation of glucose in the presence of EI, HPr and IIAGlc. It is known that IICBGlc is phosphorylated as a catalytically important intermediate at Cys421 during phosphotransfer reactions (Meins et al., 1993). IICBGlc would exist as the non-phosphorylated form in ptsI cells because the phosphorylation chain from PEP via EI, HPr, and IIAGlc to IICBGlc is not functional. In wild-type cells, it is expected that IICBGlc is predominantly phosphorylated in the absence of external glucose and dephosphorylated in the presence of glucose. In addition, disruption of the ptsG gene led to repression of the ptsH P0 promoter in the ptsI strain (Figure 2B, lanes 11 and 12) but the P0 promoter was constitutively expressed in a mlc ptsG double mutant (Figure 2B, lanes 9 and 10). Taken together, Mlc is apparently active to repress transcription of target promoters when IICBGlc is phosphorylated or when IICBGlc is absent, while it is inactive when IICBGlc is dephosphorylated.
How does IICBGlc modulate Mlc action? It had been proposed that IICBGlc may act as a sensor protein of a two-component system in the glucose-mediated regulation of the ptsHI operon because the C-terminus of IICBGlc exhibits a sequence similarity to the transmitter consensus motif of sensor proteins (De Reuse and Danchin, 1991). This raises a possibility that the activity of Mlc could be modulated by IICBGlc-dependent phosphorylation. However, Mlc shows no homology to any known regulators of the two-component system (Plumbridge, 1999). In fact, there is no evidence so far that Mlc is phosphorylated (Kim et al., 1999).
Expression and properties of IICBGlc-FLAG
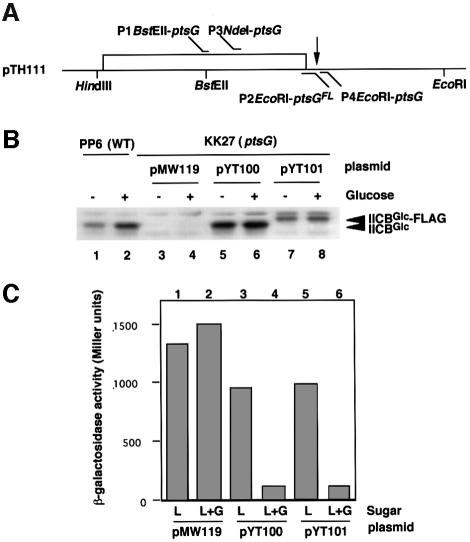
Another possibility regarding the role of IICBGlc in Mlc regulation is that IICBGlc interacts physically with Mlc depending on its phosphorylation state, thus sequestering Mlc away from the DNA (Plumbridge, 1999). To test this hypothesis, we constructed a low-copy plasmid pYT100 in which ptsG is under the control of the bla promoter. Then, the ptsG gene was manipulated to construct a variant ptsGFL gene encoding IICBGlc-FLAG fusion protein, in which the FLAG peptide was attached to the C-terminus of IICBGlc (Figure 3A). The resulting plasmid containing the ptsGFL gene was designated pYT101. As expected, IICBGlc and IICBGlc-FLAG were expressed constitutively in cells harboring pYT100 or pYT101 (Figure 3B, lanes 5–8), while the expression of IICBGlc from the chromosomal ptsG gene was stimulated by external glucose (Figure 3B, lanes 1 and 2). The expression level of IICBGlc-FLAG was significantly lower than that of IICBGlc (Figure 3B, lanes 5–8). This is presumably due to a reduced stability of mRNA caused by the lack of the Rho-independent terminator of ptsG in pYT101 (see Figure 3A).
Fig. 3. Expression and properties of IICBGlc-FLAG. (A) The ptsG gene on pTH111. The open bar represents the coding region for ptsG. The vertical arrow indicates the position of the Rho-independent terminator of ptsG. The locations of primers used for PCR amplification are shown. (B) Expression of IICBGlc and IICBGlc-FLAG. Crude extracts equivalent to an OD600 of 0.01, prepared from PP6 (lanes 1 and 2), KK27 harboring pMW119 (lanes 3 and 4), KK27 harboring pYT100 (lanes 5 and 6), KK27 harboring pYT101 (lanes 7 and 8) grown in LB medium with and without 1% glucose, were analyzed by western blotting using anti-IIBGlc antibody. Both IICBGlc and IICBGlc-FLAG are shown by arrowheads. (C) Effect of IICBGlc-FLAG on the expression of β-galactosidase. KK27 harboring pMW119 (lanes 1 and 2), pYT100 (lanes 3 and 4) and pYT101 (lanes 5 and 6) were grown in LB medium with 1% lactose (L) or with 1% lactose + 1% glucose (G). Culture samples were taken at OD600 of 0.6 and β-galactosidase activities were determined.
Before using IICBGlc-FLAG as a tool to study the possible IICBGlc–Mlc interaction, we examined whether the IICBGlc-FLAG fusion protein functions in cells. Glucose fails to inhibit lac expression in the presence of lactose in cells lacking the chromosomal ptsG gene (Figure 3C, lane 2) because glucose-specific PTS is not functional and IIAGlc remains phosphorylated. The introduction of pYT100 or pYT101 into ptsG cells restored the inducer exclusion, resulting in a large decrease in the level of β-galactosidase activity (Figure 3C, lanes 4 and 6). Thus, IICBGlc-FLAG fusion protein is functional with respect to the transport and phosphorylation of glucose, resulting in dephosphorylation of IIAGlc. In fact, the direct assay for the phosphorylation state of IIAGlc revealed that the IICBGlc-FLAG fusion protein could cause dephosphorylation of IIAGlc in the presence of glucose (data not shown).
Evidence for a physical interaction between Mlc and IICBGlc
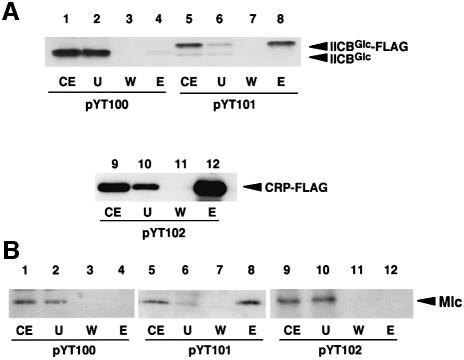
To examine a possible interaction between IICBGlc and Mlc, crude extracts were prepared from the ptsI cells harboring pYT100, pYT101 or pYT102 expressing IICBGlc, IICBGlc-FLAG or CRP-FLAG, respectively. IICBGlc or IICBGlc-FLAG is expected to exist as a non-phosphorylated form in ptsI cells because the PEP-dependent phosphorylation pathway is blocked. The crude extracts, which also contain Mlc expressed from the chromosomal mlc gene, were incubated with anti-FLAG M2–agarose affinity gel, washed extensively with a buffer, and then proteins bound to the beads were eluted with SDS sample buffer. The protein samples were first analyzed by western blotting using anti-IIBGlc antibody. As expected, a significant amount of IICBGlc-FLAG bound to anti-FLAG M2–agarose was eluted from the beads when extracts contained IICBGlc-FLAG (Figure 4A, lane 8) while no IICBGlc was detected in the eluate when extracts containing normal IICBGlc were used (Figure 4A, lane 4). A control CRP-FLAG fusion protein also effectively bound to the anti-FLAG M2–agarose and it was detected by anti-CRP antibody (Figure 4A, lane 12). We next analyzed the protein samples by western blotting using anti-Mlc antibody. Mlc was clearly detected in the eluate from the anti-FLAG M2–agarose when IICBGlc-FLAG was co-expressed (Figure 4B, lane 8). No detectable Mlc was found in the eluate when IICBGlc was co-expressed with Mlc (Figure 4B, lane 4). In addition, Mlc was not associated with a control protein CRP-FLAG (Figure 4B, lane 12), indicating that FLAG peptide itself has no ability to interact with Mlc. These results suggest strongly that IICBGlc-FLAG could interact with endogenous Mlc either directly or indirectly at least in the ptsI cells.
Fig. 4. Physical interaction between endogenous Mlc and IICBGlc. Assay for interaction between endogenous Mlc and IICBGlc was performed as described in Materials and methods. Cell extracts of YT18 harboring pYT100 (lanes 1–4), pYT101 (lanes 5–8) or pYT102 (lanes 9–12) were incubated with anti-FLAG M2–agarose, washed extensively with a buffer, and proteins bound to the beads were eluted with SDS sample buffer. The fractions were subjected to immunoblot analysis using anti-IIBGlc (A, lanes 1–8), anti-CRP (A, lanes 9–12) or anti-Mlc (B) antibodies. The protein samples used are: crude extracts (CE), 2 µl; unbound fraction (U), 10 µl, wash fraction 4 (W) 10 µl; elution fraction (E), 20 µl.
Purified Mlc binds to affinity-purified IICBGlc-FLAG
In order to examine whether exogenously added purified Mlc could bind to purified IICBGlc, we attempted to purify IICBGlc-FLAG with anti-FLAG M2–agarose from crude extracts prepared from the ptsI mlc cells (YT19) harboring pYT103 in which IICBGlc-FLAG is expressed ∼10-fold more compared with cells harboring pYT101 (Figure 5A, lane 3). Crude extracts were also prepared from the ptsI mlc cells harboring pTH111 in which IICBGlc is overexpressed (Figure 5A, lane 1). The extracts were incubated with anti-FLAG M2–agarose and the beads were washed extensively with several buffers as described in Materials and methods. The proteins bound to the beads were eluted with SDS sample buffer and analyzed by SDS–PAGE. Coomassie Blue staining of the gel revealed that a ∼50 kDa protein corresponding to IICBGlc-FLAG was eluted as a major band along with several bands when crude extracts containing IICBGlc-FLAG were used (Figure 5B, lane 4). Western blotting showed that the ∼50 kDa protein is indeed IICBGlc-FLAG (Figure 5A, lane 4). Other bands are apparently derived from anti-FLAG M2–agarose because they were also eluted from the beads alone. When crude extracts containing IICBGlc were used, neither IICBGlc nor other cellular proteins were detected (Figure 5A, lane 2 and B, lane 3). These results clearly indicate that IICBGlc-FLAG has been highly purified.
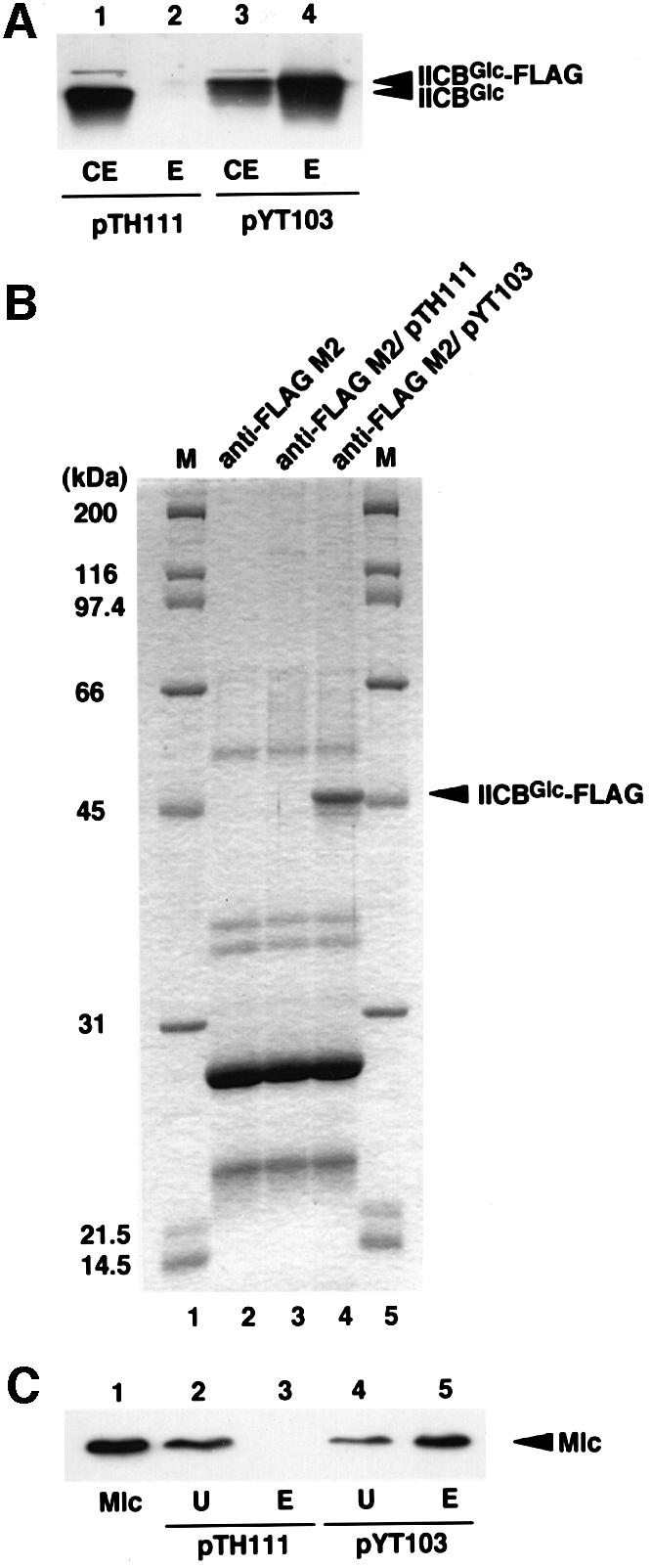
Fig. 5. Purification of IICBGlc-FLAG and binding of exogenously added Mlc to purified IICBGlc-FLAG. IICBGlc-FLAG was overproduced by introducing pYT103 into ptsI mlc cells and purified with anti-FLAG M2 affinity gel as described in Materials and methods. (A) Immunoblot analysis of IICBGlc-FLAG. Lane 1, 1 µl of crude extracts of cells harboring pTH111; lane 2, 4 µl of eluate of anti-FLAG M2–agarose treated with crude extracts of cells harboring pTH111; lane 3, 1 µl of crude extracts of cells harboring pYT103; lane 4, 4 µl of eluate of anti-FLAG M2–agarose containing purified IICBGlc-FLAG. (B) SDS–PAGE analysis of purified IICBGlc-FLAG. Lanes 1 and 5, marker proteins; lane 2, 20 µl of eluate of anti-FLAG M2–agarose alone; lane 3, 20 µl of eluate of anti-FLAG M2–agarose treated with crude extracts of cells harboring pTH111; lane 4, 20 µl of eluate of anti-FLAG M2–agarose containing purified IICBGlc-FLAG. (C) Analysis of the interaction between purified Mlc and IICBGlc-FLAG by western blotting. Purified Mlc (20 ng) was incubated with anti-FLAG M2–agarose suspensions (10 µl) with (lanes 4 and 5) or without (lanes 2 and 3) purified IICBGlc-FLAG in 50 µl of TBS buffer, washed extensively with a buffer. Proteins bound to the beads were eluted with 50 µl of SDS sample buffer. Unbound (lanes 2 and 4) and bound (lanes 3 and 5) fractions of 20 µl each were subjected to immunoblot analysis using anti-Mlc antibody. Lane 1 is 10 ng of purified Mlc.
We then tested whether the affinity-purified immobilized IICBGlc-FLAG could bind to exogenously added Mlc. The immobilized IICBGlc-FLAG was incubated with purified Mlc. The beads were separated by centrifugation and washed with a buffer. The proteins bound to the beads were eluted with SDS sample buffer and analyzed by western blotting using anti-Mlc antibody. The added Mlc was found predominantly in the eluate (Figure 5C, lanes 4 and 5). The Mlc was found in the unbound fraction but not in the eluate when control beads were used (Figure 5C, lanes 2 and 3). These results imply that the purified IICBGlc-FLAG could bind directly to purified Mlc.
Glucose promotes the Mlc–IICBGlc interaction
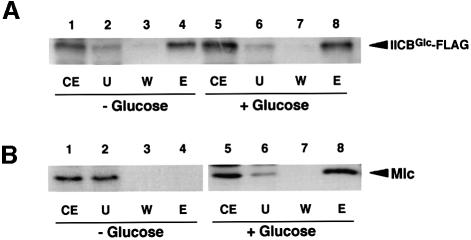
We next addressed questions of whether IICBGlc could associate with Mlc in cells where the glucose PTS is functional and if so how the phosphorylation state of IICBGlc affects the IICBGlc–Mlc interaction. For this, we introduced pYT101 expressing IICBGlc-FLAG into ptsG cells. The crude extracts of cells harboring pYT101 grown both in the presence and absence of glucose were incubated with anti-FLAG M2–agarose beads, washed extensively with a buffer, and then bound proteins were eluted with SDS sample buffer. The protein samples were analyzed by western blotting using both anti-IIBGlc and anti-Mlc antibodies. IICBGlc-FLAG was recovered in the eluate from the anti-FLAG M2–agarose beads regardless of the presence of glucose in the culture medium (Figure 6A, lanes 4 and 8). However, Mlc was detected in the eluate only when crude extracts were prepared from cells grown in the presence of glucose (Figure 6B, lanes 4 and 8). It is expected that IICBGlc is phosphorylated in the absence of external glucose while it is dephosphorylated in the presence of glucose. Thus, it is highly possible that Mlc interacts strongly with IICBGlc when IICBGlc is dephosphorylated.
Fig. 6. Effect of glucose on the formation of the Mlc–IICBGlc complex. Escherichia coli KK27 harboring pYT101 was grown in LB medium with (lanes 1–4) or without (lanes 5–8) 1% glucose. Cell extracts were prepared and binding experiments were performed as described in Materials and methods. The fractions were subjected to immunoblot analysis using anti-IIBGlc (A) or anti-Mlc (B) antibodies. The protein samples used are: crude extracts (CE), 2 µl; unbound fraction (U), 10 µl; wash fraction 4 (W) 10 µl; elution fraction (E), 20 µl.
Localization of Mlc in ptsI or ptsG cells
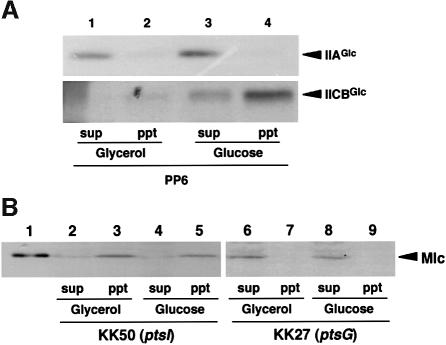
Does the Mlc–IICBGlc interaction mentioned above influence the cellular localization of Mlc? In order to answer this question, we performed cellular localization assay of proteins. First, the localization of two control proteins, cytoplasmic protein IIAGlc and transmembrane protein IICBGlc was examined with wild-type cells grown on either glucose or glycerol by western blotting using anti-IIAGlc and anti-IIBGlc antibodies. As expected, IIAGlc appeared exclusively in the soluble cytoplasm fraction regardless of the carbon source in the medium (Figure 7A, upper panel). On the other hand, IICBGlc appeared predominantly in the membrane fraction although a little IICBGlc was detected in the glycerol medium (Figure 7A, lower panel). If the interaction between IICBGlc and Mlc sequesters Mlc to the membrane, one may expect that Mlc protein is located in the membrane fraction in ptsI cells because IICBGlc is dephosphorylated. In contrast, Mlc is expected to be present in the cytoplasm in the ptsG cells lacking IICBGlc. We found that this is exactly the case. Mlc appeared predominantly in the membrane fraction in ptsI cells (Figure 7B, lanes 2–5) while it was found in the cytoplasmic soluble fraction in ptsG cells (Figure 7B, lanes 6–9). These results suggest strongly that Mlc is sequestered at the membrane via the physical interaction with the non-phosphorylated form of IICBGlc.
Fig. 7. Analysis of cellular localization of proteins. (A) Localization of IIAGlc and IICBGlc. Escherichia coli PP6 was grown in MOPS medium with 0.4% glycerol (lanes 1 and 2) or 0.2% glucose (lanes 3 and 4). The cytoplasm and membrane fractions were prepared as described in Materials and methods and 10 µl of each sample were subjected to immunoblot analysis using anti-IIAGlc or anti-IIBGlc antibody. (B) Effect of pts mutations on the localization of Mlc. KK50 (lanes 2–5) or KK27 (lanes 6–9) was grown in MOPS medium with 0.4% glycerol or 0.2% glucose. The cytoplasm and membrane fractions were prepared and 10 µl of each sample were subjected to immunoblot analysis using anti-Mlc antibody. Lane 1 corresponds to 2 ng of purified Mlc.
Glucose promotes the membrane association of Mlc
We already showed that external glucose promotes the physical interaction between IICBGlc and Mlc presumably by facilitating dephosphorylation of IICBGlc (Figure 6B). To examine if the enhanced interaction between IICBGlc and Mlc by glucose leads to membrane sequestration of Mlc, we determined the distribution of Mlc between two fractions in wild-type cells grown on either glucose or glycerol. Mlc was found mostly in the cytoplasmic fraction when cells were grown on glycerol, while it appeared in the membrane fraction when cells were grown on glucose (Figure 8A). This implies that glucose promotes the membrane association of Mlc in wild-type cells by dephosphorylating IICBGlc and therefore by enhancing the Mlc–IICBGlc interaction. It should be noted, however, that the level of IICBGlc is low in the absence of glucose in wild-type cells. This raises a possibility that Mlc is located in the cytoplasm simply due to the limited amount of IICBGlc in the absence of glucose. Then, we determined the distribution of Mlc in ptsG cells harboring pYT100 expressing IICBGlc constitutively at a moderate level. Mlc appeared again in the membrane fraction when cells were grown on glucose, while it appeared both in the cytoplasm and membrane fractions when cells were grown on glycerol (Figure 8B). Furthermore, when IICBGlc was overproduced by introducing a multicopy plasmid containing the ptsG gene, Mlc appeared predominantly in the membrane even in the glycerol medium (Figure 8C). These results suggest that not only the dephosphorylation of IICBGlc but also the increased amount of IICBGlc caused by external glucose plays a role in the effective membrane sequestration of Mlc in wild-type cells.
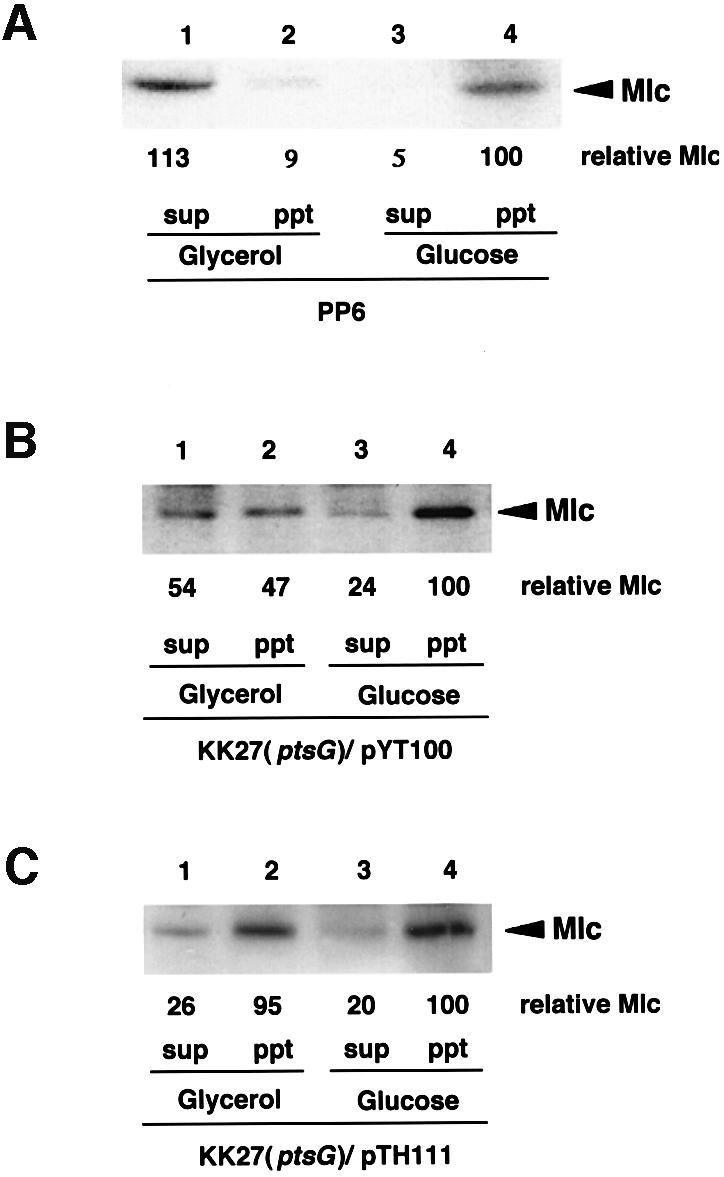
Fig. 8. Effects of glucose and IICBGlc on localization of Mlc. Escherichia coli PP6 (A), KK27 harboring pYT100 (B) and KK27 harboring pTH111 (C) were grown in MOPS medium with 0.4% glycerol (lanes 1 and 2) or 0.2% glucose (lanes 3 and 4). The cytoplasm and membrane fractions were prepared and 10 µl of each sample were subjected to immunoblot analysis using anti-Mlc antibody. Mlc levels were quantified by densitometric scanning and expressed relative to that of the membrane fraction of glucose-grown cells (=100).
Discussion
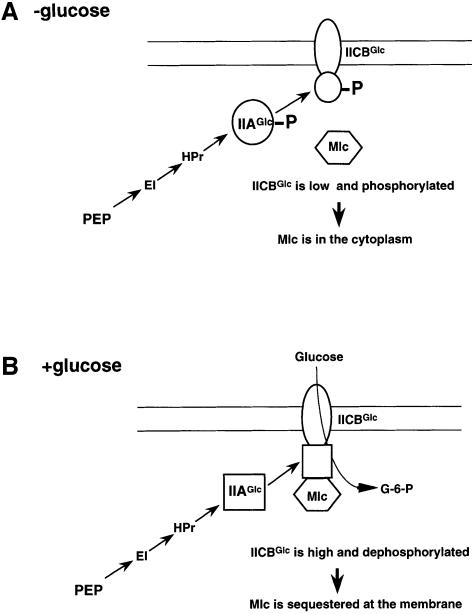
The present study demonstrates that sequestration of Mlc to the membrane via the physical interaction with IICBGlc is a key event in the glucose induction of Mlc-repressible genes. A plausible scenario of glucose induction of the Mlc-repressible promoters is depicted in Figure 9. In the absence of external glucose, the IICBGlc level is low and it exists predominantly as the phosphorylated form. The affinity of phospho-IICBGlc for Mlc is presumably not strong enough to sequester Mlc at the membrane. Thus, Mlc is mostly in the cytoplasm to bind its operators. The transport and phosphorylation of glucose through glucose-specific PTS leads to dephosphorylation of IICBGlc. The non-phosphorylated IICBGlc then associates with Mlc, which in turn prevents Mlc binding to operators resulting in derepression of Mlc-repressible operons. In addition, the increased level of IICBGlc by glucose is certainly responsible for an effective membrane sequestration of Mlc. Thus, the induction process by glucose apparently consists of a positive feedback system. These conclusions are based on the following observations: (i) endogenous Mlc is associated with IICBGlc-FLAG in ptsI cells in which IICBGlc-FLAG is expected to exist as the non-phosphorylated form (Figure 4); (ii) exogenously added Mlc binds to highly purified immobilized IICBGlc-FLAG (Figure 5); (iii) Mlc is associated with IICBGlc-FLAG in wild-type cells grown in the presence of glucose but not in the absence of glucose (Figure 6); and (iv) Mlc is found in the membrane fraction when IICBGlc is dephosphorylated either by the mutation in ptsI or by the transport of glucose in wild-type cells. Mlc is found in the cytoplasm when IICBGlc is phosphorylated due to the absence of external glucose or when it is lacking due to the mutation in the ptsG gene (Figure 7).
Fig. 9. Model for the regulation of Mlc by IICBGlc and glucose. (A) In the absence of glucose, IICBGlc is expressed at a low level and it is phosphorylated. Mlc binds the phosphorylated IICBGlc only weakly and most Mlc is in the cytoplasm to inhibit the target promoters. (B) In the presence of glucose, transport and phosphorylation of glucose leads to dephosphorylation of IICBGlc. Mlc binds strongly to the non-phosphorylated form of IICBGlc and it is trapped at the membrane resulting in derepression of target promoters including ptsG. Increased IICBGlc further contributes to the efficient sequestration of Mlc.
If IICBGlc and Mlc interact with each other, one may expect to obtain mutations in both mlc and ptsG genes that impair the physical interaction between the two proteins. It has been shown recently that some mutations in ptsG, originally called the umgC mutation (Jones-Mortimer and Kornberg, 1980), cause an increase in ptsG expression even in the absence of PTS sugars (Notley-McRobb and Ferenci, 2000; Zeppenfeld et al., 2000; J.Plumbridge, personal communication). It would be interesting to examine whether these mutations affect the IICBGlc–Mlc interaction and the cellular localization of Mlc.
How does the membrane sequestration of Mlc via the interaction with IICBGlc lead to the dissociation of Mlc from its operators? The binding of Mlc to the membrane may simply compete with its binding to DNA. Thus, the concentration of free Mlc would be expected to drop below that required for repression of the target promoters when Mlc becomes associated with the membrane. However, it remains to be studied whether the membrane association of Mlc could also cause an allosteric change in the Mlc conformation that decreases its affinity for DNA. In addition, although we have shown that a highly purified IICBGlc-FLAG could interact with purified Mlc, this does not completely exclude the possibility that additional proteins may also be involved in the IICBGlc–Mlc interaction and therefore the sequestration of Mlc to the membrane in intact cells.
While the primary functions of PTS are sugar reception, transport and phosphorylation, it is well recognized that the PTS is involved in the regulation of a variety of physiological processes acting as a major signal transduction system (Meadow et al., 1990; Postma et al., 1996; Saier et al., 1996). For example, the involvement of PTS in bacterial chemotaxis had been recognized, although how PTS works for chemoreception activity is not yet elucidated (Lux et al., 1995, 1999). The best-known regulatory function of PTS is the regulation of metabolism and uptake of various carbohydrates by IIAGlc. This cytoplasmic component of glucose PTS is known to act as both a positive effector of adenylate cyclase activity and a negative effector of the transport or use of non-PTS sugars, depending on its phosphorylation state, which is determined by the availability of external glucose (Postma et al., 1996; Takahashi et al., 1998). The BglF is an excellent example in which a membrane component of PTS plays a unique role in gene regulation. It modulates the phosphorylation state, therefore the activity of transcriptional antiterminator BglG by monitoring the availability of β-glucosides (Amster-Choder et al., 1989; Schnetz and Rak, 1990). Our study has shed light on a novel regulatory role of the membrane component of the glucose transporter IICBGlc. This is the first case in which the regulatory role of IICBGlc has been revealed.
Dynamic changes in cellular localization have been recognized as a common feature of regulatory and structural proteins even in bacteria (Shapiro and Losick, 2000). There are several known systems where the membrane association of a transcription factor plays a key role in the regulation of bacterial gene expression. For example, Pro-σE is sequestered from RNA polymerase at the cytoplasmic membrane as a membrane-associated protein in the predivisional state in Bacillus subtilis (Hofmeister, 1998). Following cell division, pro-σE is processed and σE is released into the cytoplasm of mother cells where it associates with RNA polymerase. Regulation of the proline utilization put operon in enteric bacteria is a well known example in which membrane sequestration of a transcriptional repressor was shown to be the molecular basis of the induction of the operon (Ostrovsky de Spicer and Maloy, 1993; Muro-Pastor et al., 1997). When the intracellular concentration of proline is low, PutA remains in the cytoplasm to act as a repressor, but when the intracellular concentration of proline is high, it binds to the membrane and acts as an oxidation enzyme of proline. Thus, the PutA protein shuttles between the membrane and the cytoplasm, depending on the proline concentration. Another less-characterized example is the regulation of the transcriptional activator MalT by MalK, a component of the ABC-type transporter for maltose in E.coli (Panagiotidis et al., 1998). It was shown that MalK interacts physically with MalT to downregulate its transcriptional activity although the full biological significance of this regulation was not known.
The regulation of Mlc localization by IICBGlc presented in this study has provided a clear case for dynamic and spatial regulation of transcription by a membrane protein in E.coli. A striking feature of the IICBGlc–Mlc system is that a membrane component of PTS acts as both a sensor and a modulator of a transcription factor. In other words, it determines the cellular location of a global repressor protein for genes involved in carbon metabolism depending on the availability of the sugar substrate therefore depending on the phosphorylation state of a transporter protein. It remains to be studied whether any other systems exist where the membrane sequestration of a transcription factor is used for gene regulation in response to an environmental change in E.coli.
Materials and methods
Media and growth conditions
Cells were grown aerobically at 37°C in Luria–Bertani (LB) medium or in minimal MOPS medium (Neidhardt et al., 1974). Antibiotics were used at the following concentrations: ampicillin (50 µg/ml), kanamycin (50 µg/ml) and tetracycline (15 µg/ml). Bacterial growth was monitored by determining the optical density at 600 nm.
Bacterial strains and plasmids
The E.coli K12 strains and plasmids used in this study are listed in Table I. PP6 was used as a parent wild-type strain. KK32 and KK50 were described previously. The ptsG::Tn5 region of IT1168 was transferred to PP6 and to KK50 by P1 transduction to construct KK27 and YT18, respectively. Similarly, the mlc1157::Tn10 region of KK32 was transferred to KK27 and KK50 to construct YT17 and YT19.
Table I. Bacterial strains and plasmids used in this study.
| Strain/plasmid | Relevant genotype and property | Source |
|---|---|---|
| Strain | ||
| PP6 | wild type | Kimata et al. (1998) |
| IT1168 | W3110 ptsG::Tn5 | Kimata et al. (1997) |
| KK27 | PP6 ptsG::Tn5 | this study |
| KK32 | PP6 mlc1157::Tn10 | Kimata et al. (1998) |
| KK50 | PP6 ptsI::Tn5 | Tanaka et al. (1999) |
| YT17 | KK27 mlc1157::Tn10 | this study |
| YT18 | KK50 ptsG::Tn5 | this study |
| YT19 | KK50 mlc1157::Tn10 | this study |
| Plasmid | ||
| pTH111 | derivative of pBR322 containing ptsG expressed from the bla promoter | Takahashi et al. (1998) |
| pYT100 | derivative of pMW119 containing ptsG expressed from the bla promoter | this study |
| pYT101 | derivative of pYT100 containing ptsGFL expressed from the bla promoter | this study |
| pYT103 | derivative of pBR322 containing ptsGFL expressed from the bla promoter | this study |
| pETIIB387 | derivative of pET3a containing the 3′ portion of ptsG under T7 promoter | this study |
| pHA7M | derivative of pHA7 in which a MluI site is introduced near last codon of crp | Abo et al. (2000) |
| pYT102 | derivative of pBR322 containing crpFL expressed from the bla promoter | this study |
| pTH219 | derivative of pBR322 containing crr expressed from the bla promoter | Takahashi et al. (1998) |
The 2.5 kb HindIII–EcoRI fragment containing the ptsG gene derived from pTH111 was cloned into pMW119 (Nippon Gene) to construct pYT100. A DNA fragment corresponding to the C-terminal half of IICBGlc-FLAG was amplified by PCR from pTH111 using two primers: P1BstEII–ptsG (5′-CGTTATATGGCGGGTGACCCGACTGC-3′) and P2EcoRI–ptsGFL (5′-CGCGAATTCTTACTTGTCATCGTCATCCTTG TAGTCGTGGTTACGGATGTACTCATCC-3′). The amplified fragment of ∼0.8 kb was digested with BstEII and EcoRI, and substituted for the corresponding 1.5 kb region of pYT100 to construct pYT101 carrying the ptsGFL gene encoding IICBGlc-FLAG. Similarly, the BstEII–EcoRI fragment was cloned into the corresponding site of pTH111 to construct pYT103 carrying the ptsGFL gene encoding IICBGlc-FLAG. To construct pETIIB387 expressing a cytoplasmic domain (IIBGlc) of IICBGlc, a DNA fragment encoding the C-terminal portion of IICBGlc consisting of 91 amino acid residues was amplified by PCR from pTH111 using two primers: P3NdeI–ptsG (5′-GCACTCTCACATATGGAAGACGCGA CTG-3′) and P4EcoRI–ptsG (5′-GCGCGAATTCTCAGCATTACC GGCACG-3′). The amplified fragment was digested with NdeI and EcoRI, and the resulting fragment was cloned into the corresponding sites of pET3a (Takara Shuzo, Kyoto). To construct pYT102 containing the crpFL gene encoding FLAG-tagged CRP, a DNA fragment was amplified by PCR from pHA7M using two primers: pBR–BamHI (5′-GAGCCC GATCTTCCCCATCG-3′) and MluI–crp (5′-GCGCACGCGTTACTTG TCATCGTCATCCTTGTAGTCGCCGTAAACGACGATGGTTTTA CCG-3′). The amplified fragment was digested with HindIII and MluI, and the resulting fragment was cloned into the corresponding sites of pHA7M.
Immunoblot analysis
Mlc purification was described previously (Tanaka et al., 1999). To purify the IIBGlc domain IICBGlc, BL21 cells (Takara Shuzo) harboring pETIIB387 were grown at 37°C in LB medium, and the expression of IIBGlc was induced by 0.1 mM isopropyl-β-d-thiogalactopyranoside. The IIBGlc protein was purified from extracts of the induced cells using DEAE-Sephacel and MONO-Q columns, and finally by SDS–PAGE followed by protein extraction from the gel. IIAGlc was purified from the extracts of MC4100 cells harboring pTH219 containing the crr gene, using DEAE Toyo-pearl 650 and Sephacryl HR and DEAE Toyo-pearl 650 columns. Anti-Mlc, anti-IIBGlc and anti-IIAGlc polyclonal antibodies were obtained by immunizing rabbits with purified Mlc, IIBGlc and IIAGlc proteins, respectively. Anti-CRP polyclonal antibody was described previously (Ishizuka et al., 1993). For immunoblot analysis, protein samples were loaded onto 0.1% SDS–12% polyacrylamide gels and electrophoresed. Western blotting was performed as described previously (Ishizuka et al., 1993) using polyclonal anti-IIAGlc, anti-IIBGlc, anti-Mlc and anti-CRP antibodies and a horseradish peroxidase-conjugated secondary antibody.
S1 nuclease analysis
The S1 nuclease assay was performed as described previously (Aiba et al., 1981). Total cellular RNAs were isolated from cells grown in LB medium to an OD600 of 0.6. The 741 bp HindIII–XhoI fragment 32P-labeled at its 5′ ends was used as DNA probe for pts mRNA. The DNA probe and total RNA were hybridized and treated with 40 U of S1 nuclease at 37°C for 10 min. The reaction products were analyzed on an 8% polyacryl amide–8 M urea gel.
Analysis of interaction between Mlc and IICBGlc-FLAG
Cells were grown in 20 ml of LB medium to an OD600 of 0.8, harvested and washed with 1 ml of 50 mM Tris–HCl pH 8.0, 5 mM EDTA. The cell pellets were suspended in 0.5 ml of ice-cold 50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, CompleteTM Protease inhibitor Cocktail Tablets (Boehringer Mannheim). The cells were sonicated for 5 s four times at 0°C, with 10 s rest periods in between, using a special micro tip of a Heat Systems Ultrasonics Sonicator. The disrupted cells were centrifuged at 12 000 g for 5 min at 4°C. The supernatants were used as crude extracts. Crude extracts (50 µl) were incubated with 0.2 ml of TBS buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl) and 50 µl of anti-FLAG M2–agarose suspension (Sigma) for 15 min at 4°C. The mixtures were centrifuged and the supernatants were used as unbound fractions. The pellet beads were washed four times with 0.25 ml of TBS buffer. The supernatants were used as wash fractions. The proteins bound to the beads were eluted with 50 µl of SDS sample buffer (2% SDS, 5% 2-mercaptoethanol, 62.5 mM Tris–HCl pH 6.8, 10% glycerol, 0.1% bromophenol blue) at 100°C for 5 min and used as bound fractions. The protein samples at each step were subjected to western blot analysis.
Binding of purified Mlc to purified IICBGlc-FLAG
Cells harboring pYT103 carrying the ptsGFL gene encoding IICBGlc-FLAG were grown in 20 ml of LB medium to an OD600 of 0.8. Crude extracts (0.5 ml) were prepared as mentioned above. To purify IICBGlc-FLAG, 100 µl of anti-FLAG M2–agarose suspension (50 µl bed volume) were added to the crude extracts and the mixtures were incubated for 1 h at 4°C. The mixtures were centrifuged and the beads were washed twice with 1 ml of 50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, twice with 1 ml of 50 mM Tris–HCl pH 7.5, 500 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate and twice with 1 ml of TBS buffer by centrifugation. The pellet beads were suspended with 50 µl of TBS buffer. The presence and purity of IICBGlc-FLAG in the agarose beads were examined by western blotting and SDS–PAGE followed by Coomassie Blue staining. Crude extracts were also prepared from cells harboring pTH111 carrying the wild-type ptsG gene encoding IICBGlc and treated with anti-FLAG M2–agarose. For the Mlc binding assay, 10 µl of agarose suspension with or without the purified IICBGlc-FLAG was incubated with 20 ng of purified Mlc in 50 µl of TBS buffer for 15 min at 4°C. The mixtures were centrifuged and the supernatants were used as unbound fractions. The pellet beads were washed four times with 0.25 ml of TBS buffer by centrifugation. The proteins bound to the beads were eluted with 50 µl of SDS sample buffer at 100°C for 5 min and used as bound fractions. The protein samples were subjected to western blot analysis.
Protein localization assay
Cells were grown in minimal MOPS medium containing either glycerol or glucose to an OD600 of 0.6, and 0.5 ml of cell cultures were directly sonicated for 5 s twice at 0°C, with a 10 s interval in between. The sonicated samples were centrifuged at 15 000 g (4°C) for 3 min. The pellets were dissolved in 30 µl of SDS sample buffer and used as the membrane fractions. The supernatant fraction was precipitated with cold trichloroacetic acid (5% final concentration). The precipitates were collected by centrifugation, washed with 70% acetone, dissolved in 30 µl of SDS sample buffer and used as the soluble cytoplasmic fractions. The supernatant and membrane fractions were subjected to western blot analysis.
Acknowledgments
Acknowledgements
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan.
References
- Abo T., Inada,T., Ogawa,K. and Aiba,H. (2000) SsrA-mediated tagging and proteolysis of LacI and its role in the regulation of lac operon. EMBO J., 17, 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Adhya,S. and de Crombrugghe,B. (1981) Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem., 256, 11905–11910. [PubMed] [Google Scholar]
- Amster-Choder O., Houman,F. and Wright,A. (1989) Protein phosphorylation regulates transcription of the β-glucoside utilization operon in E.coli.Cell, 58, 847–855. [DOI] [PubMed] [Google Scholar]
- Decker K., Plumbridge,J. and Boos,W. (1998) Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol. Microbiol., 27, 381–390. [DOI] [PubMed] [Google Scholar]
- De Reuse H. and Danchin,A. (1988) The ptsH, ptsI and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J. Bacteriol., 170, 3827–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuse H. and Danchin,A. (1991) Positive regulation of the pts operon of Escherichia coli: genetic evidence for a signal transduction mechanism. J. Bacteriol., 173, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuse H., Kolb,A. and Danchin,A. (1992) Positive regulation of the expression of the Escherichia coli pts operon. Identification of the regulatory regions. J. Mol. Biol., 226, 623–635. [DOI] [PubMed] [Google Scholar]
- Fox D.K., Presper,K.A., Adhya,S., Roseman,S. and Garges,S. (1992) Evidence for two promoters upstream of the pts operon: regulation by the cAMP receptor protein regulatory complex. Proc. Natl Acad. Sci. USA, 89, 7056–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister A. (1998) Activation of the proprotein transcription factor pro-σE is associated with its progression through three patterns of subcellular localization during sporulation in Bacillus subtilis. J. Bacteriol., 180, 2426–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T., Kimata,K. and Aiba,H. (1996) Mechanism responsible for glucose–lactose diauxie in Escherichia coli: challenge to the cAMP model. Genes Cells, 1, 293–301. [DOI] [PubMed] [Google Scholar]
- Ishizuka H., Hanamura,A., Kunimura,T. and Aiba,H. (1993) A lowered concentration of cAMP receptor protein caused by glucose is an important determinant for catabolite repression in Escherichia coli. Mol. Microbiol., 10, 341–350. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M.C. and Kornberg,H.L. (1980) Amino-sugar transport systems of Escherichia coli K12. J. Gen. Microbiol., 117, 369–376. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Nam,T.W., Shin,D., Koo,B.M., Seok,Y.J. and Ryu,S. (1999) Purification of Mlc and analysis of its effects on the pts expression in Escherichia coli. J. Biol. Chem., 274, 25398–25402. [DOI] [PubMed] [Google Scholar]
- Kimata K., Takahashi,H., Inada,T., Postma,P. and Aiba,H. (1997) cAMP receptor protein-cAMP plays a crucial role in glucose–lactose diauxie by activating the major glucose transporter gene in Escherichia coli. Proc. Natl Acad. Sci. USA, 94, 12914–12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K., Inada,T., Tagami,H. and Aiba,H. (1998) A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol. Microbiol., 29, 1509–1519. [DOI] [PubMed] [Google Scholar]
- Lux R., Jahreis,K., Bettenbrock,K., Parkinson,J.S. and Lengeler,J.W. (1995) Coupling the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli. Proc. Natl Acad. Sci. USA, 92, 11583–11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux R., Munasinghe,V.R., Castellano,F., Lengeler,J.W., Corrie,J.E. and Khan,S. (1999) Elucidation of a PTS-carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay. Mol. Biol. Cell, 10, 1133–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N.D., Fox,D.K. and Roseman,S. (1990) The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu. Rev. Biochem., 59, 497–542. [DOI] [PubMed] [Google Scholar]
- Meins M., Jeno,P., Muller,D., Richter,W.J., Rosenbusch,J.P. and Erni,B. (1993) Cysteine phosphorylation of the glucose transporter of Escherichia coli. J. Biol. Chem., 268, 11604–11609. [PubMed] [Google Scholar]
- Muro-Pastor A.M., Ostrovsky,P. and Maloy,S. (1997) Regulation of gene expression by repressor localization: biochemical evidence that membrane and DNA binding by the PutA protein are mutually exclusive. J. Bacteriol., 179, 2788–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F.C., Bloch,P.L. and Smith,D.F. (1974) Culture medium for enterobacteria. J. Bacteriol., 119, 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notley-McRobb L. and Ferenci,T. (2000) Substrate specificity and signal transduction pathways in the glucose-specific enzyme II (EII(Glc)) component of the Escherichia coli phosphotransferase system. J. Bacteriol., 182, 4437–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovsky de Spicer P. and Maloy,S. (1993) PutA protein, a membrane-associated flavin dehydrogenase, acts as a redox-dependent transcriptional regulator. Proc. Natl Acad. Sci. USA, 90, 4295–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotidis C.H., Boos,W. and Shuman,H.A. (1998) The ATP-binding cassette subunit of the maltose transporter MalK antagonizes MalT, the activator of the Escherichia coli mal regulon. Mol. Microbiol., 30, 535–546. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. (1998a) Control of the expression of the manXYZ operon in Escherichia coli: Mlc is a negative regulator of the mannose PTS. Mol. Microbiol., 27, 369–380. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. (1998b) Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol., 29, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. (1999) Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol., 33, 260–273. [DOI] [PubMed] [Google Scholar]
- Postma P.W., Lengeler,J.W. and Jacobson,G.R. (1996) Phosphoenol pyruvate: carbohydrate phosphotransferase systems. In Neidhardt,F.C. (ed.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 1149–1174. [Google Scholar]
- Ryu S. and Garges,S. (1994) Promoter switch in the Escherichia coli pts operon. J. Biol. Chem., 269, 4767–4772. [PubMed] [Google Scholar]
- Saier M.H. Jr, Ramseier,T.M. and Reizer,J. (1996) Regulation of carbon utilization. In Neidhardt,F.C. (ed.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 1325–1343. [Google Scholar]
- Schnetz K. and Rak,B. (1990) β-glucoside permease represses the bgl operon of Escherichia coli by phosphorylation of the antiterminator protein and also interacts with glucose-specific enzyme III, the key element in catabolite control. Proc. Natl Acad. Sci. USA, 87, 5074–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. and Losick,R. (2000) Dynamic spatial regulation in the bacterial cell. Cell, 100, 89–98. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Inada,T., Postma,P. and Aiba,H. (1998) CRP down-regulates adenylate cyclase activity by reducing the level of phosphorylated IIA(Glc), the glucose-specific phosphotransferase protein, in Escherichia coli. Mol. Gen. Genet., 259, 317–326. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Kimata,K., Inada,T., Tagami,H. and Aiba,H. (1999) Negative regulation of the pts operon by Mlc: mechanism underlying glucose induction in Escherichia coli. Genes Cells, 4, 391–399. [DOI] [PubMed] [Google Scholar]
- Zeppenfeld T., Larisch,C., Joseph,W., Lengeler,J.W. and Jahreis,K. (2000) Glucose transporter mutants of Escherichia coli K-12 with changes in substrate recognition of IICB(Glc) and induction behavior of the ptsG gene. J. Bacteriol., 182, 4443–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]